Abstract
Objective
The early diagnosis and treatment of microalbuminuria is important for preventing the progression of diabetic kidney disease in patients with diabetes. In this study, we assessed the accuracy of the semi-quantitative measurement of microalbuminuria by urine dipstick screening in patients with diabetes.
Methods
The semi-quantitative urinary albumin-to-creatinine ratio (QUACR) was used for microalbuminuria screening. A total of 291 diabetes patients with normoalbuminuria [urine albumin-to-creatinine ratio (UACR) <30 mg/g・Cre; n=205] or microalbuminuria (UACR 30-299 mg/g・Cre; n=86) were enrolled as study participants. Both the qualitative test of albumin (QUA) and the QUACR of early-morning or spot urine samples were performed at the same time. A receiver operating characteristic (ROC) analysis was performed to compare the diagnostic utility of the QUACR to that of the QUA in the detection of microalbuminuria.
Results
The sensitivity and specificity values of the QUACR were 84.9% and 76.6%, respectively. Those of the QUA were 53.5% and 84.4%, respectively. In the ROC analysis, the area under the curve values of the QUACR and QUA for the diagnosis of microalbuminuria were 0.807 (95% confidence interval: 0.752-0.863) and 0.689 (0.618-0.760), respectively.
Conclusion
These results suggest that the QUACR is a simple and efficient test-with high levels of sensitivity and specificity-for the detection of microalbuminuria in patients with diabetes.
Keywords: semi-quantitative methods for microalbuminuria, diagnostic accuracy, diabetes mellitus
Introduction
Diabetic kidney disease should be detected and treated at the microalbuminuria stage, which is potentially reversible (1). In addition to being the earliest stage of diabetic kidney disease, microalbuminuria is associated with increased cardiovascular morbidity and mortality (2). The first step in screening for microalbuminuria should be the measurement of albumin in a urine sample by a reliable method: the spot urine method (first-morning or random sample), 24-h collection, or timed collection (2). The spot urine method is the easiest way to screen for microalbuminuria (2). When using spot urine screening, the albumin-to-creatinine ratio is often also determined (2-5). The measurement of the albumin concentration along with a spot urine sample has been used by some authors (6) and recommended by others (3); however, this method is controversial (7). In the clinical setting, when a standard quantitative technique for measuring urinary albumin is unavailable, a semi-quantitative test can be used to screen for microalbuminuria (2,8). The aim of this study was to assess the performance of the qualitative urinary albumin (QUA) test and the semi-quantitative urinary albumin-to-creatinine ratio (QUACR) test in microalbuminuria screening in patients with diabetes mellitus.
Materials and Methods
This study was approved by the Ethics Committee of Hiroshima City Asa Citizens Hospital. Informed consent was obtained from patients in accordance with the conditions for approval. We prospectively assessed 291 patients with normoalbuminuria or microalbuminuria who were referred to our department at Hiroshima City Asa Citizens Hospital from January 2014 to April 2014. When patients returned to the clinic for routine consultation, a random urine specimen was collected without any specific recommendation. Using this sample, we quantitatively measured the albumin and creatinine levels, and calculated the urine albumin-to-creatinine ratio (UACR) (mg/g・Cre) using the following formula: albumin/creatinine ×100. The samples were classified into normoalbuminuria (UACR <30 mg/g・Cre), microalbuminuria (UACR 30-299 mg/g・Cre), or macroalbuminuria (UACR >300 mg/g・Cre). Patients with estimated glomerular filtration rates (eGFR) of <30 mL/min/1.73 m2 were excluded from this study because the severity of chronic kidney disease only varies according to albuminuria category in patients with an eGFR of >30 mL/min/1.73 m2 (9). We also excluded patients with macroalbuminuria, which we defined as overt diabetic kidney disease. Collectively, 291 patients (mean age: 64.3 years; male, n=164; female, n=127) were enrolled in this study.
