Abstract
There is an increasing incidence of well-differentiated thyroid cancer worldwide. Much of the increase is secondary to increased detection of small, low-risk tumors, with questionable clinical significance. This review addresses the factors that contribute to the increasing incidence and considers environmental, patient-based and clinician-led influences. Articles addressing the causes of the increased incidence were critically reviewed. A complex interplay of environmental, medical and social pressures has resulted in increased awareness of the thyroid disease risk, increased screening of thyroid cancers and increased diagnosis of thyroid cancers. Although there is evidence to suggest that the true disease incidence may be changing slightly, most of the increase is related to factors that promote early diagnosis of low-risk lesions, which is resulting in a significant phenomenon of overdiagnosis. An improved understanding of these pressures at a global level will enable healthcare policymakers to react appropriately to this challenge in the future.
Keywords: Thyroid neoplasm, incidence, screening, medical overuse, overdiagnosis
Introduction
The incidence of well-differentiated thyroid cancer (WDTC) has been increasing dramatically over the last 20 years worldwide, and WDTC is expected to be the fourth most common cancer by 2030.(1) Several studies have shown a generalized rising trend across different continents with dissimilar health systems and ethnicities.(2, 3) In 2006, Davies and Welsh (4) made an important contribution to our understanding of why these increases were occurring. Analyzing the Surveillance, Epidemiology and End Results (SEER) database from 1973-2002, these authors revealed that 87% of the increased incidence occurred in small (<2-cm) papillary cancers, without an apparent increase in thyroid cancer mortality, although recently, Lim et al. (5) found a statistically significant increase in mortality over a ten-year period (0.40/100.000 to 0.46/100.000) only for advanced stage thyroid cancers. They postulated that the main reason for the increasing incidence of thyroid cancer was overdiagnosis of the clinically occult asymptomatic tumors, accounting for 47-60% of the increase,(6-8) although overidentification is a more accurate term. Many authors have discussed this thesis, arguing that there has been an increase in clinically evident nodules, advanced tumors, aggressive molecular patterns and, exclusively, papillary histology, suggesting that the increase cannot solely be explained by overdiagnosis. (9-12) The concept of overdiagnosis describes a situation where an (asymptomatic) person is diagnosed with a condition whereby the diagnosis does not produce a net benefit for that person. The literature suggests a trend to excessive diagnosis due to increasing access to increasingly sensitive tests as the main cause of overdiagnosis. However, other factors that may explain this recent change.(13, 14) Identification of these factors would help to direct interventions in order to determine the most rational and cost-effective approach toward identifing patients who have a thyroid carcinoma and are most likely to benefit from interventions.
The aim of this review is to describe other factors that may explain the increase in incidence of thyroid carcinoma beyond the excessive use of diagnostic tools, to critically assess their effects and to attempt to organize the myriad contradictory data in a more coherent and integral frame. An epidemiological framework that evolved from the classic epidemiological triangle, named “the wheel of causation model”, was adapted; this model puts the gene/host interaction in the hub and the biological, social and physical determinants of the disease around the hub. (15)
Incidence data
Many authors have confirmed the increase incidence of thyroid carcinoma.(16-20) However, a significant discussion regarding its cause remains. Epidemiological analysis of cancer registries in Europe, (21-26) America(27, 28), the middle east(29) and Asia(30-33) have shown an increase in almost all types of thyroid tumor due to a clear birth cohort effect, while other studies have also identified a time-period effect, suggesting that both events occur simultaneously. (34-38) Identification of an increase by time period (i.e., incidence in older cohorts of approximately 1% per year versus 12% in more recent cohorts), rather than by birth date, helps to identify artificial variations in incidence due to external factors such as changes in diagnostic tools and healthcare access. Some authors have found an increase in all tumor sizes (20, 39-41) and histologies, (42) favoring a true increase in incidence, while other investigators have identified a greater increase in small-volume papillary tumors (<2 cm), favoring increased detection. (39, 43) No increase in mortality was identified in most studies. La Vecchia et al., (44) in a global analysis, found an actual decrease in mortality since 1970 in countries such as France, Italy, the Netherlands and the UK. These changes predate the recent increase in incidence, which reflects the complex interaction between risk factor exposure, diagnostic approach and disease treatment and increases in the rates of disease detection. However, Lim et al. recently found a significant increase in mortality in the US for women, individuals from Caucasian and African-American racial backgrounds, individuals who are older than 79 years, patients with papillary tumors smaller than 4 cm and patients with distant-stage tumors. (5)
Alternative factors that may explain the increasing incidence
The diagnosis of new cases (incidence) is the sum of two main causes: a true increase due to the effect of a factor (recognized or not) that produces new cases and a spurious increase due to increased detection of previously occult cases. When the first factor predominates, causality must be confirmed. However, when the predominant form is the latter, factors contributing to this spurious increase must be identified and controlled in order to classify the situation as a false causation. According to the “wheel of causation model”, four elements contribute to each new case: the patient with the condition, the physician who makes the diagnosis, the healthcare system in which the interaction between the patient and physician occurs and the environment in which the healthcare system exists. Changes in the interaction between any of these factors can have an influence on the incidence, and the complex relationship between all four elements should be considered (Figure 1).
Figure 1.
