Abstract
Objective
To analyze the neuropathic pain (NeP) components in patients with rheumatoid arthritis (RA).
Methods
The painDETECT questionnaire (PD-Q) was completed by 300 RA patients (79 men, 221 women).
Results
Nine patients (3.0%) were categorized as likely NeP, 33 (11.0%) were categorized as possible NeP, and 258 (86.0%) were categorized as unlikely NeP. When we excluded patients with diabetes mellitus, spinal diseases, neurological diseases, and herpes zoster infection (conditions associated with NeP), 5 of the patients (1.7%) had likely NeP, and 23 (7.7%) had possible NeP without any underlying conditions. Furthermore, there were no marked differences in the percentages of these underlying conditions among the patients with likely, possible, and unlikely NeP. When we compared patients with likely and possible NeP (n=42) and unlikely NeP (n=258), the body mass index (BMI), disease activity score-28 based on the erythrocyte sedimentation rate, C-reactive protein level, pain visual analogue scale (VAS), and PD-Q score were significantly higher in the patients with likely and possible NeP than in those with unlikely NeP. A multivariate analysis showed that BMI ≥22 and not being in clinical remission were associated with NeP.
Conclusion
Although RA pain has usually been classified as nociceptive pain, the present study clarified that a significant number of patients might have NeP. The present findings suggest that high disease activity and being overweight are related to NeP in RA patients.
Keywords: neuropathic pain, rheumatoid arthritis, painDETECT questionnaire, obesity
Introduction
With the introduction of biological disease-modifying antirheumatic drugs (bDMARDs), the control of rheumatoid arthritis (RA) has dramatically improved worldwide, including in Japan (1). In addition, in 2011, the approved dose of methotrexate (MTX), which is considered to be an anchor drug in the treatment of RA, was increased to 16 mg/week in Japan and was found to be effective (2). However, there are still many patients who suffer from pain when the condition develops without active inflammation.
We tried to clarify the incidence of neuropathic pain (NeP) in RA patients. The painDETECT questionnaire (PD-Q) is a tool for detecting NeP that was reported from Germany (3). Ahmed et al. reported that 33% of RA patients might have NeP components (4). Matsubayashi et al. confirmed that the PD-Q can be used in Japanese patients via a questionnaire translated into Japanese (5). In a study using the PD-Q and 2 other questionnaires, Mochizuki et al. reported that 11% of Japanese RA patients had NeP using PD-Q (6).
In the present study, we collected PD-Q responses from 300 Japanese RA patients and report the results.
Materials and Methods
The study population of this survey consisted of RA patients attending Niigata Rheumatic Center from September 2014 to March 2015. Three hundred consecutive outpatients with a diagnosis of RA who agreed to take part in this survey were enrolled. The PD-Q was completed by all 300 RA outpatients (79 men, 221 women, average age 64.6±14.3 years). Written consent was not obtained, but the publication of the results of the PD-Q was approved by our ethics committee (NO. 2017-005).
This questionnaire was deemed very easy to complete. The painDETECT was developed to screen for NeP and inquired about the following:
a) The baseline visual analogue scale (VAS): The average VAS pain (average pain intensity during the past 4 weeks), worst VAS pain (worst pain intensity during the past 4 weeks), and current VAS pain were reported.
b) Pain course pattern (4 questions)
Q1. Persistent pain with slight fluctuations: 0
Q2. Persistent pain with pain attacks: -1
Q3. Pain attacks without pain between them: +1
Q4. Pain attacks with pain between them: +1
c) Location of pain
d) Radiating pain: +2.
