Abstract
Background
The prevalence, treatment and outcomes of balloon undilatable chronic total occlusions (CTOs) have received limited study.
Methods
We examined the prevalence, clinical and angiographic characteristics, and procedural outcomes of percutaneous coronary interventions (PCI) for balloon undilatable CTOs in a contemporary multicenter US registry.
Results
Between 2012 and 2017 data on balloon undilatable lesions were available for 425 consecutive CTO PCIs in 415 patients in whom guidewire crossing was successful: 52 of 425 CTOs were balloon undilatable (12%). Mean patient age was 65±10 years and most patients were men (84%). Patients with balloon undilatable CTOs were more likely to be diabetic (67% vs. 41%, p<0.001) and have heart failure (44% vs. 28%%, p=0.027). Balloon undilatable CTOs were longer (40 mm [IQR 20-50] vs. 30 [IQR 15-40], p=0.016), more likely to have moderate/severe calcification (87% vs. 54%, p<0.001), and had higher J-CTO score (3.2±1.1 vs. 2.5±1.3, p<0.001) and PROGRESS-CTO complications score (3.9±1.7 vs. 3.1±2.0, p<0.005). They were associated with lower technical and procedural success (92% vs. 98%, p=0.024; and 88% vs. 96%, p=0.034, respectively) and higher risk for in-hospital major adverse events (8% vs. 2%, p=0.008) due to higher perforation rates. The most frequent treatments for balloon undilatable CTOs were high pressure balloon inflations (64%), rotational atherectomy (31%), laser (21%), and cutting balloons (15%).
Conclusions
Balloon undilatable CTOs are common and are associated with lower success and higher complication rates.
Clinical Trial Registration
NCT02061436, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO)
Keywords: chronic total occlusion, percutaneous coronary intervention, complex coronary intervention
Introduction
Chronic total occlusion (CTO) percutaneous coronary intervention (PCI) has been rapidly evolving with high success rates currently being achieved at experienced centers (1-6). Although failure to cross the occlusion with a guidewire is the most common mechanism of CTO PCI failure, additional technical challenges exist, such as inability to advance a balloon after successful guidewire crossing (balloon uncrossable lesions) (7-9), and inability to fully dilate the lesion despite multiple balloon inflations (balloon undilatable lesions) (Figure 1). Adequate preparation in such lesions is critical to avoid suboptimal stent expansion that can result in higher rates of stent thrombosis and in-stent restenosis (10,11). In view of continuing advancements in CTO crossing devices and techniques, the prevalence of balloon uncrossable and undilatable lesions is likely to increase. We, therefore, examined a large multicenter US CTO PCI registry to determine the frequency, treatment, and outcomes of balloon undilatable lesions.
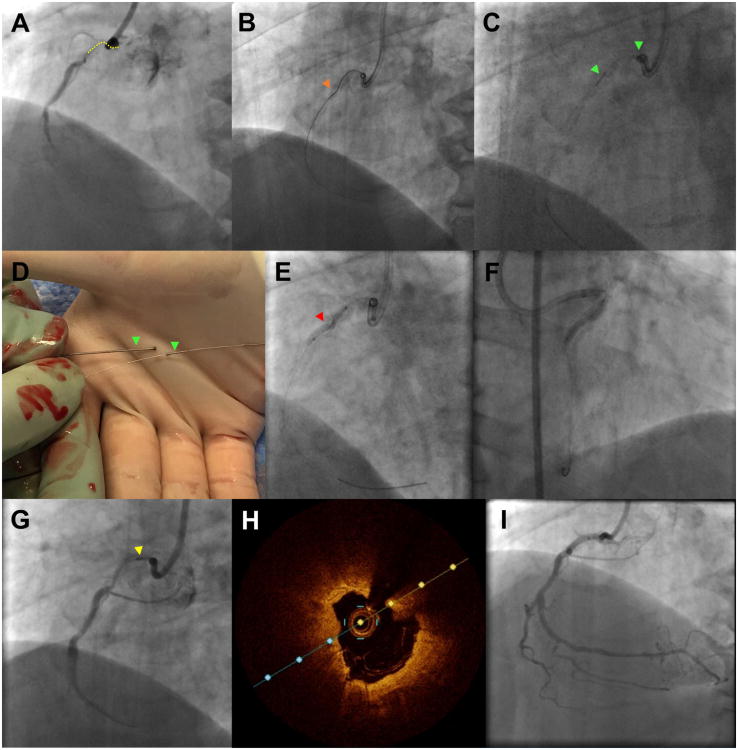
Figure 1.
Challenging PCI for balloon undilatable ostial right coronary artery (RCA) chronic total occlusion (CTO).
Panel A-B. Short (10 mm) ostial right coronary artery CTO that was crossed with a Fielder FC guidwire advanced through a Caravel microcatheter (Asahi Intecc, Nagoya, Japan).
Panel C-D. Orbital atherectomy for plaque modification (18 passes), that was complicated by crown fracture and entrapment. The fractured crown was retrieved after removal of the Viper guidewire.
Panel E. The lesion failed to dilate despited multiple balloon inflations (2.0×20 and 2.5×20 mm balloon inflated at 20-24 Atm [red arrowhead]).