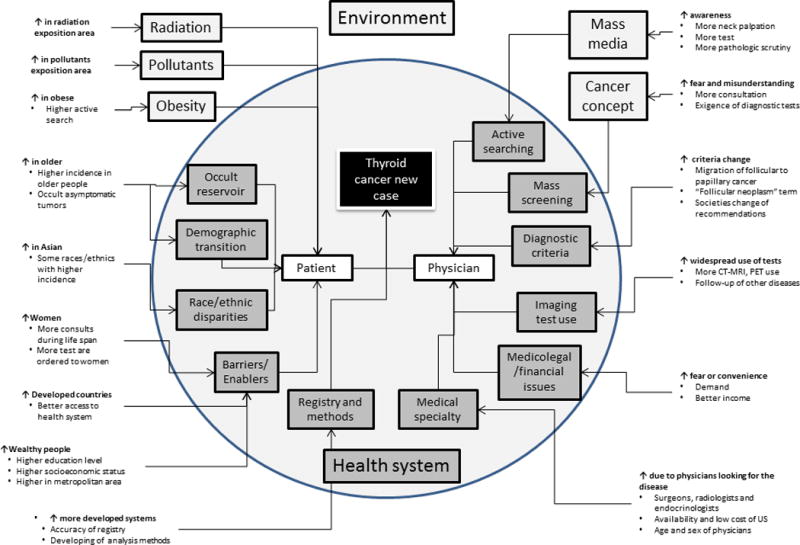
Competing factors and their influence on the incidence of thyroid cancer
Patient Factors
Prevalence and latency of the disease
Since the middle of the 20th century, the phenomenon of occult thyroid carcinoma has been recognized. Many autopsy studies of individuals who died from causes that were unrelated to the thyroid gland have identified a 2-35% rate of occult papillary carcinoma. (45) Most of these tumors were multicentric and occurred more frequently in the nodular glands and in males. The high variation in the rates between series was likely secondary to differences in the processing of the necropsy tissue (specifically the width of the section examined) and the age of the patients included. (45) Another known factor is the rate of papillary carcinoma growth, which is lower in comparison with other cancers. Therefore, if an active searching policy is adopted, many of these occult tumors will be diagnosed and will appear as new cases. Furthermore, if the population lives longer, an even higher number will appear due to the long disease latency. This phenomenon has been designated length bias and lead-time bias. (46)
Sex and ethnic differences
The increase in incidence is particularly significant in women, with a proportion of approximately 3:1. Reports from the United States and China have shown that the age-adjusted incidence increased from 6.4/100.000 to 14.8/100.000 and that this increase was most marked for women ≥45 years (8.8/100.000 to 22.9/100.000).(20, 32)
Although some authors have suggested a hormonal explanation for this difference, others have argued that women seek medical attention more often than men (as occurs during pregnancy or gynecological follow-up) and that during reproductive life, women may be more intensively screened for thyroid conditions. (47) Hall et al. (48) found a three-fold increase in the use of diagnostic imaging in women in comparison with men, and the largest increase occurred in young women. This may help to explain why the largest increase in incidence has occurred in the 20-50-year age group rather than in older women. (49)
Other authors have suggested race, ethnicity and nativity as contributing factors.(50-55) In Sweden, immigrants from the middle east, Asia and South America have an increased incidence of WDTC, (56) and in New Zealand, women from Pacific regions have a higher incidence in comparison with women of European descent. (57) However, other authors did not find differences associated with race in the US population. (58, 59) As some ethnic populations have a higher incidence of WDTC, the specific geographical locations of these populations in some countries and states could also be associated with an increase in incidence. Although gender, race and geographic factors play roles, the global trend is toward an increasing incidence worldwide.
Finally, the incidence of WDTC is higher in older people in comparison with young people. (34) However, the distribution follows an inverted U pattern, with a higher incidence for intermediate age-groups. This suggests that age alone may not be the sole factor responsible for this finding, and due to the demographic transition to an aging population, it is expected that increases in the WDTC case number are likely.
Patients’ beliefs
It has been suggested that a vicious circle exists between the information that patients receive from the media or community and the discovery of subclinical disease.(60) Distortion of patients’ perceptions that is induced by news coverage regarding the frequency and severity of the disease has been observed in patients with many types of tumor. (61) As detection of WDTC in the population increases, awareness also rises. This awareness forces further investigation, which snowballs the rates of detection further. Kahn et al. (62) found that only 40% of patients with a recent diagnosis of a thyroid tumor consulted physicians due to a nodule or “symptoms” and Brito et al., (60) and Bahl et al., (63) found that from the group of symptomatic patients, 27-48% had a thyroid carcinoma that was unrelated to the original symptoms. Self-detection of a nodule, self-detection of a lymph node, or a recent appearance of obstructive or invasive symptoms, such as dyspnea and dysphonia, are infrequent. Most neck symptoms are generally unrelated to thyroid disease, such as the symptoms that occur with globus pharyngeus or neck pain. However, patients seek explanations of their symptomatology and often focus on the thyroid as a result. This can influence the physician’s decision regarding further tests.
Simiarly, Park et al., (64) revealed that most patients are generally in favor of public screening strategies and that most consider the identification of small-volume thyroid cancer as potentially lifesaving, which supports widespread use of diagnostic tests to explain symptoms. Busco et al.(65) has proposed that some public concerns regarding the safety and health effects of environmental risk factors, such as nuclear energy, can prompt a spontaneous active surveillance. Han et al., (66) found that the most common reason for participation in WDTC screening was anxiety regarding health without symptoms, followed by workplace mass examinations, and abnormal symptoms. This suggests that a perception that WDTC screening is beneficial is the main factor that motivates patients to request testing.
Another related factor is the “snowball” effect of the index patient on the family. As family history is a risk factor for some tumors, the finding of an index case triggers panic and an active search in close family members.(67) Due to the occult disease prevalence, the possibility of finding a nodule by ultrasound is close to 70%, and approximately 5-30% of these asymptomatic nodules are ultimately confirmed as a thyroid carcinoma. For the case of WDTC, although genetic and familial associations have been shown, these are rare and difficult to differentiate from the effect of the more intense scrutiny of family members following diagnosis of the index case. (68) Finally, anxiety due to the “fear” of cancer can trigger both an initial referral and more intense investigation. Because many of the patients in whom incidental detection occurs are middle-age women, the fear of dying in young mothers can lead to pressure for physicians to arrange imaging tests to look for the “occult” cancer.
Obesity
A recent meta-analysis of observational studies found a relationship between obesity and the risk of WDTC, with an incidence increase of approximately 55% in obese people. (69) Because obesity has been growing worldwide over recent years, this association may explain the increasing incidence of WDTC. Some authors have suggested that the mechanism that explains this association is mediated by the serum levels of insulin, (70) but other studies did not find a correlation. (71) Although the relationship has been detected with all types of thyroid tumors, the recent increases are predominantly from papillary carcinoma, making the role of obesity as a cause of the increased WDTC incidence questionable. Moreover, it is possible that greater scrutiny occurs in obese people due to the wrong belief that there is a link between thyroid function and being overweight.
Physician Factors
Diagnostic approach of physicians
The awareness of physicians about WDTC can influence the rate of detection.(72) If the physician is sensitive to the disease and actively searches for nodules during the physical examination, more nodules will be detected, which leads to diagnosis of asymptomatic thyroid cancers; the physician then receives positive feedback, having identified a cancer, which serves to reinforce the active search.(60) There is evidence to suggest a trend toward higher rates of neck palpation by primary care physicians. (73) Some authors (60, 62, 74) have recognized five different patterns of identification in patients with a recent diagnosis of WDTC (40% by patient concerns/symptoms, 16% by the physician, 11% incidentally discovered in imaging tests, 26% discovered in a pathology assessment of a surgical sample from benign disease, and 7% in other patterns). Sixty to seventy percent of patients were diagnosed in the absence of symptoms, which suggests an important effect of active searching by physicians. Many studies (65, 75-78) have identified a correlation between the number of ultrasound machines, FNAB number (as an indicator of physician diagnostic activity) and the rate of WDTC. A trend toward more liberal use of FNAB for any type of nodule may partially explain the higher incidence.
