Abstract
OBJECTIVES
To determine the relationship between frailty and overall and cardiovascular mortality.
DESIGN
Longitudinal mortality analysis.
SETTING
National Health and Nutrition Examination Survey (NHANES) 1999–2004.
PARTICIPANTS
Community-dwelling older adults aged 60 and older (N = 4,984; mean age 71.1 ± 0.19, 56% female).
MEASUREMENTS
We used data from 1999–2004 cross-sectional NHANES and mortality data from the National Death Index, updated through December 2011. An adapted version of Fried’s frailty criteria was used (low body mass index, slow walking speed, weakness, exhaustion, low physical activity). Frailty was defined as persons meeting 3 or more criteria, prefrailty as meeting 1 or 2 criteria, and robust (reference) as not meeting any criteria. The primary outcome was to evaluate the association between frailty and overall and cardiovascular mortality. Cox proportional hazard models were used to evaluate the association between risk of death and frailty category adjusted for age, sex, race, smoking, education, coronary artery disease, heart failure, nonskin cancer, diabetes, and arthritis.
RESULTS
Half (50.4%) of participants were classified as robust, 40.3% as prefrail, and 9.2% as frail. Fully adjusted models demonstrated that prefrail (hazard ratio (HR) = 1.64, 95% confidence interval (CI) = 1.45–1.85) and frail (HR = 2.79, 95% CI = 2.35–3.30) participants had a greater risk of death and of cardiovascular death (prefrail: HR = 1.84, 95% CI = 1.45–2.34; frail: HR = 3.39, 95% CI = 2.45–4.70).
CONCLUSION
Frailty and prefrailty are associated with increased risk of death. Demonstrating the association between prefrail status and mortality is the first step to identifying potential targets of intervention in future studies.
Keywords: frailty, prefrailty, mortality, cardiovascular
Frailty is a decline in the body’s physiological reserve and a reduced ability to maintain homeostasis among life’s constant stressors.1 It has been associated with functional losses, disability, greater healthcare use, and higher healthcare costs.2–7 Although a standardized definition of frailty has not been agreed upon, its concept has evolved to be a complex relationship of physical, psychological, and social factors.7 Fried’s landmark study demonstrated a way to operationalize frailty collectively as a phenotype defined according to a set of variables: unintentional weight loss, self-reported exhaustion, slow gait speed, low energy expenditure and weak grip strength (frail ≥3; prefrail 2).8 By this definition the prevalence of frailty is estimated to be 10.7% while prefrailty is 41.6% in community-dwelling older adults.9 It is thought that prefrailty has similar associations with the negative outcomes of frailty. As frailty is a dynamic process, prefrail individuals are more likely to maintain prefrail status or revert back to a robust state than frail individuals.10 This makes prefrailty an intermediary, possibly reversible phase that should be investigated separately from frailty in its associations and potential interventions.
Frailty is a known risk factor for mortality.11–14 A metaanalysis demonstrated 50% greater mortality in individuals with frailty than in robust subjects and that this risk escalates with each additional phenotypic frailty component.15 Frailty is also thought to have quadruple the risk of cardiovascular mortality,16 but evidence of prefrailty’s association with overall and cardiovascular mortality is less clear, with conflicting studies demonstrating various relationships between prefrailty and survival. The aim of this study was to evaluate mortality in prefrail individuals to determine whether it is an important entity in itself. We hypothesized that the risk of all-cause and cardiovascular death in prefrail individuals would be significantly greater than robust participants.
METHODS
Study Design and Participants
Participants included in the analysis were identified from the 1999 to 2004 cross-sectional National Health and Nutrition Survey (NHANES). NHANES is a multistage probability survey conducted by the National Center for Health Statistics designed to assess the health and nutritional status of adults and children in the United States. The survey focuses on noninstitutionalized persons and oversamples non-Hispanic blacks, Mexican Americans, and persons aged 60 and older. Full details are available at http://www.cdc.gov/nchs/nhanes.html.
