Abstract
Background
It is unclear whether obesity is a risk factor for postpartum hemorrhage. We hypothesized that obese women are at greater risk of postpartum hemorrhage than women with a normal body mass index.
Methods
We conducted a cohort study of women who underwent delivery hospitalization in California between 2008 and 2012. Using multilevel regression, we examined the relationships between body mass index with hemorrhage (primary outcome), atonic hemorrhage and severe hemorrhage (secondary outcomes). Stratified analyses were performed according to delivery mode.
Results
The absolute event rate for hemorrhage was 60,604/2,176 673 (2.8%). In our cohort, 4% were underweight, 49.1% were normal body mass index, 25.9% were overweight, and 12.7%, 5.2%, and 3.1% were in obesity class I, II, and III, respectively. Compared to normal body mass index women, the odds of hemorrhage and atonic hemorrhage were modestly increased for overweight women (hemorrhage: adjusted odds ratio (aOR) 1.06; 99% confidence interval (CI) 1.04–1.08); atonic hemorrhage: aOR 1.07; 99% CI 1.05–1.09) and obesity class I (hemorrhage: aOR 1.08; 99% CI 1.05–1.11; atonic hemorrhage; aOR 1.11; 99% CI 1.08–1.15). After vaginal delivery, overweight and obese women had up to 19% increased odds of hemorrhage or atonic hemorrhage. Whereas, after cesarean delivery, women in any obesity class had up to 14% decreased odds of severe hemorrhage.
Conclusion
Our findings suggest that, at most, maternal obesity has a modest effect on postpartum hemorrhage risk. The direction of the association between hemorrhage and body mass index may differ by delivery mode.
Keywords: obesity, hemorrhage, postpartum
Introduction
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality in the United States.1–3 Furthermore, between 1994 and 2006, the rate of postpartum hemorrhage increased by 26%.4 Well-known risk factors, including multiple gestation, polyhydramnios, placenta previa, and abruption only explain a small portion of the hazard.5 As part of regional and national efforts to reduce rates of severe peripartum morbidity,6–8 clarification of less well-established risk factors for postpartum hemorrhage is needed to advance risk assessment. Risk assessment is of important clinical relevance to obstetricians and anesthesiologists. Both sets of providers play key roles in the triage, management and peripartum care planning for women at risk for postpartum hemorrhage.
In the United States, the prevalence of maternal obesity has been steadily rising, with more than half of pregnant women classified as overweight or obese.9,10 A panel of obstetric experts have speculated that the rising prevalence of maternal obesity in developed countries may explain the increase in postpartum hemorrhage incidence, and have called for more research to examine whether obesity is a key risk factor.11 However, ongoing controversy surrounds the potential association between maternal body mass index with postpartum hemorrhage. Data from several population-based studies suggest that obese women are at increased risk of postpartum hemorrhage or atonic hemorrhage.12,13 In other studies, obesity is reported to have a protective effect14 or no association with postpartum hemorrhage.15–17
Postpartum hemorrhage risk is known to vary according to mode of delivery.18,19 However, the joint effect of obesity and mode of delivery on hemorrhage risk has not been previously explored and may clarify some aspects of the previously noted controversy. Tissue injury and surgical morbidity occur more commonly in obese women than non-obese women.20,21 These factors may contribute towards a greater risk of postpartum hemorrhage for obese women undergoing cesarean delivery than for women undergoing vaginal delivery with comparable body mass index. Therefore, the risk of postpartum hemorrhage may not be uniform in each body mass index class across all modes of delivery. Examining the individual and joint contributions of obesity and mode of delivery to postpartum hemorrhage would clarify the interplay between these two potentially important risk factors and possibly allow more tailored approaches to postpartum hemorrhage prevention and management.
In this study, our primary aim was to investigate the association between maternal body mass index with postpartum hemorrhage. We hypothesized that obese women are at greater risk of postpartum hemorrhage than women with a normal body mass index. As secondary aims, we performed exploratory analyses to examine the independent associations of maternal body mass index with atonic and severe postpartum hemorrhage and, to examine for the joint effects of maternal body mass index and delivery mode on hemorrhage risk.
Materials and Methods
We performed a retrospective cohort study, analyzing linked vital statistics birth data and hospital discharge data of women who underwent delivery hospitalizations in California between January 1, 2008 and December 31, 2012. The linked dataset allows for evaluation of prepregnancy body mass index data not available in hospital discharge data. Births that occurred in military hospitals, birth centers, or at home are not reported in state hospital discharge data, thus were excluded from the study cohort. We also excluded all terminations, deliveries <20 weeks’ gestation, and women with missing prepregnancy body mass index or birthdates. Stanford University institutional review board approved the study.
The exposure of interest was prepregnancy body mass index (hereafter referred to as maternal body mass index). Maternal body mass index was categorized using World Health Organization Internal Classification.22 Specifically, categories comprise: underweight (body mass index<18.5), normal body mass index (body mass index between 18.5 and 24.9), overweight (body mass index between 25 and 29.9), obese class I (body mass index between 30 and 34.9), obese class II (body mass index between 35 and 39.9), and obese class III (body mass index≥40). Units of measurement for body mass index were kg/m2.
The primary outcome measure was postpartum hemorrhage, which was identified in our dataset using International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) diagnosis codes 666.x. In our secondary analyses, we examined the associations between maternal obesity with atonic postpartum hemorrhage and severe postpartum hemorrhage. We then evaluated the effect of delivery mode on the each hemorrhage outcome measure. Atonic hemorrhage was classified by ICD-9-CM codes 666.1, and severe hemorrhage classified jointly by ICD-9-CM codes for postpartum hemorrhage and transfusion. Transfusion was identified by ICD-9-CM codes 99.0x.
Statistical Analysis
Statistical analysis was performed with SAS 9.3 (SAS Institute Inc, Cary, NC). Prior to data analyses, all study investigators reviewed the statistical plan for the primary and secondary study aims in August 2015. The study design and analytic plan was assessed by members of the Stanford Child Health Research Institute at Stanford University in September 2015.