Panel F. An AngioSculpt balloon (Spectranetics, Fremont, CA, USA) delivered and inflated using a GuideLiner V3 (Vascular Solutions, Minneapolis, MN, USA) guide catheter extension.
Panel G. Rotational atherectomy (yellow arrowhead) was performed (8 passes, upsizing the burr diameter from 1.2 mm to 1.25 mm) over a RotaWire Floppy guidewire (Boston Scientific, Natick, MA, USA)
Panel H. Optical coherence tomography demonstrating heavy circumferential calcification in the proximal right coronary artery.
Panel I. Final angiographic result after stenting.
Methods
We examined the frequency and the baseline clinical, angiographic, and procedural characteristics and outcomes of balloon undilatable lesions in the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention, NCT02061436). Data collection on balloon undilatable lesions started in 2015. The study was approved by the institutional review board of each center.
Definitions
Coronary CTOs were defined as coronary lesions with thrombolysis in myocardial infarction (TIMI) grade 0 flow of at least 3 month duration. Estimation of the duration of occlusion was based on the first onset of angina, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Balloon undilatable CTOs were defined as lesions that could not be expanded despite multiple balloon inflations with a 1:1 sized balloon at a maximum inflation pressure up to 20 atm after successful guidewire crossing, and balloon advancement within the target lesion. Balloon uncrossable lesions were defined as lesions that could not be crossed by balloon after successful guidewire crossing into the true lumen distal to the occlusion. Balloon inflations >20 atm were defined as high-pressure. Calcification assessment was based on angiography as follows: mild (spots), moderate (involving ≤50% of the reference lesion diameter) and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least 2 bends >70° or 1 bend >90° and severe tortuosity as 2 bends >90° or 1 bend >120° in the CTO vessel. Blunt or no stump was defined as lack of tapering or lack of a funnel shape at the proximal cap. Interventional collaterals were defined as collaterals considered amenable to crossing by a guidewire and a microcatheter by the operator.
Technical success was described as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as achievement of technical success without any in-hospital complications. In-hospital major adverse cardiac events (MACE) included any of the following adverse events prior to hospital discharge: death, myocardial infarction, recurrent symptoms requiring urgent repeat target vessel revascularization with PCI or coronary artery bypass graft surgery (CABG), tamponade requiring either pericardiocentesis or surgery, and stroke. Periprocedural myocardial infarction (MI) was defined using the Third Universal Definition of Myocardial Infarction (type 4a MI)(12). Procedure time was calculated from administration of local anesthetic for vascular access to removal of the last catheter. The J-CTO score was calculated as described by Morino et al (13), the PROGRESS-CTO score as described by Christopoulos et al (14), and the PROGRESS-CTO Complications score as described by Danek et al (15).
Statistical Analysis
Categorical variables were described as percentages and were compared using Pearson's chi-square test or Fisher's exact test. Continuous variables were expressed as mean ± standard deviation or median [interquartile range, IQR] unless otherwise specified and were compared using the t-test or Wilcoxon rank-sum test, as appropriate. All statistical analyses were performed with JMP 13.0 (SAS Institute, Cary, North Carolina). A two-sided p value of 0.05 was considered statistically significant.
Results
Clinical and angiographic characteristics
Between 2015 and May 2017 data on balloon undilatable lesions was available for 425 consecutive CTO PCIs performed in 415 patients at 9 US centers. The prevalence of balloon undilatable lesions was 12 % (52 of 425). Mean patient age was 65±10 years, and most patients were men (84%). Patients with balloon undilatable lesions were more likely to have diabetes mellitus (67% vs. 41%, p<0.001), congestive heart failure (44% vs. 28%, p=0.027), and lower left ventricular ejection fraction (45±13% vs. 50±13%, p=0.015) (Table 1). However, the prevalence of prior CABG (45% vs. 35%, p=0.175), and dialysis (2% vs. 2%, p=1.000) were similar in the two groups.
Table 1.
Clinical characteristics of balloon undilatable and balloon dilatable CTO lesions.
| Clinical characteristics | Balloon undilatable lesions | Balloon dilatable lesions | P value |
|---|---|---|---|
|
| |||
| (n=52, 12%) | (n=363, 88%) | ||
| Age (years) a | 67.1±9.7 | 64.3±10.2 | 0.056 |
| Male gender, n (%) | 39 (75) | 302 (85) | 0.074 |
| BMI (kg/m2) a | 31.7±5.7 | 30.3±5.7 | 0.114 |
| Smoking (current), n (%) | 13 (27) | 82 (24) | 0.641 |
| Diabetes, n (%) | 34 (67) | 146 (41) | <0.001 |
| Dyslipidemia, n (%) | 51 (100) | 33 (95) | 0.110 |
| Hypertension, n (%) | 48 (94) | 313 (88) | 0.206 |
| Family history of CAD, n (%) | 17 (44) | 132 (42) | 0.866 |
| Prior MI, n (%) | 29 (59) | 182 (52) | 0.336 |
| Prior heart failure, n (%) | 22 (44) | 98 (28) | 0.027 |
| Prior valve surgery or procedure, n (%) | 2 (4) | 14 (4) | 0.969 |
| Prior PCI, n (%) | 35 (70) | 221 (63) | 0.336 |
| Prior CABG, n (%) | 23 (45) | 125 (35) | 0.175 |
| Baseline creatinine (mg/dL) b | 1 (1, 1) | 1 (1, 1) | 0.634 |
| Currently on dialysis, n (%) | 1 (2) | 6 (2) | 1.000 |
| Prior CVD, n (%) | 9 (18) | 41 (2) | 0.215 |
| Prior PVD, n (%) | 8 (16) | 53 (15) | 0.900 |
| Chronic lung disease, n (%) | 8 (16) | 60 (17) | 0.802 |
| Left ventricular EF (%)a | 45.2±13.4 | 50.3±13.3 | 0.015 |
Mean ± standard deviation,
Median (interquartile range)
BMI, body mass index; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; CVD, cerebrovascular disease; PVD, peripheral artery disease; EF, ejection fraction.
