Abstract
Background
In developing countries, malnutrition remains a common clinical syndrome at antiretroviral treatment (ART) initiation. Physiological changes due to malnutrition and during nutritional recovery could affect the pharmacokinetics of antiretroviral drugs.
Methods
HIV-infected children admitted with severe acute malnutrition were randomised to early or delayed initiation of lopinavir/ritonavir, abacavir and lamivudine using WHO weight-band dosage charts. Lopinavir concentrations were measured on day 1 and day 14. Thereafter patients were followed-up to week 48. The population pharmacokinetics of lopinavir was described using NONMEM v7.3. Covariates were screened to assess their influence on the pharmacokinetics of lopinavir and the relationship between pharmacokinetic variability and treatment outcomes were assessed.
Results
502 lopinavir concentrations were collected from 62 pediatric patients aged 0.1-3.9 years (median: 0.9 years). Rifampin-based ant-ituberculosis treatment and “super-boosted” lopinavir/ritonavir was prescribed in 20 patients. Lopinavir disposition was well described by a one-compartment model with first order elimination. Neither randomization to early or delayed ART, tuberculosis co-medications nor anthropometrical measurements explained the pharmcokinetic variability. Allometrically scaled fat-free-mass (FFM) influenced apparent clearance (CL/F) and volume of distribution (Vd/F). Pharmacokinetic exposure did not correlate with virologic outcomes or death at 12 or 48 weeks.
Conclusions
Lopinavir pharmacokinetics was influenced by FFM and not by timing of ART initiation or tuberculosis co-medication in severely malnourished HIV-infected children. Lopinavir pharmacokinetics was found to be highly variable and bioavailability greatly reduced, resulting in a high CL estimate in this population. The role of lopinavir dose adjustment should be further evaluated in severely malnourished children initiating ART.
Keywords: Lopinavir, Population pharmacokinetics, Severe acute malnutrition, HIV, Children
Malnutrition is common at initial HIV diagnosis in sub-Saharan African children (1,2) and is a significant risk factor for mortality (2). The causes of malnutrition in this setting are multifactorial including delay in HIV diagnosis and antiretroviral treatment (ART) with resultant increased energy expenditure and basal metabolic rate together with higher rates of opportunistic infections, diarrhea, malabsorption, food insecurity and poverty (3,4).
Severely malnourished HIV-infected children experience impaired immunologic and virologic responses and higher mortality (5,6) compared to their non-malnourished counterparts despite nutritional rehabilitation and ART (7,8). Altered pharmacokinetics of antiretroviral medications in malnourished children may be an important contributor to these poorer outcomes.
The physiologic characteristics of malnutrition and changes following nutritional recovery are particularly dynamic due to shifts in oxidative stressors, lean body mass, serum albumin levels, intestinal function and degrees of mitochondrial, hepatic or renal dysfunction (10,11). These physiological alteration may affect the pharmacokinetics of medications in malnourished children resulting in increased adverse events due to supra-therapeutic drug levels or sub-therapeutic antiretroviral drug levels, which compromise virologic efficacy and contribute to HIV drug resistance (12).
The most extensively studied antiretroviral drug in malnourished children is nevirapine (13). Malnutrition had no effect on total or unbound plasma nevirapine exposures in Malawian children, of whom 32% had mild to moderate malnutrition (13). Other studies found lower nevirapine concentrations in stunted children compared with non-stunted children (12,14). Efavirenz and lopinavir (LPV) exposures were reduced in Ugandan children, 48% of whom were malnourished, compared to historical data from children in resource-rich countries (15).
Overall, the impact of severe malnutrition on the pharmacokinetics of antiretroviral drugs is difficult to predict as reductions in drug absorption and elimination can produce opposite effects (10). There is no data evaluating the use of different ART regimens or strategies in severely malnourished HIV-infected children. This study aimed to describe the effects of nutritional rehabilitation on LPV pharmacokinetics in severely malnourished HIV-infected children and to explore the relationship between LPV pharmacokinetic exposure and virologic outcomes.
METHODS
Participants
Between June 2012 and December 2015, 82 newly diagnosed HIV-infected infants and children (ages one month to 12 years) admitted with severe acute malnutrition (weight-for-length Z-scores (WHZ) < −3, mid-upper arm circumference (MUAC) < 115 mm or peripheral edema) were enrolled in the MATCH (Malnutrition and ART Timing in Children with HIV) Study. The study was conducted at King Edward VIII Hospital, an urban referral hospital with a 100-bed pediatric unit in Durban, South Africa. The sample size was calculated to detect a mean difference in nutritional recovery between the two arms 0·5 Z–score in WHZ with a power of 80% and a significance level of 5% at 48 weeks. Of the 82 children enrolled in the MATCH study, 63 children had a LPV pharmacokinetic evaluation performed (10 children were not on LPV/rtv; 9 children had unsuitable samples).
All patients were managed with standardized re-feeding guidelines based on the WHO Guidelines for the inpatient treatment of severely malnourished children (16). Medical management of patients was at the discretion of the treating clinician.