We performed descriptive analyses to characterize the patient characteristics across increasing categories of body mass index. Cochran-Armitage trend test was used to assess the crude associations between each hemorrhage outcome with increasing BMI class. To assess the independent associations between each hemorrhage outcome with body mass index class, we performed multi-level mixed effects regression analyses adjusting for potential confounding variables as fixed effects: maternal age (<20, 20–24, 25–29, 30–34, 35–39, ≥40 years), race/ethnicity (Non-Hispanic White, Non-Hispanic Black; Non-Hispanic Asian; Hawaiian or Pacific-Islander; American Indian or Alaskan Native; Hispanic; Non-Hispanic Other), insurance (government-assisted; private; self-insured or other), highest educational level (less than high-school; high-school or graduate educational test; some college; college degree), trimester when prenatal care was commenced (first, second, third), parity (nulliparous vs. multiparous), and gestational age at delivery (20–31, 32–36, 37–40, ≥41 weeks), and mode of delivery, and the following ICD-9-CM diagnosis or procedure codes: chronic hypertension, pre-existing or gestational diabetes, multiple gestation, prior cesarean, labor prior to delivery, prolonged labor, induction of labor, chorioamnionitis, placental abruption, polyhydramnios, placenta previa, fibroids, and stillbirth (See Appendix for list of ICD-9-CM codes). Individual hospitals in California were accounted for as random effects in the multilevel model. As 5 different comparisons were made (underweight, overweight, obesity class I, II and III vs. normal body mass index) in each regression model, a conservative cut-off of P≤0.01 was chosen to minimize the chance of a type 1 error after multiple testing, with confidence intervals (CIs) of 99% to present odds ratios. In our primary analysis, we did not plan to adjust for hypertensive disorders of pregnancy (gestational hypertension, mild preeclampsia, severe preeclampsia or eclampsia) or diabetes (preexisting or gestational) because these conditions were assumed to be on the causal pathway between obesity and postpartum hemorrhage.
We examined models incorporating interaction terms to evaluate variation in the effect of body mass index class on the risk of postpartum hemorrhage, atonic hemorrhage, and severe hemorrhage according to mode of delivery. In the stratified models for vaginal and instrumental delivery, placenta previa and labor were not included as covariates because women with placenta previa invariably undergo cesarean delivery and labor precedes vaginal delivery. We also performed a sensitivity analysis to account for clustering according to the hospital where the delivery occurred using a generalized estimating equation. This approach averages the effect of body mass index class across all hospitals.
We did not perform a sample size estimation a priori. However, we performed power analysis after identifying our analytic sample and before formal data analysis. The power calculation was based on the number of women with normal body mass index (n=1,068,211) and obese class III women (n=66591) in the analytic sample, a minimum detectable and clinically relevant odds ratio of 2.0 for postpartum hemorrhage in obesity class III women compared to normal body mass index women, and an estimated postpartum hemorrhage prevalence of 2% among normal body mass index women. To address the problem of multiple testing, we applied a Bonferroni corrected alpha 0.05/5=0.01 (based on 5 body mass index classes being compared with a normal body mass index group). Based on these parameters, we determined that our analytic sample had adequate power (beta>0.999) and, therefore, was sufficiently large to detect a clinically relevant difference between study groups.
Results
A cohort flow diagram is presented in Figure 1. Of 2 475 786 women who underwent delivery hospitalization in California between 2008 and 2012, the final cohort consisted of 2 176 673 women. Deliveries occurred among 276 hospitals in California. We assumed that missing data were missing at random. The distribution of body mass index groups was: underweight (4%), normal body mass index (49.1%), overweight (25.9%), obese class I (12.7%), obese class II (5.2%), and obese class III (3.1%). There were significant differences in the distribution of patient characteristics across the body mass index groups that are described in Table 1. The absolute event rate for postpartum hemorrhage – our primary outcome - was 60,604/2,176 673 (2.8%). The overall frequency of atonic hemorrhage was 2.2% and severe hemorrhage was 0.4%. Figure 2 shows the rates of hemorrhage according to body mass index class. Rates of postpartum hemorrhage and severe postpartum hemorrhage were similar across all body mass index groups (P for trend>0.05, respectively), whereas rates of atonic hemorrhage differed across body mass index groups (P for trend=0.01).
