Abstract
The United States Environmental Protection Agency (USEPA) completed nationwide screening of six perfluoroalkyl substances in U.S. drinking water from 2013–2015 under the Third Unregulated Contaminant Monitoring Rule (UCMR3). UCMR3 efforts yielded a dataset of 36,139 samples containing analytical results from >5,000 public water systems (PWSs). This study used UCMR3 data to investigate three aspects of per- and polyfluoroalkyl substances (PFASs) in drinking water: the occurrence of PFAS and co-contaminant mixtures, trends in PFAS detections relative to PWS characteristics and potential release types, and temporal trends in PFAS occurrence. This was achieved through bivariate and multivariate analyses including categorical analysis, concentration ratios, and hierarchical cluster analysis. Approximately 50% of samples with PFAS detections contained ≥2 PFASs, and 72% of detections occurred in groundwater. Large PWSs (>10,000 customers) were 5.6 times more likely than small PWSs (≤10,000 customers) to exhibit PFAS detections; however, when detected, median total PFAS concentrations were higher in small PWSs (0.12 μg/L) than in large (0.053 μg/L). Bivariate and multivariate analyses of PFAS composition suggested PWSs reflect impacts due to firefighting foam use and WWTP effluent as compared to other source types for which data were available. Mann-Kendall analysis of quarterly total PFAS detection rates indicated an increasing trend over time (p=0.03). UCMR3 data provide a foundation for tiered design of targeted sampling and analysis plans to address remaining knowledge gaps in the sources, composition, and concentrations of PFASs in U.S. drinking water.
Keywords: Emerging contaminants, PFAS, PFOA, PFOS, drinking water, UCMR3
Graphical abstract
1. INTRODUCTION
The occurrence of per- and polyfluoroalkyl substances (PFASs) in the environment is a critical concern due to prevalence of use and release (Buck et al. 2011; Prevedouros et al. 2006), concerns about toxicity (DeWitt 2015; Lau et al. 2007; USEPA 2016b, 2016a), and known exposures (Braun et al. 2016; Frisbee et al. 2010). PFASs have been used in a wide variety of products and applications due to their unique chemistry that includes surface activity, resistance to chemical and biological degradation, and both hydro- and oleophobicity. PFAS applications and products include fluoropolymer manufacturing, stain and water repellant coatings, and certain classes of firefighting foams called aqueous film forming foams (AFFF) (Buck et al. 2011; Kissa 2001). Resistance to degradation also causes the environmental persistence of some PFASs (Krafft and Riess 2015) such perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS), which are considered recalcitrant (Merino et al., 2016; USEPA, 2016a; USEPA, 2016b). Persistent PFASs are not expected to degrade when discharged to water, air, or soil.
To date many studies have focused on perfluoroalkyl substances, particularly PFOA and PFOS. Perfluoroalkyl substances are recalcitrant and contain an alkyl tail with all carbons bonded to fluorine. There are also a wide range of polyfluoroalkyl substances, which still have at least one perfluoroalkyl moiety (CnF2n+1) but also contain carbons bonded to hydrogen, and these compounds are capable of transformation in the environment (Buck et al. 2011).
Polyfluoroalkyl substances are sometimes referred to as precursors because of their ability to transform to recalcitrant, perfluoroalkyl terminal endpoints following release in the environment (Harding-Marjanovic et al. 2015; Mejia Avendaño and Liu 2015; Knepper and Lange 2012). Release of a single precursor may result in formation of multiple intermediate PFAS transformation products with different perfluoroalkyl endpoints. This process may lead to increases in perfluoroalkyl substance concentrations with time and/or distance from the release source (Ahrens and Bundschuh 2014; Weber et al. 2017). It is unlikely that any PFAS-impacted site will be characterized by a single PFAS species; rather, it is expected that there will invariably be mixtures of PFAS involved. These PFAS mixtures will likely be found in the environment for reasons ranging from multiple sources present in a region, the general use of PFASs as mixtures even in a single product (e.g., AFFF), and finally the changes in commonly used PFASs over time such as those precipitated by the phase-out of PFOS and PFOA in the U.S. and other countries (Buck et al., 2011; CONCAWE, 2016). PFASs may also co-occur in with other (i.e. non-PFAS) contaminants. For example, application of AFFF to fuel fires has led to groundwater plumes containing PFASs and hydrocarbon constituents (McGuire et al. 2014).
It is possible to gain insights regarding sources of PFAS releases based on composition, concentrations, and temporal trends observed in occurrence data. For example, perfluoroalkyl carboxylates (PFCAs) such as PFOA have been used widely as manufacturing aids in the fluoropolymer industry, whereas perfluoroalkyl sulfonates (PFSAs) such as PFOS were a major component of some formulations of AFFF (Houtz et al. 2013; Prevedouros et al. 2006). Some AFFF formulations contain precursors that can generate PFCAs following release, and other precursors used in surface protection products may generate PFSAs (Harding-Marjanovic et al. 2015; Mejia Avendaño and Liu 2015; Rhoads et al. 2008). Additionally, PFAS use has changed over time (Houtz et al. 2013; Wang et al. 2013). Long chain PFASs, defined by USEPA as PFSAs ≥ perfluorohexane sulfonate (PFHxS) and PFCAs≥ PFOA and their precursors, have been phased out in favor of short chained PFASs and replacement products such as ADONA and GenX (Wang et al. 2013). In addition, different sources release different PFAS mixtures to different primary aqueous environments. Manufacturing releases often occur to surface water bodies, though groundwater impacts through historical disposal practices or atmospheric deposition are possible (Davis et al. 2007; Oliaei et al. 2013). Applications of AFFF occur at or near the land surface, leading to potential for migration through the subsurface to groundwater aquifers though some overland flow to surface water bodies may occur.
PFASs are unregulated at the federal level, though USEPA has issued nonenforceable lifetime health advisories (LHAs) for the sum of PFOA and PFOS of 0.07 μg/L in drinking water (USEPA, 2016a; USEPA, 2016b). To inform the need for a federal drinking water maximum contaminant levels (MCL) for unregulated compounds, USEPA compiles information on occurrence in U.S drinking water via the Unregulated Contaminant Monitoring Rule (UCMR). Under this rule, certain public water systems (PWSs) monitor for a designated list of unregulated contaminants during a three-year period. The most recent round of monitoring, labeled UCMR3, included PFOA, PFOS, and 4 additional PFASs. The publicly-accessible UCMR3 dataset was available by mid-2016. Formal regulatory determinations based on UCMR data generally occur several years after monitoring is completed (Roberson and Eaton 2014). PFASs are currently regulated at the state level in some regions (e.g. New Jersey, Vermont). Regulations typically target PFOA and/or PFOS, but have been developed for other PFASs in some areas (i.e. NCDHHS 2017). Additionally, regulation of PFAS mixtures may become more common as studies are completed on mixture toxicity. For example, only limited studies to date, have investigated the potential for synergistic effects of PFAS mixtures (Carr et al. 2013; Wolf et al. 2014).