e) Gradation of pain (7 questions)
Q1. Do you suffer from a burning sensation (e.g., stinging nettles) in the marked areas?: 0-5
Q2. Do you have a tingling or prickling sensation in the area of your pain (like crawling ants or electrical tingling)?: 0-5
Q3. Is light touching (clothing, a blanket) in this area painful?: 0-5
Q4. Do you have sudden pain attacks in the area of your pain, like electric shocks?: 0-5
Q5. Is cold or heat (bath water) in this area occasionally painful?: 0-5
Q6. Do you suffer from a sensation of numbness in the areas that you marked?: 0-5
Q7. Does slight pressure in this area, e.g. with a finger, trigger pain?: 0-5
(For each question, the responses were scored as follows: never, 0; pain was hardly noticed, 1; slight, 2; moderate, 3; strong, 4; very strong, 5)
Patients with scores of 0-12 points, 13-18 points, and ≥19 points were classified into the unlikely NeP, possible NeP, and likely NeP groups, respectively. Although we asked about the patients' VAS in the PD-Q, the patients' VAS was not determined in this study. The patients with diabetes mellitus (DM), spinal diseases, neurological diseases, and herpes zoster infection, which can induce NeP, were counted, and the number of NeP patients without underlying diseases was counted. We compared the clinical parameters of the patients with likely, possible, and unlikely NeP.
Statistical analyses
Comparisons between two groups were performed using the Mann-Whitney U test. Fisher's exact test was used to determine associations between categorical variables. The BMI, disease activity score-28 based on erythrocyte sedimentation rate (DAS28-ESR), and C-reactive protein (CRP) were dichotomized. The cut-off value for CRP was determined on receiver operating characteristic (ROC) curve analyses. ROC curve analyses indicated that the cut-off value for BMI was 21.9. Based on this value, patients were divided into two groups: BMIHigh (≥22.0) and BMILow (<22.0), because a BMI of 22 is usually used when we calculate ideal body weight. Only 2 patients belongs to 21.9≤BMI<22.0. Logistic regression analyses were performed to determine the predictive factors for NeP (possible or likely). The age, gender, existence of underlying conditions for NeP, and potential confounding factors with borderline significance (p<0.20) were included. The BMI, CRP, and DAS28-ESR did not satisfy the log linearity assumption, so we used dichotomous variables. The included variables were as follows: age, gender (female, male), clinical remission (CR) of DAS28-ESR (yes or no), BMI≥22 (yes or no), underlying disease (present or absent), painVAS (mm), ESR (mm/h), and CRP<0.6 mg/dL (yes or no) (model 1). With such a limited number of NeP patients (n=42), fewer than 5 variables should ideally be selected.
The backward stepwise selection method identified CR of DAS28-ESR and BMI≥22 as the minimal factors. We then constructed a second model with three variables (these two variables plus the existence of an underlying condition for NeP) (model 2). Statistical significance was set at p<0.05. The IBM SPSS Statistics Ver. 22.0 (IBM, Armonk, USA) software program was used for statistical calculations.
Results
Analyses of the painDETECT results
Baseline VAS
The VAS average pain was 30.3±22.6 mm, the VAS worst pain was 38.2±2.84 mm, and the VAS current pain was 28.4±23.2 mm.
Pain course pattern (4 questions)
The pain course pattern is shown in Fig. 1.
Figure 1.

The pain course pattern.
Pain location
The location of pain is shown in Fig. 2. The patients frequently responded that the pain was located in their wrists, ankles, shoulders, knees, and fingers.
Figure 2.
The location of pain.
Radiating pain
Pain was reported to radiate to other regions of the body in 14% of the patients. There were no marked sex differences (men: 26.3%, women: 73.4%).
The results are shown in Fig. 3.
Figure 3.

The gradation of pain.
Q1. Sixty-three (21.0%) of the patients felt slight, moderate, or strong pain.
Q2. Ninety-eight (32.7%) of the patients felt slight, moderate, or strong pain.
Q3. Forty-six (15.3%) of the patients felt slight, moderate, or strong pain.
Q4. Forty-seven (15.6%) of the patients felt slight, moderate, or strong pain.
Q5. Twenty-two (7.1%) of the patients felt slight, moderate, or strong pain.