The semi-quantitative measurement of albumin and creatinine was performed using a test strip (Uropaper αIII Eiken; Eiken Chemical, Tokyo, Japan), which was read by an automated device (US-3100R plus; Eiken Chemical, Tokyo, Japan). The semi-quantitative albumin results were categorized into five classes: 10 (-), 30 (±), 80 (1+), 150 mg/dL (2+), or greater (3+). The semi-quantitative creatinine results were also categorized into five classes: 10, 50, 100, 200, or 300 mg/dL.
The QUACR was calculated using the following formula: semi-quantitative albumin/semi-quantitative creatinine ×100. The patients were classified into one of four classes according to the result: dilute or normal (QUACR <30 mg/g・Cre), 1+ (QUACR 30, 80, and 150 mg/g・Cre), or 2+ (QUACR >300 mg/g・Cre). Dilute and normal QUACR were classified as normoalbuminuria. Microalbuminemia was defined as a class of 1+.
The minus and +/- classes of QUA were considered to reflect normoalbuminuria. Microalbuminemia was defined as the classes of 1+ and 2+.
Statistical analysis
The results were expressed as the mean and standard deviation or the median and 95% confidence interval (CI). A receiver operating characteristic (ROC) analysis was performed to compare the utility of the QUACR to that of the QUA for diagnosing microalbuminuria. The SPSS 19.0J software program (Windows version, SPSS, Chicago, USA) was used for all of the statistical analyses. p values of <0.05 were considered to indicate statistical significance.
Results
The clinical characteristics of patients with normoalbuminuria and microalbuminuria
The mean baseline values of the study subjects are shown in the Table. The patients were divided into two groups: the normoalbuminuria group (n=205) and the microalbuminuria group (n=86). As shown in the Table, the UACR of the microalbuminuria group was significantly higher than that of the normoalbuminuria group (p<0.001). Moreover, the duration of diabetes was also significantly longer in the microalbuminuria group than in the normoalbuminuria group (p=0.003). The other categories, such as age, gender, BMI, and eGFR, did not differ between the two groups to a statistically significant extent.
Table.
The Clinical Characteristics of Study Patients.
| Normoalbuminuria | Microalbuminuria | p value | |
|---|---|---|---|
| N (male/female) | 205 (117/88) | 86 (47/39) | 0.796 |
| Age (years) | 63.9±12.8 | 66.3±12.7 | 0.074 |
| BMI (kg/m2) | 24.6±4.8 | 25.4±4.9 | 0.187 |
| Duration of diabetic therapy | 8.0 (3.0-15.0) | 14.5 (5.0-22.0) | 0.001 |
| sBP | 129±16 | 138±16 | <0.001 |
| dBP | 76±12 | 76±12 | 0.982 |
| eGFR (mL/min/1.73m2) | 71.9 (59.4-82.6) | 66.8 (53.2-83.4) | 0.143 |
| HbA1c (%) | 7.1±1.2 | 7.4±1.2 | 0.063 |
| UACR (mg/g·Cre) | 7.1 (3.0-12.6) | 69.2 (46.2-129.2) | <0.001 |
Data are expressed as mean values±SD or median values (interquartile). p values were determined by Student’s t test or Mann- Whitney U test. Categorized data were analyzed by χ2 test. BMI: body mass index, eGFR: estimated glomerular filtration rate, UACR: urinary albumin-to-creatinine ratio, sBP: systolic blood pressure, dBP: diastolic blood pressure
The diagnostic performance of the QUACR in the diagnosis of microalbuminuria
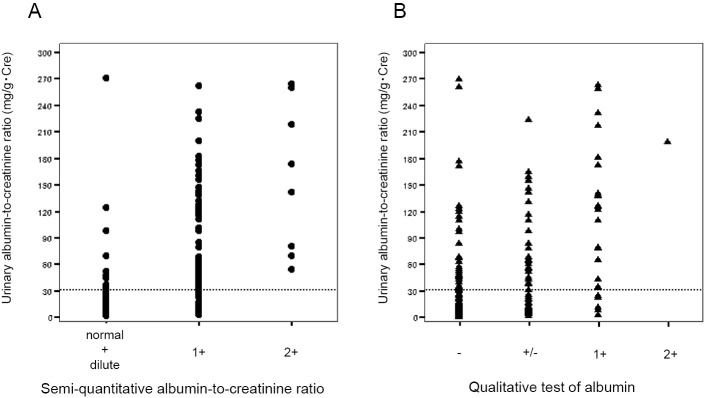
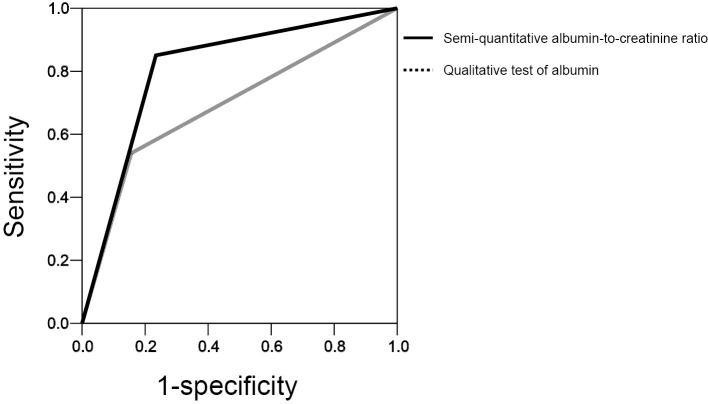
