Abstract
Aims
DCCT showed that intensive type 1 diabetes management reduces complication incidence but did not focus on other cardiovascular disease risk factors, whose control in type 1 diabetes has not been well-studied. We assessed trends in cardiovascular risk factors in type 1 diabetes and attainment of concurrent American Diabetes Association (ADA) guidelines/recommendations (for HbA1c, blood pressure, LDL cholesterol, triglycerides) for complication prevention.
Methods
Individuals with childhood-onset type 1 diabetes (n=658; 49.4% women; baseline (1986–1988) median age 27 and duration 19 years) were followed biennially for up to 25 years, with surveys and/or examinations.
Results
At the latest recorded follow-up, achievement of concurrent ADA recommendations increased for HbA1c (from 9.7 to 25.6%, p<0.0001); was unchanged for blood pressure (from 89.7% to 87.4%, p=0.36); and decreased for LDL cholesterol (from 62.3 to 39.7%, p<0.0001). Adoption of intensive insulin therapy (from 5.9 to 64.4%, p<0.0001) and hypercholesterolemia (from 67.3 to 78.9%, p=0.0006) also increased. Overall, the proportion meeting all four recommendations was essentially unaltered (from 6.8% to 7.6%) (p=0.69). Results were similar by gender.
Conclusions
Although the adoption of intensive insulin therapy and obtaining ADA HbA1c recommendations are increasing, overall cardiovascular risk factor compliance remains low and merits further intervention.
Keywords: Cardiovascular risk factors, management, trends, type 1 diabetes, sex
1. Introduction
The incidence of type 1 diabetes continues to increase annually1 and with that comes a growing concern for the burden posed by diabetes-associated microvascular and macrovascular complications.2 Increased risk of cardiovascular disease (CVD) has been repeatedly recognized as a complication of type 1 diabetes, and recent data still suggest a 10-fold greater risk.3 There are multiple modifiable risk factors, such as HbA1c, blood pressure, lipids, smoking, and obesity, which can be intervened upon to reduce CVD risk in type 1 diabetes.4 Indeed, results from the Diabetes Control and Complications Trial (DCCT) demonstrated that intensive management of hyperglycemia greatly reduces the development and progression of micro5- and macro-vascular6 disease in type 1 diabetes, transforming care for these individuals. Unfortunately, years after the advent of intensive insulin therapy, the rates of CVD are still higher in type 1 diabetes compared to the general population, suggesting that tighter control of non-glycemic factors might be beneficial. However, to what extent improvements in cardiovascular risk factor control have occurred in the general type 1 diabetes population and whether they may differ between men and women, is not clear. An earlier study suggested poor adherence.7 In the present study, we aimed to assess trends in CVD risk factors among men and women over the 25-year follow-up of a cohort study of individuals with childhood-onset type 1 diabetes.
For more than 20 years, the American Diabetes Association (ADA) has disseminated key recommendations for diabetes care standards and guidelines in an attempt to improve management.8 Understanding how well these guidelines are attained may give insight into what areas of care are needed to potentially reduce the continuing high CVD rates. We therefore aimed to determine the proportion of individuals within this type 1 diabetes cohort falling within the guidelines set by the ADA for the prevention and management of diabetes complications. We additionally assessed trends over time in the proportions adopting intensive insulin therapy as well as in the proportions of overweight/obesity, hypercholesterolemia and hypertension, given their close interrelation with ADA goal attainment.
2. Subjects, materials and methods
2.1. The Pittsburgh Epidemiology of Diabetes Complications (EDC) study
The EDC study is a historical prospective cohort study of risk factors for complications resulting from childhood-onset (<17 years old) type 1 diabetes. Participants were either diagnosed, or seen within 1 year of diagnosis, at Children’s Hospital of Pittsburgh between 1950 and 1980. The cohort, which has been shown to be representative of the Allegheny County, Pennsylvania, type 1 diabetes population,9 has been described in detail elsewhere.10, 11 Briefly, participants have been followed by biennial surveys since initial examination in 1986–1988 and clinical examinations, occurring biennially for the first 10 years and again at 18 and 25 years of followup. The study protocol was approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent.
At each biennial follow-up, information was collected by questionnaire concerning demographic characteristics, health care, self-care, and medical history information. At each exam, sitting blood pressures were measured using a random-zero sphygmomanometer (except for the 18 and 25 year exams when aneroid sphygmomanometer was used), according to the Hypertension Detection and Follow-up Program protocol.12 The mean of the second and third blood pressures was determined and hypertension was defined as blood pressure >140/90 mmHg or use of antihypertensive medication. This definition for hypertension was used in the analysis regardless of the ADA guidelines since the latter refer to treatment goals rather than diagnostic criteria. Fasting blood samples were taken for measurement of lipids. HDL-cholesterol was determined by a precipitation technique (heparin and manganese chloride) with a modification13 of the Lipid Research Clinics method.14 Total cholesterol and triglycerides were measured enzymatically15, 16 and low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.17 Hypercholesterolemia was defined as LDL-C ≥100 mg/dL or use of statins. Fasting blood samples were analyzed for HbA1 (microcolumn cation-exchange; Isolab, Akron, OH) for the first 18 months, after which automated high-performance liquid chromatography (Diamat; Bio-Rad, Hercules, CA; the correlation over a full range of A1c values observed among study participants (approximately 6.38%–10.13%) was r=0.95) was performed for 10 years. For follow-up beyond the 10 years, HbA1c was measured with the DCA 2000 analyzer (Bayer, Tarrytown, NY, USA). The DCA and Diamat assays were highly correlated (r=0.95). Original HbA1 (1986–1998) and HbA1c values (1998–2004) were converted to DCCT aligned standard HbA1c values using regression formulae derived from duplicate assays (DCCT HbA1c = (0.83 × DIAMAT HbA1) + 0.14 and DCCT HbA1c = (DCA HbA1c − 1.13)/0.81). Height and weight were measured during clinic visits and BMI was calculated as weight (kg)/height (m)2. Intensive insulin therapy was defined as the participant’s use of multiple daily insulin injections, which included three or more shots daily or the use of an insulin pump, as well as checking glucose 28 times or more weekly. Overt CVD was defined as a participant having a history of or experiencing incident claudication, amputation due to vascular causes, coronary artery disease, and/or stroke during follow-up.