Q6. Seventy-seven (25.7%) of the patients felt slight, moderate, or strong pain.
Q7. One hundred and sixteen (38.7%) of the patients felt slight, moderate, or strong pain, and the PD-Q results indicated that 9 patients (3.0%, 3 men and 6 women) had likely NeP, 33 (11.0%, 6 men and 27 women) had possible NeP, and 258 (86.0%, 70 men and 188 women) had unlikely NeP.
Patients' background characteristics and a comparison of the patients with likely and possible NeP and unlikely NeP
Patients' background characteristics and the results of a comparison of the patients with likely and possible NeP and unlikely NeP are shown in Table 1. The average disease duration was 11.5±10.2 years. The average ESR and CRP level and DAS28-ESR were 20.0±19.8, 0.67±1.60, and 2.87±1.19, respectively. The current pain VAS determined using a regular questionnaire (at a regular visit, not using the PD-Q) is also shown in Table 1
Table 1.
The Background Information of the Patients with Likely and Possible NeP and with Unlikely NeP.
| All | Likely and possible | Unlikely | p value | |
|---|---|---|---|---|
| n | 300 | 42 | 258 | |
| Age (years old) | 64.6±14.3 | 64.8±15.3 | 64.5±14.1 | 0.682 |
| BMI (kg/m2) | 22.6±3.8 | 23.7±3.7 | 22.4±3.8 | 0.0343 |
| <18.5(%) | 45 (15.0%) | 3 (7.14%) | 42 (16.2%) | 0.162* |
| <22 (%) | 137 (45.7%) | 12 (28.5%) | 125 (48.4%) | 0.0191* |
| <25 (%) | 223 (74.3%) | 28 (66.6%) | 195 (75.6%) | 0.253* |
| Duration (years) | 11.5±10.2 | 11.9±11.3 | 11.6±10.1 | 0.766 |
| Stage of RA (I/II/III/IV) | 87/82/37/94 | 16/9/6/11 | 71/73/31/83 | 0.47* |
| Class of RA (1/2/3/4) | 94/116/85/5 | 14/14/14/0 | 80/102/71/5 | 0.748* |
| DAS-28-ESR | 2.87±1.19 | 3.49±1.06 | 2.77±1.18 | <0.001 |
| Clinical remission (%) | 139 (46.3%) | 9 (21.4%) | 130 (50.4%) | <0.001* |
| CR or LDA (%) | 194 (64.6%) | 16 (38.1%) | 178 (69.0%) | <0.001* |
| CR or LDA or MDA | 284 (94.6%) | 39 (92.9%) | 245 (95.0%) | 0.477* |
| Pain VAS (mm) | 22.1±20.1 | 31.56±22.04 | 20.55±19.39 | 0.00161 |
| CRP (mg/dL) | 0.67±1.6 | 0.92±1.4 | 0.63±1.59 | 0.0484 |
| CRP<0.6 mg/dL | 227 (75.7%) | 26 (61.9%) | 201 (77.9%) | 0.0325 |
| ESR (mm/h) | 20.0±19.8 | 23.3±18.7 | 19.44±19.92 | 0.075 |
| MMP-3 (ng/mL) | 117.3±99.3 | 114.34±95.68 | 114.34±95.68 | 0.713 |
| Male MMP-3 (ng/mL) | 149.7±116.4 | 132.01±76.98 | 151.97±120.81 | 0.660 |
| Female MMP-3 (ng/mL) | 105.8±89.97 | 135.20±131.19 | 100.60±79.99 | 0.473 |
| Elevated MMP-3 (%) | 117 (39.0%) | 18 (42.9%) | 99 (38.4%) | 0.611* |
| PD-Q score | 6.70±5.27 | 16.23±3.54 | 5.13±3.58 | <0.001 |
| Orthopedic surgery (%) | 75 (25.0) | 13 (28.6) | 195 (75.5) | 0.567* |
| The numbers of orthopedic surgeries | 2.01±1.20 | 2.17±1.02 | 1.98±1.23 | 0.378 |
| Years after the first surgery | 9.43±6.66 | 13.00±7.66 | 8.74±6.29 | 0.0513 |
| Years after the last surgery | 6.11±4.51 | 7.58±6.02 | 5.83±4.16 | 0.425 |
| ACPA-positive(%) | 226 (75.3) | 29 (69.1) | 197 (75.5) | 0.336* |