The total sample in NHANES consisted of 38,077 participants. Of these, 29,402 were interviewed and examined in a mobile examination center. We aimed to include only those aged 60 and older (n = 7,729) who had full body composition data (n = 4,984) in our secondary analysis. Trained field staff interviewed participants in English or Spanish, and data collection was automated. Respondents completed questionnaires directly or, if unable, by proxy. The local institutional review board at Dartmouth college exempted this study from review because of the de-identified nature of the data.
Mortality Data
Mortality was evaluated using the 2015 public-use linked mortality files, which include mortality data from the time of the study through December 31, 2011. These data were obtained from the National Death Index, a service of the National Center for Health Statistics that serves as a centralized database of death record information in state vital statistics offices. These data are linked to the NHANES data using a unique study identifier. Full details are available at https://www.cdc.gov/nchs/ndi/index.htm. Time to death was calculated in days from the date of death, and overall and cardiovascular mortality were assessed. Cardiovascular mortality was defined using International Classification of Disease codes.
Study Variables
We applied the Fried definition of frailty to our study sample using data from participant self-reported questionnaires and objective measures. The 5 criteria of frailty from the Cardiovascular Health Study8,17 were adapted, as has been done previously with NHANES data18: unintentional weight loss of 10 pounds or more in a year: low body mass index (BMI)<18.5 kg/m2; self-reported exhaustion: difficulty walking between rooms; weakness: difficulty lifting or carrying 10 lbs; slow walking speed: gait speed <0.8 m/s; and low physical activity: reduced physical activity compared to others your age. Frailty was defined as meeting 3 or more of the five criteria, prefrailty as meeting 1 or 2 criteria, and robust as meeting 0 of the 5 criteria.
Covariates
Demographic variables included self-reported age, sex, race, marital status, education, smoking status, and ethnicity. We categorized respondents as non-Hispanic white, non-Hispanic black, Hispanic, or other. We ascertained self-reported comorbidities such as diabetes, arthritis, coronary artery disease, congestive heart failure, and non-skin cancer if participants answered the question “Has a doctor ever told you have [disease state]?” Participants were classified as smokers if they had smoked at least 100 cigarettes in their lifetime.
Weight was measured in kilograms on an electronic digital scale. Height was measured using a stadiometer. Subjects were asked a number of self-perception questions regarding limitations in activities of daily living (ADLs) and instrumental activities of daily living (IADLs). All activities were self-reported, and subjects noted on scale from 1 to 4 their degree of difficulty in performing these tasks. We classified subjects as having difficulty if they indicated a response of anything other than “no difficulty.” Of the 7 well-accepted IADLs,19 NHANES included managing money, performing household chores, preparing meals, and handling routine needs. We defined ADL disability as difficulty getting in and out of bed or inability to dress or eat. NHANES did not include assessment of bathing or toileting.20
Statistical Analysis
All data were merged into one file for this analysis. All analyses accounted for the complex, stratified nature of NHANES and incorporated primary sampling units, weighting, and strata, according to analytical guidelines. Continuous variables are represented as means and standard errors and categorical variables as counts and weighted percentages. Analysis of variance and chi-square tests were used to assess differences in baseline characteristics according to frailty group. Because gait speed was not assessed in NHANES 2003–04, we used multiple imputation analyses to account for missing values. Multiple imputations were conducted using R version 3.3.2 and the package mice, which operates by creating plausible data values from a distribution specifically designed for each data point. We generated 5 imputed data sets using predictive mean matching. The correction variables used were age, sex, education, protein, race, diabetes, arthritis, congestive heart failure, cancer, and lean mass percentage. The 5 data sets were averaged, resulting in a final imputed data set used for analysis. To test the quality of the imputation, analyses were run on the full imputed data set and on a subset excluding the imputed variables.