Figure 1.
Flow Diagram
Table 1.
Patient Characteristics
| Characteristic | BMI<18.5 (n=86,252) |
BMI=18.5–25 (n=1,068,211) |
BMI=25–29.9 (n=564,495) |
BMI=30–34.9 n=277,306) |
BMI=35–39.9 (n=113,818) |
BMI≥40 (n=66,591) |
P value |
|---|---|---|---|---|---|---|---|
| Age (years) | <0.001 | ||||||
| < 20 | 12,760 (14.8%) | 105,585 (9.9%) | 41,996 (7.5%) | 16,877 (6.1%) | 5401 (4.8%) | 2229 (3.3%) | |
| 20–24 | 21,278 (24.6%) | 216,606 (20.3%) | 119,755 (21.2%) | 61,273 (22.1%) | 25,286 (22.2%) | 14,003 (21%) | |
| 25–29 | 21,529 (25%) | 272,278 (25.5%) | 156,544 (27.7%) | 79,216 (28.6%) | 34,018 (29.9%) | 21,095 (31.7%) | |
| 30–34 | 18,965 (22%) | 278,241 (26%) | 143,258 (25.4%) | 69,335 (25%) | 29,313 (25.7%) | 17,823 (26.8%) | |
| 35–39 | 9654 (11.2%) | 155,924 (14.6%) | 80,858 (14.3%) | 39,716 (14.3%) | 15,696 (13.8%) | 9235 (13.9%) | |
| ≥ 40 | 2066 (2.4%) | 39,577 (3.7%) | 22,084 (3.9%) | 10,889 (3.9%) | 4104 (3.6%) | 2206 (3.3%) | |
| Race | <0.001 | ||||||
| White Non-Hispanic | 25,457 (29.5%) | 347,983 (32.6%) | 137,628 (24.4%) | 60,848 (21.9%) | 27,568 (24.2%) | 16,799 (25.2%) | |
| Black Non-Hispanic | 4,842 (5.6%) | 50,530 (4.7%) | 32,780 (5.8%) | 18,507 (6.7%) | 9201 (8.1%) | 7809 (11.7%) | |
| Asian Non-Hispanic | 22,033 (25.5%) | 150,880 (14.1%) | 33,704 (6%) | 8727 (3.2%) | 1967 (1.7%) | 536 (0.8%) | |
| Hawaiian/Pacific Islander | 3,329 (3.9%) | 42,961 (4%) | 17,635 (3.1%) | 7302 (2.6%) | 2719 (2.4%) | 1384 (2.1%) | |
| Hispanic | 30,222 (35%) | 471,398 (44.1%) | 339,796 (60.2%) | 180,081 (64.9%) | 71,414 (62.7%) | 39,291 (59%) | |
| American Indian/Alaska Native | 305 (0.4%) | 3591 (0.4%) | 2,509 (0.4%) | 1642 (0.6%) | 878 (0.8%) | 725 (1.1%) | |
| Other | 64 (0.1%) | 868 (0.1%) | 443 (0.1%) | 199 (0.1%) | 71 (0.1%) | 47 (0.1%) | |
| Insurance | <0.001 | ||||||
| Government | 40,778 (47.3%) | 463,565 (43.4%) | 304,309 (53.9%) | 161,586 (58.3%) | 64,858 (57%) | 38,814 (58.3%) | |
| Private | 41,757 (48.4%) | 570,085 (53.4%) | 245,013 (43.4%) | 109,159 (39.3%) | 46,605 (40.9%) | 26,508 (39.8%) | |
| Self-insured/Other | 3717 (4.3%) | 34,561 (3.2%) | 15,173 (2.7%) | 6561 (2.4%) | 2355 (2.1%) | 1269 (1.9%) | |
| Education | <0.001 | ||||||
| Less than High School | 17,323 (20.1%) | 214,850 (20.1%) | 157,770 (28%) | 81,048 (29.2%) | 29,057 (25.6%) | 15,118 (22.7%) | |
| High school/GED | 22,098 (25.6%) | 248,563 (23.3%) | 152,889 (27.1%) | 83,226 (30%) | 36,648 (32.2%) | 22,722 (34.1%) | |
| Some college | 14,911 (17.3%) | 182,488 (17.1%) | 106,728 (18.9%) | 58,095 (21%) | 27132 (23.8%) | 17708 (26.6%) | |
| College degree(s) | 31,920 (37%) | 422,310 (39.5%) | 147,108 (26%) | 54,937 (19.8%) | 20981 (18.4%) | 11043 (16.6%) | |
| Onset of Prenatal Care | <0.001 | ||||||
| First Trimester | 40,778 (47.3%) | 463,565 (43.4%) | 304,309 (53.9%) | 227,612 (82.1%) | 93,302 (82%) | 54,284 (81.5%) | |
| Second Trimester | 41,757 (48.4%) | 570,085 (53.4%) | 245,013 (43.4%) | 40,204 (14.5%) | 16,762 (14.7%) | 9970 (15%) | |
| Third Trimester | 3,717 (4.3%) | 34,561 (3.2%) | 15,173 (2.7%) | 9,490 (3.4%) | 3754 (3.3%) | 2337 (3.5%) | |
| Gestational Age at Delivery (weeks) | <0.001 | ||||||
| < 32 | 891 (1%) | 9,705 (0.9%) | 6,062 (1.1%) | 3,558 (1.3%) | 1699 (1.5%) | 1076 (1.6%) | |
| 32–36+6 | 6,454 (7.5%) | 66,940 (6.3%) | 36,306 (6.4%) | 19,286 (6.9%) | 8502 (7.5%) | 5523 (8.3%) | |
| 37–40+6 | 73,499 (85.2%) | 908,960 (85.1%) | 478,860 (84.8%) | 234,303 (84.5%) | 95,331 (83.7%) | 55,314 (83.1%) | |
| ≥ 41 | 5,408 (6.3%) | 82,606 (7.7%) | 43,267 (7.7%) | 20,159 (7.3%) | 8286 (7.3%) | 4678 (7%) | |
| Parity | <0.001 | ||||||
| Nulliparous | 47,142 (54.7%) | 486,349 (45.5%) | 193,897 (34.4%) | 83,269 (30%) | 33,335 (29.3%) | 19,301 (29%) | |
| Multiparous | 39,110 (45.3%) | 581,862 (54.5%) | 370,598 (65.6%) | 194,037 (70%) | 80,483 (70.7%) | 47,290 (71%) | |
| Plurality | <0.001 | ||||||
| Singleton | 85,104 (98.7%) | 1,051,255 (98.4%) | 556,037 (98.5%) | 273045 (98.5%) | 111,885 (98.3%) | 65,368 (98.2%) | |