A similar situation can occur with pathologists. They could use different thresholds to define malignancy and apply increasing levels of scrutiny to their specimens.(79, 80) An increasing number of thyroidectomies are performed every year; most are for benign conditions, and 7 to 13% of specimens have incidental microcarcinomas that are less than 1 cm. Some authors (60, 62) have shown a proportion of 14-26% of incidentally discovered tumors in benign thyroidectomy samples, and it has been demonstrated that the number of blocks examined from thyroidectomy specimens for retrosternal goiters increased from 2.5 in 1966 to 9.1 in 2005.(81) With the current improvement of pathologic techniques, which allow examinations of thinner tissue slices, the resultant increase in the identification of small tumors reinforces the behavior, leading to a further increase in active searching. (73, 82)
Finally, media information about environmental radioactive exposition after nuclear accidents and situations such as “the Angelina Jolie” effect in breast cancer, reinforces active searching by physicians. (74)
Diagnostic criteria
The change in diagnostic criteria may also explain an increase in the incidence of papillary carcinoma. The re-categorization of some cases of follicular carcinoma to the follicular variant of papillary carcinoma that occurred in 1988 can explain part of the increased slope. Otto et al.,(83) and Verkooijen et al., (84) described a re-categorization of approximately 12-20% of follicular tumors to papillary tumors.
Another factor may be the terms used to classify nodules and tumors. While the histological classification of tumors does not typically have gray areas, the cytological diagnosis of thyroid nodules (with potentially alarming terms such as follicular neoplasm)(85) and pathological reports of small clinically insignificant microtumors, such as carcinomas, are strong instigators of active searching for WDTC. Experience with other tumors, such as breast ductal carcinoma, prostate carcinoma, kidney masses and low-grade sarcomas, for which histologic definitions were changed to other less alarming terms, has demonstrated that simple refinement of nomenclature can lead to decreases in incidence of cancer without changes in prognosis.
Incidental finding during follow-up of other diseases and tumors
Technological improvements with better discrimination power and more widespread use of diagnostic tests for myriad situations can incidentally identify thyroid nodules. Despite the fact that most of these tumors are asymptomatic, all patients then require adequate diagnosis.(60, 62) Hoang et al. (35) found a strong correlation between the numbers of CT scans made in a specific geographic area and the incidence of thyroid carcinoma. In addition to index nodules having a tendency toward occult malignancy, the process of investigating these nodules with high-definition ultrasonography can lead to the identification of further lesions (86).
Many studies have found an association between other tumors and thyroid carcinoma, with no biological explanation. (87) This phenomenon may be explained by the intensive follow-up screening that survivors from other tumors undergo, which involves a higher number of tests. With the increased effectiveness and availability of therapies for cancer, the number of survivors is elevated and the possibility of finding an incidental tumor is higher.
A similar situation occurred when iodine supplementation programs were initiated in conjunction with goiter monitoring strategies. The increased level of scrutiny for one condition led to a consequential increase in the WDTC incidence. (30, 77)
Medical education and specialty
Some authors have proposed that some characteristics of physicians who are located in specific geographic areas may increase the rate of detection. If the physicians have a particular interest or training in thyroid pathology or are influenced by governmental or social policies, it is expected that more patients with WDTC will be identified. Udelsman and Zhang(88) found a correlation between the US states with a higher increase in WDTC incidence and the proportion of endocrinologists and surgeons and number of ultrasound tests in these states. In South Korea, primary care physicians frequently own an ultrasound machine (89) and order an ultrasound as an opportunistic screening test during a routine exam. (64) Nagar et al.,(90) explored the relationship between the age of the physician and incidence of WDTC and found that geographic areas with a high proportion of physicians older than 55 years had a lower increase in incidence compared with areas with a high proportion of physicians younger than 55 years; the correlation was explained by the varying rate of technological adoption. Rosen et al., (91) found a correlation between the female sex of the physician and a higher number of imaging studies, suggesting that the style of female physicians is more prone to active searching. An increase in the incidence of WDTC has been demonstrated in women after hysterectomy. (92) However, this only occurs in the first two years after surgery, which suggests that screening of nodules by the gynecologist is an important factor in this population.
The consensus opinions and guidelines developed by medical societies could also influence the detection of thyroid nodules and the use of diagnostics tools. The introduction of an ultrasonographic-specific size to indicate a FNAB could increase the rate of biopsy with a consequently higher identification rate of asymptomatic tumors, an observation that has already been made for other diseases including diabetes and hypertension. (93) Additionally, changes in the approach to thyroid disease that are suggested by scientific associations can cause an increase in incidence; for example, the recommendations for treating benign thyroid diseases with thyroidectomy produces more specimens, leading to a progressive increase in the detection of thyroid cancer. (81) Van den Bruel et al.,(76) found that in regions with a high cancer incidence, patients with thyrotoxicosis and goiter were most frequently treated with surgery.
Training in neck palpation could be another factor influencing the WDTC incidence. There has been a trend away from teaching physical examination skills in medical school curricula, and much of the physical exam has been replaced with the use of imaging tests. Studies comparing the accuracy of physical examination by expert clinicians with ultrasound found that sensitivity and specificity of the physical exam was 22% and 94%, respectively, with a poor kappa value for agreement of 0.18; nodules larger than 2 cm were only identified by clinical exam in 48% of patients; and nodules larger than 2 cm were confirmed by ultrasound in only 32% of patients with a clinical suspicion of a thyroid nodule.(94, 95) These findings help explain why the incidence of thyroid carcinomas that are larger than 2 cm can also be a product of increased use of imaging tests.
Economic and medicolegal issues
Economic and financial factors related to medical practice have also been highlighted. Ahn and Welch (96), described the exaggerated reactions of scientific societies to the Physician Coalition for Prevention of Overdiagnosis of WDTC in South Korea after a call to avoid screening in WDTC. Lee et al., (89) examined the relationship between the incidence of WDTC and the financial characteristics of healthcare systems between countries and found a negative correlation with a low public health expenditure. The authors suggest that a higher proportion of private health financing means a greater individual ability to pay for health services with the effect of induced demand by providers and a consequential overuse of services, such as medical tests.
Some authors have also described the influence of medicolegal issues. In fear of litigation in the event of a missed diagnosis, many pathologists have increased the number of reports that indicate indeterminate or malignant tumors, even after second revisions by experts who discard this diagnosis. (97) In the case of radiologists, a failure to report findings on an imaging study would carry adverse liability if the nodule became clinically significant in the future. The 3-tiered system proposed by the American College of Radiology to recommend follow-up or intervention have shown a decrease of 35-46% in interventions on incidental nodules with an approximate miss of 1.3% of malignancies.(98) A similar situation occurs for other specialists such as endocrinologists and surgeons.