The primary outcome was mortality risk, and the primary predictor was frailty category. Three separate Cox proportional hazard models were created to evaluate the risk of death. Model 1 was unadjusted and included frailty categories as the sole predictors; Model 2 included age, sex, race, education, and smoking; and Model 3 included the covariates in Model 2 and additionally adjusted for comorbidities such as diabetes, heart failure, cancer, coronary artery disease, and arthritis. All-cause and cardiovascular mortality were assessed and are presented in the results as hazard ratios (HRs) with 95% confidence intervals (CIs). Kaplan-Meier survival curves for overall and cardiovascular mortality are presented in Figure 1. As an exploratory analysis, mortality modeling was stratified according to age group (60–69, 70–79, ≥80). As a comparison to our imputed gait speed model, frailty rates were calculated using a 4-component model by removing the gait speed criteria: robust meeting 0 criteria, prefrail meeting 1–2 criteria, frail meeting 3–4 criteria. The above models were replicated (not shown). Data analysis was conducted using Stata version 12 (Stata Corp., College Station, TX). P < .05 was considered statistically significant.
Figure 1.
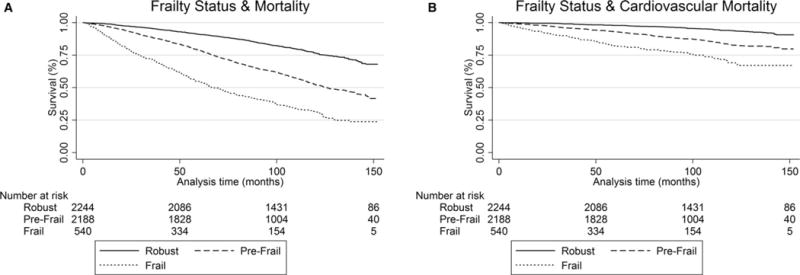
Kaplan Meier survival curve for (A) all-cause and (B) cardiovascular mortality of robust, prefrail, and frail participants demonstrating a significant trend toward greater death from all-cause and cardiac causes with greater frailty status.
RESULTS
The mean age of the selected study sample of 4,984 participants was 71.1 ± 0.19, and they had a mean BMI of 28.2 ± 0.10 kg/m2. The majority of the participants were non-Hispanic white (Table 1). Participants with higher frailty status were more likely to be female, have a higher BMI, and be older. Frail and prefrail participants were more likely to have concurrent comorbidities such as diabetes, coronary artery disease, arthritis, stroke, chronic kidney disease, and chronic obstructive pulmonary disease and significantly more likely to have dysfunction in at least one IADL or ADL. There were few observed differences in baseline characteristics between those with complete frailty variable data and those without complete frailty data. Those with incomplete data were slightly older, had lower BMI, and more ADL and IADL limitations at baseline (Table S1).
Table 1.