Patients were randomized to either initiate ART within 14 days from admission (early arm) or delay ART initiation (delayed arm) until nutritional recovery (WHZ of –2, achieved at least 15% weight gain or demonstrated resolution of edema and return of appetite) and more than 14 days from admission. A pre–determined computer generated randomization table was used, weighted to ensure equal numbers of patients with tuberculosis (TB) in each arm.
Antiretroviral treatment was administered as per the South African national ART guidelines (2012-2015) (17). Children less than age three years or less than 10kgs received abacavir, lamivudine and liquid formulation of lopinavir/ritonavir (LPV/rtv). Drug were dosed according to country specific guidelines and WHO weight band-based dosage charts; drugs were obtained from the South African National ART program. Patients requiring rifampicin-containing anti-tuberculosis treatment received “super-boosted” LPV/rtv (LPV:rtv ratio of 1:1) as per the weight band dosage table (17). Adherence to medication during hospitalization was verified by review of the hospital prescription chart.
Written informed consent was obtained from the caregivers of all children enrolled in the MATCH study (Clinical trial registry number: PACTR 21609001751384). The trial was approved by the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal (BFC126/11), King Edward VIII Hospital and the Kwa-Zulu Natal Department of Health.
Samples and Assay
Blood samples were drawn on the day of ART initiation and day 14 post ART initiation according to the following sampling schedules; on day 1, samples were drawn at the following nominal time points: 1.3 - 1.8 hrs post dose, 3 – 4 hrs post dose, 5 - 7 hrs post dose and 8 – 10 post dose. On day 14, one sample was drawn 30 minutes prior to dosing (measurement related to the 2nd dose on day 13), and 1.3 - 1.8 hrs post dose, 3 – 4 hrs post dose, 5 - 7 hrs post dose and 8 - 10 hrs post dose. Exact sampling times post dose were recored and used in the analysis. Whole blood was transported to the laboratory on ice within an hour of being drawn and centrifuged at 2,000 rpm for 10 minutes using a refrigerated centrifuge. Plasma (500uL) was aliquoted into cryotubes and stored at −70 °C before being shipped on dry ice for measurement of drug concentrations. Blood samples were analysed for LPV using a validated liquid chromatography-mass spectrometry method as described previously (18). The lower limit of quantification (LoQ) for LPV was 0.0195ug/mL. HIV viral loads (Cobas Ampliprep/ Cobas TaqMan system supplied by Roche) were measured at 12 and 48 weeks following study entry. Treatment failure was defined as death or viral load (VL) >1000 copies/mL (17).
Pharmacokinetic Modeling
The population modeling was conducted using NONMEM® version 7.3 [19], Intel FORTRAN compiler and PsN® version 4.1 [20]. Structural model parameter estimates, inter-individual variability (IIV) and residual unexplained variability (RUV) were obtained by first-order conditional estimation with interaction (FOCE+I). The pharmacokinetic structural base model for LPV was initially explored followed by stochastic model evaluation, covariate model development and model evaluation steps. The IIV was modeled exponentially and inter-occasion variability (IOV) was modeled by an additional random effects parameter, as described previously (19).
where Pjj represents the estimate of a parameter P for subject i on occasion j about the typical population value (Ppop). Parameter i,P is a random variable distributed with a mean value of 0 and variance of 2P which represents the IIV variability of P in the population. Parameter К is a random variable, was assumed to be sampled from a normal distribution of mean value 0 and a variance of π2, representing the variability of P on different occasions. An occasion was defined as a dose followed by at least one observation. Here the maximum possible number of occasions per patient was three, (i) day 1 observations grouped together, (ii) day 13 trough observation and (iii) day 14 observations grouped together. The RUV was estimated using proportional, additive and combined error models. First LPV concentration reported as below LoQ values within a dosing interval was set to a value of ½ LoQ, others discarded.
Allometric exponents were fixed to 0.75 for CL/F and 1 for Vd/F, when testing use of weight or fat-free mass (FFM) (20) to account for size. Further covariates screened to assess their influence on the pharmacokinetics of lopinavir included: early versus delayed ART initiation, study day, time since LPV start, presence/absence of edema, age, cholesterol, triglyceride, and anthropometrical measurements, the combined effect of rifampicin and extra ritonavir (in children on tuberculosis treatment and “super-boosted” LPV/rtv) and start of rifampicin and extra ritonavir. Each of the covariates were added in a univariate step and the covariate with the largest drop in OFV was retained in the model. Afterwards, a multivariate step was performed and again covariates were retained in the model when they: improved the fit of the model to the data; if biologically plausible, and; if a significant decrease in the objective function value (OFV) generated by NONMEM was noted. For nested models, the difference between a pair of OFV values, for a covariate model and the base model, approximates to the Chi-square (X2) statistic which can be tested for significance (X21, 0.05 = 3.84). The Akaike’s information criterion (AIC) was used for non-nested models. Covariates representing continuous data items were screened separately using linear, power and exponential functions in which the parameterization was centered on a standard covariate value. For model evaluation diagnostics goodness-of-fit (GOF) plots and prediction- and variance corrected visual predictive checks (pred-var VPC) were used. The percentile bootstrap 95% confidence intervals around the final population model parameters were obtained using an automated nonparametric bootstrap with sample replacement (n=500 runs). SAS Version 9·2 was used for for statistical analyses.