Fig. 1 are scatter diagrams showing the relationship between the UACR and the QUACR and QUA, respectively. The sensitivity, specificity, false-positive, and false-negative values for the QUACR and QUA were 84.9%, 76.6%, 23.4%, and 14.9%, respectively, and 53.5%, 84.4%, 15.6%, and 45.8%, respectively. The area under the ROC curve values of the QUACR and QUA for the diagnosis of microalbuminuria were 0.807 (0.752-0.863) and 0.689 (0.618-0.760), respectively (Fig. 2). The ROC analysis revealed that QUACR showed superior diagnostic accuracy to the QUA.
Figure 1.
The correlations among the parameters. (A) A scatter diagram comparing the urinary albumin-to-creatinine ratio (UACR) and the semi-quantitative albumin-to-creatinine ratio (QUACR). (B) A scatter diagram comparing the UACR and qualitative tests of albumin (QUA).
Figure 2.
The area under the ROC curve values of the urinary dipstick test for the diagnosis of microalbuminuria. The area under the ROC curve values of the semi-quantitative albumin-to-creatinine ratio (QUACR) and the qualitative test of albumin (QUA) were 0.807 (95% confidence interval: 0.752-0.863) and 0.689 (0.618-0.760), respectively. Solid line, QUACR; dotted line, QUA
Discussion
This study demonstrated the superior accuracy and sensitivity of the QUACR in comparison to the QUA in the detection of microalbuminuria. Thus, the QUACR was found to be a simple, rapid, low-cost, and reliable test for microalbuminuria screening.
The urinary albumin excretion rate measured in a 24-hour collection cycle is considered to be the gold standard for assessing the urinary albumin level and diagnosing microalbuminuria (9). However, 24-hour urine collection is cumbersome and errors may occur due to inaccurate timing and/or incomplete testing. The American Diabetes Association (ADA) guidelines for the detection of microalbuminuria suggest the use of 24-hour collection, timed specimens taken over a period of <24 hours, or untimed random specimens (10). Researchers have typically used the UACR to diagnose microalbuminuria from spot or random collection; however, some have also used the albumin concentration (11). The measurement of the UACR in a diurnal random urine specimen is an accurate method for microalbuminuria screening. However, the measurement of the UACR is not simpler and the test is significantly more expensive than the qualitative and semi-quantitative methods. The costs of the QUACR and UACR were 230 JPY and 1,080 JPY, respectively. On the other hand, although tests of the albumin concentration might be affected by the dilution or concentration of the urine sample, this option is still accurate and less expensive than the UACR (12). The assessment of the albumin concentration using the QUACR can be performed using a urine dipstick. This semi-quantitative method of measuring the urinary albumin concentration is also affected by the dilution or concentration of the urine sample. However, the determination of the albumin-to-creatinine ratio based on the measurement of the creatinine concentration using the same sample minimized the effect of the urine volume. Previous reports have indicated that the QUACR provides an immediate and reliable method for detecting microalbuminuria (13,14). Thus, the application of the albumin-to-creatinine ratio with the use of the QUACR, as outlined in this study, is considered to be an appropriate method for accurately diagnosing microalbuminuria.