2.2. ADA guidelines/recommendations
The annual ADA recommendations for appropriate HbA1c, blood pressure, LDL-C, and triglyceride levels for people with diabetes were compiled from 199018 to 2008.19 Although the guidelines are not always specific to type 1 diabetes, those appropriate for any adult with diabetes were used. EDC participants were subsequently classified as falling within, or not, the recommendations at each clinical assessment during the study follow-up, using those recommendations that more closely corresponded to the specific study period. Thus, for each component of the recommendations, a dichotomous variable was created at each time point, which took the value of one if goals were attained and the value of zero if not. However, the 1995 recommendations5 were also used as guidelines for EDC assessments from study entry to the 1994–1996 examination period for all risk factors of the previous years, excluding blood pressure, as it was the first year complete guideline information was made available. Blood pressure guidelines (prior to 1995) were based on those from the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.20–22
2.3. Statistical analysis
Descriptive statistics, including means and frequencies, were used to examine the distribution and counts of the data. Differences in demographic and clinical characteristics between men and women were evaluated using the Wilcoxon two-sample test for continuous variables and the χ2 test for categorical variables. A χ2 test was also used for the analysis of trends from 1986–2014. Differences were considered statistically significant at the p<0.05 level. SAS version 9.4 (SAS Institute, Cary, NC) and the Open Source Epidemiologic Statistics for Public Health calculators23 were used for analyses.
3. Results
The baseline characteristics for the total cohort of childhood-onset type 1 diabetes (n=658, 49.4% female), as well as the characteristics stratified by gender, are presented in Table 1. Overall, the median (interquartile range) age and duration of diabetes were 27 (21.9, 33.3) years and 18.5 (13.2, 25.5) years, respectively. HbA1c was high for both men and women, with an overall median of 8.6% (7.7%, 9.7%) or 70 mmol/mol (61 mmol/mol, 83 mmol/mol). The cohort had a median body mass index (BMI) of 23.3 (21.4, 25.4) kg/m2. A greater proportion of women compared to men were obese (4.3 vs. 1.8%, respectively, p=0.06), but the waist to hip ratio (WHR) was significantly higher, as expected, in men (0.87 (0.84, 0.90)) than in women (0.77 (0.74, 0.81), p<0.0001). Men also had significantly higher systolic and diastolic blood pressures compared to women (p<0.0001), and as a result, the proportion of men who had hypertension was greater as well (36.6 vs. 20.3%, respectively, p<0.0001). The proportion using ACE/ARB medications, which might affect blood pressure target achievement, was low and did not differ by gender. Triglyceride concentrations were higher in men (89 (64, 135) mg/dl) compared to women (77 (59, 111) mg/dl), whereas women, as in the general population, had significantly higher HDL cholesterol concentrations than men (p<0.0001).
Table 1.
Participant Characteristics at Study Entry
| Characteristics | Total cohort (n=658) |
Men (n=333) |
Women (n=325) | p-value |
|---|---|---|---|---|
| Age (years)* | 27 (21.9, 33.3) | 27.3 (21.9, 33.1) | 26.9 (21.9, 33.4) | 0.91 |
| Age at onset (years)* | 8.4 (5.1, 11.5) | 8.2 (4.6, 11.5) | 8.6 (5.7, 11.5) | 0.10 |
| Diabetes duration (years)* | 18.5 (13.2, 25.5) | 19.0 (13.9, 25.4) | 18.2 (12.5, 25.6) | 0.48 |
| Percent female (n) | 49.4% (325) | -- | -- | |
| BMI (kg/m2)* | 23.3 (21.4, 25.4) | 23.6 (21.6, 25.6) | 22.8 (21.2, 25.3) | 0.16 |
| Percent overweight (n) | 29.8% (196) | 30.9% (102) | 28.7% (93) | 0.53 |
| Percent obese (n) | 3.0% (19) | 1.8% (5) | 4.3% (13) | 0.06 |
| Waist to hip ratio* | 0.83 (0.77, 0.87) | 0.87 (0.84, 0.90) | 0.77 (0.74, 0.81) | <0.0001 |
| HbA1c (%)* | 8.6 (7.7, 9.7) | 8.6 (7.8, 9.7) | 8.5 (7.7, 9.7) | 0.60 |
| HbA1c (mmol/mol)* | 70 (61, 83) | 70 (62, 83) | 69 (61, 83) | 0.60 |
| Systolic blood pressure (mmHg)* | 111 (104, 121) | 114 (107, 123) | 108 (100, 115) | <0.0001 |
| Diastolic blood pressure (mmHg)* | 72 (66, 79) | 76 (69, 82) | 69 (64, 75) | <0.0001 |
| Hypertension (%, n) | 28.6% (188) | 36.6% (121) | 20.3% (65) | <0.0001 |
| Blood pressure medication use (%, n) | 10.8% (71) | 14% (46) | 7.6% (24) | 0.01 |
| HDL cholesterol (mg/dl)* | 52.2 (45.1, 60.8) | 48.1 (42.6, 55.6) | 57.2 (49.2, 65.8) | <0.0001 |
| LDL cholesterol (mg/dl)* | 114.0 (93.7, 144.0) | 115.0 (94.3, 151.0) | 112.0 (91.8, 137.0) | 0.10 |
| Triglycerides (mg/dl)* | 82.0 (61.0, 126.0) | 89.0 (64.0, 135.0) | 77.0 (59.0, 111.0) | 0.002 |
| ACE/ARB use (%, n) | 3.5% (23) | 3.5% (11) | 3.5% (11) | 1.0 |
Data are median (interquartile range) or percent (n) unless otherwise indicated. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density cholesterol.