Multiple studies examined PFAS occurrence data generated by UCMR3 monitoring (Hu et al. 2016; Suthersan et al. 2016). One study concluded that 6 million U.S residents were being served by PWSs with PFOA and PFOS concentrations that exceeded the LHA in one or more samples (Hu et al. 2016). Additionally, positive correlations were identified between numbers of potential PFAS point sources within specific regions and PFAS detections within those areas (Hu et al. 2016). This work concluded that industrial sources represented the highest per source contribution, but minor sources, including wastewater treatment plants (WWTPs) that are more numerous, likely also represent a significant cumulative contribution. A recent evaluation of UCMR3 data focused on 1,4-dioxane occurrence concluded PFAS detections were significantly correlated with 1,4-dioxane detections. However, detection rates for all PFASs were much lower than the detection rate for 1,4-dioxane and several other UCMR3 contaminants (Adamson et al. 2017).
Knowledge gaps remain regarding sources, occurrence, and fate of PFASs in the environment, and UCMR3 data can be further leveraged towards providing insights into some of these key questions. The UMCR3 dataset for PFASs is unique because it includes data on PFAS mixtures from PWSs of various sizes, from multiple source water types (e.g. surface water, groundwater), over a broad geographic distribution, and over a three-year period. The objective of the current study was to use UCMR3 data to advance understanding of PFASs in U.S drinking water by addressing three primary hypotheses: 1) due to complex chemistry and usage history for PFAS-containing products, multiple PFASs will be present whenever detected in a drinking water supply, and are likely to be correlated with other co-contaminants; 2) concentration and relative abundance of PFASs detected in drinking water are a function of PWS characteristics (i.e. source water type, system size) and serve as indicators of the source of PFAS contamination; 3) temporal trends in PFAS occurrence reflect their recalcitrance and changes in use and source control of these compounds. Lastly, the results of the study were used to investigate the extent to which the UCMR3 dataset is a general representative proxy for PFAS occurrence in U.S. drinking water.
2. METHODS
2.1 Data Sources
This study relied on publicly available data collected to support USEPA’s UCMR3 efforts (USEPA 2012). UCMR3 data contains PWS information and compound analytical results for >73,000 drinking water samples collected 2013–2015 from >5000 PWSs. System information includes PWS identification, state, zip code, system size, and water source type for each drinking water sample collected. PWSs size was designated as either large (serving populations >10,000) or small (serving populations ≤10,000). Water sources were specified as groundwater, groundwater under the influence of surface water, surface water, or mix. Sampling and analysis of 6 PFASs (Table 1) and 15 additional compounds was mandatory for all large PWSs in the U.S., but only a subset of smaller PWSs. Each PWS sampled for at least one year, yielding 36,139 samples analyzed for PFASs. Minimum reporting limits (MRLs) were specified to ensure each PWS generated uniform occurrence data (Table 1). A complete description of the UCMR3 sampling and analysis protocol can be found in USEPA (2016c), and a summary of the process is provided in Adamson et al. (2017). Information regarding population size served by specific PWSs was identified in the USEPA Safe Drinking Water Information System (SDWIS, https://ofmpub.epa.gov/apex/sfdw/f?p=108:200::::::).
Table 1.
UCMR3 PFASs, MRLs, and regulatory limits
| Compound | MRL (μg/L) | Advisory or State Standard (ng/L)1 | Source |
|---|---|---|---|
| Perfluoroheptanoate (PFHpA) | 0.01 | 702 | CTDPH |
| Perfluorooctanoate (PFOA) | 0.02 | 702, 703, 202, 14 | LHA, CTDPH, VTDOH, NJDWQI |
| Perfluorononanoate (PFNA) | 0.02 | 702, 10 | CTDPH, NJ |
| Perfluorobutane sulfonate (PFBS) | 0.09 | – | – |
| Perfluorohexane sulfonate (PFHxS) | 0.03 | 702 | CTDPH |
| Perfluorooctane sulfonate (PFOS) | 0.04 | 702, 202 | LHA, VTDOH |
Notes: CTDPH = Connecticut Department of Public Health, LHA = USEPA lifetime health advisory, VTDOH = Vermont Department of Health, NJDWQI = New Jersey Drinking Water Quality Institute. (1) Includes only regulatory limits used in this study to evaluate the UCMR3 data set and is not a comprehensive review of U.S. state-level PFAS regulatory limits. It should be noted that regulatory limits are subject to change. (2) Regulatory limit applies to the sum of PFHpA, PFOA, PFNA, PFHxS, and PFOS (3) Regulatory limit applies to the sum of PFOS and PFOA because their reference doses are numerically identical and based on similar developmental effects (USEPA 2016b, 2016a).
2.2 Regulatory Limits
To provide perspective on PFAS impacts in U.S. drinking water, data were evaluated vs. relevant advisories and regulatory limits. The LHAs for PFOA and PFOS were used to evaluate potential PFAS exceedances of health-based standards, along with select drinking water regulatory limits established by individual states (e.g., Connecticut, Vermont, New Jersey) (Table 1). State limits were used to evaluate the national data set, but this was done solely to provide perspective on the impact of applying different drinking water standards and not to imply that these standards apply outside of regions for which they were developed.
2.2 Statistical Analysis
A combination of methods was used to assess patterns in co-occurrence and relationships between parameters. To assess if differences between two groups (e.g., concentration distribution of one PFAS vs. a different PFAS) were significant, the Mann Whitney U test was used (two-tailed test, p≤0.05). Bivariate analyses of relationships between PFAS pairs were performed with odds ratios (correlates likelihood of detection) and linear regression of concentrations, using log-transformed data when needed to account for non-normal data distributions. Many parameters were categorical in nature (e.g., detection vs. non-detection; small vs. large system size), so co-occurrence was evaluated using categorical analysis methods (chi square). Results were reported as an odds ratio that quantifies the increase in likelihood (i.e. odds) of a particular outcome based on association with another particular occurrence (e.g., PFOS detection in samples with PFOA detection vs. no PFOA). The Mann-Kendall test was used as a nonparametric assessment of temporal trends in detection rates over time. These analyses were performed using custom Microsoft Excel tools or ProUCL (Version 5.0.00, USEPA, 2013).
Cluster analysis was used as a multivariate method for determining relationships between PFASs based on detection and concentration. The primary goal was to establish the hierarchy between each of the 6 PFASs as a measure of collective co-occurrence. Concentrations in samples with detections of one or more PFASs were entered into ClustVis (http://biit.cs.ut.ee/clustvis/), and pareto scaling was used as an initial data processing step to minimize the effects of large concentration ranges. Clustering was performed using Euclidean distances, and Ward’s Method was selected for linking clusters. Detailed methods associated with this tool for cluster analysis are described elsewhere (Metsalu and Vilo 2015).