Healthcare system factors
Access to healthcare system
Some authors have proposed that better access to healthcare and subsequent increased availability of diagnostic technology may explain the increase in incidence. Epidemiologic evidence from cancer registry analyses showed that the increase in WDTC was higher in developed countries (12/100.000 in Israel and 10/100.000 in the United States vs 1.5/100.000 in Uganda and 2.6/100.000 in Egypt) where access to the healthcare system is better.(3) A relationship between race, higher education level, urban residence, location around a metropolitan area and higher socioeconomic status, which all allow better healthcare access, and a higher incidence of WDTC has been found, even in healthcare systems with no barriers to access.(65, 99-102) In South Korea, the incidence of WDTC is 55% lower in the low-income group in comparison with high income. (103) Morris et al.,(104, 105) found that 25% of the incidence can be explained by variables associated with better healthcare access. A higher incidence of WDTC in comparison with the national average has also been found in specific populations with better access to healthcare, such as the military in the US. (106) However, other authors have failed to find a correlation between access to healthcare and socioeconomic characteristics with the incidence of WDTC. (43, 49, 107)
Registry accuracy and methods of analysis
Improvements in the identification of patients with thyroid tumors can be a potential cause of increasing incidence. Veiga et al.,(108) found a decrease of 25% in the poorly specified histology rate in the Sao Paulo cancer registry and an increase in papillary thyroid carcinomas over the same time scale, possibly due to the re-categorization. Another factor could be under-registration of cases due to the type of information source. Most cancer registries initially collected information from large reference centers. As the registries become more inclusive, information from non-reference centers, where less advanced cases were treated, could artificially increase the incidence of low-risk tumors. (109)
Methods of analysis of epidemiological data may also contribute to the increase. Some studies have demonstrated that data analyzed with newer methods can artificially increase the incidence. (110, 111)
Environmental element
Denomination, screening and overdiagnosis
A historical definition of cancer accepted by most physicians indicates that cancer is a neoplastic disease that can be fatal. (112) According to this definition, cancer would inevitably result in death. However, since the middle of the 20th century, it has been demonstrated that cancer can be cured in many patients, and in others, the disease may be rendered a chronic condition, with which it is possible to live for years. In addition, it is accepted today that many humans can live with malignancies that never become clinically relevant, as occurs with prostate carcinoma in older men.
The strongest evidence favoring thyroid carcinoma as a latent tumor is provided by studies from Japan (113, 114) that have demonstrated the slow growth rate and low rate of metastasis of papillary microcarcinomas and their unique biological behavior, which is very different from other common tumors. Nonetheless, in the minds of governments, physicians and patients, WDTC maintains its potential for a fatal outcome.(115) For these reasons, many interventions are focused on screening and early detection, attempting to “control” the disease, assuming that the oncological advantages of early diagnosis and treatment that have been demonstrated in other tumors can be translated to WDTC.(116, 117) Screening by definition is the “identification of an unrecognized disease by the application of tests…and has as its aim the early detection in presumptive healthy individuals of specifics diseases that can be controlled early in their natural history”. (118) With this in mind, a more focused neck examination together with ultrasonography can be used as a screening tool with the result that a large number of individuals are diagnosed with asymptomatic thyroid nodules and require FNAB per the recommendations. However, long-term studies have been unable to demonstrate a significant decrease in the already extremely low rates of mortality as a result of such an approach. The most extreme example of this was seen in South Korea, where the incidence has grown by almost 3% per year in the last decade. This has resulted in WDTC becoming the most common cancer in women, aged 15 to 64 years, in 2013, without any significant change in mortality and where a strong correlation between screening penetration and WDTC incidence was found. (112, 116, 119) Similar patterns have been described worldwide.(6, 36, 84) The problem with this strategy is the impossibility of knowing which of the detected cancers will develop into a clinically relevant condition that threatens the life of the patient. This problem obliges clinicians to treat all detected disease. Data from South Korea showed that smaller tumors were detected more frequently after the adoption of a national screening program and that a dramatic reduction of new cases occurred after recognition of the problem by medical professionals and government agencies. (96)
Radiation
Exposure to ionizing radiation is an accepted risk factor for the development of WDTC. Many sources of radiation that could affect the thyroid gland have been identified, with nuclear energy and diagnostic or therapeutic X-ray exposure being the most common.
The accidents in Chernobyl and Fukushima have been responsible for an acute increase in WDTC in areas of contamination.(120-122) Nonetheless, the evidence of effects in areas far away from the accident, under the path of the “radioactive cloud”, is weak. Even the elevated rate of WDTC around the nuclear plants is debated.(123, 124)
The influence of radiation has also been recognized when the exposure occurs in children. Case-control studies have shown a two-fold increase in incidence when children had a history of exposure to dental X-rays. There is also information that the number of imaging tests that cover the thyroid bed is increasing worldwide, and due to the sensitivity of the thyroid gland to radiation, some authors have suggested that this exposure may be responsible for the increasing incidence of WDTC. (125-127) It is clear that today, children are more exposed to radiation from medical imaging tests than in the past. However, the effect in adults is smaller, so the size of the effect and latency of radiation from these sources may not explain the increase in adults in areas without access to X-ray exposure. The number of CT and X-ray dental exams in the US dramatically increased since 1970, which has accompanied the rising incidence. Other factors include the use of 131I (which has decreased progressively) and radiotherapy for the treatment of malignancies in the head and neck, breast and lymphomas, which may involve the thyroid bed in the treatment field. (128) It is very difficult to isolate these chronic effects from factors related to an increase in screening and active searching for coincident malignancies in patients who are already under oncological surveillance.(129)
Exposure to pollutants
Many studies have suggested a relationship between environmental pollutants (e.g., nitrate, hydrocarbons, pesticides, and heavy metal) and WDTC.(130) Since the 1970s, several flame retardant materials have been used in textiles, rugs, furniture, airplanes, cars, electronic equipment and construction. Among this group of chemical substances, the polybrominated diphenyl ethers (PBDEs) are particularly dangerous for humans because they persist in the environment and can be ingested, inhaled or absorbed through the skin. (131) PBDE was recently associated with the risk of thyroid disease in postmenopausal American women. (132) However, a recent case-control study failed to demonstrate a significant association between PBDE exposition and thyroid cancer. (133) Nitrate is known to affect the metabolism of iodine in the thyroid, and chronic exposure to high levels of nitrate might result in the development of goiter through TSH stimulation. It has been suggested that interaction between high doses of radiation and high levels of nitrate in water can explain the increased number of cases in areas surrounding nuclear plants accidents. (134, 135) However, most studies lack strong epidemiological data, and therefore, the association remains unproven. Although, there may be a relationship between exposure to pollutants and increases in the WDTC incidence, the effect would be located to specific regions with ubiquitous exposure and would therefore not explain a worldwide increase.
Iodine supplementation
Other authors (136) have found an association between excessive dietary iodine and chronic thyroiditis, which is itself said to be associated with an increased rate of papillary cancer, but other authors did not find an association when salt iodization was introduced. (137-139) This excess is limited to specific countries and, again, not sufficiently widespread to explain the worldwide increase.