Our study is associated with some limitations. The excretion of urinary creatinine is affected by aging. The progressive reduction of muscle mass with age could result in the reduced excretion of urinary creatinine, leading to a higher UACR with increasing age (7); this might make it necessary to use an age-specific UACR value. Furthermore, the QUACR was only measured once in the present study. In patients with early-stage diabetic kidney disease, the excretion of urinary albumin varies due to hydration-based variations in the urine concentration. The frequent examination within a 3-to-6-month period would be important for detecting the significant excretion of albumin and/or the progression of albuminuria (10). Repeated measurements may be required to confirm the accuracy of the QUACR based on the individual coefficients of variation for each parameter.
This study demonstrated that the QUACR is a simple and efficient test that is superior to the QUA for detecting microalbuminuria. The measurement of the QUACR using random urine specimens may therefore be useful in screening for microalbuminuria in patients with diabetes.
The QUACR, which was used in this study, was a simple test that can be applied in screening for microalbuminuria. Any positive results from QUACR testing should be confirmed by a quantitative analysis of the urine albumin and creatinine levels.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This work was carried out with cooperation from the Clinical Examination Unit, Hiroshima City Asa Citizens Hospital.
References
- 1.Gross JL, Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention and treatment. Diabetes Care 28: 176-188, 2005. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Nephropathy in diabetes. Diabetes Care 27: S79-S83, 2004. [DOI] [PubMed] [Google Scholar]
- 3.European Diabetes Policy Group A desktop guide to type 2. Diabetic Med 16: 716-730, 1984. [PubMed] [Google Scholar]
- 4.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 48: 436-472, 2002. [PubMed] [Google Scholar]
- 5.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive kidney diseases (NIDDK). Am J Kidney Dis 42: 617-622, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Zelmanovitz T, Gross JL, Oliveira JR, Paggi A, Tatsch M, Azevedo MJ. The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy. Diabetes Care 20: 516-519, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bakker AJ. Detection of macroalbuminuria - Receiver operating curve analysis favors albumin-to-creatinine ratio over albumin concentration. Diabetes Care 22: 307-313, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen CE, Viberti GC, Peheim E, et al. Multicenter evaluation of the Micral-Test II test strip, an immunologic rapid test for the detection of microalbuminuria. Diabetes Care 20: 1642-1646, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Special issue: clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi 54: 1034-1191, 2012. (in Japanese). [PubMed] [Google Scholar]
- 10.Standards of Medical Care in Diabetes-2014. Diabetes Care 37: S14-S80, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Dyer AR, Greenland P, Elliott P, INTERMAP Research Group, et al. Evaluation of measures of urinary albumin excretion in epidemiologic studies. Am J Epidemiol 160: 1122-1131, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross JL, Zelmanovitz T, Oliveira J, de Azevedo MJ. Screening for diabetic nephropathy: is measurement of urinary albumin-to-creatinine ratio worthwhile? Diabetes Care 22: 1599-1600, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Mogensen CE, Viberti GC, Peheim E, et al. Multicenter evaluation of the Micral-Test II test strip, an immunologic rapid test for the detection of microalbuminuria. Diabetes Care 20: 1642-1646, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Incerti J, Zelmanovitz T, Camargo JL, Gross JL, de Azevedo MJ. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant 20: 2402-2407, 2005. [DOI] [PubMed] [Google Scholar]