The angiographic characteristics of the study lesions are presented in Table 2. The most common CTO target vessel was the right coronary artery (54%), followed by the left anterior descending artery (24%), and left circumflex (21%). As compared with balloon dilatable lesions, balloon undilatable lesions were longer (median length was 40 mm [interquartile range (IQR) 20-50] vs. 30 mm [IQR 15-40], p=0.016), and more likely to be associated with moderate or severe calcification (87% vs. 54%, p<0.001). They also had higher J-CTO score (3.2±1.1 vs. 2.5±1.3, p<0.001), and PROGRESS-CTO complications score (3.9±1.7 vs. 3.1±2.0, p<0.005), but similar PROGRESS CTO score (1.5±1.2 vs. 1.5±1.0, p=0.881).
Table 2.
Angiographic characteristics of the study CTO lesions, classified according to whether they were balloon undilatable or not.
| Angiographic characteristics | Balloon undilatable lesions | Balloon dilatable lesions | P value |
|---|---|---|---|
|
| |||
| (n=52, 12%) | (n=373, 88%) | ||
| CTO target vessel, n (%) | 0.239 | ||
| RCA (%) | 25 (48) | 191 (55) | |
| LCX (%) | 8 (17) | 75 (23) | |
| LAD (%) | 18 (35) | 77 (22) | |
| Other (%) | 1 (2) | 6 (2) | |
| CTO length (mm) b | 40 (20, 50) | 30 (15, 40) | 0.016 |
| Vessel diameter (mm) b | 3 (3, 3) | 3 (3, 3) | 0.092 |
| Proximal cap ambiguity, n (%) | 19 (38) | 107 (35) | 0.632 |
| Side branch at proximal cap, n (%) | 28 (55) | 146 (48) | 0.342 |
| Blunt stump/no stump, n (%) | 30 (60) | 162 (51) | 0.251 |
| Interventional collaterals, n (%) | 41 (43) | 174 (56) | 0.083 |
| Moderate/severe calcification, n (%) | 41 (87) | 169 (54) | <0.001 |
| Moderate/severe tortuosity, n (%) | 25 (53) | 125 (40) | 0.089 |
| In-stent restenosis, n (%) | 12 (25) | 59 (19) | 0.324 |
| Prior failed CTO PCI, n (%) | 13 (26) | 67 (21) | 0.477 |
| J-CTO score a | 3.2±1.1 | 2.5±1.3 | <0.001 |
| PROGRESS-CTO score a | 1.5±1.2 | 1.5±1.0 | 0.881 |
| PROGRESS-CTO Complications score a | 3.9±1.7 | 3.1±2.0 | 0.005 |
Mean ± standard deviation,
Median (interquartile range)
CTO, chronic total occlusion; RCA, right coronary artery; LCX, left circumflex artery; LM segment; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; J, Japan; PROGRESS, Prospective Global Registry of Chronic Total Occlusion Interventions.
Procedural techniques
The techniques used for treating balloon undilatable lesions and the clinical outcomes are shown in Table 3. Guidewire crossing was achieved in 44% with antegrade wire escalation, 21% with antegrade dissection reentry, and 35% with the retrograde approach. Bilateral injections were used in 75% and most cases were performed via femoral access (79% left femoral access and 52% right femoral access). Intravascular ultrasound was used more frequently in balloon undilatable lesions (65% vs. 42%, p<0.003). The overall prevalence of balloon uncrossable lesions was 11.6% (n=49), of which 47% (n=23) were also balloon undilatable as compared with 8% among balloon crossable lesions (p<0.001). Conversely, 44% (n=23) of balloon undilatable lesions were also balloon uncrossable.
Table 3.
Technical characteristics of the study CTO lesions, classified according to whether they were balloon undilatable or not.