Conclusion
A high proportion of the increase in the WDTC incidence is due to exaggerated screening and a consequent overdiagnosis. However, other factors are also important and contribute to this phenomenon. Overdiagnosis is the final consequence of the interplay of factors including demographic transition, disparities in healthcare access, quality of the registry, mass-media influence, economic and medicolegal incentives and medical education. Many of these factors are interrelated, and the weight of each individual effect on the overall picture is unknown. Most of the available literature focuses on individual factors, which makes the overall influence of these interrelated individual pressures impossible to quantify. Nonetheless, an improved understanding of these pressures at a global level will allow healthcare policymakers to react appropriately to this challenge in the future.
In this review, we have described these factors and attempted to explain why they lead to overdiagnosis. However, we also attempted to understand the whole picture. Most information about this subject is fractured, being presented in different studies from different academic areas and in numerous journals, so it is difficult to integrate the data. This difficulty induces extreme positions between stakeholders, each favoring only one factor without considering that a single causal explanation for such a complex problem as the higher incidence of thyroid carcinoma is improbable.
Acknowledgments
This study didn’t received any grant support
Footnotes
“This article was written by members of the International Head and Neck Scientific Group (www.IHNSG.com)”
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–28. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 3.Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20(5):525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 5.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 7.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid. 2015;25(10):1127–36. doi: 10.1089/thy.2015.0116. [DOI] [PubMed] [Google Scholar]
- 8.O’Grady TJ, Gates MA, Boscoe FP. Thyroid cancer incidence attributable to overdiagnosis in the United States 1981-2011. Int J Cancer. 2015;137(11):2664–73. doi: 10.1002/ijc.29634. [DOI] [PubMed] [Google Scholar]
- 9.Leenhardt L, Grosclaude P, Cherie-Challine L, Thyroid Cancer C. Increased incidence of thyroid carcinoma in france: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14(12):1056–60. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 10.Ward EM, Jemal A, Chen A. Increasing incidence of thyroid cancer: is diagnostic scrutiny the sole explanation? Future Oncol. 2010;6(2):185–8. doi: 10.2217/fon.09.161. [DOI] [PubMed] [Google Scholar]
- 11.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–91. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathur A, Moses W, Rahbari R, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer. 2011;117(19):4390–5. doi: 10.1002/cncr.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocr Pract. 2015;21(6):686–96. doi: 10.4158/EP14466.DSCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fos PJ. Epidemiology foundations The science of public health. 1st. Jossey-Bass; 2011. pp. 43–50. [Google Scholar]
- 16.Burgess JR. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982-1997) Thyroid. 2002;12(2):141–9. doi: 10.1089/105072502753522374. [DOI] [PubMed] [Google Scholar]
- 17.Colonna M, Grosclaude P, Remontet L, et al. Incidence of thyroid cancer in adults recorded by French cancer registries (1978-1997) Eur J Cancer. 2002;38(13):1762–8. doi: 10.1016/s0959-8049(02)00110-7. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds RM, Weir J, Stockton DL, Brewster DH, Sandeep TC, Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 2005;62(2):156–62. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 19.Montanaro F, Pury P, Bordoni A, Lutz JM, Swiss Cancer Registries N Unexpected additional increase in the incidence of thyroid cancer among a recent birth cohort in Switzerland. Eur J Cancer Prev. 2006;15(2):178–86. doi: 10.1097/01.cej.0000197450.94980.36. [DOI] [PubMed] [Google Scholar]
- 20.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115(16):3801–7. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 21.Akslen LA, Haldorsen T, Thoresen SO, Glattre E. Incidence pattern of thyroid cancer in Norway: influence of birth cohort and time period. Int J Cancer. 1993;53(2):183–7. doi: 10.1002/ijc.2910530202. [DOI] [PubMed] [Google Scholar]
- 22.dos Santos Silva I, Swerdlow AJ. Thyroid cancer epidemiology in England and Wales: time trends and geographical distribution. Br J Cancer. 1993;67(2):330–40. doi: 10.1038/bjc.1993.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olaleye O, Ekrikpo U, Moorthy R, et al. Increasing incidence of differentiated thyroid cancer in South East England: 1987-2006. Eur Arch Otorhinolaryngol. 2011;268(6):899–906. doi: 10.1007/s00405-010-1416-7. [DOI] [PubMed] [Google Scholar]
- 24.McNally RJ, Blakey K, James PW, Gomez Pozo B, Basta NO, Hale J. Increasing incidence of thyroid cancer in Great Britain, 1976-2005: age-period-cohort analysis. Eur J Epidemiol. 2012;27(8):615–22. doi: 10.1007/s10654-012-9710-x. [DOI] [PubMed] [Google Scholar]
- 25.Londero SC, Krogdahl A, Bastholt L, et al. Papillary thyroid carcinoma in Denmark 1996-2008: an investigation of changes in incidence. Cancer Epidemiol. 2013;37(1):e1–6. doi: 10.1016/j.canep.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Husson O, Haak H, van Steenbergen L, et al. Rising incidence, no change in survival and decreasing mortality from thyroid cancer in The Netherlands since 1989. Endocrine-Related Cancer. 2013;20:263–71. doi: 10.1530/ERC-12-0336. [DOI] [PubMed] [Google Scholar]
- 27.Zheng T, Holford TR, Chen Y, et al. Time trend and age-period-cohort effect on incidence of thyroid cancer in Connecticut, 1935-1992. Int J Cancer. 1996;67(4):504–9. doi: 10.1002/(SICI)1097-0215(19960807)67:4<504::AID-IJC7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Semenciw R, Ugnat AM, Mao Y. Increasing thyroid cancer incidence in Canada, 1970-1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85(9):1335–9. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubina A, Cohen O, Barchana M, et al. Time trends of incidence rates of thyroid cancer in Israel: what might explain the sharp increase. Thyroid. 2006;16(10):1033–40. doi: 10.1089/thy.2006.16.1033. [DOI] [PubMed] [Google Scholar]
- 30.Burgess JR, Dwyer T, McArdle K, Tucker P, Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978-1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab. 2000;85(4):1513–7. doi: 10.1210/jcem.85.4.6554. [DOI] [PubMed] [Google Scholar]
- 31.Saika K, Matsuda T, Sobue T. Incidence rate of thyroid cancer by histological type in Japan. Jpn J Clin Oncol. 2014;44(11):1131–2. doi: 10.1093/jjco/hyu179. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Wang W. Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia Pac J Public Health. 2015;27(2):NP223–9. doi: 10.1177/1010539512436874. [DOI] [PubMed] [Google Scholar]