Treatment Outcomes versus Pharmacokinetics
Treatment outcomes were related to LPV concentrations on each study day (maximum 3 days/patient), by comparing proportions of patients with any sample below LoQ versus patients without any sample below the LoQ using a Chi-squared test.
RStudio (Version 0.99.484) was used to investigate an association between individual estimates of apparent LPV clearance (CL/F) and LPV exposure (AUC0-12 h∙mg/L) for all study days and treatment success and failure at 12 and 48 weeks using (i) a one-way analysis of variance (ANOVA) test followed by Tukey’s test for post hoc analysis and (ii) a binary logistic regression, with p <0.01 considered as statistically significant.
RESULTS
Of 62 patients who had samples performed on day 1, four patients died and two were transferred between day 1 and day 14, resulting in 56 patients having samples drawn on Day 14. LPV concentrations (8% of them reported as below LoQ, half of them were discarded) from 502 time points were available for analysis. Treatment outcomes were available for 37 patients at weeks 12 and 50 patients at week 48. Table 1 summarizes the patient characteristics of patients for the early and the delayed ART start groups.
Table 1.
Demographic and pharmacokinetic data of the study population at baseline
| Early (mean±SD) |
Delayed (mean±SD) |
p-value | |
|---|---|---|---|
| Patients assessed on day1:day14 | 34:31 | 29:27 | – |
| Age (months) | 15.5 (16.3) | 14.59 (10.8) | 0·57 |
| Sex (M:F) | 19:15 | 17:12 | 0·52 |
| Edema at admission (N) | 5 | 7 | 0.65 |
| Rifampicin co-administration (N) | 10 | 10 | 0·19 |
| Time to ART initiation (days) | 6.2 | 23.7 | 0·0001 |
| Weight for Age Z-score | −3.6 (1.2) | −3.2 (1.6) | 0.12 |
| Weight (kg) | 6.5 (2.8) | 6.6 (2.6) | 0.86 |
| Height (cm) | 67.1 (14.6) | 67.9 (12.01) | 0.82 |
| BMI Z-score | −2.5 (1.8) | −1.8 (2.0) | 0.15 |
| Fat free mass (FFM) | 5.1 (1.8) | 5.5 (1.9) | 0.41 |
| Mid-upper arm circumference (cm) | 11.1 (1.7) | 19.0 (2.3) | 0.15 |
| Hemoglobin (g/dL) | 8.9 (2.1) | 8.8 (1.9) | 0.74 |
| Total Protein (g/dL) | 65.0 (17.8) | 65.2 (16.5) | 0.99 |
| Albumin (g/dL) | 22.7 (8.0) | 21.6 (6.4) | 0.34 |
| Creatinine | 33.9 (34.2) | 35.4 (31.6) | 0.87 |
| Cholesterol | 2.7 (1.2) | 2.9 (1.1) | 0.48 |
| Triglyceride | 3.2 (2.4) | 2.3 (1.5) | 0.28 |
SD: Standard deviation, M: Male, F: Female, N: number, ART: antiretroviral treatment, BMI: Body mass index
Lopinavir Pharmacokinetics
The time-course of LPV disposition was well described by a one-compartment model with first-order elimination. Typical population parameter estimates (BOV (%CV)) were CL/F (L/h/5.6kg): 3.1 (126%), apparent volume of distribution (Vd/F, L/5.6kg): 9.6 and absorption rate (ka, h−1): 0.385 (56.8%). Using IOV to estimate variability on CL/F and ka was superior to IIV. Estimation of IIV for the relative bioavailability (F; IIV= 69.5%) resulted in model improvement, with the individual estimates of F being constrained between 0 and 1 using logit- transformation. The IIV estimated for F consequently is reflective of variability for apparent CL/F and Vd/F estimates. The proportional RUV (%CV) was 37.7% for samples taken within the first 5 hours after the dose and 27.2% with a BSV of 15.5%, allowing the RUV magnitude to vary from patient to patient (21). Final parameter estimates are shown in Table 2.
Table 2.
Parameter estimates and bootstrap results for the final model
| Parameter | Units | Parameter estimates | IIV (%) [Shrinkage (%)] | IOV (%) [Shrinkage (%)] | Bootstrap median (95%CI) | ||
|---|---|---|---|---|---|---|---|
| Parameter estimate | IVV % | IOV (%) | |||||
| Clearance (CL/F) | L/h/5.6 kg | 3.1 | – | 126.5 [23] | 3.0 (2.9 – 3.5) | – | 122.3 (101.1 – 147.4) |
| Volume of distribution (Vd/F) | L/5.6 kg | 9.6 | – | – | 9.7 (9.1 – 11.7) | – | |
| Absorption rate constant (ka) | /h | 0.39 | – | 56.8 [16] | 0.40 (0.28 – 0.48) | – | 55.6 (26.5 – 75.8) |
| Reduction of relative bioavailability (F) for non-study days | -fold | 3.2 | 69.5 [18] | – | 3.2 (2.3 – 3.8) | 70.8 (32.6 – 86.4) | – |
| Increase of F with increasing cholesterol above 3 mmol/L | % | 20.7 | 12.8 (4.0 – 45.3) | ||||
| Proportional for <5 h | % | 37.7 | 15.5 [20] | – | 37.8 (30.7 – 44.8) | 14.5 (5.7 – 22.6) | – |
| Proportional for >5 h | % | 27.2 | – | 26.7 (18.8 – 35.6) | – | ||
IOV: inter-occasion variability; IIV: inter-individual variability; CL/F: apparent clearance; Vd/F: apparent volume of distribution; CI: confidence interval
Where, FFM (kg): fat-free mass (20); and CHOL (mmol/L): cholesterol concentration.