3. RESULTS AND DISCUSSION
3.1 Overview of Data and Regulatory Comparison
A total of 36,977 samples from 4,920 PWSs were analyzed for PFASs as part of the UCMR3 monitoring, and detections of PFASs occurred in 1.6% of samples and 4% of PWSs in the 2013–2015 period. PFAS detections ranged from 0.01 (PFHpA) to 7 μg/L (PFOS). The number of detections of each compound varied from 19 (PFNA and PFBS) to 379 (PFOA, Figure S.1). Within detections (i.e. non-detects eliminated), average PFAS concentrations ranged from 0.025 (PFHpA) – 0.18 (PFBS) μg/L. Elevated average concentration and potentially the low frequency of detection of PFBS are partially a function of the 0.09 μg/L MRL, which was the highest of the 6 PFASs studied (Table 1). The low frequency of detection for PFNA cannot be attributed to the MRL as the limit of 0.02 μg/L was the same as that of PFOA, the most frequently detected compound. MRLs of 0.01-0.02 μg/L for PFCAs (PFHpA, PFOA, and PFNA) were lower than MRLs of 0.03 – 0.09 μg/L for PFSAs (PFBS, PFHxS, and PFOS).
Results of UCMR3 monitoring results were compared to select health-based water quality standards to gain perspective on the number of systems representing potentially-elevated exposures. From 2013–2015, 64 PWSs exceeded the non-enforceable LHA of 0.07 μg/L for PFOS and PFOA. Previous work noted six million people in the U.S. are served by these PWSs (Hu et al. 2016). Multiple states have established health-based, enforceable (at the state level) PFAS drinking water quality standards that differ from the LHA. Though not applicable nationally, these standards illustrate how changing regulatory standards influence an exposure assessment. For example, over 8 million people are served by the 124 UCMR3 PWSs with PFOA and PFNA measured in excess of New Jersey regulations, and approximately 14 million are served by the 153 PWSs that exceeded the Vermont PFOA regulation (Table 1). This demonstrates that continued evolution of PFAS regulation, based in part on continued study of toxicity, leads to uncertainty regarding the number of PWSs in the U.S. that will be judged to pose health concerns for exposed populations.
In 598 samples with detectable levels of PFASs, approximately 50% contained mixtures of 2 or more PFASs (Figure 1). As mentioned, PFAS co-occurrence may reflect release from multiple PFAS-containing products or applications, changes in PFAS use over time, and/or precursor transformation. PFAS mixtures are therefore consistent with complex use, release, and chemistry, as well as previous studies where multiple PFASs were measured in surface water and groundwater at PFAS-impacted sites such as military installations, landfills, and PFAS or fluoropolymer manufacturing facilities (Anderson et al. 2016; Bach et al. 2017; Houtz et al. 2013; McGuire et al. 2014; Oliaei, F. et al. 2006; Oliaei et al. 2013; Weber et al. 2017).
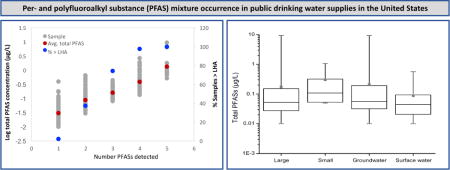
Figure 1.
Summary of total PFAS concentrations (log μg/L) in each sample (left y-axis) sorted by the number of PFASs detected in each sample and percentage of samples in each category exceeding the USEPA LHA (right y-axis). Note calculated averages and LHA exceedances consider only samples in which detections occurred.
Concentration distributions illustrate that increases in the number of PFASs detected in drinking water samples are associated with higher average total PFAS levels and increased likelihood of exceeding the LHA (Figure 2). Considering only samples with measureable PFAS concentrations, there was a log linear increase (R2=0.97) in average total PFAS concentration with increasing number of compounds detected. In samples with 3-5 PFASs detected, 75-100% exceeded the LHA. These trends suggest samples with more than five PFASs present will contain still greater total PFAS concentrations and will exceed the LHA. Recent studies have identified numerous additional PFASs that co-occur with UCMR3 PFASs in surface water and groundwater (e.g. Backe et al. 2013; Barzen-Hanson et al. 2017; Strynar et al. 2015). Occurrence of these additional PFASs in UCMR3 was not evaluated, but results of previous studies suggest there will be instances where they occur.
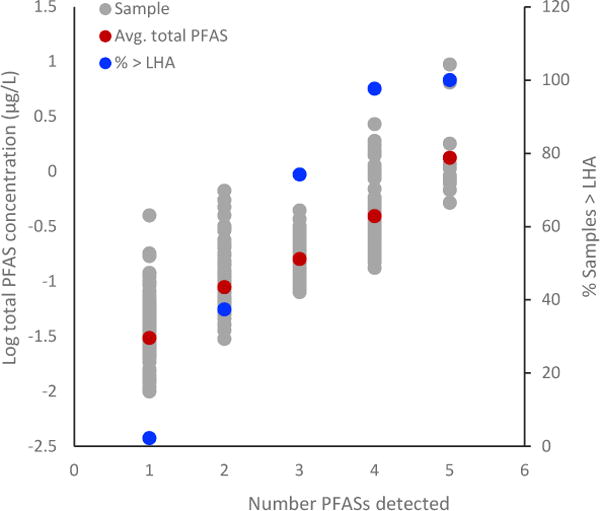
Figure 2.
Logs of ratios of a) PFHxS:PFOS, b) PFHxS:PFOA, and c) PFBS:PFHpA compared to ratios in previous studies. Lines represent the range of ratio values (where available) and circles denote median values. Squares denote ratios of maximum groundwater concentrations for sites where ranges were not reported. Sources types are AFFF-impacted groundwater (AFFF (GW)) (Anderson et al. 2016; Houtz et al. 2013; Hull et al. 2017), wastewater treatment plant effluent (WWTP) (Ahrens et al. 2009), and fluoropolymer manufacturing (FP) (Bach et al. 2017; Dauchy et al. 2012; Oliaei et al. 2013).
3.2 Occurrence of Compound Mixtures in UCMR3
3.2.1 Odds Ratios
The occurrence of PFAS mixtures was investigated by calculating the odds ratio for all pairs of PFASs (Table S.1). The odds ratio quantifies how the detection of one compound increases the likelihood of detecting a second compound. Higher odds ratios are associated with increased co-occurrence. The odds ratio considers correlations between detections and not concentrations. As mentioned, patterns in PFAS use lead to potential for commingled releases and/or simultaneous release of multiple PFASs, so it is not surprising that there was a high degree of association between most pairs of PFASs. Odds ratios ranged from 46 (PFOS and PFNA) to 876 (PFOS and PFHxS). Use of odds ratios to further elucidate release type is limited because multiple sources may explain co-occurrence of a single pair. For example, PFOS and PFHxS are documented to co-occur at sites impacted by AFFF, fluoropolymer manufacturing, and landfills (Bach et al. 2017; Dauchy et al. 2012; McGuire et al. 2014; NHDES 2017; Oliaei et al. 2013).