The Wilcoxon two-sample test was used for non-normally distributed variables.
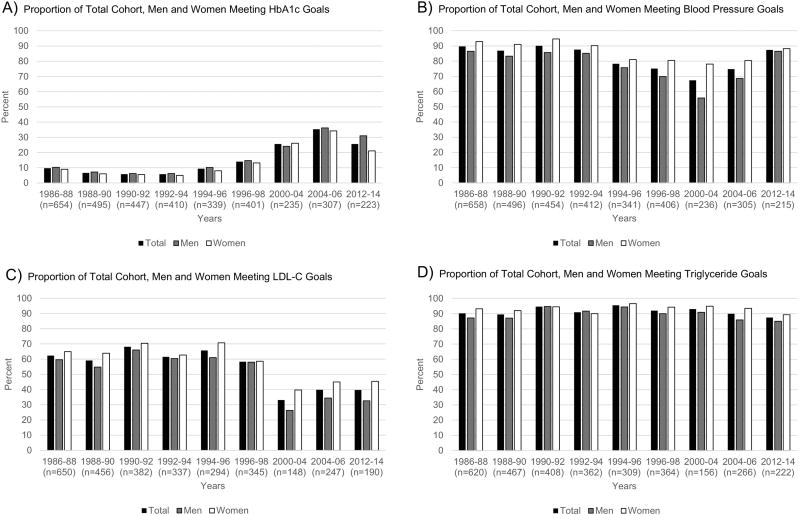
The guidelines set by the ADA for the prevention and management of diabetes complications are presented in Supplemental Table 1. The ADA recommendations for HbA1c, blood pressure, LDL-C with and without overt CVD, and triglycerides are listed from 1995 to 2014. The proportion of the total cohort, as well as for men and women separately, meeting recommendations is shown in Figure 1 and Table 2. As full exams were not conducted in the 1998–2000 and 2006–2008 cycles, they were excluded from analyses.
Figure 1.
Proportion of Total Cohort, Men and Women Meeting Recommended Goals
A. Proportion of Total Cohort, Men and Women Meeting HbA1c Goals
B. Proportion of Total Cohort, Men and Women Meeting Blood Pressure Goals
C. Proportion of Total Cohort, Men and Women Meeting LDL-C Goals
D. Proportion of Total Cohort, Men and Women Meeting Triglyceride Goals
Table 2.
Proportion (%, 95% Confidence Intervals) of Total Cohort, Men, and Women who are Overweight, Obese, using Intensive Insulin Therapy, have Hypercholesterolemia or Hypertension*
| Time period |
Intensive Insulin Therapy |
Overweight | Obese | Hypercholesterolemia | Hypertension | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | Total | Men | Women | |
|
| |||||||||||||||
| 1986–88 (n=308–333) | 5.9 (4.3, 8.0) | 3.2 (1.7, 5.7) | 8.6 (6.0, 12.2) | 29.8 (26.5, 33.4) | 30.9 (26.2, 36.1) | 28.7 (24.0, 33.9) | 3.0 (2.0, 4.7) | 1.8 (0.8, 3.9) | 4.3 (2.6, 7.1) | 67.3 (63.7, 70.8) | 68.5 (63.3, 73.22) | 66.2 (60.9, 71.1) | 16.1 (13.5, 19.1) | 19.4 (15.5, 24.0) | 12.7 (9.6, 16.8) |
| 1988–90 (n=218–271) | 12.7 (10.1, 15.8) | 8.5 (5.7, 12.4) | 17.2 (13.0, 22.4) | 36.0 (31.9, 40.4) | 37.4 (31.8, 43.4) | 34.5 (28.7, 40.8) | 4.5 (3.0, 6.7) | 2.3 (1.1, 4.9) | 6.9 (4.3, 10.9) | 67.3 (63.1, 71.3) | 69.6 (63.8, 74.8) | 64.8 (58.5, 70.7) | 21.7 (18.4, 25.37) | 24.9 (20.2, 30.3) | 18.2 (14.0, 23.4) |
| 1990–92 (n=188–253) | 4.8 (3.2, 7.1) | 4.2 (2.3, 7.6) | 5.3 (3.1, 8.9) | 36.1 (31.8, 40.7) | 41.5 (35.3, 48.1) | 30.6 (24.9, 37.0) | 5.2 (3.5, 7.6) | 4.5 (2.4, 8.0) | 5.9 (3.5, 9.8) | 63.6 (58.9, 68.2) | 67.8 (61.2, 73.8) | 59.3 (52.4, 65.9) | 21.4 (17.89, 25.31) | 26.2 (21.0, 32.1) | 16.5 (12.2, 21.8) |
| 1992–94 (n=153–235) | 8.4 (6.2, 11.4) | 5.7 (3.3, 9.6) | 11.0 (7.5, 15.7) | 42.7 (38.0, 47.5) | 45.7 (39.0, 52.5) | 39.6 (33.1, 46.5) | 8.5 (6.2, 11.6) | 8.2 (5.2, 12.7) | 8.9 (5.7, 13.6) | 69.7 (64.8, 74.3) | 72.3 (65.6, 78.2) | 66.9 (59.5, 73.5) | 26.0 (22.1, 30.4) | 27.8 (22.2, 34.1) | 24.2 (18.9, 30.4) |
| 1994–96 (n=140–254) | 8.5 (6.3, 11.4) | 5.8 (3.4, 9.6) | 11.2 (7.8, 15.9) | 44.3 (39.8, 49.0) | 47.8 (41.3, 54.3) | 40.9 (34.6, 47.5) | 9.5 (7.1, 12.6) | 8.6 (5.5, 13.0) | 10.5 (7.1, 15.2) | 66.5 (61.1, 71.5) | 72.0 (64.6, 78.3) | 60.4 (52.4, 67.9) | 30.1 (25.7, 34.9) | 34.2 (27.9, 41.1) | 25.6 (19.7, 32.5) |