| Technical characteristics | Balloon undilatable lesions | Balloon dilatable lesions | P value |
|---|---|---|---|
|
| |||
| (n=52, 12%) | (n=373, 88%) | ||
| Bilateral injection, n (%) | 36 (75) | 248 (73) | 0.763 |
| Crossing strategies used | |||
| • AWE, n (%) | 41 (79) | 312 (84) | 0.387 |
| • ADR, n (%) | 18 (35) | 114 (31) | 0.554 |
| • Retrograde technique, n (%) | 24 (46) | 124 (33) | 0.067 |
| Final crossing strategy | 0.215 | ||
| • AWE, n (%) | 23 (44) | 104 (55) | |
| • ADR, n (%) | 11 (21) | 72 (21) | |
| • Retrograde, n (%) | 18 (35) | 84 (24) | |
| First crossing strategy | 0.084 | ||
| • AWE, n (%) | 36 (71) | 293 (83) | |
| • ADR, n (%) | 4 (7) | 23 (7) | |
| • Retrograde, n (%) | 11 (22) | 39 (11) | |
| IVUS use overall | 31 (65) | 155 (42) | 0.003 |
| • Proximal cap identification, n (%) | 2 (7) | 11 (7) | 1.000 |
| • Guide wiring, n (%) | 8 (26) | 31 (20) | 0.474 |
| • Stent sizing., n (%) | 10 (32) | 88 (57) | 0.001 |
| • Guide reverse CART reentry, n (%) | 1 (3) | 3 (2) | 0.521 |
| • Stent optimization, n (%) | 22 (71) | 87 (56) | 0.126 |
| Balloon uncrossable lesions, n (%) | 23 (44) | 26 (7) | <0.001 |
| Access site | |||
| • Right femoral access site, n (%) | 41 (79) | 294 (79) | 1.000 |
| • Left femoral access site, n (%) | 27 (52) | 172 (46) | 0.432 |
| • Right radial access site, n (%) | 22 (42) | 128 (34) | 0.259 |
| • Left radial access site, n (%) | 17 (33) | 94 (25) | 0.249 |
| Technical success, n (%) | 48 (92) | 367 (98) | 0.024 |
|
| |||
| Procedural characteristics* | (n=52, 12%) | (n=363, 88%) | |
|
| |||
| Procedural success, n (%) | 45 (88) | 346 (96) | 0.034 |
| Procedure time (min) a | 195 (115, 262) | 141 (97, 205) | 0.007 |
| Contrast volume (mL) a | 284 (185, 315) | 262 (200, 350) | 0.642 |
| Fluoroscopy time (min) a | 67 (40, 104) | 49 (30, 76) | 0.007 |
| Patient AK dose (Gray) a | 3 (2, 4) | 3 (2, 4) | 0.083 |
Median (interquartile range)
Mean ± standard deviation
ADR, antegrade dissection reentry; AK, air kerma; AWE, antegrade wire escalation;CART, controlled antegrade and retrograde subintimal tracking; IVUS, intravascular ultrasound.
Several techniques were used for lesion preparation, such as high-pressure balloon inflations (64%), rotational atherectomy (31%), laser (21%), cutting balloon (15%), and AngioSculpt (Spectranetics, Fremont, CA, USA) (14%) (Table 4). Two or more techniques were used in 48% of the undilatable lesions with higher overall technical (100% vs. 85%, p=0.112) and procedural (96% vs. 85%, p=0.350) success, and lower major complication rate (0% vs. 15%, p=0.112) as compared with cases in which only one technique was used.
Table 4.
Outcomes of various techniques used to treat balloon undilatable lesions.
| Technique | Use/lesion | Technical success | Procedural success | MACE overall | Perforation* |
|---|---|---|---|---|---|
| High-pressure balloon inflation, n (%) | 33 (64) | 33 (100) | 31 (94) | 2 (6) | 3 (9) |
| AngioSculpt, n (%) | 7 (14) | 7 (100) | 7 (100) | 0 (0) | 0 (0) |
| Cutting balloon, n (%) | 8 (15) | 8 (100) | 8 (100) | 0 (0) | 3 (20) |
| Laser atherectomy, n (%) | 11 (21) | 11 (100) | 10 (91) | 0 (0) | 3 (14) |
| Rotational atherectomy, n (%) | 16 (31) | 14 (88) | 14 (88) | 1 (6) | 2 (13) |
| Orbital atherectomy, n (%) | 3 (6) | 3 (100) | 3 (100) | 0 (0) | 0 (0) |
| Other, n (%) | 4 (8) | 4 (100) | 4 (100) | 1 (25) | 0 (0) |
Other technique refers for use of buddy wire and Chocolate balloon (Trireme Medical Inc., Pleasanton, CA, USA)
MACE, major adverse cardiac events.
Among CTO PCI for undilatable lesions 6 perforation were detected (in 4 cases 2 or more techniques were used, whereas in the remaining 2 cases only one technique was used[cutting balloon; rotational atherectomy]).
Procedural outcomes
The overall technical and procedural success rates were 98% and 95%, respectively and were lower in balloon undilatable lesions: technical success: 92% vs. 98%, (p=0.024); procedural success: 88% vs. 96% (p=0.034) (Table 3). The median procedural (195 min [IQR 115-262] vs. 141 min [IQR 97-205], p<0.007) and fluoroscopy time (67 min [IQR 40-104] vs. 49 min [IQR 30-76], p<0.007) were longer in the balloon undilatable group, but air kerma radiation dose (3 Gray [IQR 2-4] vs. 3 Gray [2-4], p=0.083) and contrast volume (284 ml [IQR 185-315] vs. 262 ml [IQR 200-350], p=0.642) were similar in the two groups.
Procedural complications are presented in Table 5. Balloon undilatable lesions were associated with higher incidence of in hospital MACE (8% vs. 2%, p=0.008), due to higher incidence of coronary perforations, including perforations causing tamponade and requiring pericardiocentesis (5.8% vs. 0.3%, p=0.007). Perforations were most commonly treated with prolonged balloon inflation (67% of all cases of perforations), anticoagulation reversal (33%), covered stent implantation (33%), emergency surgical evacuation (17%) and pericardiocentesis (17%).