Participant Baseline Characteristics
| Characteristics | Total, N = 4,984 |
Robust, n = 2,246 |
Prefrail, n = 2,195 |
Frail, n = 541 |
P-Value |
|---|---|---|---|---|---|
| Age, mean (SE) | 71.1 (0.19) | 68.7 (0.22) | 73.3 (0.23) | 74.9 (0.45) | <.001 |
| Sex, n (%) | |||||
| Female | 2,531 (56.6) | 949 (47.2) | 1,244 (65.6) | 336 (68.1) | <.001 |
| Male | 2,453 (43.5) | 1,297 (52.8) | 951 (34.4) | 205 (31.9) | <.001 |
| Race and ethnicity, n (%) | <.001* | ||||
| Non-Hispanic white | 2,846 (81.2) | 1,387 (86.1) | 1,203 (77.4) | 256 (70.5) | |
| Non-Hispanic black | 811 (8.3) | 281 (5.5) | 403 (10.3) | 127 (15.8) | |
| Hispanic | 1,202 (7.2) | 533 (5.9) | 522 (8.1) | 146 (10.8) | |
| Other | 125 (3.2) | 45 (2.4) | 67 (4.2) | 12 (3.0) | |
| ≥College education, n (%) | 1,676 (40.6) | 986 (50.4) | 585 (32.3) | 105 (23.4) | <.001 |
| Smoker, n (%) | .02* | ||||
| Never | 2,327 (46.7) | 1,004 (44.2) | 1,052 (49.1) | 271 (50.3) | |
| Former | 2,035 (41.4) | 948 (43.9) | 889 (39.6) | 198 (35.5) | |
| Current | 611 (11.9) | 288 (11.9) | 254 (11.4) | 69 (14.3) | |
| Body mass index, kg/m2, mean (SE) | 28.2 (0.10) | 27.8 (0.12) | 28.3 (0.18) | 30.7 (0.49) | <.001 |
| ≥1 activity of daily living limitations | 2,423 (47.3) | 576 (25.3) | 1,320 (63.5) | 527 (96.8) | <.001 |
| ≥1 instrumental activity of daily living limitations | 1,751 (32.6) | 248 (10.2) | 988 (46.4) | 515 (94.5) | <.001 |
| Comorbidities, n (%)a | |||||
| Diabetes mellitus | 1,060 (18.3) | 356 (13.2) | 499 (21.0) | 205 (34.5) | <.001 |
| Heart failure | 373 (7.1) | 47 (1.9) | 211 (10.1) | 115 (22.9) | .34 |
| Nonskin cancer | 916 (21.7) | 418 (22.1) | 395 (20.8) | 103 (22.9) | .49 |
| Coronary artery disease | 870 (18.3) | 297 (14.2) | 421 (20.1) | 152 (30.9) | <.001 |
| Arthritis | 2,379 (50.2) | 786 (38.3) | 1,228 (59.8) | 363 (73.6) | <.001 |
| Hypertension | 2,326 (87.7) | 892 (87.0) | 1,123 (87.7) | 311 (90.1) | .34 |
| Stroke | 405 (7.6) | 101 (4.5) | 192 (8.3) | 112 (21.1) | <.001 |
| Chronic obstructive pulmonary disease | 496 (11.8) | 130 (6.8) | 269 (15.9) | 97 (21.4) | <.001 |
| Chronic kidney disease | 81 (4.2) | 22 (2.5) | 39 (4.8) | 20 (11.5) | .03 |
Self-reported at initiation of screening.
SE = standard error.
P value for overall in group.
Table 2 presents a breakdown of each frailty component; the number of components participants in the study fulfilled; and overall rates of participants who were robust, prefrail, and frail. Robust and frail rates differed between our 5- and 4-component models mostly at the extremes, with 50.4% and 65.4% classified as robust and 9.2% and 4.5% as frail, respectively. The frailty component seen at the highest rate was low walking speed (31.3%), followed by weakness (27.1%). Few participants met all 5 criteria (<1%).
Table 2.
Frailty Components and rates
| Frailty Components | Five-Component Model | Four-Component Modela |
|---|---|---|
| n (weighted %) | ||
| Difficulty walking between rooms | 586 (10.0) | |
| Body mass index <18.5 kg/m2 | 59 (1.3) | |
| Less activity than peers | 735 (14.1) | |
| Gait speed <0.8 m/sb | 1,865 (31.3) | n/a |
| Difficulty lifting 10 pounds | 1,455 (27.1) | |
| Number of components | ||
| 0 | 2,246 (50.4) | 3,063 (67.0) |
| 1 | 1,486 (27.7) | 1,026 (21.7) |
| 2 | 709 (12.6) | 414 (7.8) |
| 3 | 371 (6.4) | 189 (3.4) |
| 4 | 167 (2.7) | 5 (0) |
| 5 | 3 (0) | n/a |
| Frailty status | ||
| Robust | 2,246 (50.4) | 3,158 (65.4) |
| Prefrail | 2,195 (40.3) | 1,561 (30.2) |
| Frailty | 541 (9.2) | 263 (4.5) |
Defining frailty according to 4-component model: robust = 0 criteria, prefrail = 1–2 criteria, frailty = 3–4 criteria.