| 1996–98 (n=166–281) | 13.0 (10.3, 16.3) | 11.2 (7.9, 15.8) | 14.8 (10.9, 19.8) | 45.6 (41.4, 49.9) | 49.1 (43.1, 55.0) | 41.9 (36.0, 48.0) | 10.9 (8.5, 13.9) | 8.9 (6.1, 12.9) | 13.0 (9.4, 17.7) | 72.5 (67.8, 76.8) | 75.8 (69.3, 81.2) | 68.9 (61.8, 75.2) | 34.4 (30.2, 38.9) | 36.6 (29.7, 42.0) | 33.2 (27.3, 39.6) |
| 2000–04 (n=74–221) | 42.4 (37.5, 47.4) | 36.2 (29.4, 43.5) | 48.0 (41.1, 54.9) | 56.7 (51.5, 61.7) | 61.9 (54.4, 68.9) | 52.1 (45.0, 59.1) | 20.1 (16.3, 24.6) | 17.9 (12.8, 24.3) | 22.1 (16.8, 28.5) | 81.8 (76.2, 86.3) | 88.3 (81.4, 92.9) | 74.3 (65.2, 81.7) | 44.6 (39.1, 50.2) | 47.9 (39.9, 56.0) | 41.5 (34.1, 49.3) |
| 2004–06 (n=127–199) | 46.8 (41.6, 52.1) | 43.3 (35.9, 50.9) | 50.0 (42.7, 57.3) | 64.4 (59.4, 69.0) | 66.7 (59.6, 73.1) | 62.2 (55.3, 68.7) | 22.4 (18.5, 26.9) | 22.4 (17.0, 29.0) | 22.5 (17.2, 28.8) | 77.5 (72.5, 81.8) | 83.2 (76.6, 88.3) | 71.5 (63.9, 78.1) | 36.1 (31.2, 41.4) | 42.1 (34.7, 49.9) | 30.9 (24.3, 37.8) |
| 2012–14 (n=236–306) | 64.4 (58.4, 69.9) | 58.7 (49.8, 67.0) | 69.2 (61.2, 76.2) | 64.4 (58.9, 69.5) | 64.5 (56.3, 71.9) | 64.2 (56.7, 71.1) | 31.7 (26.7, 37.1) | 31.9 (24.8, 40.0) | 31.5 (24.9, 39.0) | 78.9 (73.4, 83.6) | 85.6 (77.9, 90.9) | 73.5 (65.5, 80.2) | 31.2 (25.5, 37.4) | 35.2 (26.8, 44.7) | 27.8 (20.7, 36.2) |
The same individuals were not assessed in each cycle.
Figure 1A illustrates that from baseline to the 2012–2014 cycle, the proportion of individuals meeting the HbA1c recommendations increased from 9.7% to 25.6% (p<0.0001), as did the proportion on intensive insulin therapy (5.9% to 64.4%, p<0.0001), as seen in Table 2. Interestingly, although women were more likely to be on intensive insulin therapy compared to men, the proportion meeting HbA1c recommendations was similar by gender, with overlapping 95% confidence intervals as presented in Supplemental Table 2.
The proportion with hypercholesterolemia was high throughout the follow-up period and increased over time from 67.3% to 78.9% (p=0.0006), a trend that retained significance only among men (p=0.0005 versus p=0.12 among women) (Table 2). This increase was accompanied by a decrease in meeting LDL-C guidelines (Figure 1C), with goal achievement dropping from 65.7% to 39.7% (p<0.0001). Over the 25-year follow-up, adherence to LDL-C guidelines thus decreased from 59.6% to 32.6% (p<0.0001) in men and from 64.9% to 45.4% (p=0.0003) in women. Women were more likely to meet LDL-C guidelines than men though, generally, confidence intervals overlapped, and thus observed gender differences were not statistically significant (Supplemental Table 2). This reduction in goal achievement for LDL-C guidelines may rather reflect the lowering of the targets in 2002 and 2005. In fact, assessing the proportion of the cohort meeting only the 2008-guidelines suggested decreasing trends from 1986–88 to 2000–04 (from 31% to 25%) but an increase in goal attainment thereafter, being 39% in 2012–14 (not shown). The proportion of individuals meeting triglyceride guidelines remained consistently high from baseline to the 2012–2014 cycle, with a non-significant trend in both genders (Figure 1D, Supplemental Table 2).