Table 5.
Procedural complications during the study CTO interventions, classified according to whether the target lesion was balloon undilatable or not.
| Procedural Complications | Balloon undilatable lesions | Balloon dilatable lesions | P value |
|---|---|---|---|
|
| |||
| (n=52, 12%) | (n=363, 88%) | ||
| In-hospital MACE, n (%) | 4 (7.7) | 6 (1.7) | 0.008 |
| Death | 0 (0.0) | 1 (0.8) | 0.120 |
| Acute myocardial infarction | 0 (0.0) | 1 (0.3) | 1.000 |
| Stroke | 0 (0.0) | 1 (0.3) | 1.000 |
| Repeat PCI | 1 (1.9) | 1 (0.3) | 0.235 |
| Repeat CABG | 1 (1.9) | 0 (0.0) | 0.008 |
| Pericardial tamponade | 3 (5.8) | 1 (0.3) | 0.007 |
| Tamponade requiring pericardiocentesis | 3 (5.8) | 1 (0.3) | 0.007 |
| Perforation, n (%) | 6 (11.5) | 7 (1.9) | 0.003 |
| Perforation of CTO target vessel, n (%) | 5 (9.6) | 3 (0.8) | <0.001 |
| Perforated collateral, n (%) | 1 (1.9) | 1 (0.3) | 0.235 |
| Perforation type, n (%) | <0.001 | ||
| Ellis Class 1 | 0 (0.0) | 0 (0.0) | |
| Ellis Class 2 | 2 (3.8) | 1 (0.3) | |
| Ellis Class 3 | 2 (3.8) | 3 (0.8) | |
| Ellis Class 3 – Cavity spilling | 2 (3.8) | 0 (0.0) | |
| Vascular Access Complication, n (%) | 1 (1.9) | 2 (0.6) | 0.332 |
| Donor vessel dissection/thrombosis, n (%) | 2 (3.9) | 6 (1.6) | 0.264 |
| Bleeding, n (%) | 0 (0.0) | 5 (1.4) | 1.000 |
MACE, major adverse cardiac events; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery.
Discussion
To the best of our knowledge, this is the first study describing the prevalence and outcomes of balloon undilatable CTOs, The main findings were that balloon undilatable CTOs: (a) are common (12% of all CTOs); (b) often require use of aggressive vessel preparation techniques; and (c) are associated with lower technical and procedural success and higher in-hospital complication rates.
Balloon undilatable CTOs are lesions that fail to expand despite multiple balloon inflations after successful guidewire crossing, and balloon advancement within the target lesion. Stenting such lesions should be avoided until after adequate dilation has been achieved to prevent stent underexpansion, which in turn may predispose to in-stent restenosis and stent thrombosis (10,11,16). As anticipated, balloon undilatable lesions were more likely to also be balloon uncrossable (in 44%), and to have longer length and heavy calcification.
The frequency of balloon undilatable lesions was high in our cohort, likely in part due to treatment of increasingly complex CTOs over time, such as lesions with severe calcification. Fernandez et al. investigated 6,882 consecutive PCIs in a single center study between 2007 and 2011 and reported 58 ‘balloon failure’ cases (0.84%). Balloon failure was defined as balloon failure to cross in 36 patients [16 of whom had CTOs], and balloon failure to expand in 22 cases [2 of which were CTOs]). Balloon failure cases were treated with the combination of laser and/or rotablation atherectomy, with 91% overall success rate (17). In the ELLEMENT (Excimer Laser LEsion Modification to Expand Non-dilatable sTents) multicenter pilot study 28 consecutive cases were enrolled with stent underexpansion treated with excimer laser atherectomy (ELCA) between 2009 and 2011, however the study focused on the technical approach and not the prevalence of these lesions (18).
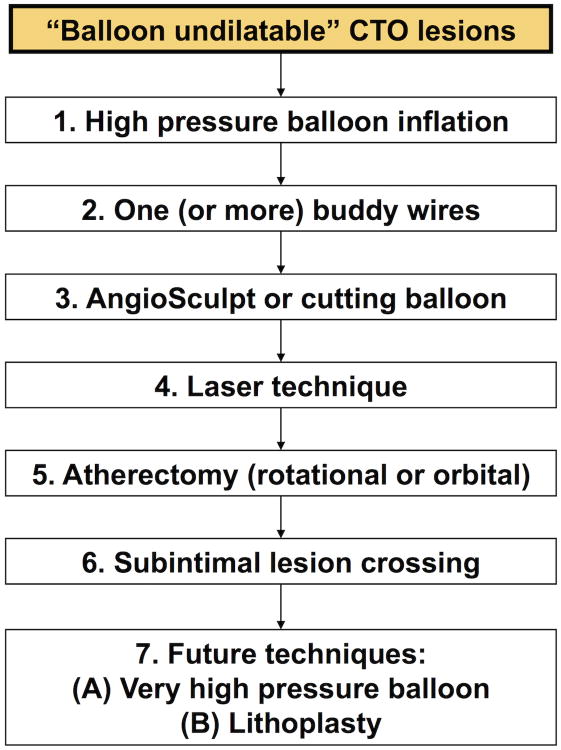
Balloon undilatable lesions had lower technical and procedural success and higher risk for complications. There are several treatment options for balloon undilatable lesions, which can be applied in an algorithmic fashion (Figure 2).(19) The first step usually involves high-pressure inflation with a 1:1 sized non-compliant balloon (the median maximum inflation pressure was 25 atm [IQR 20-30] in our cohort)(20). High-pressure balloon inflation is the simplest and most widely available technique that can be repeated multiple times, however it carries risk for balloon rupture and/or vessel perforation. If balloon rupture occurs, coronary angiography should be immediately performed after removal of the ruptured balloons to determine whether coronary perforation has occurred. Occasionally two smaller balloons can be inflated side-by-side within the undilatable coronary segment to facilitate vessel expansion. Balloon inflation can also be repeated after inserting one (or more) buddy wire(s) through the lesion (21-23). Another option is to use an AngioSculpt (Spectranetics, Fremont, CA, USA), or a cutting balloon(24) to create controlled incisions in the vessel wall that may assist with vessel expansion. However, these devices may be challenging to deliver to the lesion due to lack of flexibility caused by the wires or cutting blades and can be facilitated by using strong guide catheter support, for example by use of one or more guide catheter extensions (mother-daughter-granddaughter technique(25)) or by using side branch anchoring (24,26,27). The AngioSculpt, and the cutting balloon were utilized in 10% and 9% of our cases, respectively.