| Multiparous | 1148 (1.3%) | 16,956 (1.6%) | 8,458 (1.5%) | 4261 (1.5%) | 1933 (1.7%) | 1223 (1.8%) | |
| Chronic Hypertension | 398 (0.5%) | 8599 (0.8%) | 10,221 (1.8%) | 9111 (3.3%) | 6337 (5.6%) | 6536 (9.8%) | <0.001 |
| Gestational Hypertension | 923 (1.1%) | 16,743 (1.6%) | 13,790 (2.4%) | 9257 (3.3%) | 4894 (4.3%) | 3572 (5.3%) | <0.001 |
| Mild Preeclampsia | 953 (1.1%) | 14,863 (1.4%) | 11,444 (2.0%) | 7423 (2.7%) | 3720 (3.3%) | 2629 (3.9%) | <0.001 |
| Severe Preeclampsia or Eclampsia | 755 (0.9%) | 10,255 (1.0%) | 6962 (1.2%) | 4085 (1.5%) | 2029 (1.8%) | 1379 (2.1%) | <0.001 |
| Fibroids | 682 (0.8%) | 13,149 (1.2%) | 7937 (1.4%) | 4188 (1.5%) | 1838 (1.6%) | 1171 (1.8%) | <0.001 |
| Diabetes Mellitus | 133 (0.1%) | 3801 (0.4%) | 4885 (0.9%) | 4678 (1.7%) | 3054 (2.7%) | 2819 (4.2%) | <0.001 |
| Previous Cesarean | 7913 (9.2%) | 140,855 (13.2%) | 104,677 (18.5%) | 62,263 (22.4%) | 29164 (25.6%) | 20134 (30.2%) | <0.001 |
| Stillbirth | 58 (0.1%) | 808 (0.1%) | 444 (0.1%) | 227 (0.08%) | 107 (0.09%) | 69 (0.1%) | 0.04 |
| Previa | 604 (0.7%) | 7526 (0.7%) | 3651 (0.7%) | 1,606 (0.6%) | 585 (0.5%) | 302 (0.5%) | <0.001 |
| Prolonged Labor | 1171 (1.3%) | 13,852 (1.3%) | 5107 (0.9%) | 1,912 (0.7%) | 730 (0.6%) | 397 (0.6%) | <0.001 |
| Labor | 31,935 (37%) | 396,640 (37.1%) | 206,787 (36.6%) | 102,272 (36.9%) | 42168 (37.1%) | 25104 (37.7%) | <0.001 |
| Chorioamnionitis | 1801 (2.1%) | 24,976 (2.3%) | 12,615 (2.2%) | 5,532 (2%) | 2140 (1.9%) | 1198 (1.8%) | <0.001 |
| Abruption | 903 (1.1%) | 9,355 (0.9%) | 4699 (0.8%) | 2,356 (0.9%) | 978 (0.9%) | 544 (0.8%) | <0.001 |
| Polyhydramnios | 236 (0.3%) | 4087 (0.4%) | 2758 (0.5%) | 1,744 (0.6%) | 876 (0.8%) | 633 (0.9%) | <0.001 |
| Induction | 10,888 (12.6%) | 152,078 (14.2%) | 86,149 (15.3%) | 45,403 (16.4%) | 20,322 (17.9%) | 12,473 (18.7%) | <0.001 |
| Mode of Delivery | <0.001 | ||||||
| Vaginal | 61,438 (71.2%) | 721,708 (67.6%) | 355,529 (63%) | 161,243 (58.1%) | 59,932 (52.6%) | 29,791 (44.7%) | |
| Assisted Vaginal | 4887 (5.7%) | 46,242 (4.3%) | 16,840 (3%) | 6264 (2.3%) | 2119 (1.9%) | 1043 (1.6%) | |
| Cesarean | 19,927 (23.1%) | 300,261 (28.1%) | 192,126 (34%) | 109,799 (39.6%) | 51,767 (45.5%) | 35,757 (53.7%) |
Data presented as n (%)
BMI = body mass index; GED = graduate educational test.
Figure 2.
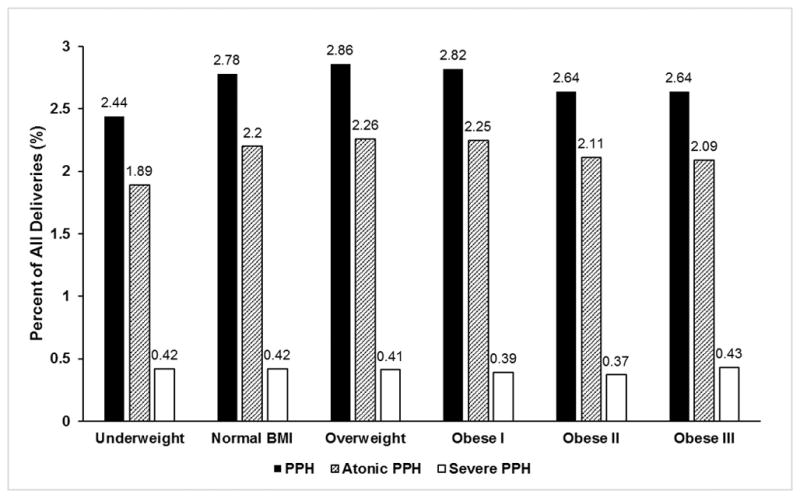
PPH = postpartum hemorrhage. Obesity class I, II, and III refer to World Health Organization obesity classes I, II, and III.
Table 2 presents the results of the main effects multivariable analyses assessing the independent effects of obesity on postpartum hemorrhage, atonic hemorrhage, and severe hemorrhage. Compared to women with a normal body mass index, the odds of postpartum hemorrhage and atonic hemorrhage were increased for overweight women (postpartum hemorrhage: adjusted odds ratio (aOR)=1.06; 99% (confidence intervals) CI=1.04 – 1.08); atonic hemorrhage: aOR=1.07; 99% CI=1.05 – 1.09) and for women with obesity class I (postpartum hemorrhage: aOR=1.08; 99% CI=1.05 – 1.11; atonic hemorrhage; aOR=1.11; 99% CI=1.08–1.15). However, women with obesity class II and III were not at increased risk of postpartum hemorrhage or atonic hemorrhage, whereas being underweight was associated with a reduced odds of postpartum hemorrhage (aOR=0.92; 99% CI=0.87–0.96) and atonic hemorrhage (aOR=0.89; 99% CI=0.84–0.94). Being overweight or obese class I, II, or III was associated with a reduced odds of severe postpartum hemorrhage (e.g., for obesity class III, severe PPH aOR=0.85; 99% CI=0.76 – 0.97). We explored whether adjustment for diabetes and hypertensive disorders of pregnancy further influenced the relations between body mass index class and postpartum hemorrhage (data presented in the Appendix). Addition of these covariates further modestly attenuated the associations between overweight (aOR=1.04; 99% CI=1.01–1.07) and obesity class I (aOR=1.05; 99% CI=1.01–1.09) with postpartum hemorrhage suggesting potential association of these comorbidities with enhancement in hemorrhage risk. We also ran a generalized estimating equation model for our primary outcome to assess whether the point estimates differed compared to those obtained in our mixed effects logistic regression model. The results of this model clustering on hospital were very similar to those of our main findings (data not presented), with no substantial changes in the strength or direction of association between body mass index classes with postpartum hemorrhage.
Table 2.