| NSAID (%) | 127 (42.3) | 15 (35.7) | 112 (43.4) | 0.402* |
| Pregabalin (%) | 22 (7.3) | 5 (11.9) | 17 (6.5) | 0.21* |
| Opioid (%) | 28 (9.3) | 7 (16.6) | 21 (8.1) | 0.0881* |
| Tricyclic antidepressant | 0 (0) | 0 (0) | 0 (0) | 1* |
| Duloxetine (%) | 0 (0) | 0 (0) | 0 (0) | 1* |
| PSL (%) | 208 (69.3) | 23 (54.8) | 183 (70.9) | 0.0477* |
| PSL dose (mg/day) | 2.68±2.76 | 2.49±2.79 | 2.71±2.76 | 0.496 |
| csDMARDs(%) | 250 (83.3) | 34 (81.0) | 216 (83.7) | 0.657* |
| bDMARDs(%) | 105 (35.0) | 14 (33.3) | 91 (35.3) | 0.863* |
BMI: body mass index, RA: rheumatoid arthritis, DAS: disease activity score, VAS: visual analogue scale, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, MMP-3: matrix metalloproteinase-3, PD-Q: painDETECT questionnaire, SD: standard deviation, ACPA: anti-citrullinated protein antibody. NSAIDs: non-steroidal anti-inflammatory drugs, MTX: methotrexate, PSL: prednisolone, csDMARDs: conventional synthetic disease-modifying antirheumatic drugs, bDMARDs: biological DMARDs
Statistical analyses were performed using the Mann-Whitney U test except for those marked with *(Fisher’s exact test); p values were evaluated using the Mann-Whitney U test†or Fisher’s exact test§.
A total of 25% of patients had a history of orthopedic surgery. The class and stage were classified using Steinbrocker's classification (7). The history of orthopedic surgeries is shown in Supplementary material 1.
We compared the patients with likely and possible NeP (n=42) and unlikely NeP (n=258) (Table 1). The BMI (23.7±3.7 vs. 22.4±3.8), DAS28-ESR value (3.49±1.06 vs. 2.77±1.18), CRP level (0.92±1.40 vs. 0.63±1.59), VAS value (31.6±22.0 vs. 20.6±19.4), and PD-Q score (16.2±3.54 vs. 5.13±3.58) were significantly higher in patients with likely and possible NeP than in patients with unlikely NeP. The proportion of CR in patients with unlikely NeP was significantly higher (50.4%) than in patients with likely or possible NeP (21.4%, p<0.001). However, of note: nine patients with CR had likely or possible NeP (all were possible NeP). One of these patients had DM, and another had a history of compression fracture and cerebral infarction. However, the other seven patients with CR had possible NeP without any underlying conditions.
The percentage of orthopedic surgery, number of surgeries, and years after surgery were not markedly different between the two groups. The proportions of positive ACPA, usage of pregabalin, opioid, tricyclic antidepressant, duloxetine, methotrexate, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), bDMARDs, and non-steroidal anti-inflammatory drugs (NSAIDs) were also not markedly different between the two groups. Although there were differences in the usage of PSL between the two groups, the doses of PSL were not markedly different between the two groups.
BMI>25 was considered to indicate obesity, but when we set the cut-off index at 25, no statistical significance was achieved.