Positive associations also occurred between detection of PFASs and other UCMR3 contaminants, including 1,4-dioxane, HCFC-22, hexavalent chromium, chlorate, and 1,1-dichloroethane. Odds ratios were lower than those associated with PFAS pairs (1.2-14.2). Strongest odds ratios were between PFASs and 1,4-dioxane. PFASs and 1,4-Dioxane are both documented to co-occur with trichloroethylene, particularly in groundwater (Adamson et al. 2014; Anderson et al. 2012; McGuire et al. 2014), but the direct association of PFASs with 1,4-Dioxane has not been investigated outside of UCMR3 data. These patterns of co-occurrence do not provide definitive evidence of co-located usage or release of PFASs with these other contaminants, and environmental distribution is further influenced by factors such as differential transport, attenuation, or treatment.
3.2.2 Bivariate Concentration Correlations
Odds ratios provided limited understanding regarding potential release types leading to PFAS detections in UCMR3 data, so concentrations of PFASs were examined to provide additional insights into co-occurrence and potential sources. Whereas odds ratios considered strictly PFAS detections, bivariate concentration correlations examined ratios of concentrations in samples where at least two PFASs occurred. Cross plots of concentrations for each pair of PFASs indicate if consistent correlations are present in the ratios of two PFASs over the range of detection. The relationships based on the UCMR3 data exhibit a significant degree of variability (R2 = 0.00-0.89, Figure S.2), which likely reflects factors such as multiple possible PFAS sources in drinking water and differing fate and transport characteristics. Despite this variability, significant (p<0.05) positive correlations in concentrations of six PFAS pairs (PFOS/PFOA, PFOS/PFHxS, PFOS/PFHpA, PFOA/PFHxS, PFOA/PFHpA, and PFBS/PFHpA) did occur. These six pairs were further examined by comparing ranges of ratios that occurred within samples to ratios measured in AFFF-impacted groundwater, WWTP effluent, and fluoropolymer manufacturing (Figure 2).
The highest odds ratio was observed for PFOS and PFHxS and this pair also exhibited a significant, positive correlation in concentrations (Figure S.2). PFOS and PFHxS were both major components of historic AFFF formulations and are commonly reported to occur at AFFF-impacted sites (Anderson et al. 2016; Houtz et al. 2013; Hull et al. 2017; McGuire et al. 2014); however, they are also documented to co-occur at sites impacted by manufacturing activity (Bach et al. 2017; Dauchy et al. 2012; Oliaei et al. 2013) and in WWTP effluent (Ahrens et al. 2009). Ratios PFHxS to PFOS in data from UCMR3, and each of these source types were similar (Figure 2a), making it difficult to use this specific pair to better understand predominant sources of PFASs in UCMR3.
Conversely, ratios of PFOS to PFOA and PFHxS to PFOA measured in previous studies are more likely reflect differences based on release type (Figure 2b and Figure S.3). PFOA was the primary PFAS historically used for manufacturing some types of polytetrafluoroethylene (Prevedouros et al. 2006). So not surprisingly, aqueous data from previous studies of sites impacted by historical fluoropolymer manufacturing practices exhibit ratios reflective of high PFOA concentrations relative to other PFASs (Bach et al. 2017; Dauchy et al. 2012; Oliaei et al. 2013). PFAS ratios in WWTP effluent data also reflect elevated levels of PFOA (Ahrens et al. 2009). PFOA is not documented to be a major component in foams that have been analyzed (i.e. mg/L of PFOA vs. g/L of PFOS) (Houtz et al. 2013). PFOA occurs in AFFF-impacted water, but in comparison to water impacted by fluoropolymer manufacturing and WWTP effluent, ratios of PFOS to PFOA and PFHxS to PFOA reflect relatively lower PFOA concentrations (Anderson et al. 2016; Houtz et al. 2013; Hull et al. 2017). Ratios of PFOS and PFHxS to PFOA in UCMR3 appear more consistent with AFFF-impact scenarios as opposed to other release types for which data are available (Figure 2b and Figure S.3).
The remaining three PFAS pairs that had significant concentration correlations in the UCMR3 dataset involve PFHpA. Interestingly, PFHpA, has no known instances of intentional production or use that could be identified in the literature, and likely occurs as an impurity or due to precursor transformation (Hanari et al. 2014; Harding-Marjanovic et al. 2015; Houtz and Sedlak 2012). In UCMR3 data, ratios of PFOS to PFHpA, PFOA to PFHpA, and PFBS to PFHpA are different from the majority of fluoropolymer manufacturing ratios. They are consistent with AFFF-impacted groundwater and, to some degree, WWTP effluent (Figure 2c and Figure S.3). The similarity of PFBS to PFHpA ratios in UCMR3 to WWTP effluent is particularly interesting because WWTP effluent is expected to release primarily to surface water bodies and the majority of the limited PFBS detections in UCMR3 data were in surface water. Differences between source water types in UCMR3 are discussed further in Section 3.3.1.
3.2.3 Multivariate Analysis
To further utilize the full distribution of concentration data generated during UCMR3 monitoring, hierarchical cluster analysis was performed using the set of samples with detections of one or more PFASs (Figure S.4). Relative to pair-wise evaluations, this method further integrates information from the 6 different PFASs measured in each sample. Resulting clusters support results of bivariate concentration correlations. Specifically, PFOA and PFHpA cluster together and are distinct from an adjacent cluster of PFOS and PFHxS. PFBS did not cluster with either of these two groups, but it was more closely related to the PFOA/PFHpA and PFOS/PFHxS clusters than PFNA.
The dissimilarity of PFNA and PFBS is related to low detection rates (which impacts the concentration-based cluster analyses) but also may reflect other factors. PFNA and PFBS are present at AFFF-impacted sites, but at lower concentrations relative to other UCMR3 PFASs (Anderson et al. 2016; Houtz et al. 2013; Hull et al. 2017). The primary known use of PFNA is for manufacturing of polyvinylidene fluoride (Buck et al. 2011; Prevedouros et al. 2006), which is not known to be associated with other PFASs and may explain the dissimilarity from clusters in the analysis. Separate clustering of PFBS may be the result of multiple factors. Production of PFASs by some manufacturers has shifted towards perfluorobutane sulfonyl based chemistry for applications such as stain resistant surface treatments and fluorosurfactants (Buck et al. 2011; Renner 2006), which may serve as unique sources of PFBS. As noted, detections of PFBS were primarily in surface water, and PFBS is the only UCMR3 PFAS where the rate of detection was higher in surface water vs. groundwater. This is consistent with transport characteristics of PFBS, which exhibits weaker sorption than PFOA, PFNA, PFHxS, and PFOS (Guelfo and Higgins 2013; Higgins and Luthy 2006), and therefore less likely to be retained in aquifer sediments or the solid phase (e.g. sludge) of waste treatment processes. Notably this is consistent with PFBS to PFHpA ratios, which were similar in composition to WWTP effluent. Collectively, the separate clustering (i.e. weaker relationships) of PFBS and PFNA suggests the influence of both separate source types and environmental distribution.