Results of the Multilevel Logistic Regression Analyses Showing the Relation Between Maternal Body Mass Index and Postpartum Hemorrhage Outcomes.
| PPH* | Atonic PPH* | Severe PPH* | |
|---|---|---|---|
| BMI | Adjusted OR (99% CI) | Adjusted OR (99% CI) | Adjusted OR (99% CI) |
| <18.5 | 0.92 (0.87 – 0.96) | 0.89 (0.84 – 0.94) | 1.05 (0.94 – 1.17) |
| 18.5 – 24.9 | Reference | Reference | Reference |
| 25 – 29.9 | 1.06 (1.04 – 1.08) | 1.07 (1.05 – 1.09) | 0.94 (0.89 – 0.99) |
| 30 – 34.9 | 1.08 (1.05 – 1.11) | 1.11 (1.08 – 1.15) | 0.85 (0.8 – 0.91) |
| 35 – 39.9 | 1.01 (0.97 – 1.05) | 1.04 (1.0 – 1.09) | 0.78 (0.71 – 0.87) |
| ≥ 40 | 1.01 (0.96 – 1.07) | 1.03 (0.98 – 1.09) | 0.85 (0.76 – 0.97) |
BMI = body mass index; PPH = postpartum hemorrhage; OR = odds ratio
Hierarchical models adjusted for Maternal Age, Race/Ethnicity, Insurance, Education, Chronic Hypertension, Trimester when Prenatal Care was initiated, Gestational Age at Delivery, Parity, Plurality, Previous cesarean section, Labor, Prolonged Labor, Induction of Labor, Chorioamnionitis, Placental Abruption, Polyhydramnios, Previa, Fibroids, Stillbirth, Mode of Delivery; Hospital site was considered as a random effect, to account for patient clustering.
Because we observed evidence of interaction between obesity and mode of delivery (P<0.2) in models with postpartum hemorrhage, atonic hemorrhage, and severe hemorrhage as dependent variables, we performed stratified analyses according to delivery mode (Table 3). The study cohort comprised 1 389 641 (63.8%) vaginal deliveries, 77 395 (3.6%) instrumental deliveries, and 709 637 (32.6%) cesarean deliveries. The presence, strength, and magnitude of the relations between body mass index class and each hemorrhage outcome differed according to delivery mode. Among those undergoing vaginal delivery, overweight and women of any class of obesity class had up to 19% increased odds of postpartum hemorrhage or atonic hemorrhage compared to normal body mass index women. In contrast, no associations were found between being overweight and any obesity class status with postpartum hemorrhage, atonic hemorrhage, or severe hemorrhage in the instrumental delivery cohorts. In the cesarean delivery cohort, belonging to obesity class III had a 13 % decreased odds of atonic hemorrhage compared to normal body mass index women. The odds of severe hemorrhage were even lower for obesity class I (aOR=0.77; 99% CI=0.67–0.87), class II (aOR=0.76; 99% CI=0.64–0.91), and class III (aOR=0.76; 99% CI=0.62–0.94) compared to normal body mass index women.
Table 3.
Adjusted Odds Ratios for the Relation Between Maternal Body Mass Index and Postpartum Hemorrhage Outcomes, Stratified by Mode of Delivery
| PPH | Atonic PPH | Severe PPH | ||||
|---|---|---|---|---|---|---|
| Vaginal Delivery* | ||||||
| BMI | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) |
| <18.5 | 1504 (2.4%) | 0.92 (0.86 – 0.99) | 1164 (1.9%) | 0.90 (0.83 – 0.97) | 203 (0.3%) | 1.1 (0.91 – 1.33) |
| 18.5 – 24.9 | 20,432 (2.8%) | Reference | 16,036 (2.2%) | Reference | 2173 (0.3%) | Reference |
| 25 – 29.9 | 11,090 (3.1%) | 1.09 (1.06 – 1.13) | 8720 (2.4%) | 1.1 (1.06 – 1.14) | 1050 (0.3%) | 0.97 (0.88 – 1.08) |
| 30 – 34.9 | 5325 (3.3%) | 1.16 (1.12 – 1.21) | 4233 (2.6%) | 1.19 (1.14 – 1.25) | 479 (0.3%) | 0.97 (0.85 – 1.11) |
| 35 – 39.9 | 1953 (3.3%) | 1.12 (1.05 – 1.19) | 1524 (2.5%) | 1.13 (1.05 – 1.21) | 151 (0.2%) | 0.81 (0.65 – 1.01) |
| ≥ 40 | 1042 (3.5%) | 1.15 (1.06 – 1.25) | 805 (2.7%) | 1.15 (1.05 – 1.27) | 99 (0.3%) | 1.02 (0.78 – 1.34) |
| Instrumental Delivery* | ||||||
| BMI | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) |
| <18.5 | 177 (3.6%) | 0.96 (0.81 – 1.12) | 134 (2.7%) | 0.85 (0.67 – 1.08) | 36 (0.8%) | 1.23 (0.78 – 1.97) |
| 18.5 – 24.9 | 1913 (4.1%) | Reference | 1585 (3.4%) | Reference | 291 (0.6%) | Reference |
| 25 – 29.9 | 743 (4.4%) | 1.09 (0.99 – 1.19) | 617 (3.7%) | 1.1 (0.97 – 1.25) | 100 (0.6%) | 0.91 (0.67 – 1.23) |
| 30 – 34.9 | 274 (4.4%) | 1.13 (0.99 – 1.3) | 223 (3.6%) | 1.12 (0.92 – 1.37) | 38 (0.6%) | 0.91 (0.58 – 1.44) |
| 35 – 39.9 | 80 (3.8%) | 0.97 (0.77 – 1.23) | 70 (3.3%) | 1.05 (0.75 – 1.45) | 12 (0.6%) | 0.84 (0.39 – 1.82) |
| ≥ 40 | 54 (5.2%) | 1.29 (0.96 – 1.72) | 42 (4.0%) | 1.22 (0.79 – 1.86) | 9 (0.9%) | 1.32 (0.54 – 3.24) |
| Caesarean Delivery* | ||||||
| BMI | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) | N (%) | Adjusted OR (99% CI) |
| <18.5 | 333 (1.7%) | 0.96 (0.78 – 1.18) | 127 (0.6%) | 0.88 (0.76 – 1.02) | 98 (0.5%) | 0.94 (0.74 – 1.19) |
| 18.5 – 24.9 | 5910 (2.0%) | Reference | 2048 (0.7%) | Reference | 1605 (0.5%) | Reference |
| 25 – 29.9 | 3427 (1.8%) | 1.09 (0.97 – 1.23) | 1186 (0.6%) | 0.99 (0.93 – 1.05) | 902 (0.5%) | 0.91 (0.83 – 1.00) |
| 30 – 34.9 | 1782 (1.6%) | 1.13 (0.95 – 1.36) | 553 (0.5%) | 0.95 (0.88 – 1.02) | 415 (0.4%) | 0.77 (0.67 – 0.87) |
| 35 – 39.9 | 809 (1.6%) | 0.97 (0.71 – 1.32) | 257 (0.5%) | 0.91 (0.82 – 1.00) | 200 (0.4%) | 0.76 (0.64 – 0.91) |
| ≥ 40 | 543 (1.5%) | 1.28 (0.88 – 1.88) | 180 (0.5%) | 0.87 (0.79 – 0.98) | 134 (0.4%) | 0.76 (0.62 – 0.94) |
BMI = body mass index; PPH = postpartum hemorrhage; aOR = adjusted odds ratios
Rates of PPH were row percentages with the denominator based on the number of women in each body mass index class. Models adjusted for maternal age, race/ethnicity, insurance, education, chronic hypertension, trimester when prenatal care was initiated, gestational age at delivery, parity, plurality, previous cesarean delivery, prolonged labor, induction of labor, chorioamnionitis, placental abruption, polyhydramnios, fibroids, stillbirth. Hospital site was considered as a random effect, to account for patient clustering within site. Labor and placenta previa were also adjusted for in the models for cesarean delivery.