Figure 2.
Treatment algorithm for balloon undilatable lesions.
Modified with permission from(19)
Additional strategies for expanding balloon undilatable lesions include laser (used in 13% in our cohort), or atherectomy (rotational atherectomy was used in 19% and orbital atherectomy in 4% of lesions in our study) (17,28,29). Laser is easy to use and can be advanced over any standard 0.014 inch guidewire, whereas both orbital and rotational atherectomy require use of a specialized, thinner guidewire. Laser can be used even in previously stented lesions, whereas rotational or orbital atherectomy are avoided in this setting. In “balloon undilatable” lesions due to in-stent restenosis, laser can be activated with simultaneous contrast injection to modify the calcified plaque (30). The LEONARDO study (Early outcome of high energy Laser [Excimer] facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions (31)) analyzed 80 patients with 100 lesions of treated with high (60-80 mJ/mm2, 80 Hz) and standard (60 mJ/mm2, 40 Hz) energy laser. As primary indication for laser therapy, 37% was balloon failure and 11% were chronic total occlusions. The overall technical success rate was 93.7%, without perforations, no reflow phenomenon, target vessel dissection, or acute vessel closure. With use of higher laser energy, the initial technical (42.7% to 93.7%, p<0.001) and procedural (42.7% to 91.7%, p<0.001) success improved significantly. Use of laser with simultaneous contrast injection should in general be avoided in de novo lesions due to high rate of perforation or dissection (32).
Insertion of specialized guidewires for atherectomy should be performed with caution, ideally using the trapping technique that can be performed with a standard balloon, a dedicated balloon (Trapper balloon, Boston Scientific, Natick, MA, USA), or guide extension catheter with integrated trapping balloon (TrapLiner, Vascular Solutions, Minneapolis, MN, USA). Tian et al. compared the short and long term outcomes of rotational atherectomy (RA), plain old balloon angioplasty (POBA), and cutting balloon angioplasty (CBA) before stent implantation in heavily calcified lesions (33). In contrast to our study, they found no difference in the incidence of perforation (0.0% vs. 0.0% vs. 0.0%) or no reflow phenomenon (0.0% vs. 0.0% vs. 0.3%, p>0.99) with atherectomy. Similarly, there was no difference in the incidence of major adverse cardiac events (14.6% vs. 12.3% vs. 8.3%, p=0.2) all-cause death (9.8% vs. 8.2% vs. 4.5%, p=0.18), and target lesion revascularization (5.2% vs. 3.5% vs. 3.9%, p=0.76) at 12 months follow-up. As a last resort, subintimal crossing could be considered, “crushing” the plaque from the subintimal space, but such techniques are dependent on high level of expertise in dissection/reentry techniques.
Novel technologies for treating balloon undilatable lesions are in development or available outside the US. One such technology, currently available in Europe, is the high-pressure balloon (OPN NC High-Pressure PTCA Balloon, SIS Medical AG; Winterthur, Switzerland) that can be inflated up to 35 atmospheres. Also, the lithoplasty balloon (Shockwave Medical, Fremont, California) can deliver ultrasound shockwaves (8 pulses/10 seconds) achieving tissue modification. Lithoplasty is currently approved in the US only for peripheral arterial interventions, but initial application for PCI has been promising (34).
Our study has limitations. First, the study was observational without patient randomization to various treatment modalities. The selection of applied strategies was based upon the clinical and angiographic characteristics as assessed by the operator. Second, long-term follow up of the study patients was not available. Third, there was no core laboratory assessment of the study angiograms or clinical event adjudication. Fourth, the procedures were performed at dedicated, high volume CTO centers, by experienced operators, potentially limiting extrapolation to less experienced operators and centers. Fifth, evaluation of calcification was based on angiography, which is known to underestimate the presence and severity of calcification as compared with intravascular imaging.
Conclusions
In conclusion, balloon undilatable lesions are common in contemporary CTO PCI, often require use of advanced treatment strategies and are associated with worse clinical outcomes than balloon dilatable lesions. Additional comparative studies are needed to identify optimal treatment strategies and upcoming new technologies are likely to have a catalytic impact on optimizing the outcomes of these complex lesions and patients.