3.3 Influence of System Characteristics
3.3.1 Source Water Relationships
In general groundwater was predominant in UCMR3 data, representing 72% of all samples where PFAS detections occurred, and the average total PFAS concentration was higher (p<0.0001, Mann-Whitney U test) in groundwater (0.21 μg/L) than in surface water (0.09 μg/L, Figure 3). As mentioned, rates of occurrence and concentrations of the individual UCMR3 PFASs were also higher in groundwater vs. surface water with the exception of PFBS (Figure S.5), and this likely reflects both sources and transport properties. PFHpA is the only UCMR3 PFAS that exhibits weak sorption similar to that of PFBS (Guelfo and Higgins 2013), and though it has higher occurrence and average concentration in groundwater, the differences were minor as compared to the segregation by source water type seen for other groundwater dominant PFASs. In order to determine if other key differences were present in the composition of PFASs in surface water vs. groundwater in UCMR3 data, concentrations of total PFSAs (PFOS, PFHxS, and PFBS) and PFCAs (PFHpA, PFOA, and PFNA) in UCMR3 were further studied to see if trends were apparent.
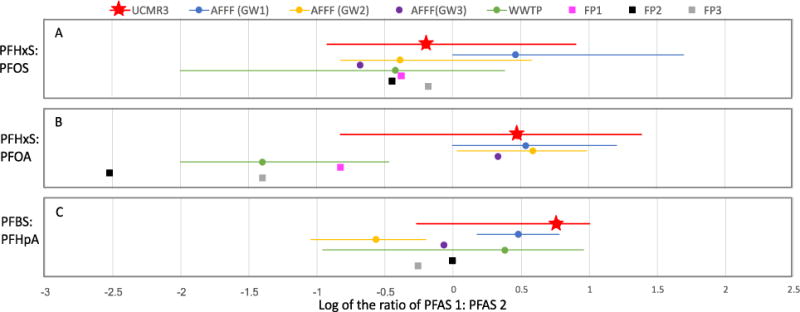
Figure 3.
PFAS concentrations as function of system size and source water type in all samples with detections of one of more PFASs. The lower and upper ends of the whisker represent minimum and maximum detections, respectively. Grey squares represent the average value. Lower and upper ends of the box represent the 25th and 75th percentile, respectively, and the center line is the median value.
Total PFSA and total PFCA composition of UCMR3 samples illustrate key differences based on source water type. This was evaluated using the ratio of total PFSAs (PFOS+PFHxS+PFBS) to total PFASs (sum of all 6 UCMR3 PFASs). The remaining fraction of total PFAS is comprised by total PFCAs (PFHpA+PFOA+PFNA). The total PFSA to total PFASs ratio exhibits a clear shift towards higher percentages of PFSAs in samples from groundwater relative to samples from surface water (Figure S.6). For example, 61% of groundwater samples had total PFSA to total PFASs ratios greater than 0.5. In surface water, only 37% of samples with detections were dominated by PFSAs and the remaining 63% had PFSA to total PFASs ratios of less than 0.5 (i.e. were dominated by PFCAs). There was a statistically-significant difference between the groundwater and surface water sample sets (Mann-Whitney U test; p<0.01). These data confirm that total PFAS concentrations in groundwater-based drinking water supplies in UCMR3 were more likely to contain mixtures dominated by PFSAs.
The composition of total PFASs in surface water vs. groundwater is largely consistent with the types of releases more prevalent in each type of source water. For example, PFSAs are primarily associated with AFFF, mist suppressants, and surface protection products (Favreau et al., 2017). AFFF poses particular relevance to groundwater because it is primarily land applied leading to potential for subsurface migration, and as discussed, multiple PFAS ratios in UCMR3 were consistent with ratios measured in groundwater at AFFF-impacted sites. Using data from two of these sites for the six UCMR3 PFASs (i.e. eliminating other PFASs measured at these sites for which data are not available in UCMR3), the median percentage of PFSAs in the sum of 6 UCMR3 PFASs was 82-96% in groundwater (Houtz et al. 2013; Hull et al. 2017). This range is higher than the median of 74% PFSAs observed in UCMR3 groundwater samples with PFAS detections, possibly reflecting different AFFF formulations, associated precursor transformation, or contributions of additional PFAS sources such as fluoropolymer manufacturing that may lead to higher fractions of PFCAs.
In contrast, PWSs sourced from surface water were more likely to be dominated by PFCAs. The median fraction of PFCAs comprising total PFASs in UCMR3 surface water systems was 100% (i.e. the majority of surface water samples with detections contained PFCAs but not PFSAs). As discussed, environmental samples with higher percentages of PFCAs have been attributed to sources including fluoropolymer manufacturing, landfills, and WWTP effluent (Ahrens et al. 2009; Arvaniti and Stasinakis 2015; Bach et al. 2017; Dauchy et al. 2012; Houtz et al. 2016; Oliaei et al. 2013; Weber et al. 2017), which all have relevance to surface water scenarios. Manufacturing facilities may impact surface water through surface runoff, storm water outfalls, or industrial WWTP effluent. WWTPs typically discharge effluent to surface water, they receive wastes from numerous potential sources including landfill leachate, and are generally ineffective at removing PFASs (Merino et al. 2016). Considering only UCMR3 PFASs, previous work has found raw drinking water sources impacted by fluoropolymer manufacturing were comprised of a median of 79% PFCAs (Dauchy et al. 2012), compared to the median value of 100% in UCMR3 surface water samples with detections. PFCAs in prior studies of WWTP comprised a median value of 75% of total PFASs (Ahrens et al. 2009). The median value of 100% in UCMR3 surface water samples is elevated relative to these sources. PFASs in surface water discharges are likely subject to dilution in receiving waters before uptake by downstream PWSs. In prior studies of WWTP effluent, 88% of PFAS detections were below the MRLs for the respective UCMR3 compounds (Ahrens et al. 2009). In general, the combination of elevated MRLs and dilution is likely an underlying cause of low surface water detection frequency in UCMR3 relative to groundwater.
3.3.2 System Size
The UCMR3 monitoring program included all large PWSs in the U.S. and a subset (800) of small PWSs. The frequency of detection for all UCMR3 PFASs was higher in large PWSs than in small (Figure S.5). The detection frequency for ≥1 PFASs in samples from small systems was low (9 of 2830 samples, or 0.3%). Based on a categorical analysis of sample data for total PFASs (sum of the 6 UCMR3 PFASs), water samples supplied from large PWSs were 5.6 times more likely than small PFASs to have a detection of one or more PFASs (p<0.0001; 95% confidence limits of 2.9 - 10.8 on the odds ratio). However, when detected, total PFAS concentrations were higher (p=0.026, Mann-Whitney U test) in small PWSs (average = 0.30 μg/L) than in large (average = 0.17 μg/L) (Figure 3). Given that only a subset of small PWSs were sampled as part of UCMR3 efforts, these results raise questions regarding the potential for PFAS impacts in the remainder of small systems. The representativeness of the data set is further discussed in Section 3.5.