Discussion
In this large population-based cohort study, we observed only a very small effect of maternal body mass index on the risk of postpartum hemorrhage. We did not find strong evidence of positive ‘dose-response’ relationships between body mass index class with postpartum hemorrhage, atonic hemorrhage, or severe hemorrhage. These findings suggest that maternal obesity is not an important risk factor for postpartum hemorrhage.
There is a notable lack of clarity in the association between body mass index class and postpartum hemorrhage reported in observational studies.11 In a number of population-based studies from Denmark, Canada, Finland, and the United States comparing perinatal outcomes between obese and non-obese women with singleton pregnancies, obesity was not associated with postpartum hemorrhage.15–17,23 Data from other studies suggest that obese women are at reduced risk of hemorrhage and morbidity. Among 743 630 pregnant women who delivered in Washington State between 2004 and 2013, obese class III had a 30% decreased odds of severe PPH compared with normal body mass index.24 In a single-center study, Paglia et al. reported that non-obese women had a 1.8-fold increased odds of severe hemorrhage compared to obese women.14 Butwick et al. reported that, among women experiencing uterine atony during cesarean delivery, obese women were at reduced risk of hemorrhage-related morbidity compared to non-obese women.25 These findings are consistent with the inverse association we observed between maternal obesity and severe hemorrhage, especially among women undergoing cesarean delivery.
In contrast to our main findings, two population-wide studies have reported a positive association between obesity and postpartum hemorrhage. In a study examining 1,114,071 Swedish women with singleton pregnancies, the risk of atonic hemorrhage was increased by 14%, 47%, and 114% in women from obesity classes I, II, and III, respectively compared to non-obese women.12 In a Japanese study of 97, 157 women with singleton pregnancies, obese women had 1.1-fold and 1.9-fold increased risk of postpartum hemorrhage compared to non-obese women after vaginal and cesarean delivery, respectively.13 Residual confounding instead of a true effect of obesity may explain at least part of the increased risk of hemorrhage observed in these studies. In our study, maternal obesity was more prevalent and we accounted for a larger set of relevant confounders to provide more clarity about the associations between maternal obesity and postpartum hemorrhage. Although we had adequate power to detect a minimum odds ratio of 2, the point estimates in our primary analysis were substantially lower despite being statistically significant for overweight and obesity class I women. These findings suggest that the relations between maternal BMI and PPH are of questionable clinical significance. We acknowledge that differences between medical care systems and practices, racial compositions, and national obesity rates may also explain why the reported associations between body mass index class and postpartum hemorrhage vary across different populations.
The small effect of body mass index on postpartum hemorrhage risk has important clinical ramifications. Risk assessment is a critical aspect of postpartum hemorrhage prevention. By identifying at-risk patients, providers can ensure that adequate resources and staff are available to manage hemorrhage prior to delivery. This key aspect of care is described in the consensus bundle for obstetric hemorrhage published by The National Partnership for Maternal Safety.8 Similarly, in their latest postpartum hemorrhage guidelines, the American College of Obstetricians and Gynecologists suggest that a risk assessment tool be considered.26 Well-established risk factors, such as chorioamnionitis, multiple gestation, and hypertensive disorders of pregnancy,18,19,27 are likely to be considered for inclusion in these tools. However, based on our findings, consideration of obesity in these risk assessment tools may not be warranted.
Several potential explanations can be offered for the small effect of body mass index on postpartum hemorrhage risk and the reduced risk of severe hemorrhage. Firstly, the findings of our sensitivity analysis suggest that effect of obesity may be partly influenced by the presence of diabetes and/or hypertensive disorders. The addition of these factors to our regression models modestly attenuated the weak positive association between obesity and postpartum hemorrhage. Secondly, uterine atony is recognized as the leading etiology for postpartum hemorrhage.18,19 In our study, we observed no monotonic increase in the rate of atonic hemorrhage with increasing body mass index. This may be because uterine contractility or oxytocin signaling does not differ markedly across body mass index classes. Data from in-vitro studies suggest that no differences in contraction strength or frequency in myometrial strips exist between obese vs non-obese women,28 and oxytocin receptor gene and protein expression in myometrial strips are not related to maternal body mass index.29 However, other work suggests that myometrial samples taken from obese women contract with less force and less frequently compared to those from non-obese women.30 Thirdly, compared to non-obese patients, obese patients have a hypercoagulable state (manifest by higher plasma fibrinogen, factor VII, factor VIII, von Willebrand factor, and plasminogen activator inhibitor levels),31 which may mitigate the severity of blood loss and the need for transfusion during a major bleed. Fourthly, because obese women have larger blood volumes than non-obese women,32 patients may tolerate more blood loss (assuming isovolemic hemodilution) before reaching a transfusion trigger or experiencing the consequences of hemorrhagic shock.