Attainment of blood pressure guidelines also slightly, but non-significantly (p=0.36), declined from baseline to the 2012–2014 cycle (Figure 1B and Supplemental Table 2), and the presence of hypertension increased over time (p<0.0001, Table 2). These trends did not differ by gender. Women, however, appeared to meet blood pressure recommendations more often than men (Supplemental Table 2).
In the total cohort (Table 2), both overweight (p<0.0001) and obesity (p<0.0001) status increased throughout the follow up period. The proportion of men and women who were overweight increased from 30.9% to 64.5% (p<0.0001) and from 28.7% to 64.2% (p<0.0001), respectively. Likewise, the proportion of men who were obese increased from 1.8% to 31.9% (p<0.0001), compared to the proportion of women, which increased from 4.3% to 31.5% (p<0.0001).
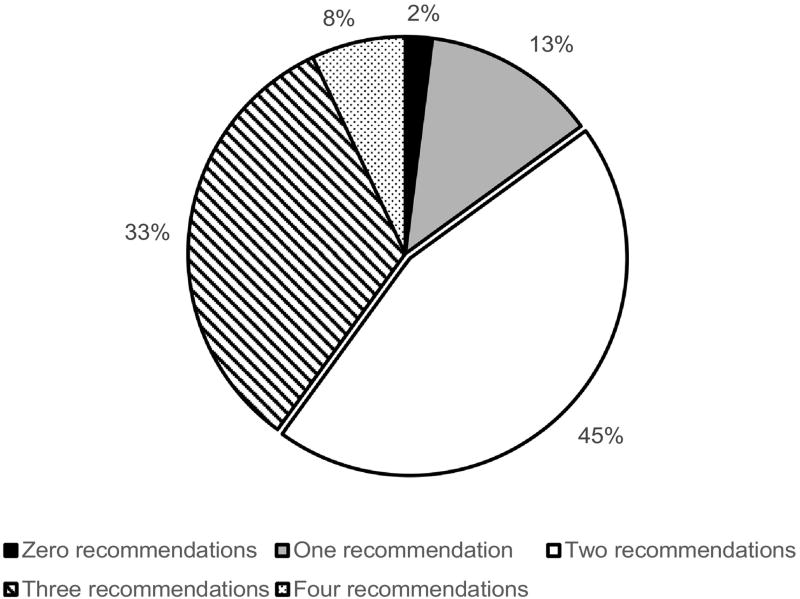
Overall, the proportion of individuals in this cohort who were able to meet all four ADA recommendations showed little change, from 6.8% at baseline to 7.2% by the end of the follow-up period (p=0.69). Although not statistically significant, women were slightly more likely to meet all recommendations compared to men (8.1% vs 7.0% respectively, p=0.79). Figure 2 depicts the overall proportion meeting zero, one, two, three, or all four ADA guidelines at the last cycle (2012–2014). At baseline, most participants met three out of the four recommendations (52.4%), but only 6.8% met all four, and 3.6% failed to meet any recommendations. During the last cycle, about a 45% of participants met two recommendations, and about one-third of the participants met three recommendations, but 2% still did not meet any of the guidelines.
Figure 2.
Proportion Meeting Zero, One, Two, Three, and Four Recommendations at Last Cycle (2012–2014). Solid black = zero recommendations; Checkered = one recommendation; Solid white = two recommendations; Stripes = three recommendations; Polka dots = four recommendations.
To account for the aging of the cohort, and thus, the increasing or decreasing trends of particular modifiable factors as part of normal aging, we repeated analyses restricting the cohort to those aged 35–45 years old at each cycle. Our findings suggested that, as in the entire cohort, the proportion of 35–45 year olds meeting HbA1c recommendations increased over time (Supplemental Table 3), as did the proportion on intensive insulin therapy and of overweight or obesity (all p<0.0001) (not shown). Also similar to the total cohort, there were no significant trends in the proportion meeting blood pressure recommendations among the 35–45 age group. Unlike in the total cohort, however, the presence of hypertension did not significantly change over the 25-year follow-up in the 35–45 age group. As in the overall cohort, the proportion meeting guidelines for LDL-C declined, while hypercholesterolemia increased, over time, although the p-value for trend did not reach significance. The proportion meeting guidelines for triglycerides remained high (Supplemental Table 3).
4. Discussion
In this cohort of individuals with childhood-onset type 1 diabetes, we observed that the proportion falling within the ADA recommendations for HbA1c increased, whereas the proportion meeting the ADA recommendations for LDL-C gradually decreased over the 25-year follow up period, reflecting the lowering of LDL-C goals. On the contrary, attainment of blood pressure and triglyceride goals remained high throughout follow-up. When analyses were restricted to individuals age 35–45 years at each time point, similar findings were obtained. In terms of trends over time in cardiovascular factors that may affect ADA goal attainment, overall, adoption of intensive insulin therapy increased over time, as did the proportion with hypercholesterolemia, hypertension and the percentage classified as overweight or obese.
Nonetheless, at the latest follow-up examination cycle (2012–2014), the highest proportion of ADA recommendations met for the cohort was for blood pressure and triglycerides, both at 87.4%, while the lowest proportion of recommendations met was for HbA1c at 25.6%. These findings suggest, as discussed later, that the “goals” for blood pressure and triglycerides (based mainly on non-type 1 diabetes data) may be too lax. Less than 10% of the cohort met all four recommendations, with most individuals meeting either two or three recommendations. Thus, our findings suggest that a very small proportion of EDC study participants currently fall within the recommendations for all risk factors evaluated, suggesting that more needs to be done in terms of improving control of individual risk factors.