Imputed and nonimputed.
Over the course of follow-up 1,901 participants died, with 521 (27.4%) dying from cardiovascular causes. Median follow-up time was 95.8 months (interquartile range 78–124). Overall and cardiovascular mortality analyses along the frailty spectrum are outlined in Table 3. The adjusted models suggest a 64% higher mortality rate (HR = 1.64, 95% CI = 1.45–1.85) in prefrail individuals than in those who were robust and a rate nearly 3 times as high in frail individuals (HR = 2.79, 95% CI = 2.35–3.30). Similar estimates were observed for cardiovascular mortality (prefrail: HR = 1.84, 95% CI = 1.45–2.34; frail: HR = 3.39, 95% CI = 2.45–4.70). Figures 1 and 2 depict the Kaplan Meier survival curves for overall mortality and cardiovascular mortality. As an exploratory analysis, we stratified trail participants mortality rates according to age group (Table S2). Prefrailty rates and overall mortality according to age group demonstrated a trend toward greater risk than robust for all geriatric age groups (60–69: HR = 1.65, 95% CI = 1.31–2.09; 70–79: HR = 1.77, 95% CI = 1.45–2.17; ≥80: HR = 1.57, 95% CI = 1.28–1.92). We found similar results examining prefrailty and cardiovascular mortality compared to robust (60–69: HR = 1.52, 95% CI = 0.95–2.44; 70–79: HR = 2.42, 95% CI = 1.61–3.65; ≥80: HR = 1.88, 95% CI = 1.25–2.81).
Table 3.
Association Between Frailty and Overall and Cardiovascular Mortality
| Mortality | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | |||
| Overall | |||
| Prefrail | 2.40 (2.16–2.67) | 1.79 (1.60–2.01) | 1.64 (1.45–1.85) |
| Frail | 4.97 (4.34–5.69) | 3.89 (3.36–4.51) | 2.79 (2.35–3.30) |
| Cardiovascular | |||
| Prefrail | 2.82 (2.28–3.48) | 2.07 (1.65–2.60) | 1.84 (1.45–2.34) |
| Frail | 3.72 (2.85–4.87) | 4.79 (3.61–6.34) | 3.39 (2.45–4.70) |
Model 1: unadjusted.
Model 2: adjusted for age, sex, race, education, smoking.
Model 3: adjusted for Model 2 covariates and diabetes, heart failure, cancer, coronary artery disease, arthritis.
Reference: robust.
DISCUSSION
This study demonstrates that, although frailty has a larger association with overall and cardiovascular mortality than prefrailty, the association between prefrailty and mortality is meaningful and noteworthy. Specifically, prefrail participants were 64% more likely than robust participants to die.
Few studies have focused on the relationship between prefrailty and mortality. A systematic review15 evaluating the association between mortality and frailty did not examine the relationship specifically with prefrailty. The Cardiovascular Health Study demonstrated the relative risk of mortality in prefrail individuals over a 4 year time period to be 1.67, (95% CI = 1.29–2.15),17 and another study21 that evaluated men aged 65 and older found prefrail participants were 36% more likely to die than robust individuals. Other studies such as the Crystal study22 and The Three-City Study6 challenge this association, with their findings failing to show statistically significant greater mortality in prefrail participants, making this relationship unclear.