- 33.Xie SH, Chen J, Zhang B, et al. Time trends and age-period-cohort analyses on incidence rates of thyroid cancer in Shanghai and Hong Kong. BMC Cancer. 2014;14:975. doi: 10.1186/1471-2407-14-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu C, Zheng T, Kilfoy BA, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973-2004. Thyroid. 2009;19(10):1061–6. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoang JK, Choudhury KR, Eastwood JD, et al. An exponential growth in incidence of thyroid cancer: trends and impact of CT imaging. AJNR Am J Neuroradiol. 2014;35(4):778–83. doi: 10.3174/ajnr.A3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colonna M, Guizard AV, Schvartz C, et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983-2000) Eur J Cancer. 2007;43(5):891–900. doi: 10.1016/j.ejca.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Dal Maso L, Lise M, Zambon P, et al. Incidence of thyroid cancer in Italy, 1991-2005: time trends and age-period-cohort effects. Ann Oncol. 2011;22(4):957–63. doi: 10.1093/annonc/mdq467. [DOI] [PubMed] [Google Scholar]
- 38.Meza R, Chang JT. Multistage carcinogenesis and the incidence of thyroid cancer in the US by sex, race, stage and histology. BMC Public Health. 2015;15:789. doi: 10.1186/s12889-015-2108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–52. doi: 10.1016/j.surg.2010.10.016. discussion 1152-3. [DOI] [PubMed] [Google Scholar]
- 40.Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010;200(4):454–61. doi: 10.1016/j.amjsurg.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):1357–61. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aschebrook-Kilfoy B, Grogan RH, Ward MH, Kaplan E, Devesa SS. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23(8):1015–21. doi: 10.1089/thy.2012.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horn-Ross PL, Lichtensztajn DY, Clarke CA, et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1067–79. doi: 10.1158/1055-9965.EPI-13-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–95. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 45.Furuya-Kanamori L, Bell KJ, Clark J, Glasziou P, Doi SA. Prevalence of Differentiated Thyroid Cancer in Autopsy Studies Over Six Decades: A Meta-Analysis. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.7419. [DOI] [PubMed] [Google Scholar]
- 46.Pelikan S, Moskowitz M. Effects of lead time, length bias, and false-negative assurance on screening for breast cancer. Cancer. 1993;71(6):1998–2005. doi: 10.1002/1097-0142(19930315)71:6<1998::aid-cncr2820710613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 47.Kilfoy BA, Devesa SS, Ward MH, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1092–100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall SF, Walker H, Siemens R, Schneeberg A. Increasing detection and increasing incidence in thyroid cancer. World J Surg. 2009;33(12):2567–71. doi: 10.1007/s00268-009-0226-9. [DOI] [PubMed] [Google Scholar]
- 49.Hanley JP, Jackson E, Morrissey LA, et al. Geospatial and Temporal Analysis of Thyroid Cancer Incidence in a Rural Population. Thyroid. 2015;25(7):812–22. doi: 10.1089/thy.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corsten MJ, Hearn M, McDonald JT, Johnson-Obaseki S. Incidence of differentiated thyroid cancer in Canada by City of residence. J Otolaryngol Head Neck Surg. 2015;44:36. doi: 10.1186/s40463-015-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finlayson A, Barnes I, Sayeed S, McIver B, Beral V, Ali R. Incidence of thyroid cancer in England by ethnic group, 2001-2007. Br J Cancer. 2014;110(5):1322–7. doi: 10.1038/bjc.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitz MR, Sider JG, Katz RL, Pollack ES, Newell GR. Ethnic patterns of thyroid cancer incidence in the United States, 1973-1981. Int J Cancer. 1988;42(4):549–53. doi: 10.1002/ijc.2910420413. [DOI] [PubMed] [Google Scholar]
- 53.Horn-Ross PL, Chang ET, Clarke CA, et al. Nativity and papillary thyroid cancer incidence rates among Hispanic women in California. Cancer. 2012;118(1):216–22. doi: 10.1002/cncr.26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horn-Ross PL, McClure LA, Chang ET, et al. Papillary thyroid cancer incidence rates vary significantly by birthplace in Asian American women. Cancer Causes Control. 2011;22(3):479–85. doi: 10.1007/s10552-010-9720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu GP, Li JC, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20(5):465–73. doi: 10.1089/thy.2008.0281. [DOI] [PubMed] [Google Scholar]
- 56.Mousavi SM, Brandt A, Sundquist J, Hemminki K. Risks of papillary and follicular thyroid cancer among immigrants to Sweden. Int J Cancer. 2011;129(9):2248–55. doi: 10.1002/ijc.25867. [DOI] [PubMed] [Google Scholar]
- 57.Meredith I, Sarfati D, Atkinson J, Blakely T. Thyroid cancer in Pacific women in New Zealand. N Z Med J. 2014;127(1395):52–62. [PubMed] [Google Scholar]
- 58.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol. 2013;20(8):2746–53. doi: 10.1245/s10434-013-2892-y. [DOI] [PubMed] [Google Scholar]
- 59.Magreni A, Bann DV, Schubart JR, Goldenberg D. The effects of race and ethnicity on thyroid cancer incidence. JAMA Otolaryngol Head Neck Surg. 2015;141(4):319–23. doi: 10.1001/jamaoto.2014.3740. [DOI] [PubMed] [Google Scholar]
- 60.Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The Impact of Subclinical Disease and Mechanism of Detection on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Olmsted County, Minnesota During 1935 Through 2012. Thyroid. 2015;25(9):999–1007. doi: 10.1089/thy.2014.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen JD, Scherr CL, Brown N, Jones C, Christy K, Hurley RJ. Public estimates of cancer frequency: cancer incidence perceptions mirror distorted media depictions. J Health Commun. 2014;19(5):609–24. doi: 10.1080/10810730.2013.837551. [DOI] [PubMed] [Google Scholar]
- 62.Kahn C, Simonella L, Sywak M, Boyages S, Ung O, O’Connell D. Pathways to the diagnosis of thyroid cancer in New South Wales: a population-based cross-sectional study. Cancer Causes Control. 2012;23(1):35–44. doi: 10.1007/s10552-011-9852-2. [DOI] [PubMed] [Google Scholar]
- 63.Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients. World J Surg. 2014;38(6):1312–7. doi: 10.1007/s00268-013-2407-9. [DOI] [PubMed] [Google Scholar]
- 64.Park SH, Lee B, Lee S, et al. A qualitative study of women’s views on overdiagnosis and screening for thyroid cancer in Korea. BMC Cancer. 2015;15:858. doi: 10.1186/s12885-015-1877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busco S, Giorgi Rossi P, Sperduti I, Pezzotti P, Buzzoni C, Pannozzo F. Increased incidence of thyroid cancer in Latina, Italy: a possible role of detection of subclinical disease. Cancer Epidemiol. 2013;37(3):262–9. doi: 10.1016/j.canep.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Han MA, Choi KS, Lee HY, Kim Y, Jun JK, Park EC. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pac J Cancer Prev. 2011;12(7):1657–63. [PubMed] [Google Scholar]