Results of multivariate analyses
We constructed two models. In both models, BMI≥22 and not being in CR of DAS28-ESR were associated with NeP after adjustment for the existence of underlying conditions that might cause NeP (Table 2).
Table 2.
A Multivariate Analysis of Factors that might have been Related to NeP in the Patients of the Present Study.
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | ||
| DAS28-ESR non CR vs. CR (reference) | 3.40 | 1.34-8.64 | 0.0101 | 3.87 | 1.76-8.51 | <0.001 | |
| BMI≥22 vs. others (reference) | 2.68 | 1.26-5.69 | 0.0101 | 2.48 | 1.129-5.17 | 0.0150 | |
| Underlying disease + vs. – (reference) | 0.999 | 0.462-2.16 | 0.997 | 1.040 | 0.502-2.16 | 0.914 | |
| Age | 0.989 | 0.964-1.01 | 0.392 | ||||
| Pain VAS | 1.010 | 0.993-1.03 | 0.220 | ||||
| CRP<0.6 mg/dL | 0.414 | 0.156-1.10 | 0.077 | ||||
| ESR | 0.982 | 0.958-1.01 | 0.154 | ||||
| Male gender | 0.761 | 0.328-1.77 | 0.526 | ||||
CI: confidential interval, DAS28-ESR: disease activity score-28 based on erythrocyte sedimentation rate, CR: clinical remission, PSL: prednisolon, BMI; body mass index, VAS: visual analogue scale, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate
Table 3.
Underlying Conditions that might have been Related to NeP in the Patients of the Present Study.
| All | Likely | Possible | Unlikely | |
|---|---|---|---|---|
| n | 300 | 9 | 33 | 258 |
| 1. Spinal diseases | 23 | 1 | 2 | 20 |
| 2. Diabetes mellitus | 42 | 2 | 5 | 35 |
| 3. Neurological diseases | 5 | 0 | 0 | 5 |
| 4. Herpes zoster infection | 2 | 0 | 1 | 1 |
| 1 and 2 | 13 | 1 | 2 | 10 |
| 1 and 3 | 4 | 0 | 0 | 4 |
| 2 and 3 | 6 | 0 | 1 | 5 |
| 1, 2 and 3 | 4 | 0 | 0 | 4 |
Neurological diseases: brain tumors, Parkinson’s disease, restless legs syndrome, stroke, epilepsy, and chronic subdural hematoma.
Analyses of patients without underlying conditions of NeP
We tried to rule out the effects of DM, spinal disease, neurological disease, and herpes zoster infection, which seemed to be related to NeP (Table 2). In a sub-analysis in which we excluded the patients who were categorized as having these conditions, we found that in 201 patients, 5 (2.5%) and 23 patients (11.4%) without these underlying conditions were categorized as likely and possible NeP, respectively.
Thus, 28/300 (9.3%) RA patients had either likely or possible NeP without underlying conditions associated with NeP (Table 2)
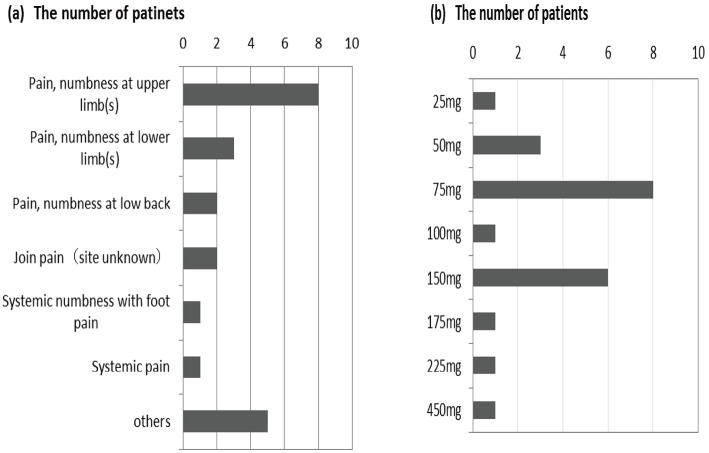
In Japan, pregabalin was approved for NeP in 2013. Pregabalin was already prescribed to 22 patients [likely NeP, n=2 (22.2%), possible NeP, n=3 (8.8%), and unlikely NeP, n=17 (6.6%); Supplementary material 2]. The reasons for the prescription of pregabalin are shown in Fig. 4a, while the dose of pregabalin is shown in Fig. 4b.