Our study has several strengths. In this large population based study, which included more than 2 million women, we were able to characterize a diverse obstetric cohort to study an outcome with a low prevalence. The overall prevalences of postpartum hemorrhage, atonic hemorrhage, and severe hemorrhage are in line with those reported in other population-wide studies.4,5,19 Through the use of linked administrative discharge data and vital statistics data, we had access to detailed patient information, including prepregnancy body mass index, to examine the association between maternal obesity and postpartum hemorrhage. Prior epidemiological studies of postpartum hemorrhage in the United States lack maternal body mass index data.4,5,19 Our study has a number of limitations. A potential limitation is the accuracy and validity of maternal discharge data and birth certificate data for classifying key variables. Although postpartum hemorrhage was classified using administrative data, validation studies report high positive predictive values (>80%) for ICD-9 codes.33,34 Several studies have assessed the reliability of body mass index data on birth certificates. In a study comparing birth certificate to medical record data in 1204 births, Bodnar et al. demonstrated good agreement in the prepregnancy body mass index categorization for normal body mass index, overweight, and obese class 2 and 3 women.35 Using data from the Women, Infants, and Children Program in Florida, Park et al. also found that prepregnancy weight, height, and body mass index from birth certificates are reliable and are valid for use in population-based studies.36 Chen et al., using data from the 1988 National Maternal and Infant Health Survey, observed a high correlation (0.9) between self-reported and recorded prepregnancy body mass index data.37 We did not account for weight gain during pregnancy. Because gestational weight gain decreases with increasing body mass index,38 this may partially explain why the differences in postpartum hemorrhage risk were modest between obese and normal body mass index women. Further studies are needed to examine the influence of gestational weight gain on hemorrhage risk. Prolonged labor and induction of labor may be on the causal pathway between maternal obesity and postpartum hemorrhage, therefore adjustment for these variables may have influenced the strength of our observed associations. We could not account for residual confounders in our analysis, such as exposure to antenatal anemia, antenatal anticoagulation, mode of analgesia or anesthesia. Therefore, residual confounding may partially explain the weak positive association between being overweight and obesity class I with postpartum hemorrhage. In our stratified analyses, variation in the size of each delivery cohort and resultant type 2 error may explain the differential findings according to delivery mode. Because blood loss data are not captured in our datasource, we used transfusion codes as a proxy for severe hemorrhage, an approach also used in prior population-wide studies.1,2,39 We could not determine whether transfusion was given during the acute period of active blood loss or for treating postpartum anemia after arrest of bleeding. Therefore, it is unclear whether transfusion is a marker of bleeding severity versus anemia severity after postpartum hemorrhage.
In summary, our findings demonstrate that obesity is not a strong risk factor for postpartum hemorrhage. A detrimental effect on postpartum hemorrhage from obesity is likely to be much lower than previously reported in selected analyses and may only have modest clinical relevance.
Summary Statement.
Our study observed a minor increase in the risk of postpartum hemorrhage associated with being overweight and class I obesity. This association is much weaker than previously reported and has uncertain clinical relevance.
Acknowledgments
Funding Statement: This work was supported by funding from the Child Health Research Institute, Lucile Packard Foundation for Children’s Health and the Stanford Clinical and Translational Science Award (grant number: UL1 TR001085). A.J.B. is also supported by an award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD070972). Dr Stephansson is supported by the Swedish Research Council (2013-2429) and the Strategic Research Program in Epidemiology at Karolinska Institutet, Stockholm, Sweden.
APPENDIX
Table 1.
ICD-9-CM codes used to identify diagnoses and procedures
| Chronic Hypertension = 401.x-405.x; 642.0x-642.2x; 642.7x; 642.9x |
| Diabetes = 249.xx-250.xx, 648.0x |
| Multiple gestation = V27.2–V27.8, 651.x |
| Prior Cesarean = 654.2x |
| Labor prior to delivery = 644.x, 656.3x, 660–663.x, 665.1x, |
| Prolonged Labor = 662.x |
| Induction of Labor = 73.01; 73.1; 73.4, 659.0x-659.1x |
| Chorioamnionitis = 658.4x |
| Placental Abruption = 641.2x |
| Polyhydramnios = 657.x |
| Placenta Previa = 641.0x-641.1x |
| Fibroids = 218.x, 654.1x |
| Stillbirth = V27.1, V27.3, V27.4, V27.6, V27.7 |
Table 2.
Multilevel Logistic Regression Analysis Showing the Relation Between Maternal Body Mass Index and Postpartum Hemorrhage including Diabetes and Hypertensive Disorders of Pregnancy as Co-Variates.
| PPH* | |
|---|---|
| BMI | Adjusted OR (99% CI) |
| <18.5 | 0.92 (0.87 – 0.98) |
| 18.5 – 24.9 | Reference |
| 25 – 29.9 | 1.04 (1.01 – 1.07) |
| 30 – 34.9 | 1.05 (1.01 – 1.09) |
| 35 – 39.9 | 0.97 (0.92 – 1.02) |
| ≥ 40 | 0.96 (0.9 – 1.03) |
BMI = body mass index; PPH = postpartum hemorrhage; OR = odds ratio
Hierarchical model adjusted for Maternal Age, Race/Ethnicity, Insurance, Education, Chronic Hypertension, Trimester when Prenatal Care was initiated, Gestational Age at Delivery, Parity, Multiple Gestation, Previous cesarean section, Labor, Prolonged Labor, Induction of Labor, Chorioamnionitis, Placental Abruption, Polyhydramnios, Previa, Fibroids, Diabetes (pre-existing or gestational), Gestational hypertension, Pre-eclampsia, Stillbirth, Mode of Delivery; Hospital site was considered as a random effect, to account for patient clustering.
Footnotes
Prior Presentation: Presented at the best research paper session at the 49th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology, Seattle, May 2017.
Conflicts of Interests: The authors declare no competing interests.