Reduced adherence, based on the pre-dose sample taken on day 14, which is linked to the dose on the previous day, was identified as influential on F of LPV and reduced F 3.2 fold (ΔOFV = −19.3). Inclusion of FFM, allometrically scaled, into the model resulted in a better model fit (ΔOFV = −8.7) than allometric scaling by total body weight (ΔOFV = −6.6). Further, increased cholesterol was linearly related to F, with a 20.7% increase in F for every 1 mmol/L increase in cholesterol above 3 mmol/L (ΔOFV = −4.8). None of the other tested covariates including randomisation to early or delayed ART, or being on tuberculosis treatment (with the combined effect of rifampicin and extra ritonavir) nor anthropometrical measurements, explained the variability on CL, Vd or ka.
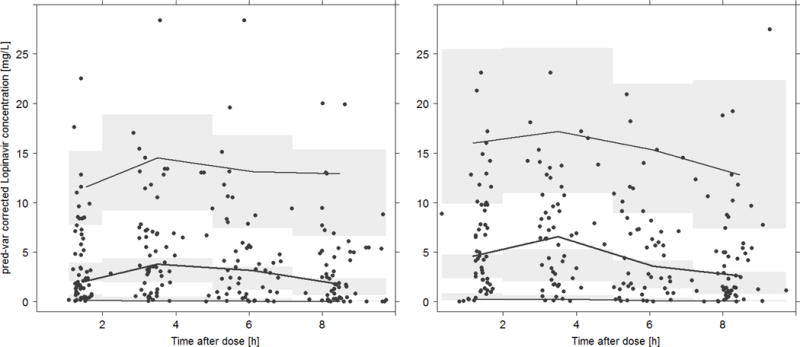
The final LPV model was evaluated using a pred-varVPC and a bootstrap. Figure 1 demonstrates that the model describes the data well, particularly for day 1. However an under-prediction of the median peak concentrations on day 14 was noted, which could not be improved upon after intensive evaluation of differences between day 1 and day 14 and no further covariate inclusion. The bootstrap produced similar parameters estimates to the final model (Table 2), indicating that the estimates for the population PK parameters in the final model are robust and stable.
Figure 1.
Treatment Outcomes versus Pharmacokinetics
Twenty-three patients had one or more of the nine samples which were measured below the lower LoQ; 13 of these patients had one sample below LoQ, five patients had two samples measured below LoQ, two patients had three samples below LoQ and three patients had four samples with concentrations below LoQ. Comparison of frequency of LoQ samples for patients with treatment failure to patients with treatment success showed no statistical difference at 12 weeks (p=0.41) or 48 weeks (p=0.44).
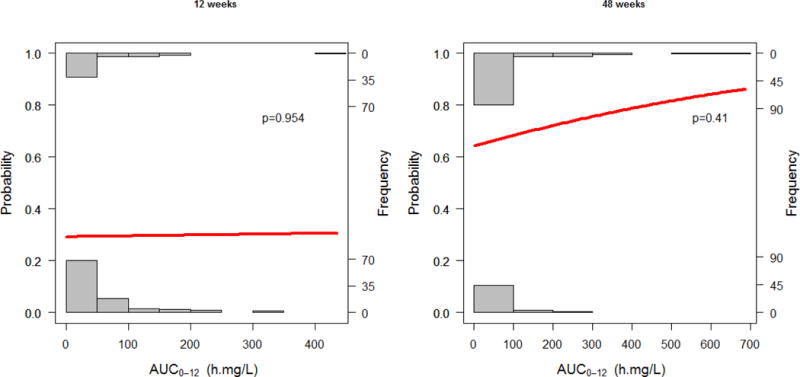
Comparison between patients with treatment failure to patients with treatment success of the individual estimates of apparent LPV clearance (CL/F) and LPV exposure (AUC0-12 h∙mg/L) showed no statistical difference at 12 weeks (p=0.89, p=0.95) and 48 weeks (p=0.92, p=0.65) using the Wilcox-rank test, respectively. No association as found between LPV exposure (AUC0-12 h∙mg/L) and treatment outcomes at 12 weeks (p=0.954) and 48 weeks (p=0.41) using binary logistic regression. Results are displayed in Table 3 and Figure 2.
Table 3.