Evidence for a strong association between frailty and cardiovascular disease exist while prefrailty’s relationship is less defined.11,23–26 The Progetto Veneto Anziani Study found that low energy expenditure, exhaustion, and slow gait speed were predictive of new cardiovascular events, and prefrailty appeared to be an independent predictor of cardiovascular disease.25 A metaanalysis examining the relationship between frailty and mortality found that prefrail participants were 3 times as likely to die from cardiovascular disease over a median 4.4-year follow-up,16 although that analysis was based on 2 studies not representative of community-dwelling elderly adults; one focused on posthospital discharge mortality after a myocardial infarction,24 and the other evaluated individuals without baseline cardiovascular disease.25
Determining this association is important in clarifying prefrailty as an important diagnostic entity in itself versus another early disease state. Although some preconditions such as prehypertension have been well defined, no association has been found between prehypertension and overall mortality.27 This is unlike other precondition states such as prediabetes, for which a metaanalysis demonstrated an associated greater risk of all-cause and cardiovascular mortality when defined according to fasting glucose as low as 5.6 mmol/L.28 This demonstrates that not all preconditions are equal in their associated risks.
Another important argument for early recognition of prefrailty outside its own associated risks is its higher likelihood of reversibility. One study of the transitions of frailty found that 57.6% of participants had at least one transition in the 54-month follow-up.10 Transition to greater frailty was 43.3%, versus 23% to less frailty. Rates of transition from frail to robust were negligible (≤0.9%), and the longer participants were classified as frail, the greater their chance of remaining frail and the higher their mortality, but if participants entered the study prefrail, the chances of remaining prefrail or transitioning to robust over 36 months was 78.4%. Those starting prefrail had higher rates of transitioning back to robust in the first 36 months, although chances were lower, and mortality was greater for those remaining prefrail over this 54-month period. This demonstrates that recognizing prefrailty and intervening reduces not only the chances of frailty progression, but also the higher mortality risk of remaining prefrail.
Our study of a large, nationally representative sample provides further support for a significant association between mortality and prefrailty. These results highlight the importance of identifying individuals across the frailty spectrum. Few studies have explored the most mutable risk factors aimed at preventing transitions to higher frailty states. One trial29 assessed the effect of an exercise and nutritional intervention on quality of life and physical performance over 12 weeks in prefrail women. Significant improvements in handgrip strength, subjective pain, and emotional well-being with exercise were observed but not maintained at 6-month follow-up. A larger study, the Lifestyle Interventions and Independence for Elders Pilot Study,30 completed over 12 months, examined older adults at risk of disability and demonstrated that 150 min/wk of activity plus balance training led to faster walking speed. These studies indicate that interventions can reverse prefrailty, but studies showing maintenance of these benefits over time are lacking.
Our study had a number of strengths, including large sample size and use of self-reported and biometric measurements and of a validated tool for frailty classification, which makes our findings relevant. Or study also had several important limitations. First, the variables present in NHANES to operationalize Fried’s frailty criteria required us to modify some of the original definitions, but the prevalence rates of each component were comparable with those in other studies.8,15,31 Second, because walking speed was missing for 3,645 participants, we performed multiple imputations to maximize our data analysis. We used multivariate imputation by chained equations for our missing data, a robust method that generates multiple predictions for each missing value, taking the uncertainty of the imputations into account and yielding accurate standard errors.32 We did not use data without imputed gait speed for conclusion data, but baseline characteristics of those with gait speed information were compared with characteristics of those with imputed gait speed to show that they were similar populations. Third, a number of the frailty components are based on self-reported data and are subject to recall bias. Fourth, although we demonstrated greater mortality risk by age, we caution the reader on generating conclusions from this data because of the sample sizes in the age subgroups. Because our study sample included only community-dwelling adults, this could have led to some selection bias, particularly for those aged 80 and older, which probably made our results more conservative than if institutionalized elderly adults had been included. Also, because of the increasing literature on the association between valvular heart disease and frailty, we caution the reader on the limitation of our dataset to correct for this variable in our analysis.33,34 In addition, medical care and culture have changed since early the 2000s, and mortality rates continue to decline from heart disease, stroke, and cancer, whereas diabetes and obesity rates have increased, according to several U.S. studies.35,36 The decline in rates cardiovascular mortality seems to have slowed, probably because of incremental advances in prevention and treatment, along with the increasing aging population’s high on rates of heart disease and mortality.37,38 A limitation in Fried’s frailty model is its lack of cognitive and psychosocial factors in determining frailty status. There is increasing evidence that inclusion of these factors could improve predictive ability for adverse health outcomes.39 The deficit accumulation model that Rockwood and colleagues defined encompasses cognitive changes as part of defining frailty. Although frailty rates vary according to definition (9.9% for physical frailty and 13.6% for physical and psychosocial frailty),9 when evaluating mortality, as we aimed to do here, both models have shown comparable results.40,41 Lastly, with any epidemiology-based analysis, causality cannot be inferred, and future longitudinal, interventional studies are needed.