References
- 1.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Meikle SF, Jamieson DJ, Whiteman MK, Barfield WD, Hillis SD, Posner SF. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353.e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, Joseph KS. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449.e1–7. doi: 10.1016/j.ajog.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123:978–81. doi: 10.1097/AOG.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123:973–7. doi: 10.1097/AOG.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 8.Main EK, Goffman D, Scavone BM, Low LK, Bingham D, Fontaine PL, Gorlin JB, Lagrew DC, Levy BS National Parternship for Maternal S, Council for Patient Safety in Women’s Health C. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Anesth Analg. 2015;121:142–8. doi: 10.1097/AOG.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 9.Branum AM, Kirmeyer SE, Gregory EC. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65:1–11. [PubMed] [Google Scholar]
- 10.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, Joseph KS, Lewis G, Liston RM, Roberts CL, Oats J, Walker J. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118:561–8. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto K, Aoki S, Toma R, Fujiwara K, Sakamaki K, Hirahara F. Pregnancy Outcomes Based on Pre-Pregnancy Body Mass Index in Japanese Women. PLoS One. 2016;11:e0157081. doi: 10.1371/journal.pone.0157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paglia MJ, Grotegut CA, Johnson LN, Thames B, James AH. Body mass index and severe postpartum hemorrhage. Gynecol Obstet Invest. 2012;73:70–4. doi: 10.1159/000329335. [DOI] [PubMed] [Google Scholar]
- 15.Kim SS, Zhu Y, Grantz KL, Hinkle SN, Chen Z, Wallace ME, Smarr MM, Epps NM, Mendola P. Obstetric and Neonatal Risks Among Obese Women Without Chronic Disease. Obstet Gynecol. 2016;128:104–12. doi: 10.1097/AOG.0000000000001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118:305–12. doi: 10.1097/AOG.0b013e3182245d49. [DOI] [PubMed] [Google Scholar]
- 17.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125:133–43. doi: 10.1097/AOG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 19.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 20.Alanis MC, Villers MS, Law TL, Steadman EM, Robinson CJ. Complications of cesarean delivery in the massively obese parturient. Am J Obstet Gynecol. 2010;203:271.e1–7. doi: 10.1016/j.ajog.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 21.Girsen AI, Osmundson SS, Naqvi M, Garabedian MJ, Lyell DJ. Body mass index and operative times at cesarean delivery. Obstet Gynecol. 2014;124:684–9. doi: 10.1097/AOG.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization; Organization WH, editor. Report of a WHO consultation, World Health Organ Technical Report Series. Geneva: 2000. Obesity: preventing and managing the global epidemic; pp. i–xii.pp. 1–253. [PubMed] [Google Scholar]
- 23.Pallasmaa N, Ekblad U, Aitokallio-Tallberg A, Uotila J, Raudaskoski T, Ulander VM, Hurme S. Cesarean delivery in Finland: maternal complications and obstetric risk factors. Acta Obstet Gynecol Scand. 2010;89:896–902. doi: 10.3109/00016349.2010.487893. [DOI] [PubMed] [Google Scholar]
- 24.Lisonkova S, Muraca GM, Potts J, Liauw J, Chan WS, Skoll A, Lim KI. Association Between Prepregnancy Body Mass Index and Severe Maternal Morbidity. JAMA. 2017;318:1777–1786. doi: 10.1001/jama.2017.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butwick AJ, Carvalho B, El-Sayed YY. Risk factors for obstetric morbidity in patients with uterine atony undergoing caesarean delivery. Br J Anaesth. 2014;113:661–8. doi: 10.1093/bja/aeu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American College of Obstetericians and Gynecologists. ACOG Practice Bulletin No 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168–e186. doi: 10.1097/AOG.0000000000002351. [DOI] [PubMed] [Google Scholar]
- 27.Mehrabadi A, Hutcheon JA, Lee L, Kramer MS, Liston RM, Joseph KS. Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG. 2013;120:853–62. doi: 10.1111/1471-0528.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins CA, Martin W, Anderson L, Blanks AM, Norman JE, McConnachie A, Nelson SM. Maternal obesity and its relationship with spontaneous and oxytocin-induced contractility of human myometrium in vitro. Reprod Sci. 2010;17:177–85. doi: 10.1177/1933719109349780. [DOI] [PubMed] [Google Scholar]
- 29.Grotegut CA, Gunatilake RP, Feng L, Heine RP, Murtha AP. The influence of maternal body mass index on myometrial oxytocin receptor expression in pregnancy. Reprod Sci. 2013;20:1471–7. doi: 10.1177/1933719113488446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114:343–8. doi: 10.1111/j.1471-0528.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- 31.Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez JS, Alexander JM, Sarode R, McIntire DD, Leveno KJ. Calculated blood loss in severe obstetric hemorrhage and its relation to body mass index. Am J Perinatol. 2012;29:557–60. doi: 10.1055/s-0032-1310528. [DOI] [PubMed] [Google Scholar]
- 33.Lain SJ, Roberts CL, Hadfield RM, Bell JC, Morris JM. How accurate is the reporting of obstetric haemorrhage in hospital discharge data? A validation study. Aust N Z J Obstet Gynaecol. 2008;48:481–4. doi: 10.1111/j.1479-828X.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 34.Romano PS, Yasmeen S, Schembri ME, Keyzer JM, Gilbert WM. Coding of perineal lacerations and other complications of obstetric care in hospital discharge data. Obstet Gynecol. 2005;106:717–25. doi: 10.1097/01.AOG.0000179552.36108.6d. [DOI] [PubMed] [Google Scholar]
- 35.Bodnar LM, Abrams B, Bertolet M, Gernand AD, Parisi SM, Himes KP, Lash TL. Validity of birth certificate-derived maternal weight data. Paediatr Perinat Epidemiol. 2014;28:203–12. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC program: Florida, 2005. Matern Child Health J. 2011;15:851–9. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- 37.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20:74–81. doi: 10.1097/EDE.0b013e3181878645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu SY, Callaghan WM, Bish CL, D’Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol. 2009;200:271.e1–7. doi: 10.1016/j.ajog.2008.09.879. [DOI] [PubMed] [Google Scholar]
- 39.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199:133.e1–8. doi: 10.1016/j.ajog.2007.12.020. [DOI] [PubMed] [Google Scholar]