3.4 PFAS Persistence Over Time
Despite efforts to phase out PFOS, PFOA, and their precursors, there is concern about their long-term fate in the environment due to the lack of attenuation mechanisms. Additionally, other PFASs are still broadly used, leading to questions about overall PFAS emissions into the environment. UCMR3 generated three years of data that may provide insights into temporal trends in PFAS concentrations. Concentration data were not suitable for temporal analysis (Supplementary data, Section S.1). Instead, for each quarter when monitoring occurred, a single detection rate was established representative of all PFASs analyzed during that period. Detection rates were also established individually for PFOS and PFOA. This method is considered valid because >99% samples were analyzed for all 6 PFASs simultaneously and the same MRLs were used throughout the UCMR3 monitoring period. The quarterly detection rates for all PFASs, PFOA, and PFOS over time were evaluated using both linear regression and the Mann Kendall test (Figure 4, Figure S.7) (Connor et al. 2014). For overall PFAS detections, the slope of the best-fit regression line was increasing but not statistically different from zero (p=0.26), while Mann-Kendall analysis indicated an increasing trend in detection rates over time (p=0.03). Similar results were obtained for analysis of individual detection rates for PFOA (increasing, p=0.01) and PFOS (probably increasing, p=0.1). This provides moderate evidence of increasingly-frequent detection rates over the course of UCMR3 monitoring for PFAS overall and for PFOA and PFOS individually.
Figure 4.
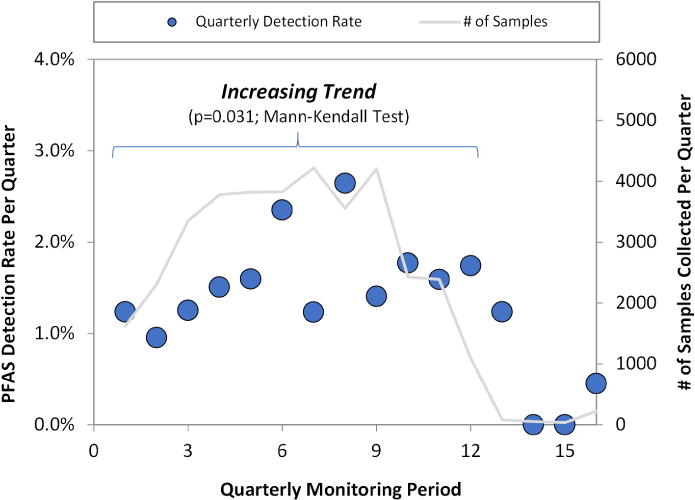
PFAS Occurrence Trend Using Quarterly Results for All Samples. Data from all UCMR3 samples with analysis of one or more PFAS were grouped by monitoring period (quarterly). The Mann-Kendall test was used to evaluate the trend in detection frequency for data from the first 12 quarters (2013–2015). Data from the last 4 quarters (2016) were not used due to the low number of samples per quarter (<2% of total). Circles are detection rates and correspond to the left y-axis; lines are the number of samples per quarter and correspond to the right y-axis.
Temporal trends in UCMR3 data for PFOA and PFOS do not provide evidence that the 2002 phase-out of PFOS and gradual phase-out of PFOA beginning in 2006 had an impact on detection rates during the period of UCMR3 monitoring. Though based on detections, these results support a previous study that found concentrations of PFOS and PFOA in a North Carolina watershed remained similar between 2006 and 2013 (Sun et al. 2016). Each PFAS included in UCMR3 is a perfluoroalkyl substance, so results in North Carolina and UCMR3 may partially reflect a lack of destructive attenuation mechanisms that would accelerate decreasing PFAS concentrations and detections over time. Additionally, use of replacement PFASs that may serve as precursors after release into the environment may contribute to consistent or increased detections of PFASs. Precursors of PFOA and PFOS have been phased out in the U.S., but generation may be ongoing from legacy releases, and precursors of shorter UCMR3 PFASs may still be in use. Based on these results, recent efforts at PFAS source control of new or legacy PFASs are not clearly reflected in occurrence rates in drinking water data.
4. IMPLICATIONS OF UCMR3 FOR PFAS IN US DRINKING WATER
The UCMR3 monitoring program included only a subset of environmentally-relevant PFASs and drinking water sources in the U.S. Nevertheless, the resulting dataset is large and includes a broad distribution of PWSs in the U.S. and its territories. An important question is whether data are representative of PFASs in U.S. drinking water in terms of distribution, composition, and concentrations.
UCMR3 captured all large PWSs in the U.S. but only 800 small PWSs and no private drinking water wells. When selected in 2010, the PWSs sampled in UCMR3 served ~79% (248 million) of the US population (Adamson et al., 2017). Previous studies estimate that in 2010, 44.5 million U.S. residents used private wells as a major source of drinking water (Hu et al. 2016; Maupin, Molly A. et al. 2014). Private, state, and local monitoring efforts have identified PFAS impacts in small PWSs and private wells not involved in UCMR3 (e.g., Hoosick Falls, NY and Bennington, VT). To better understand resulting knowledge gaps, data from small PWSs using groundwater were assumed to be representative of private wells. The detection rate for at least one PFAS in small systems using groundwater was 0.4% as compared to a 1.9% detection rate in all groundwater samples. If small groundwater systems are considered a proxy for private wells, then private wells are 4.5 times less likely to detect one or more PFASs relative to other systems.
While the rate of PFAS detection in small PWSs and private wells is likely to be lower than other system types, there may be relatively elevated PFAS levels in these supplies when impacts do occur. Analyses of the UCMR3 dataset based on PWS characteristics revealed higher average, total PFAS concentrations in groundwater and in small systems (Figure 3, Figure S.5). Only a small set of 8 samples with PFAS detections were collected at PWSs that were both small and sourced from groundwater (i.e. the surrogate for private wells). However, these small, groundwater PWSs were also characterized by higher total PFAS concentrations (median = 0.18 μg/L) relative to all other detections (median = 0.053 μg/L) (p=0.01). These results suggest per capita PFAS exposure risks are potentially greater at impacted small, groundwater PWSs and private wells as compared to other impacted systems. This may be attributable to less dilution at small-system wells with relatively modest pumping rates and capture zones, a trait that may be further exacerbated at private wells that serve household demand.
Low rates of detection in surface water PWSs may be related to PFAS dilution to concentrations below compound MRLs (Table 1). Additionally, transient detections of PFASs at concentrations near the MRL were observed in some PWSs (Supplementary data, Section S.2, Figure S.8), raising questions regarding the influence of compound MRLs on the representativeness of UCMR3 data. Combined, the MRLs for PFOA (0.02 μg/L) and PFOS (0.04 μg/L) are 0.06 μg/L, which is approximately 14% lower than the 0.07 μg/L LHA. A round robin study of PFAS analysis measured aqueous PFOA concentrations of 0.021 – 0.083 μg/L in a sample with a known concentration of 0.025 μg/L, resulting in a relative standard deviation (RSD) of 32% (van Leeuwen et al. 2009). Similarly, aqueous PFOS measurements were 0.02-0.06 μg/L in sample with a known concentration 0.023 μg/L, resulting in a RSD of 29% (van Leeuwen et al. 2009). Considering an average RSD of 31% for PFOS and PFOA, these results suggest drinking water with a true concentration of 0.07 μg/L for the sum of PFOA and PFOS may yield laboratory measured concentrations of 0.049-0.091 μg/L. Based on these results, it is possible that some PWSs with exceedances of the LHA yielded non-detect results for one or both compounds, and some PWSs measured as exceeding the LHA may have true concentrations that are lower. This raises questions regarding how well UCMR3 data capture LHA exceedances especially at levels approaching the advisory. Additionally, for those states (e.g. New Jersey, Vermont) adopting regulatory limits for PFASs at levels less than the LHA, UCMR3 data may not provide adequate information regarding the compliance of PWSs with local standards.