Indeed, although glycemic control is getting better, it is still suboptimal. This is concerning given findings from DCCT/EDIC that intensive diabetes control is associated with decreases in the incidence of both microvascular and CVD complications.5, 6 The reduction in the incidence of macrovascular events in DCCT/EDIC was still 38% after 28 years follow up in the original intensive compared to conventional group therapy even though for the majority of the follow up both groups pursued intensive therapy.24
Despite the encouraging clinical trial results, however, the incidence of cardiovascular disease in the general type 1 diabetes population has not declined as sharply as the incidence of microvascular complications. As shown in the EDC study, notwithstanding a major decline in total mortality and renal failure rates in individuals deceased after 1965, and in neuropathy for those diagnosed in the 1970s, rates of decline for cardiovascular disease were low.25 These results would suggest that management of other cardiovascular risk factors, in addition to glycemic control, may be required to observe a greater decline in CVD rates.
Generally, hyperlipidemia is not a feature of type 1 diabetes; indeed, numeric lipid values are generally better than seen in the general population.26 This is reflected in the current results, suggesting that the proportion with triglyceride concentrations within the recommended levels was high and not altered over the follow-up period, although LDL-C management appeared to worsen over time, reflecting tightening of goals. The general drop in LDL-C recommendations met over time experienced by the total cohort paralleled a slower, yet statistically significant, increasing trend for hypercholesterolemia. Restricting the analysis to individuals between the ages of 35 and 45 at each time point, showed non-significant downward trends for LDL-C goal attainment, suggesting that the observed decline in the proportion meeting guidelines in the total cohort may be driven either by the smaller sample size and/or increasing LDL-C levels related to aging.
Although no significant differences were observed by gender in the proportion meeting LDL-C guidelines, men were slightly more likely to be hypercholesterolemic compared to women, mirroring the generally better lipid concentrations in women compared to men. The DCCT/EDIC study, however, has previously shown that CVD reducing interventions are underused in women compared to men with type 1 diabetes, even when women presented with a more adverse risk factor profile.27 Conversely, Kautzky-Willer et al. found that women had a lower adherence to pharmacological intervention than men, including the prescription of statins and the achievement of lipid goals, among individuals younger than 50 years at increased cardiovascular risk.28 The reasons for this discrepancy are not clear, though differences in the populations (USA vs. Europe), socioeconomic status (including health insurance status) and the nature of assessment (self-report) could have contributed.
Blood pressure control also appeared to worsen over time overall, as well as among 35–45 year olds, although these trends did not reach statistical significance. It was the only ADA recommended modifiable factor that suggested gender differences, with women meeting recommendations more often than men. The presence of hypertension increased in the cohort over time, paralleling the lower attainment of blood pressure goals. Women were less likely to have hypertension than their male counterparts over time, although these differences were not significant. DCCT/EDIC investigators have previously suggested that male sex is associated with an increased hypertension risk.29 a finding we were unable to fully replicate in the EDC cohort.
Of great concern is the increasing prevalence of overweight and obesity in the type 1 diabetes population, since excess weight gain plays a major role in CVD risk. We have previously reported a temporal increase in overweight and adiposity in this cohort,30 which the current results confirm. It is possible that these increasing trends in overweight and obesity may reflect increased adoption of intensive insulin therapy. Indeed, according to DCCT study findings, intensive insulin therapy is associated with weight gain, which when excessive, promotes adverse changes in lipid levels and blood pressure despite the concurrent improvement in glycemic levels.31
It is nevertheless clear that intensive insulin therapy cannot be solely responsible for the dramatic weight gain in the type 1 diabetes population, as suggested by a study comparing the prevalence of overweight among children with newly onset type 1 diabetes at two time points approximately 10 years apart. Investigators observed that the prevalence of overweight increased 5-fold, and is now similar to the prevalence of overweight in the general population at the most recent follow-up.32 Finally, weight gain in the company of concurrent improvements in glycemic control has been shown to positively affect the cardiovascular risk profile of individuals with type 1 diabetes.33
Previously published data from the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study confirm that those with type 1 diabetes actually have a similar or better (i.e. lipids) risk factor profile when compared to the general population. Indeed, both men and women with type 1 diabetes had significantly lower total cholesterol, LDL-C and triglyceride levels, as well as higher HDL levels when compared to controls without diabetes.34 Nevertheless, despite this apparent similar, or better, risk factor profile, there is still a greater incidence of CVD among those with type 1 diabetes. These data suggest that, given the stress posed by the increased glycemic levels in type 1 diabetes, tighter control of CVD risk factors, with potentially stricter cut-off points, may be required to reduce cardiovascular disease risk among these individuals. Thus, in a prior publication35 from this cohort comparing two subgroups diagnosed 10 years apart but matched for age and duration, we showed that though the impact of glycemia and hyperlipidemia had decreased as a prediction of major outcomes, hypertension remained a major predictor suggesting inadequate treatment. Analyses of the 10-year EDC follow-up also suggested gradients of risk well below the current blood pressure and triglyceride recommendations.36 Similarly, FinnDiane investigators showed that the recommended goal for triglycerides (<150 mg/dL) failed to predict CAD in normoalbuminuric patients with type 1 diabetes, whereas the median cutoff (83 mg/dL) predicted incident CAD events.37 Given the current findings of high compliance with goals, it therefore seems likely the blood pressure and triglyceride goals are inappropriately high for this population.