- 67.Bresner L, Banach R, Rodin G, Thabane L, Ezzat S, Sawka AM. Cancer-related worry in Canadian thyroid cancer survivors. J Clin Endocrinol Metab. 2015;100(3):977–85. doi: 10.1210/jc.2014-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nixon IJ, Suarez C, Simo R, et al. The impact of family history on non-medullary thyroid cancer. Eur J Surg Oncol. 2016;42(10):1455–63. doi: 10.1016/j.ejso.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16(12):1042–54. doi: 10.1111/obr.12321. [DOI] [PubMed] [Google Scholar]
- 70.Marcello MA, Cunha LL, Batista FA, Ward LS. Obesity and thyroid cancer. Endocr Relat Cancer. 2014;21(5):T255–71. doi: 10.1530/ERC-14-0070. [DOI] [PubMed] [Google Scholar]
- 71.Luo J, Phillips L, Liu S, Wactawski-Wende J, Margolis KL. Diabetes, Diabetes Treatment, and Risk of Thyroid Cancer. J Clin Endocrinol Metab. 2016;101(3):1243–8. doi: 10.1210/jc.2015-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leenhardt L, Bernier MO, Boin-Pineau MH, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150(2):133–9. doi: 10.1530/eje.0.1500133. [DOI] [PubMed] [Google Scholar]
- 73.Leux C, Colonna M, Guizard AV, et al. Time trends in the geographic variation of thyroid cancer incidence by tumor size from 1983 to 2000 in France. Rev Epidemiol Sante Publique. 2009;57(6):403–10. doi: 10.1016/j.respe.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Evans DG, Barwell J, Eccles DM, et al. The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014;16(5):442. doi: 10.1186/s13058-014-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall SF, Irish J, Groome P, Griffiths R. Access, excess, and overdiagnosis: the case for thyroid cancer. Cancer Med. 2014;3(1):154–61. doi: 10.1002/cam4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van den Bruel A, Francart J, Dubois C, et al. Regional variation in thyroid cancer incidence in Belgium is associated with variation in thyroid imaging and thyroid disease management. J Clin Endocrinol Metab. 2013;98(10):4063–71. doi: 10.1210/jc.2013-1705. [DOI] [PubMed] [Google Scholar]
- 77.Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16(1):47–53. doi: 10.1089/thy.2006.16.47. [DOI] [PubMed] [Google Scholar]
- 78.Zevallos JP, Hartman CM, Kramer JR, Sturgis EM, Chiao EY. Increased thyroid cancer incidence corresponds to increased use of thyroid ultrasound and fine-needle aspiration: a study of the Veterans Affairs health care system. Cancer. 2015;121(5):741–6. doi: 10.1002/cncr.29122. [DOI] [PubMed] [Google Scholar]
- 79.Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130(5):736–44. doi: 10.1309/AJCPKP2QUVN4RCCP. [DOI] [PubMed] [Google Scholar]
- 80.Franc B, de la Salmoniere P, Lange F, et al. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol. 2003;34(11):1092–100. doi: 10.1016/s0046-8177(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 81.Grodski S, Brown T, Sidhu S, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144(6):1038–43. doi: 10.1016/j.surg.2008.08.023. discussion 1043. [DOI] [PubMed] [Google Scholar]
- 82.Sakorafas GH, Stafyla V, Kolettis T, Tolumis G, Kassaras G, Peros G. Microscopic papillary thyroid cancer as an incidental finding in patients treated surgically for presumably benign thyroid disease. J Postgrad Med. 2007;53(1):23–6. doi: 10.4103/0022-3859.30323. [DOI] [PubMed] [Google Scholar]
- 83.Otto KJ, Lam JS, MacMillan C, Freeman JL. Diminishing diagnosis of follicular thyroid carcinoma. Head Neck. 2010;32(12):1629–34. doi: 10.1002/hed.21373. [DOI] [PubMed] [Google Scholar]
- 84.Verkooijen HM, Fioretta G, Pache JC, et al. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14(1):13–7. doi: 10.1023/a:1022593923603. [DOI] [PubMed] [Google Scholar]
- 85.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19(11):1159–65. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 86.Uppal A, White MG, Nagar S, et al. Benign and Malignant Thyroid Incidentalomas Are Rare in Routine Clinical Practice: A Review of 97,908 Imaging Studies. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1327–31. doi: 10.1158/1055-9965.EPI-15-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Canchola AJ, Horn-Ross PL, Purdie DM. Risk of second primary malignancies in women with papillary thyroid cancer. Am J Epidemiol. 2006;163(6):521–7. doi: 10.1093/aje/kwj072. [DOI] [PubMed] [Google Scholar]
- 88.Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24(3):472–9. doi: 10.1089/thy.2013.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee T, Kim S, Cho H, Lee JH. The Incidence of Thyroid Cancer Is Affected by the Characteristics of a Healthcare System. J Korean Med Sci. 2012;27:1491–98. doi: 10.3346/jkms.2012.27.12.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagar S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Grogan RH. Age of diagnosing physician impacts the incidence of thyroid cancer in a population. Cancer Causes Control. 2014;25(12):1627–34. doi: 10.1007/s10552-014-0467-2. [DOI] [PubMed] [Google Scholar]
- 91.Rosen MP, Davis RB, Lesky LG. Utilization of outpatient diagnostic imaging. Does the physician’s gender play a role? J Gen Intern Med. 1997;12(7):407–11. doi: 10.1046/j.1525-1497.1997.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luoto R, Grenman S, Salonen S, Pukkala E. Increased risk of thyroid cancer among women with hysterectomies. Am J Obstet Gynecol. 2003;188(1):45–8. doi: 10.1067/mob.2003.121. [DOI] [PubMed] [Google Scholar]
- 93.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237(3):794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 94.Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17(8):487–96. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 95.Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992;20(1):37–42. doi: 10.1002/jcu.1870200107. [DOI] [PubMed] [Google Scholar]
- 96.Ahn HS, Welch HG. South Korea’s Thyroid-Cancer “Epidemic”–Turning the Tide. N Engl J Med. 2015;373(24):2389–90. doi: 10.1056/NEJMc1507622. [DOI] [PubMed] [Google Scholar]
- 97.Renshaw AA, Gould EW. Why there is the tendency to “overdiagnose” the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002;117(1):19–21. doi: 10.1309/CJEU-XLQ7-UPVE-NWFV. [DOI] [PubMed] [Google Scholar]
- 98.Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol. 2015;12(2):143–50. doi: 10.1016/j.jacr.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 99.Guay B, Johnson-Obaseki S, McDonald JT, Connell C, Corsten M. Incidence of differentiated thyroid cancer by socioeconomic status and urban residence: Canada 1991-2006. Thyroid. 2014;24(3):552–5. doi: 10.1089/thy.2013.0308. [DOI] [PubMed] [Google Scholar]