An increasing number of studies have identified a variety of precursors and replacement products for compounds such as PFOA in both groundwater and surface water (Backe et al. 2013; Barzen-Hanson et al. 2017; Strynar et al. 2015; Sun et al. 2016). Additionally, precursor transformation is a potential explanation for relatively high rate of PFHpA occurrence and increasing trends in quarterly PFAS detection rates in UCMR3 data. The state of North Carolina recently adopted a drinking water health goal of 0.14 μg/L for the PFOA replacement product perfluoro-2-propoxypropanoic acid (PFPrOPrA, trade name GenX) (NCDHHS 2017), so it is evident that concerns regarding PFASs in the environment extend beyond the UCMR3 PFASs. Collectively this suggests that the UCMR3 dataset does not fully represent the composition of total PFASs present in U.S. drinking water, and that future monitoring of additional PFASs may be warranted.
UCMR3 data represent significant progress in understanding occurrence of PFASs in U.S. drinking water and provide insights that may be applied to informed design of future monitoring efforts. Specifically, results from small PWSs (low occurrence rate but higher concentrations) suggest a need for targeted evaluation of small PWSs and private wells. Risk-based geospatial approaches that consider factors such as PFAS sources, system location, and source water vulnerability can help in effective design of sampling programs (Guelfo, Jennifer et al. 2018). Results also suggest a need for improved understanding of the total PFAS composition in drinking water. A tiered scheme that analyzes UCMR3 PFASs as an initial screening, then revisits samples with detections for further analysis using methods to quantify total PFAS load (Barzen-Hanson et al. 2017; Houtz and Sedlak 2012) would help to understand relevant exposures and provide more information regarding sources. This approach would help identify priority compounds for efforts centered around development of treatment techniques or toxicological and epidemiological studies, leading to more efficient mitigation of risks associated with PFASs in drinking water.
Supplementary Material
HIGHLIGHTS.
Analysis of data from nearly 37,000 drinking water samples collected across the U.S. and analyzed for per- and polyfluoroalkyl substances (PFASs).
Samples nationwide contain measureable levels of PFAS mixtures and other co-contaminants such as 1,4-Dioxane.
Data highlight a need for additional, targeted evaluation of small public water systems, private wells, and occurrence of a broader suite PFASs.
CAPSULE.
PFASs occur in public water systems throughout the U.S.; concentrations and composition are dominated by mixtures of PFASs and other co-contaminants.
Acknowledgments
We acknowledge financial support from the Superfund Research Program of the National Institute of Environmental Health Sciences, grant 2P42 ES013660. Technical support and other valuable feedback were provided by Sharon Rauch, (GSI Environmental), Elizabeth Pina (formerly GSI Environmental, currently CH2M), Eric Suuberg (Brown University) and Abigail Cartwright and Jose Alberto Maldonado (former GSI Environmental interns).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson DT, Mahendra S, Walker KL, Rauch SR, Sengupta S, Newell CJ. A Multisite Survey To Identify the Scale of the 1,4-Dioxane Problem at Contaminated Groundwater Sites. Environ Sci Technol Lett. 2014;1:254–258. doi: 10.1021/ez500092u. [DOI] [Google Scholar]
- Adamson DT, Piña EA, Cartwright AE, Rauch SR, Hunter Anderson R, Mohr T, et al. 4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Sci Total Environ. 2017;596–597:236–245. doi: 10.1016/j.scitotenv.2017.04.085. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Bundschuh M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review: Fate and effects of polyfluoroalkyl and perfluoroalkyl substances. Environ Toxicol Chem. 2014;33:1921–1929. doi: 10.1002/etc.2663. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Felizeter S, Sturm R, Xie Z, Ebinghaus R. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Mar Pollut Bull. 2009;58:1326–1333. doi: 10.1016/j.marpolbul.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Anderson JK, Bower PA. Co-occurrence of 1,4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: Fact or fiction. Integr Environ Assess Manag. 2012;8:731–737. doi: 10.1002/ieam.1306. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Long GC, Porter RC, Anderson JK. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere. 2016;150:678–685. doi: 10.1016/j.chemosphere.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Arvaniti OS, Stasinakis AS. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci Total Environ. 2015;524–525:81–92. doi: 10.1016/j.scitotenv.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Bach C, Dauchy X, Boiteux V, Colin A, Hemard J, Sagres V, et al. The impact of two fluoropolymer manufacturing facilities on downstream contamination of a river and drinking water resources with per- and polyfluoroalkyl substances. Environ Sci Pollut Res. 2017;24:4916–4925. doi: 10.1007/s11356-016-8243-3. [DOI] [PubMed] [Google Scholar]
- Backe WJ, Day TC, Field JA. Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environ Sci Technol. 2013;47:5226–5234. doi: 10.1021/es3034999. [DOI] [PubMed] [Google Scholar]
- Barzen-Hanson KA, Roberts SC, Choyke S, Oetjen K, McAlees A, Riddell N, et al. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ Sci Technol. 2017;51:2047–2057. doi: 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study: Prenatal PFAS Exposure and Child Adiposity. Obesity. 2016;24:231–237. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CK, Watkins AM, Wolf CJ, Abbott BD, Lau C, Gennings C. Testing for departures from additivity in mixtures of perfluoroalkyl acids (PFAAs) Toxicology. 2013;306:169–175. doi: 10.1016/j.tox.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Farhat SK, Vanderford M. GSI Mann-Kendall Toolkit for Quantitative Analysis of Plume Concentration Trends. Groundwater. 2014;52:819–820. doi: 10.1111/gwat.12277. [DOI] [PubMed] [Google Scholar]
- Dauchy X, Boiteux V, Rosin C, Munoz J-F. Relationship Between Industrial Discharges and Contamination of Raw Water Resources by Perfluorinated Compounds. Part I: Case Study of a Fluoropolymer Manufacturing Plant. Bull Environ Contam Toxicol. 2012;89:525–530. doi: 10.1007/s00128-012-0704-x. [DOI] [PubMed] [Google Scholar]
- Davis KL, Aucoin MD, Larsen BS, Kaiser MA, Hartten AS. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere. 2007;67:2011–2019. doi: 10.1016/j.chemosphere.2006.11.049. [DOI] [PubMed] [Google Scholar]
- DeWitt JC. Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances. Springer International Publishing; Switzerland: 2015. [Google Scholar]