Percent of patients with LPC concentrations below the limit of quantification (LoQ), median individual apparent LPV clearance and LPV exposure (AUC0-12) versus virologic treatment outcomes at week 12 and 48.
| Patients with concentrations below LoQ (%) | p -value* | CL/F (L/h) (median [IQR]) | p-value* | AUC0-12 (h∙mg/L) (median [IQR]) | p-value* | |
|---|---|---|---|---|---|---|
| Treatment outcome week 12 (n= 54), LTFU=4, Missing VL=4 | ||||||
| Failure (n=38) | 28% | 0.41 | 4.5 [1.9 – 11.9] | 0.79 | 23.6 [10.2 – 62.8] | 0.98 |
| Success (n=16) | 42% | 4.3 [2.1 – 12.6] | 28.4 [ 9.0 – 61.8] | |||
| Treatment outcome week 48 (n=58), LTFU =8, Missing VL=4 | ||||||
| Failure (n=17) | 41% | 0.44 | 3.8 [1.7 – 13.9] | 0.95 | 25.5 [ 8.7 – 69.0] | 0.90 |
| Success (n=33) | 30% | 3.5 [2.0 – 10.4] | 31.4 [11.8 – 60.8] | |||
compared to ‘Failure’ category, n - number of patients included, LoQ - Limit of Quantification, LPV – lopinavir, CL/F – apparent LPV clearance (l/h)
Figure 2.
DISCUSSION
In this pharmacokinetic evaluation of LPV, severely malnourished children displayed significant pharmacokinetic variability, reduced bioavailability and consequently greater CL/F estimate of 0.6 L/h/kg in comparison to reports from other studies in non-malnourished children of LPV CL/F ranging from 0.2 to 0.4 L/h/kg (15,22,23). The findings are in keeping with the results from a recently published study conducted in Ugandan children, where there was a trend towards lower LPV bioavailability in malnourished compared to non-malnourished children. However in that study only 8% of the Ugandan study participants were wasted and the evaluations were performed at least 14 days after ART initiation (24). In comparison, in this study, all patients were severely malnourished and exposures were measured on day 1 and 14 of ART initiation. We have found that observed peak concentrations were slightly increased on day 14 compared to those predicted by the model (see Figure 1); however, the difference could not be explained by any of the available explanatory factors collected in the study. The increase could be due to recovery of the children in regards to their clinical condition as well as their nutritional status. Since LPV in serum is highly protein bound to albumin and alpha-1-acid glycoprotein (AAG), changes in AAG following nutritional rehabilitation - not measured in this study - could account for this finding. A similar lower-than-predicted peak concentration for LPV was noted in the study of Ugandan children (15).
LPV/rtv-based treatment has been demonstrated to result in superior virologic suppression rates in children less than age 3 years(25,26), and therefore remains part of the WHO recommended first line regimen in young children (27). LPV pharmacokinetics have been well described in non-malnourished children and have formed the basis for the development of the WHO weight-band dosages for LPV that were used in this study (28). As the majority of young children requiring ART reside in countries where up to 42% of children are malnourished at ART initiation (29), using standard weight-based dosing may result in sub-optimal drug concentrations in a significant proportion of children initiated on ART. The role of LPV dose adjustment to achieve therapeutic dose during the acute phase of malnutrition needs to be further evaluated.
This study showed that FFM was superior to total body weight in describing variability around CL/F and Vd/F, which may be due to the relatively high FFM proportion of total weight. Initial weight gain in malnourished children, especially if associated with stunting, is predominately due to an initial increase in fat mass with later increase in lean body mass (30). The average time to ART initiation in the delayed arm was significantly longer (mean 6.2 vs. 23.7 days; p=0.0001). It is likely that a more prolonged delay in ART initiation is required for lean body weight to normalize. However delaying ART initiation comes at a potential risk of excess mortality and morbidity, (31) especially in children with advanced HIV disease. The small statistically significant advantage of including FFM instead of total body weight may have limited clinical relevance on exposure, given the overall large remaining unexplained IIV, as long as weight-based dosing is applied. We failed to demonstrate that improved LPV exposure (AUC) during the acute phase of malnutrition predicted virologic failure or death at 12 and 48 weeks. However, sub-therapeutic plasma concentrations in children initiating ART early during nutritional recovery when the HIV viral load is high may permit development of drug resistance mutations that impact longer-term treatment outcomes. Dose adjustment of LPV may facilitate earlier ART initiation while achieving adequate LPV exposures.
Therapeutic drug monitoring (TDM) in hair or blood samples to detect sub-optimal drug concentrations has been used as a marker of virologic treatment failure and development of HIV drug resistance (32,33). In a pediatric study of LPV TDM from samples collected between 10-80 weeks post ART initiation, sub-therapeutic LPV levels were linked with HIV drug resistance. The authors postulated that the sub-therapeutic LPV levels were most likely a surrogate marker of prolonged poor adherence resulting in the evolution HIV drug resistance (33). The sampling in our study was performed soon after ART initiation and sub-therapeutic plasma levels were most likely a result of altered pharmacokinetics of LPV associated with malnutrition, the effect of FFM and other factors such as inflammatory processes related to high burden of HIV and other opportunistic infections that might affect metabolic pathways. The altered pharmacokinetics due to these factors may reverse following nutritional recovery, returning the LPV plasma level to their normal while poor adherence may not, accounting for the lack of association in our study.