- 100.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980-2008. Thyroid. 2013;23(1):103–10. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sprague BL, Warren Andersen S, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19(6):585–93. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 102.Reitzel LR, Nguyen N, Li N, Xu L, Regan SD, Sturgis EM. Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity. Thyroid. 2014;24(3):556–67. doi: 10.1089/thy.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JM, Kim HM, Jung BY, Park EC, Cho WH, Lee SG. The association between cancer incidence and family income: analysis of Korean National Health Insurance cancer registration data. Asian Pac J Cancer Prev. 2012;13(4):1371–6. doi: 10.7314/apjcp.2012.13.4.1371. [DOI] [PubMed] [Google Scholar]
- 104.Morris LG, Sikora AG, Myssiorek D, DeLacure MD. The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol. 2008;15(4):1169–76. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 105.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23(7):885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Enewold LR, Zhou J, Devesa SS, et al. Thyroid cancer incidence among active duty U.S. military personnel, 1990-2004. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2369–76. doi: 10.1158/1055-9965.EPI-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lise M, Franceschi S, Buzzoni C, et al. Changes in the incidence of thyroid cancer between 1991 and 2005 in Italy: a geographical analysis. Thyroid. 2012;22(1):27–34. doi: 10.1089/thy.2011.0038. [DOI] [PubMed] [Google Scholar]
- 108.Veiga LH, Neta G, Aschebrook-Kilfoy B, Ron E, Devesa SS. Thyroid cancer incidence patterns in Sao Paulo, Brazil, and the U.S. SEER program, 1997-2008. Thyroid. 2013;23(6):748–57. doi: 10.1089/thy.2012.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andreoni GI, Veneziano DB, Giannotti Filho O, Marigo C, Mirra AP, Fonseca LA. Cancer incidence in eighteen cities of the State of Sao Paulo, Brazil. Rev Saude Publica. 2001;35(4):362–7. doi: 10.1590/s0034-89102001000400005. [DOI] [PubMed] [Google Scholar]
- 110.Pickle LW, Hao Y, Jemal A, et al. A new method of estimating United States and state-level cancer incidence counts for the current calendar year. CA Cancer J Clin. 2007;57(1):30–42. doi: 10.3322/canjclin.57.1.30. [DOI] [PubMed] [Google Scholar]
- 111.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–82. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 113.Oda H, Miyauchi A, Ito Y, et al. Incidences of Unfavorable Events in the Management of Low-Risk Papillary Microcarcinoma of the Thyroid by Active Surveillance Versus Immediate Surgery. Thyroid. 2016;26(1):150–5. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34(6):1222–31. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 115.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15(6):e234–42. doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–7. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 117.Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet. 2014;384(9957):1848. doi: 10.1016/S0140-6736(14)62242-X. [DOI] [PubMed] [Google Scholar]
- 118.A Dictionary of Epidemiology. 5th. New York: Oxford University Press; 2008. [Google Scholar]
- 119.Oh CM, Won YJ, Jung KW, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res Treat. 2016;48(2):436–50. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tronko M, Mabuchi K, Bogdanova T, et al. Thyroid cancer in Ukraine after the Chernobyl accident (in the framework of the Ukraine-US Thyroid Project) J Radiol Prot. 2012;32(1):N65–9. doi: 10.1088/0952-4746/32/1/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ostroumova E, Gudzenko N, Brenner A, et al. Thyroid cancer incidence in Chornobyl liquidators in Ukraine: SIR analysis, 1986-2010. Eur J Epidemiol. 2014;29(5):337–42. doi: 10.1007/s10654-014-9896-1. [DOI] [PubMed] [Google Scholar]
- 122.Katanoda K, Kamo K, Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011–a potential overdiagnosis? Jpn J Clin Oncol. 2016;46(3):284–6. doi: 10.1093/jjco/hyv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bollaerts K, Sonck M, Simons K, et al. Thyroid cancer incidence around the Belgian nuclear sites: surrogate exposure modelling. Cancer Epidemiol. 2015;39(1):48–54. doi: 10.1016/j.canep.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 124.Giardina PA, Laurita MJ, Shah SK. A Review of Joseph J. Mangano’s Study on the Variation in Thyroid Cancer Incidence. Health Phys. 2015;109(3):258–64. doi: 10.1097/HP.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 125.Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31(5):756–73. doi: 10.1210/er.2010-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baker SR, Bhatti WA. The thyroid cancer epidemic: is it the dark side of the CT revolution? Eur J Radiol. 2006;60(1):67–9. doi: 10.1016/j.ejrad.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 127.Williams ED. Chernobyl and thyroid cancer. J Surg Oncol. 2006;94(8):670–7. doi: 10.1002/jso.20699. [DOI] [PubMed] [Google Scholar]
- 128.Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62(2):75–100. doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jargin SV. Validity of thyroid cancer incidence data following the Chernobyl accident. Health Phys. 2011;101(6):754–7. doi: 10.1097/HP.0b013e3182166780. [DOI] [PubMed] [Google Scholar]
- 130.Leux C, Guenel P. Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev Epidemiol Sante Publique. 2010;58(5):359–67. doi: 10.1016/j.respe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 131.Linares V, Belles M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89(3):335–56. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- 132.Allen JG, Gale S, Zoeller RT, Spengler JD, Birnbaum L, McNeely E. PBDE flame retardants, thyroid disease, and menopausal status in U.S. women. Environ Health. 2016;15(1):60. doi: 10.1186/s12940-016-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aschebrook-Kilfoy B, DellaValle CT, Purdue M, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol. 2015;181(11):883–8. doi: 10.1093/aje/kwu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Drozd VM, Saenko VA, Brenner AV, et al. Major Factors Affecting Incidence of Childhood Thyroid Cancer in Belarus after the Chernobyl Accident: Do Nitrates in Drinking Water Play a Role? PLoS One. 2015;10(9):e0137226. doi: 10.1371/journal.pone.0137226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21(3):389–95. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Camargo RY, Tomimori EK, Neves SC, et al. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. Eur J Endocrinol. 2008;159(3):293–9. doi: 10.1530/EJE-08-0192. [DOI] [PubMed] [Google Scholar]
- 137.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–95. doi: 10.1016/S2213-8587(14)70225-6. [DOI] [PubMed] [Google Scholar]
- 138.Clero E, Doyon F, Chungue V, et al. Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid. 2012;22(4):422–9. doi: 10.1089/thy.2011.0173. [DOI] [PubMed] [Google Scholar]
- 139.Poljak NK, Kontic M, Colovic Z, Jeroncic I, Luksic B, Mulic R. Iodine intake and epidemiological characteristics of thyroid cancer: comparison between inland and littoral Croatia. Acta Clin Croat. 2011;50(3):329–39. [PubMed] [Google Scholar]