Figure 4.
(a) Reasons for pregabalin prescription. (b) The dose of pregabalin.
Discussion
With improvements in therapy, especially the introduction of bDMARDs, the control and prognosis of RA has improved (1). However, there are still some RA patients in whom the disease activity is well controlled, but they nevertheless continue to experience severe pain. Pain is classified into nociceptive pain, NeP, and mixed pain (8). Trauma, osteoarthritis, and RA are classified as nociceptive pain; post-herpetic neuralgia, diabetic neuropathy, and sciatic pain are classified as NeP; and spinal canal stenosis, lumbar disc hernia, and post-operative pain are classified as mixed pain (8). However, we hypothesized that a certain number of RA patients in whom RA is well controlled have NeP.
During our study, two papers were published on NeP in RA patients (3,5). Ahmed used the PD-Q to investigate 100 RA patients (3) and found that 33% of RA patients might have NeP components. In their study, Mochizuki used the PD-Q and two other questionnaires (a Japan-original neuropathic pain screening questionnaire: NPSQ; and The Leeds Assessment of Neuropathic Symptoms and Signs Pain Scale: LANSS) (3) and reported that 11% of RA patients might have NeP components. We investigated the PD-Q responses of 300 RA patients. Based on our analysis of the results, 3.0% of the patients were classified as having likely NeP, and 11.3% of the patients were classified as having possible NeP. Even though we excluded patients with conditions that might have affected NeP, a certain number of RA patients still might have NeP components. Neither Ahmed nor Mochizuki analyzed these underlying conditions. Thus, our report might have some significance. Furthermore, there were no differences in the complication of these underlying conditions among the groups of patients with likely, possible, and unlikely NeP, which might indicate that these underlying conditions are not contributing to the high rate of NeP in RA patients. It is possible that, in addition to nociceptive pain, the pain of RA patients includes mixed pain, which has a component of NeP. Thus far, pain in RA patients has been considered to be nociceptive pain due to the presence of inflammation, but patients with joint deformity without inflammation might have mixed pain or NeP. However, we detected no marked differences in the disease duration or the history of surgery between the patients with likely and possible NeP, and those with unlikely NeP. In addition, the disease activity of the patients with likely and possible NeP was higher than that of patients with unlikely NeP. Since patients' VAS is included in DAS28-ESR, it might be difficult to determine whether or not high disease activity is due solely to inflammation. Of note, nine patients who achieved CR had possible NeP.
A number of patients in this study were already taking pregabalin. Since pregabalin is indicated for the treatment of NeP, the patients with unlikely NeP who were taking pregabalin might have been classified into the likely or possible NeP groups if the painDETECT had been performed prior to the use of pregabalin. Thus, it is possible that the percentage of NeP in RA patients might be higher. The Japan Society of Pain Clinicians Guidelines for the Pharmacologic Management of Neuropathic Pain recommends the use of a tricyclic antidepressant (amitriptyline), pregabalin, and duloxetine as first-line drugs and tramadol and an extract from the inflamed cutaneous tissue of rabbits inculcated with vaccinia virus as second-line drugs (9). However, at present, only pregabalin has been approved for treating NeP in Japan. Since weight gain and edema are adverse effect of pregabalin, we should pay attention to weight gain and edema when administering this agent to patients with NeP.