Tuberculosis was a common diagnosis, requiring co-administration of “super-boosted” LPV/rtv (LPV/rtv with additional ritonavir to achieve a ratio of 1:1) in rifampicin-treated patients (34). In our study population of severely malnourished children, the approach using “super-boosted” LPV/rtv in children on tuberculosis treatment resulted in similar LPV exposures to children without tuberculosis, thus providing supporting data for the continued recommendation of this regimen with similar dose adjustments as described above (35, 36, 37). A population pharmacokinetic model developed by Zhang et al. suggested that the recommend doses of LPV/rtv needed to be increased in malnourished children with and without concomitant rifampicin-based tuberculosis treatment. The model predicted the doses of “super-boosted” LPV/rtv needed to maintain LPV trough concentrations > 1mg/L in 95% of children. Children in the 3-5.9 kg weight band needed close to twice the dose per kilogram of body weight (LPV/rtv 22/22 mg/kg) compared to the 14-19.9 kg weight band (LPV/rtv 12/12 mg/kg) (21). The weight band dosage table used in our study achieved similar doses (LPV/rtv 20/25mg/kg for children between 3-5.9 kg and 11.7/12 mg/kg for children between 14-19.9 kg) however still resulted in lower LPV exposures .
The estimated bioavailability of LPV was greatly reduced on days when no full pharmacokinetic sampling was undertaken (day 13, pre-dose), which is likely due to poor adherence on these ‘non-observed’ days. All study patients remained in-patients between day 1 and day 14, the caregiver under the supervision of clinical staff administered ART. The poor palatability of LPV/rtv syrup makes administration of this formulation difficult (38) and is the likely cause for this finding. Alternative formulations of LPV/rtv in young children have been studied with variable success. Crushed LPV/rtv tablets do not result in adequate plasma levels (39,40), and while LPV/rtv pellets result in adequate plasma levels, the palatability of the formulation is still sub-optimal (41). A granule formulation with adequate taste masking is under development by Drugs for Neglected Diseases Initiative (DNDi) and in the future may result in a formulation with good palatability and tolerability (42).
The inability to detect effects of TB co-treatment, and timing of ART initiation may be due to lack of power to do so given the extreme variability encountered in the data. Furthermore, the variable adherence of the in-patient cohort despite verification of administration in hospital prescription charts was not anticipated. The design therefore did not allow definitive accounting for the effect of adherence on LPV variability. Adherence could also have potentially confounded the evaluation of timing of ART initiation (delayed ART) and TB co-treatment. Finally, evaluation of the association between LPV exposure and treatment outcome was limited by the low frequency of outcomes and the cumulative effects of adherence and other drugs in the ART regimen on outcomes.
Conclusions
LPV pharmacokinetics were not affected by the timing of ART initiation. No relationship with the use of rifampicin and super-boosted LPV/rtv in TB co-infected patients was found in this study, likely due to limited number of co-infected patients. Only FFM and cholesterol were found to explain some of the varibility in the pharmacokinetics of LPV in severely malnourished HIV-infected pediatric patients. This supports the use of weight-based dosing for LPV, as currently recommended by WHO. The substantial remaining varibility in LPV’s pharmacokinetics was not explained by the factors evaluated in this study. Bioavailability was markedly reduced in this patient population, resulting in a somewhat higher CL/F estimate in comparion to other studies, which should be considered when dosing LPV/rtv in malnourished infants and young children initiating ART.
Acknowledgments
The MATCH Study team (Thobekile Sibaya, Micheal Healy, Alejandro Palma, Edem Binka, Diana Cristina and Leora Sewnarain), KRITH (John Adamson and Thembelihle Ngotho), the parents and study subjects for participation in the study, and the Australian Centre of Pharmacometrics (http://www.therapeuticinnovation.com.au/Infrastructure/Pharmacometrics11.aspx) is acknowledged for the NONMEM software license and hardware.
Funding: The drug assays were supported by the National Research Foundation of South Africa (Grant Number 90729) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1 AI068634, UM1 AI068636 and UM1AI106701, U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (AI068632).