The large number of prefrail and frail individuals will continue to grow as our society continues to age.42 Prefrailty and frailty should be ascertained because an estimated 3% to 5% of deaths of older adults could be delayed with frailty prevention.43 The implementation of interventions targeting the most common deficits in the frailty spectrum (gait speed and weakness) has a large potential to improve function: reduce hospitalization, institutionalization, and unneeded healthcare spending while improving quality of life.8,15,26 Recognizing prefrailty as its own entity is essential because of its association with overall and cardiovascular mortality, as well as its window of opportunity for reversibility in delaying frailty progression. Knowledge of prefrailty’s association of mortality allows providers to see the value of aggressive primary and secondary prevention through optimizing medication management and health promotion efforts. With this relationship known, research efforts can examine potential interventions and their ability to improve long-term outcomes.
Supplementary Material
Table S1. Baseline Characteristics of Study Participants with Complete versus Incomplete Data
Table S2. Relative Hazard Rations of Mortality by Baseline Frailty Status Extrapolated by Age
Acknowledgments
Presented at the American Geriatrics Society Annual Meeting, San Antonio, Texas, May 2017.
Financial Disclosure: Dr. Crow’s research reported in this publication was supported by Dartmouth centers for Health and Aging- Geisel School of medicine at Dartmouth and the Department of Medicine.
Dr. Bartels receives funding from the National Institute of Mental Health (K12 HS0217695 (AHRQ), T32 MH073553, R01 MH078052, R01 MH089811, R01 MH102325, R24 MH102794), the Centers for Disease Control and Prevention (U48 DP005018), and the Health Resources and Services Administration (U1 QHP28718, T32 HP30036).
Dr. Batsis’ research reported in this publication was supported in part by National Institute on Aging, National Institutes of Health (NIH) Award K23AG051681. Alexander Titus’ research reported in this publication was supported in part by NIH Award T32LM012204. Research reported in this publication was supported by Dartmouth Clinical and Translational Science Institute Award UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of NIH. This work was also supported by Dartmouth Health Promotion and Disease Prevention Research Center Cooperative Agreement U48DP005018 from the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the NIH or CDC.
Footnotes
Conflict of Interest: There are no conflicts of interest pertaining to this manuscript.
Author Contributions: Crow, Lohman, Bruce, Bartels, Batsis: Study concept and design, data analysis and interpretation, preparation of manuscript. Titus, Mackenzie: Data analysis and interpretation, preparation of manuscript.