There are few other studies in type 1 diabetes that have assessed adherence to guidelines, and these are mainly focused on children, adolescents, and young adults. Also, these studies mainly monitored compliance of clinical guidelines regarding the number of examinations, measurements, and medical practitioner visitation for individuals with type 1 diabetes. The SEARCH for Diabetes in Youth study observed that children and youth with diabetes are lacking in glycemic control and often do not receive treatment for dyslipidemia. They found that children reported having recommended eye exams and tests for HbA1c less than other measurements, which increases their risk for complications.38 In a prospective cohort study assessing ADA compliance in youth and young adults, only about one-third of participants met the criteria for optimal compliance to guidelines, whereas more than half had poor compliance to set guidelines.39 Also, as the duration of diabetes increased, the proportion meeting recommendation goals significantly decreased each year. In assessing compliance of HbA1c goals in adolescents, Clements et al. observed that less than 20% of their cohort achieved an HbA1c goal of less than 7.5% (58 mmol/mol). Interestingly, a greater proportion of adolescents who thought that the International Society for Pediatric and Adolescent Diabetes (ISPAD) goal was lower than 7.5% met this target,40 suggesting that awareness and/or perception of set recommendations are important factors in achieving adherence to guidelines.
A limitation of our analyses is that they represent cross-sectional analyses at different time points and therefore the same individuals were not necessarily studied from cycle to cycle. In addition, there was a reduction in the sample size examined over the 25-year follow-up, largely reflecting mortality (now n=206) and moving out of the Pittsburgh area (n=80) as well as unwillingness to participate for other reasons. Thus, results could have been affected by survival bias and/or the aging of the population rather than represent true trends in the type 1 diabetes population. However, we obtained similar results when analyses were restricted to individuals aging 35–45 years at each time point. Another weakness of this study is that the ADA recommendations used were not specific to those with type 1 diabetes. Guidelines given were for the general standards of medical care in type 2 diabetes, and not specifically for type 1 diabetes. Inferring treatment guidelines and goals from type 2 diabetes studies may be inappropriate given the major differences in age, disease etiology and comorbidities between the two diabetes types. This weakness of our study therefore stresses the need for research data derived specifically from the type 1 diabetes population. Moreover, due to the absence of available recommendations prior to 1989, we used the guidelines from 1995 for that year and all previous years. Finally, though we present data on goal achievement, space does not permit analysis in proportions at risk for treatment, and meeting goals on treatment.
In conclusion, our findings suggest an increase in the proportion of HbA1c recommendations met, intensive insulin therapy use, statin use, the presence of hypertension, overweight and obesity status, but a decrease in the proportion meeting LDL-C goals. The generally high adherence to blood pressure and triglyceride recommendations remained stable, which suggests these goals may be too lax, supporting other such evidence. These results illustrate the importance of making patients aware of their blood pressure and lipid profiles, as well as the probable need for stricter recommended targets for adult type 1 diabetes with childhood onset, given continuing high rates despite good blood pressure and triglyceride compliance with current targets.
Supplementary Material
Acknowledgments
We thank all study participants for their invaluable contributions as well as the Epidemiology of Diabetes Complications (EDC) study staff.
Funding sources
This research was supported by NIH grant DK34818 and the Rossi Memorial Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
KS conducted the analysis and wrote the manuscript; TC researched, analyzed data, and wrote/edited the manuscript; TJO designed the EDC study and reviewed/edited the manuscript. This study was part of author KS' Master’s essay.
Conflict of interest statement
The authors declare no conflicts of interest.
Prior Presentation
Preliminary results were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7. Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 2.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of Type 1 Diabetes. Endocrinol Metab Clin North Am. 2010;39:625–640. doi: 10.1016/j.ecl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2016;39:2296–2303. doi: 10.2337/dc16-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, Eckel RH. Type 1 Diabetes and Associated Cardiovascular Risk and Disease. In: McGuire DK, Marx N, editors. Diabetes in Cardiovascular Disease: a Companion to Braunwald’s Heart Disease. New York, NY: Elsevier; 2014. pp. 127–135. [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group. The Effect of Long-Term Intensified Insulin Treatment on the Development of Microvascular Complications of Diabetes Mellitus. N Engl J Med. 1993;329:304–309. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zgibor JC, Wilson RR, Orchard TJ. Has Control of Hypercholesterolemia and Hypertension in Type 1 Diabetes Improved Over Time? Diabetes Care. 2005;28:521–526. doi: 10.2337/diacare.28.3.521. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of Medical Care for Patients with Diabetes Mellitus: Position Statement. Diabetes Care. 1995;18:8–15. [Google Scholar]
- 9.Wagener DK, Sacks JM, LaPorte RE, MacGregor JM. The Pittsburgh Study of Insulin-dependent Diabetes Mellitus: Risk for Diabetes Among Relatives of IDDM. Diabetes. 1982;31:136–144. doi: 10.2337/diab.31.2.136. [DOI] [PubMed] [Google Scholar]
- 10.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. The prevalence of complications in insulin-dependent diabetes mellitus by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study-II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 11.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Ellis D, LaPorte RE, Kuller LH, Wolfson S, Drash AL. Factors associated with the avoidance of severe complications after 25 years of insulin-dependent diabetes mellitus: Pittsburgh Epidemiology of Diabetes Complications Study-I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 12.Borhani NO, Kass EH, Langford HG, Payne GH, Reminton RD, Stamler J. The hypertension detection and follow-up program. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR, Albers JJ. Heparin-Mn2+ quantitation of high-density lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–904. [PubMed] [Google Scholar]
- 14.U.S. Department of Health, Education and Welfare, Public Health Service. Manual of Laboratory Operations: Lipid Research Clinics Program. Washington, DC: U.S. Govt. Printing Office; 1975. (DHEW publ. no. NIH 75-628) [Google Scholar]
- 15.Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 16.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.American Diabetes Association. Role of Cardiovascular Risk Factors in Prevention and Treatment of Macrovascular Disease in Diabetes: Consensus Statement. Diabetes Care. 1990;13:53–59. doi: 10.2337/diacare.12.8.573. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of Medical Care in Diabetes 2008: Position Statement. Diabetes Care. 2008;31:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 20.The 1984 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1984;144:1045–1057. [PubMed] [Google Scholar]
- 21.The 1988 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. 1988 Joint National Committee. Arch Intern Med. 1988;148:1023–1038. [PubMed] [Google Scholar]
- 22.The Fifth Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1993;153:154–183. [PubMed] [Google Scholar]
- 23.Dean A, Sullivan K, Soe M. [Accessed July 31, 2017];OpenEpi: Open source epidemiologic statistics for public health [Internet] 2015 Available from http://www.openepi.com/Menu/OE_Menu.htm.