Footnotes
Author contributions
MA – study design, data collection, data analysis, manuscript writing; HM - study design, manuscript review ; RB - study design, manuscript review; PLR - study design, manuscript review; TS - data collection ; LW - sample processing ; SH - data analysis, manuscript writing and review
References
- 1.Madec Y, Germanaud D, Moya-Alvarez V, Alkassoum W, Issa A, Amadou M, et al. HIV prevalence and impact on renutrition in children hospitalised for severe malnutrition in Niger: an argument for more systematic screening. PLoS One. 2011;6(7):e22787. doi: 10.1371/journal.pone.0022787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fergusson P, Tomkins A. HIV prevalence and mortality among children undergoing treatment for severe acute malnutrition in sub-Saharan Africa: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103:541–8. doi: 10.1016/j.trstmh.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child [Internet] 2014;99(6):546–51. doi: 10.1136/archdischild-2012-303348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody A, Bartz S, Hornik CP, Kiyimba T, Bain J, Muehlbauer M, et al. Effects of HIV infection on the metabolic and hormonal status of children with severe acute malnutrition. PLoS One. 2014;9(7):1–11. doi: 10.1371/journal.pone.0102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argemi X, Dara S, You S, Mattei JF, Courpotin C, Simon B, et al. Impact of malnutrition and social determinants on survival of HIV-infected adults starting antiretroviral therapy in resource-limited settings. AIDS. 2012;26(9):1161–6. doi: 10.1097/QAD.0b013e328353f363. [DOI] [PubMed] [Google Scholar]
- 6.Marazzi MC, De Luca S, Palombi L, Scarcella P, Ciccacci F, Ceffa S, et al. Predictors of Adverse Outcomes in HIV-1 Infected Children Receiving Combination Antiretroviral Treatment: Results from a DREAM Cohort in Sub-Saharan Africa. Pediatr Infect Dis J. 2013;33(3):295–300. doi: 10.1097/INF.0b013e3182a0994b. [DOI] [PubMed] [Google Scholar]
- 7.Hughes SM, Amadi B, Mwiya M, Nkamba H, Mulundu G, Tomkins A, et al. CD4 counts decline despite nutritional recovery in HIV-infected Zambian children with severe malnutrition. Pediatrics. 2009;123(2):e347–51. doi: 10.1542/peds.2008-1316. [DOI] [PubMed] [Google Scholar]
- 8.Fergusson PL. Severe acute malnutrition and HIV in children in Malawi. Ugeskr Laeger. 2010;172(39):2671–2674. [PubMed] [Google Scholar]
- 9.Rose AM, Hall CS, Martinez-Alier N. Aetiology and management of malnutrition in HIV-positive children. Arch Dis Child. 2014;99(6):546–51. doi: 10.1136/archdischild-2012-303348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol. 2010;66(10):1025–35. doi: 10.1007/s00228-010-0851-0. [DOI] [PubMed] [Google Scholar]
- 11.Krishnaswamy K, Ushasri V, Naidu NA. The effect of malnutrition on the pharmacokinetics of phenylbutazone. Clin Pharmacokinet. 1981;6(2):152–9. doi: 10.2165/00003088-198106020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan S, Ramachandran G, Agibothu Kupparam HK, Mahalingam V, Soundararajan L, Perumal Kannabiran B, et al. Factors influencing plasma nevirapine levels: a study in HIV-infected children on generic antiretroviral treatment in India. J Antimicrob Chemother. 2011;66(6):1354–9. doi: 10.1093/jac/dkr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock L, Else L, Poerksen G, Molyneux E, Moons P, Walker S, et al. Pharmacokinetics of nevirapine in HIV-infected children with and without malnutrition receiving divided adult fixed-dose combination tablets. J Antimicrob Chemother. 2009;64(6):1251–9. doi: 10.1093/jac/dkp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis JC, L’Homme RF, Ewings FM, Mulenga V, Bell F, Chileshe R, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12(2):253–60. [PubMed] [Google Scholar]
- 15.Bartelink IH, Savic RM, Dorsey G, Ruel T, Gingrich D, Scherpbier HJ, et al. The effect of malnutrition on the pharmacokinetics and virologic outcomes of lopinavir, efavirenz and nevirapine in food insecure HIV-infected children in Tororo, Uganda. Pediatr Infect Dis J. 2015;34(3):e63–70. doi: 10.1097/INF.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organisation. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children. Available on: http://apps.who.int/iris/bitstream/10665/95584/1/9789241506328_eng.pdf [Accessed on 25 August 2016] [PubMed]
- 17.Department of Health, Republic of South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. 2014 Dec 24; Available on: http://www.kznhealth.gov.za/family/HIV-Guidelines-Jan2015.pdf [Accessed on 04 November 2016]
- 18.Court R, Gordon M, Cohen K, Stewart A, Gosnell B, Wiesner L, et al. Random lopinavir concentrations predict resistance on lopinavir-based antiretroviral therapy. Int J Antimicrob Agents. Elsevier BV. 2016;48(2):158–62. doi: 10.1016/j.ijantimicag.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993 Dec;21(6):735–50. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 20.Al-Sallami HS, Goulding A, Grant A, Taylor R, Holford N, Duffull SB. Prediction of Fat-Free Mass in Children. Clin Pharmacokinet. 2015 Nov 5;54(11):1169–78. doi: 10.1007/s40262-015-0277-z. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson MO, Jonsson EN, Wiltse CG, Wade JR. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998 Apr;26(2):207–46. doi: 10.1023/a:1020561807903. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, McIlleron H, Ren Y, Van Der Walt JS, Karlsson MO, Simonsson USH, et al. Population pharmacokinetics of lopinavir and ritonavir in combination with rifampicin-based antitubercular treatment in HIV-infected children. Antivir Ther. 2012;17(1):25–33. doi: 10.3851/IMP1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crommentuyn KML, Kappelhoff BS, Mulder JW, Mairuhu ATA, van Gorp ECM, Meenhorst PL, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005 Oct;60(4):378–89. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruel T, Kakura A, Ikilezi G, Mwangwa F, Dorsey G, Rosenthal Philip, et al. Virologic and immunologic outcomes of HIV-infected Ugandan children randomized to lopinavir-ritonavir or nonnucleoside- reverse-transcriptase-inhibitor therapy. J Acquir Immune Defic Syndr. 2014;15(65(5)):535–41. doi: 10.1097/QAI.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow-Mosha L, Angelidou K, Lindsey J, Archary M, Cotton M, Dittmer S, et al. Nevirapine- Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy in HIV-Infected Infants and Young Children: Long-term Follow-up of the IMPAACT P1060 Randomized Trial. Clin Infect Dis. 2016 Oct;63(8):15. 1113–21. doi: 10.1093/cid/ciw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus Ritonavir-Boosted Lopinavir for HIV-Infected Children. N Engl J Med. 2012 Jun 21;366(25):2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach, 2010. Geneva: 2010. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23741772 [Accessed on 18 November 2016] [PubMed] [Google Scholar]
- 28.Bouazza N, Foissac F, Fauchet F, Burger D, Kiechel JR, Treluyer JM, et al. Lopinavir/ritonavir plus lamivudine and abacavir or zidovudine dose ratios for paediatric fixed-dose combinations. Antivir Ther. 2015;20(2):225–33. doi: 10.3851/IMP2876. [DOI] [PubMed] [Google Scholar]
- 29.Bachou H, Tylleskar T, Downing R, Tumwine JK. Severe malnutrition with and without HIV-1 infection in hospitalised children in Kampala, Uganda: differences in clinical features, haematological findings and CD4+ cell counts. Nutr J. 2006;5:27. doi: 10.1186/1475-2891-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins PA, Hoffman DJ, Fernandes MTB, Nascimento CR, Roberts SB, Sesso R, et al. Br J Nutr. 5. Vol. 92. Cambridge University Press; 2004. Nov 9, Stunted children gain less lean body mass and more fat mass than their non-stunted counterparts: a prospective study; p. 819. [DOI] [PubMed] [Google Scholar]
- 31.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013 Nov;382(9904):1555–63. doi: 10.1016/S0140-6736(13)61409-9. [cited 2016 Nov 1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouazza N, Urien S, Blanche S, Hirt D, Foissac F, Benabound S, et al. Concentration-response model of Lopinavir/Ritonavir in HIV-1-infected pediatric patients. Pediatr Infect Dis J. 2014;33(8):e213–8. doi: 10.1097/INF.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 33.Moholisa RR, Schomaker M, Kuhn L, Meredith S, Coovadia A, Strehlau R, et al. Plasma lopinavir concentrations predict virological failure in a cohort of South African children initiating a protease-inhibitor-based regimen. Antivir Ther. 2014;19(4):399–406. doi: 10.3851/IMP2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Guidelines Guideline on When To Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. World Heal Organ. 2015 Sep;:78. Available on: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf [Accessed on 04 November 2016]. [PubMed]
- 35.Elsherbiny D, Ren Y, McIlleron H, Maartens G, Simonsson USH. Population pharmacokinetics of lopinavir in combination with rifampicin-based antitubercular treatment in HIV-infected South African children. Eur J Clin Pharmacol. 2010 Oct 16;66(10):1017–23. doi: 10.1007/s00228-010-0847-9. [DOI] [PubMed] [Google Scholar]
- 36.Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2008 Apr 15;47(5):566–9. doi: 10.1097/QAI.0b013e3181642257. [DOI] [PubMed] [Google Scholar]
- 37.Rabie H, Denti P, Lee J, Masango M, Coovadia A, Pillay S, et al. Conference of Retroviruses and Opportunistic Infections. Seattle: 2017. Lopinavir/ritonavir 1:1 super-boosting overcomes rifampicin interactions in children. Abstract 29LB. [Google Scholar]
- 38.Penazzato M, Prendergast A, Tierney J, Cotton M, Gibb D. Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age. In: Penazzato M, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2012. [DOI] [PubMed] [Google Scholar]
- 39.Best B, Capparelli EV, Diep H, Rossi SS, Farrell MJ, Williams E, et al. J Acquir Immune Defic Syndr. 2011;58(4):385–91. doi: 10.1097/QAI.0b013e318232b057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastiaans DET, Forcat S, Lyall H, Cressey TR, Hansudewechakul R, Kanjanavanit S, et al. Pharmacokinetics of Pediatric Lopinavir/ Ritonavir Tablets in Children When Administered Twice Daily According to FDA Weight Bands. Pediatr Infect Dis J. 2014;33(3):301–5. doi: 10.1097/INF.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 41.Musiime V, Fillekes Q, Kekitiinwa a, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66(2):148–54. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 42.Drugs for Neglected Disease Initiative (DNDi) Child-Friendly Formulation of WHO-Recommended Treatment Now Approved by the US FDA for Children Living with HIV. Available on: http://www.dndi.org/2015/media-centre/press-releases/pr-phti-fda-approval-pellets/ [Accessed on 04 November 2016].