- Favreau P, Poncioni-Rothlisberger C, Place BJ, Bouchex-Bellomie H, Weber A, Tremp J, et al. Multianalyte profiling of per- and polyfluoroalkyl substances (PFASs) in liquid commercial products. Chemosphere. 2017;171:491–501. doi: 10.1016/j.chemosphere.2016.11.127. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. Perfluorooctanoic Acid, Perfluorooctanesulfonate, and Serum Lipids in Children and Adolescents: Results From the C8 Health Project. Arch Pediatr Adolesc Med. 2010;164 doi: 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo Jennifer, Marlow Thomas, Klein David, Savitz David, Frickel Scott, Crimi Michelle, et al. Evaluation and management strategies for per- and polyfluoroalkyl substances (PFASs) in drinking water aquifers: perspectives from impacted U.S. Northeast communities. Environ Health Perspect. 2018 doi: 10.1289/EHP2727. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Higgins CP. Subsurface Transport Potential of Perfluoroalkyl Acids at Aqueous Film-Forming Foam (AFFF)-Impacted Sites. Environ Sci Technol. 2013;47:4164–4171. doi: 10.1021/es3048043. [DOI] [PubMed] [Google Scholar]
- Hanari N, Yamazaki T, Itoh N, Fujiki N, Aoyagi Y, Takatsu A, et al. Evaluation of perfluorooctanoic acid purity based on potentiometric titration. Anal Methods. 2014;6:3177–3182. doi: 10.1039/C3AY42154F. [DOI] [Google Scholar]
- Harding-Marjanovic KC, Houtz EF, Yi S, Field JA, Sedlak DL, Alvarez-Cohen L. Aerobic Biotransformation of Fluorotelomer Thioether Amido Sulfonate (Lodyne) in AFFF-Amended Microcosms. Environ Sci Technol. 2015;49:7666–7674. doi: 10.1021/acs.est.5b01219. [DOI] [PubMed] [Google Scholar]
- Higgins CP, Luthy RG. Sorption of perfluorinated surfactants on sediments. Env Sci Technol. 2006;40:7251–7256. doi: 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Higgins CP, Field JA, Sedlak DL. Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil. Environ Sci Technol. 2013;47:8187–8195. doi: 10.1021/es4018877. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sedlak DL. Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff. Environ Sci Technol. 2012;46:9342–9349. doi: 10.1021/es302274g. [DOI] [PubMed] [Google Scholar]
- Houtz EF, Sutton R, Park J-S, Sedlak M. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016;95:142–149. doi: 10.1016/j.watres.2016.02.055. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett. 2016 doi: 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R, Barber LB, LeBlanc DR, Weber AK, Vecitis CD. Poly- and perfluoalkyl substances in contaminated groundwater. Cape Cod, Massachusetts: 2017. pp. 2014–2016. [Google Scholar]
- Kissa E. In: Fluorinated Surfactants and Repellents, Second Edition Revised and Expanded. Hubbard AT, editor. Marcel Dekker, Inc; New York, NY: 2001. [Google Scholar]
- Knepper TP, Lange FT, editors. Polyfluorinated Chemicals and Transformation Products. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. [Google Scholar]
- Krafft MP, Riess JG. Selected physicochemical aspects of poly- and perfluoroalkylated substances relevant to performance, environment and sustainability—Part one. Chemosphere. 2015;129:4–19. doi: 10.1016/j.chemosphere.2014.08.039. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Maupin Molly A, Kenny Joan F, Hutson Susan S, Lovelace John K, Barber Nancy L, Linsey Kristin S. Estimated Use of Water in the United States in 2010 Circular 1405. U.S. Geological Survey; Reston, VA: 2014. [Google Scholar]
- McGuire ME, Schaefer C, Richards T, Backe WJ, Field JA, Houtz E, et al. Evidence of Remediation-Induced Alteration of Subsurface Poly- and Perfluoroalkyl Substance Distribution at a Former Firefighter Training Area. Environ Sci Technol. 2014;48:6644–6652. doi: 10.1021/es5006187. [DOI] [PubMed] [Google Scholar]
- Mejia Avendaño S, Liu J. Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere. 2015;119:1084–1090. doi: 10.1016/j.chemosphere.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Merino N, Qu Y, Deeb RA, Hawley EL, Hoffmann MR, Mahendra S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ Eng Sci. 2016;33:615–649. doi: 10.1089/ees.2016.0233. [DOI] [Google Scholar]
- Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCDHHS. Questions and Answers Regarding North Carolina Department of Health and Human Services Updated Risk Assessment for GenX (Perfluoro-2-propoxypropanoic acid) 2017. [Google Scholar]
- NHDES. NHDES PFAS Sampling. 2017 Available: http://nhdes.maps.arcgis.com/home/item.html?id=66770bef141c43a98a445c54a17720e2 [accessed 23 July 2017]
- Oliaei F, Kriens D, Weber R, Watson A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA) Environ Sci Pollut Res. 2013;20:1977–1992. doi: 10.1007/s11356-012-1275-4. [DOI] [PubMed] [Google Scholar]
- Oliaei F, Kriens Don, Kessler Katrina. Investigation of Perfluorochemical (PFC) Contamination in Minnesota Phase One. Report to Senate Environment Committee 2006 [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, Fate and Transport of Perfluorocarboxylates. Environ Sci Technol. 2006;40:32–44. doi: 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- Renner R. The long and the short of perfluorinated replacements. Environ Sci Technol. 2006;40:12–13. doi: 10.1021/es062612a. [DOI] [PubMed] [Google Scholar]
- Rhoads KR, Janssen EML, Luthy RG, Criddle CS. Aerobic biotransformation and fate of N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE) in activated sludge. Env Sci Technol. 2008;42:2873–2878. doi: 10.1021/es702866c. [DOI] [PubMed] [Google Scholar]
- Roberson JA, Eaton A. Retrospective Analysis of Mandated National Occurrence Monitoring and Regulatory Decision-Making. J - Am Water Works Assoc. 2014;106:E116–E128. doi: 10.5942/jawwa.2014.106.0040. [DOI] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS) Environ Sci Technol. 2015;49:11622–11630. doi: 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett. 2016;3:415–419. doi: 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Suthersan S, Quinnan J, Horst J, Ross I, Kalve E, Bell C, et al. Making Strides in the Management of “Emerging Contaminants”. Groundw Monit Remediat. 2016;36:15–25. doi: 10.1111/gwmr.12143. [DOI] [Google Scholar]
- USEPA. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS) 2016a [Google Scholar]
- USEPA. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA) 2016b [Google Scholar]
- USEPA. The Third Unregulated Contaminant Monitoring Rule 2012 [Google Scholar]
- van Leeuwen SPJ, Swart CP, van der Veen I, de Boer J. Significant improvements in the analysis of perfluorinated compounds in water and fish: Results from an interlaboratory method evaluation study. J Chromatogr A. 2009;1216:401–409. doi: 10.1016/j.chroma.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int. 2013;60:242–248. doi: 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Weber AK, Barber LB, LeBlanc DR, Sunderland EM, Vecitis CD. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ Sci Technol. 2017;51:4269–4279. doi: 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Rider CV, Lau C, Abbott BD. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology. 2014;316:43–54. doi: 10.1016/j.tox.2013.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