- 24.Lachin JM, Orchard TJ, Nathan DM for the DCCT/EDIC Research Group Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pambianco G, Costacou T, Ellis D, Becker DJ, Orchard TJ. The 30-Year Natural History of Type 1 Diabetes Complications: The Pittsburgh Epidemiology of Diabetes Complications Study Experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 26.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 27.Larkin ME, Backlund JY, Cleary P, Bayless M, Schaefer B, Canady J, Nathan DM, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Disparity in management of diabetes and coronary heart disease risk factors by sex in DCCT/EDIC. Diabet Med. 2010;27(4):451–8. doi: 10.1111/j.1464-5491.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 28.Kautzky-Willer A, Stich K, Hintersteiner J, Kautzky A, Kamyar MR, Saukel J, Johnson J, Lemmens-Gruber R. Sex-specific-differences in cardiometabolic risk in type 1 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;12:78. doi: 10.1186/1475-2840-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Boer IH, Kestenbaum B, Rue TC, Steffes MW, Cleary PA, Molitch ME, Lachin JM, Weiss NS, Brunzell JD, Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Insulin Therapy, Hyperglycemia, and Hypertension in Type 1 Diabetes Mellitus. Arch Intern Med. 2008;168:1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW, Orchard TJ. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med. 2010;27:398–404. doi: 10.1111/j.1464-5491.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of Excessive Weight Gain with Intensive Therapy of Type 1 diabetes on Lipid Levels and Blood Pressure: Results from the DCCT. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing Prevalence of Overweight Children and Adolescents at Onset of Insulin-Treated Diabetes. Diabetes. 2003;26:2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 33.Williams KV, Erbey JR, Becker D, Orchard TJ. Improved glycemic control reduces impact of weight gain on cardiovascular risk factors in type 1 diabetes: The Epidemiology of Diabetes Complications Study. Diabetes Care. 1999;22:1084–1091. doi: 10.2337/diacare.22.7.1084. [DOI] [PubMed] [Google Scholar]
- 34.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M, Coronary Artery Calcification in Type 1 Diabetes Study Effect of Type 1 Diabetes on the Gender Difference in Coronary Artery Calcification: a Role for Insulin Resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 35.Miller RG, Secrest AM, Ellis D, Becker DJ, Orchard TJ. Changing impact of modifiable risk factors on the incidence of major outcomes of type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2013;36:3000–3006. doi: 10.2337/dc13-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orchard TJ, Forrest KY, Kuller LH, Becker DJ, Pittsburgh Epidemiology of Diabetes Complications Study Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2001;24:1053–1059. doi: 10.2337/diacare.24.6.1053. [DOI] [PubMed] [Google Scholar]
- 37.Tolonen N, Forsblom C, Mäkinen VP, Harjutsalo V, Gordin D, Feodoroff M, Sandholm N, Thorn LM, Wadén J, Taskinen MR, Groop H, FinnDiane Study Group Different lipid variables predict incident coronary artery disease in patients with type 1 diabetes with or without diabetic nephropathy: the FinnDiane study. Diabetes Care. 2014;37:2374–2382. doi: 10.2337/dc13-2873. [DOI] [PubMed] [Google Scholar]
- 38.Waitzfelder B, Pihoker C, Klingensmith G, Case D, Anderson A, Bell RA, Lawrence JM, Mayer-Davis EJ, Imperatore G, Standiford D, Rodriguez BL, Dabelea D, Seid M, SEARCH for Diabetes in Youth Study Group Adherence to Guidelines for Youths with Diabetes Mellitus. Pediatrics. 2011;128:531–538. doi: 10.1542/peds.2010-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements SA, Anger MD, Bishop FK, McFann KK, Klingensmith GJ, Maahs DM, Wadwa RP. Lower A1c among adolescents with lower perceived A1c goal: a cross-sectional survey. Int J Pediatr Endocrinol. 2013;17:1–6. doi: 10.1186/1687-9856-2013-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabák AG, Tamás G, Zgibor J, Wilson R, Becker D, Kerényi Z, Orchard TJ. Targets and Reality: A Comparison of Health Care Indicators in the U.S. (Pittsburgh Epidemiology of Diabetes Complications Study) and Hungary (DiabCare Hungary) Diabetes Care. 2000;23:1284–1289. doi: 10.2337/diacare.23.9.1284. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association. Standards of Medical Care in Diabetes-2014: Position Statement. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.