Acknowledgments
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Texas Southwestern Medical Center, Dallas, TX, USA. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding: The Progress CTO registry has received support from the Abbott Northwestern Hospital Foundation.
Research reported in this publication was supported by the Clinical and Translational Science Awards Program of the National Institutes of Health (Bethesda, MD, USA) under grant number UL1-RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Rangan: Research grants from InfraReDx, Inc., and The Spectranetics Corporation.
Dr. Banerjee: research grants from Gilead and the Medicines Company; consultant/speaker honoraria from Covidien and Medtronic; ownership in MDCARE Global (spouse); intellectual property in HygeiaTel.
Footnotes
Disclosures: Dr. Tajti: nothing to disclose.
Dr. Karmpaliotis: Speaker honoraria: Abbott Vascular, Boston Scientific, Medtronic, Vascular Solutions.
Dr. Alaswad: consulting fees from Terumo and Boston Scientific; consultant, no financial, Abbott Laboratories.
Dr. Toma: nothing to disclose.
Dr. Choi: nothing to disclose.
Dr. Jaffer: Consultant: Abbott Vascular and Boston Scientific. Research grant: Canon, Siemens and National Institutes of Health.
Dr. Doing: nothing to disclose.
Dr. Patel: speakers' bureau for Astra Zeneca.
Dr. Mahmud: consulting fees from Medtronic and Corindus; speaker's fees from Medtronic, Corindus, and Abbott Vascular; educational program fees from Abbott Vascular; and clinical events committee fees from St. Jude.
Dr. Uretsky: nothing to disclose.
Dr. Karatasakis: nothing to disclose.
Dr. Karacsonyi: nothing to disclose.
Dr. Danek: nothing to disclose.
Dr. Brilakis: consulting/speaker honoraria from Abbott Vascular, ACIST, Amgen, Asahi, CSI, Elsevier, GE Healthcare, Medicure, and Nitiloop; research support from Boston Scientific and Osprey. Board of Directors: Cardiovascular Innovations Foundation. Board of Trustees: Society of Cardiovascular Angiography and Interventions
References
- 1.Brilakis ES, Banerjee S, Karmpaliotis D, et al. Procedural Outcomes of Chronic Total Occlusion Percutaneous Coronary Intervention: A Report From the NCDR (National Cardiovascular Data Registry) JACC Cardiovasc Interv. 2015;8:245–53. doi: 10.1016/j.jcin.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Christopoulos G, Karmpaliotis D, Alaswad K, et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–228. doi: 10.1016/j.ijcard.2015.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galassi AR, Sianos G, Werner GS, et al. Retrograde Recanalization of Chronic Total Occlusions in Europe: Procedural, In-Hospital, and Long-Term Outcomes From the Multicenter ERCTO Registry. J Am Coll Cardiol. 2015;65:2388–400. doi: 10.1016/j.jacc.2015.03.566. [DOI] [PubMed] [Google Scholar]
- 4.Wilson WM, Walsh SJ, Yan AT, et al. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102:1486–93. doi: 10.1136/heartjnl-2015-308891. [DOI] [PubMed] [Google Scholar]
- 5.Maeremans J, Walsh S, Knaapen P, et al. The Hybrid Algorithm for Treating Chronic Total Occlusions in Europe: The RECHARGE Registry. J Am Coll Cardiol. 2016;68:1958–1970. doi: 10.1016/j.jacc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Habara M, Tsuchikane E, Muramatsu T, et al. Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. 2016;87:1027–35. doi: 10.1002/ccd.26354. [DOI] [PubMed] [Google Scholar]
- 7.Sapontis J, Christopoulos G, Grantham JA, et al. Procedural failure of chronic total occlusion percutaneous coronary intervention: Insights from a multicenter US registry. Catheter Cardiovasc Interv. 2015;85:1115–22. doi: 10.1002/ccd.25807. [DOI] [PubMed] [Google Scholar]
- 8.Patel SM, Pokala NR, Menon RV, et al. Prevalence and treatment of “balloon-uncrossable” coronary chronic total occlusions. J Invasive Cardiol. 2015;27:78–84. [PubMed] [Google Scholar]
- 9.Karacsonyi J, Karmpaliotis D, Alaswad K, et al. Prevalence, indications and management of balloon uncrossable chronic total occlusions: Insights from a contemporary multicenter US registry. Catheter Cardiovasc Interv. 2016 doi: 10.1002/ccd.26780. [DOI] [PubMed] [Google Scholar]
- 10.Kim BK, Shin DH, Hong MK, et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ Cardiovasc Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 11.Kang J, Cho YS, Kim SW, et al. Intravascular Ultrasound and Angiographic Predictors of In-Stent Restenosis of Chronic Total Occlusion Lesions. PLoS One. 2015;10:e0140421. doi: 10.1371/journal.pone.0140421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–21. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Christopoulos G, Kandzari DE, Yeh RW, et al. Development and Validation of a Novel Scoring System for Predicting Technical Success of Chronic Total Occlusion Percutaneous Coronary Interventions: The PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) Score. JACC Cardiovasc Interv. 2016;9:1–9. doi: 10.1016/j.jcin.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Danek BA, Karatasakis A, Karmpaliotis D, et al. Development and Validation of a Scoring System for Predicting Periprocedural Complications During Percutaneous Coronary Interventions of Chronic Total Occlusions: The Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) Complications Score. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian NL, Gami SK, Ye F, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409–17. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9:243–50. doi: 10.4244/EIJV9I2A40. [DOI] [PubMed] [Google Scholar]