We initially hypothesized that NeP was more common in patients with long-term RA, with more joint destruction, and in patients with a history of joint surgery. Unexpectedly, however, there were no differences in these parameters between the patients with likely and possible NeP and those with unlikely NeP. The BMI values of the patients with likely and possible NeP were significantly higher than those in the patients with unlikely NeP. In addition, a multivariate analysis also showed that BMI>22 was associated with NeP. Ahmed also reported that the BMI in patients with NeP was higher than in patients without NeP. Obesity has already been reported to be related to pain (10). Obesity is thought to represent a pro-inflammatory state, and adipocytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and leptin cause inflammation. In contrast, chronic pain may lead to a sedentary lifestyle, resulting in obesity. Recent reports have shown that obesity and pain have a complex relationship, and the relationship between obesity and neuropathic pain is still unclear. It seems that obesity is also related to NeP in RA patients. Ahmed showed that NeP is more common in patients with BMI values of >30 (3). We tried to perform the same analysis, but there were only 9 patients with BMI values of >30 in the present study. It was therefore difficult to determine the statistical significance of the results. The population of obese RA patients in Japan is lower than that in Western countries.
Our results suggest that, in order to reduce NeP, we should control the disease activity of RA and the body weight of the patients. However, we should bear in mind that even with a good control of disease activity, NeP can occur in RA patients, since 9 patients with RA had possible NeP with CR.
Complication of osteoarthritis, Sjögren's syndrome, enthesis, fibromyalgia or depression might affect the results of PD-Q, but it was difficult to collect these data from the medical charts. Some limitations associated with the present study warrant mention. One limitation is the relatively small number of patients who developed NeP. As a result, we were only able to include a limited number of confounding factors in the logistic regression analysis.
Conclusion
Although the pain associated with RA patients is classified as nociceptive pain, substantial numbers of patients might also have NeP. High disease activity and being overweight are thought to be related to NeP in RA patients. We should control the RA activity and body weight to reduce NeP.
Author's disclosure of potential Conflicts of Interest (COI).
Satoshi Ito: Honoraria, Abbvie Japan, Eisai, Mitsubishi Tanabe Pharma, Chugai Pharmaceutical and Bristol-Myers Squibb.
Supplementary Materials
The history of orthopedic surgeries
Underlying conditions that might have been related to NeP in the patients who were treated with Pregabalin
References
- 1. Yamanaka H, Seto Y, Tanaka E, et al. Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol 23: 1-7, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Ito S, Unno M, Kobayashi D, et al. Dose escalation of methotrexate in rheumatoid arthritis patients. J New Rem Clin 63: 1302-1315, 2014. [Google Scholar]
- 3. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 22: 1911-1920, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed S, Magan T, Vargas M, Harrison A, Sofat N. Use of the painDETECT tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reporting. J Pain Res 14: 579-588, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsubayashi Y, Takeshita K, Sumitani M, et al. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One 8: e68013, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mochizuki T, Yano K, Hiroshima R, et al. The study of neuropathic pain using the three diagnostic tools in osteoarthritis of the knee and rheumatoid arthritis. J Musculoskelet Pain Res 6: 19-23, 2014. (in Japanese). [Google Scholar]
- 7. Steinbrocker O, Traeger CH, Robert C, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA 140: 659-662, 1949. [DOI] [PubMed] [Google Scholar]
- 8. Devor M. Response of nerve to injury in relation to neuropathic pain. In: Textbook of Pain, E-edition. McMahon S, Koltzenburg M, Wall PD, Eds. Churchill Livingstone, Philadelphia, 2005: 905-924. [Google Scholar]
- 9.Japan Society of Pain Clinicians Guidelines for the Pharmacologic Management of Neuropathic Pain. 2nd ed. Shinko Trading, Tokyo, 2016. [Google Scholar]
- 10. McVinnie DS. Obesity and pain. Br J Pain 27: 163-170, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The history of orthopedic surgeries
Underlying conditions that might have been related to NeP in the patients who were treated with Pregabalin