Sponsor Role: None.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson TN, Wu DS, Stiegmann GV, et al. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202:511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock JO, Konig HH, Brenner H, et al. Associations of frailty with health care costs—results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makizako H, Shimada H, Doi T, et al. Impact of physical frailty on disability in community-dwelling older adults: A prospective cohort study. BMJ Open. 2015;5:e008462. doi: 10.1136/bmjopen-2015-008462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comans TA, Peel NM, Hubbard RE, et al. The increase in healthcare costs associated with frailty in older people discharged to a post-acute transition care program. Age Ageing. 2016;45:317–320. doi: 10.1093/ageing/afv196. [DOI] [PubMed] [Google Scholar]
- 6.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: The Three-City Study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 7.Chong E, Ho E, Baldevarona-Llego J, et al. Frailty and risk of adverse outcomes in hospitalized older adults: A comparison of different frailty measures. J Am Med Dir Assoc. 2017;18:638.e637–638.e611. doi: 10.1016/j.jamda.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: A systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 10.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 11.Klein BE, Klein R, Knudtson MD, et al. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Graham JE, Snih SA, Berges IM, et al. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55:644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D, Song X, Mitnitski A, et al. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17:465–471. doi: 10.1007/BF03327413. [DOI] [PubMed] [Google Scholar]
- 14.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 15.Shamliyan T, Talley KM, Ramakrishnan R, et al. Association of frailty with survival: A systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Veronese N, Cereda E, Stubbs B, et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev. 2017;35:63–73. doi: 10.1016/j.arr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: Lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blodgett J, Theou O, Kirkland S, et al. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The Index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 22.Turusheva A, Frolova E, Korystina E, et al. Do commonly used frailty models predict mortality, loss of autonomy and mental decline in older adults in northwestern Russia? A prospective cohort study. BMC Geriatr. 2016;16:98. doi: 10.1186/s12877-016-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purser JL, Kuchibhatla MN, Fillenbaum GG, et al. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791. doi: 10.1016/j.ahj.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: The Pro.V.A. study. J Am Coll Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Zhang X, Zheng L, et al. Prehypertension is not associated with all-cause mortality: A systematic review and meta-analysis of prospective studies. PLoS ONE. 2013;8:e61796. doi: 10.1371/journal.pone.0061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Cai X, Chen P, et al. Associations of prediabetes with all-cause and cardiovascular mortality: A meta-analysis. Ann Med. 2014;46:684–692. doi: 10.3109/07853890.2014.955051. [DOI] [PubMed] [Google Scholar]
- 29.Kwon J, Yoshida Y, Yoshida H, et al. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: A randomized controlled trial. J Am Med Dir Assoc. 2015;16:263.e261–e268. doi: 10.1016/j.jamda.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Rejeski WJ, King AC, Katula JA, et al. Physical activity in prefrail older adults: Confidence and satisfaction related to physical function. J Gerontol B Psychol Sci Soc Sci. 2008;63B:P19–P26. doi: 10.1093/geronb/63.1.p19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Garrido J, Ruiz-Ros V, Buigues C, et al. Clinical features of prefrail older individuals and emerging peripheral biomarkers: A systematic review. Arch Gerontol Geriatr. 2014;59:7–17. doi: 10.1016/j.archger.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mack M. Frailty and aortic valve disease. J Thorac Cardiovasc Surg. 2013;145:S7–S10. doi: 10.1016/j.jtcvs.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 35.Hoyert DL. 75 years of mortality in the United States, 1935–2010. NCHS Data Brief. 2012;88:1–8. [PubMed] [Google Scholar]
- 36.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: Possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bureau; WDUC. The next four decades: The older population in the United States: 2010 to 2050. Curr Popul Rep. 2010:P25–P1138. [Google Scholar]
- 39.Aubertin-Leheudre M, Woods AJ, Anton S, et al. Frailty clinical phenotype: A physical and cognitive point of view. Nestle Nutr Inst Workshop Ser. 2015;83:55–63. doi: 10.1159/000382061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al’s clinical frailty scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19:1043–1048. doi: 10.1007/s12603-015-0667-9. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Thabane L, Ioannidis G, et al. Comparison between frailty index of deficit accumulation and phenotypic model to predict risk of falls: Data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Hamilton cohort. PLoS ONE. 2015;10:e0120144. doi: 10.1371/journal.pone.0120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knickman JR, Snell EK. The 2030 problem: Caring for aging baby boomers. Health Serv Res. 2002;37:849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the Women’s Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61A:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Study Participants with Complete versus Incomplete Data
Table S2. Relative Hazard Rations of Mortality by Baseline Frailty Status Extrapolated by Age