- 18.Latib A, Takagi K, Chizzola G, et al. Excimer Laser LEsion modification to expand non-dilatable stents: the ELLEMENT registry. Cardiovasc Revasc Med. 2014;15:8–12. doi: 10.1016/j.carrev.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Brilakis ES, editor. Manual of Coronary Chronic Total Occlusion Interventions A Step-By-Step Approach. Waltham, MA: Elsevier; 2013. [Google Scholar]
- 20.Raja Y, Routledge HC, Doshi SN. A noncompliant, high pressure balloon to manage undilatable coronary lesions. Catheter Cardiovasc Interv. 2010;75:1067–73. doi: 10.1002/ccd.22430. [DOI] [PubMed] [Google Scholar]
- 21.Burzotta F, Trani C, Mazzari MA, et al. Use of a second buddy wire during percutaneous coronary interventions: a simple solution for some challenging situations. J Invasive Cardiol. 2005;17:171–4. [PubMed] [Google Scholar]
- 22.Meerkin D. My buddy, my friend: focused force angioplasty using the buddy wire technique in an inadequately expanded stent. Catheter Cardiovasc Interv. 2005;65:513–5. doi: 10.1002/ccd.20259. [DOI] [PubMed] [Google Scholar]
- 23.Lindsey JB, Banerjee S, Brilakis ES. Two “buddies” may be better than one: use of two buddy wires to expand an underexpanded left main coronary stent. J Invasive Cardiol. 2007;19:E355–8. [PubMed] [Google Scholar]
- 24.Wilson A, Ardehali R, Brinton TJ, Yeung AC, Lee DP. Cutting balloon inflation for drug-eluting stent underexpansion due to unrecognized coronary arterial calcification. Cardiovasc Revasc Med. 2006;7:185–8. doi: 10.1016/j.carrev.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Finn MT, Green P, Nicholson W, et al. Mother-Daughter-Granddaughter Double GuideLiner Technique for Delivering Stents Past Multiple Extreme Angulations. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.116.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Secco GG, Foin N, Viceconte N, Borgia F, De Luca G, Di Mario C. Optical coherence tomography for guidance of treatment of in-stent restenosis with cutting balloons. EuroIntervention. 2011;7:828–34. doi: 10.4244/EIJV7I7A130. [DOI] [PubMed] [Google Scholar]
- 27.Lee MS, Singh V, Nero TJ, Wilentz JR. Cutting balloon angioplasty. J Invasive Cardiol. 2002;14:552–6. [PubMed] [Google Scholar]
- 28.Kobayashi Y, Teirstein P, Linnemeier T, Stone G, Leon M, Moses J. Rotational atherectomy (stentablation) in a lesion with stent underexpansion due to heavily calcified plaque. Catheter Cardiovasc Interv. 2001;52:208–11. doi: 10.1002/1522-726x(200102)52:2<208::aid-ccd1049>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Sunew J, Chandwaney RH, Stein DW, Meyers S, Davidson CJ. Excimer laser facilitated percutaneous coronary intervention of a nondilatable coronary stent. Catheter Cardiovasc Interv. 2001;53:513–7. doi: 10.1002/ccd.1212. discussion 518. [DOI] [PubMed] [Google Scholar]
- 30.Karacsonyi J, Danek BA, Karatasakis A, Ungi I, Banerjee S, Brilakis ES. Laser Coronary Atherectomy During Contrast Injection for Treating an Underexpanded Stent. JACC Cardiovasc Interv. 2016;9:e147–8. doi: 10.1016/j.jcin.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 31.Ambrosini V, Sorropago G, Laurenzano E, et al. Early outcome of high energy Laser (Excimer) facilitated coronary angioplasty ON hARD and complex calcified and balloOn-resistant coronary lesions: LEONARDO Study. Cardiovasc Revasc Med. 2015;16:141–6. doi: 10.1016/j.carrev.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Deckelbaum LI, Natarajan MK, Bittl JA, et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. The Percutaneous Excimer Laser Coronary Angioplasty (PELCA) Investigators. J Am Coll Cardiol. 1995;26:1264–9. doi: 10.1016/0735-1097(95)00330-4. [DOI] [PubMed] [Google Scholar]
- 33.Tian W, Mahmoudi M, Lhermusier T, et al. Comparison of Rotational Atherectomy, Plain Old Balloon Angioplasty, and Cutting-Balloon Angioplasty Prior to Drug-Eluting Stent Implantation for the Treatment of Heavily Calcified Coronary Lesions. J Invasive Cardiol. 2015;27:387–91. [PubMed] [Google Scholar]
- 34.De Silva K, Roy J, Webb I, et al. A Calcific, Undilatable Stenosis: Lithoplasty, a New Tool in the Box? JACC Cardiovasc Interv. 2017;10:304–306. doi: 10.1016/j.jcin.2016.11.048. [DOI] [PubMed] [Google Scholar]