Abstract
Glycated hemoglobin (HbA1c) is a widely used marker of glycemic control but can be affected by hemolytic anemia. Glycated albumin (GA) is also affected in patients with liver cirrhosis. We herein report the assessment of glycemic control in a 41-year-old man with dehydrated hereditary stomatocytosis and a PIEZO1 gene mutation complicated by diabetes mellitus and liver cirrhosis due to hemochromatosis. The estimated HbA1c calculated from the average glucose level obtained by continuous glucose monitoring or by self-monitoring of blood glucose was useful for evaluating the glycemic control in this patient, as HbA1c and GA were unreliable due to the coexisting conditions.
Keywords: diabetes mellitus, average glucose level, continuous glucose monitoring, estimated HbA1c, hemolytic anemia, dehydrated hereditary stomatocytosis with PIEZO1 gene mutation
Introduction
Glycated hemoglobin (HbA1c) is widely used for the assessment of glycemic control in patients with diabetes mellitus. HbA1c reflects the average glucose level over the previous 1 to 2 months, since the mean erythrocyte lifespan is 120 days. Regarding individualized glycemic control goals, the clinical practice guidelines of the Japan Diabetes Society recommend setting a target HbA1c and corresponding glucose range, with the aim of preventing the development of microangiopathic complications (1). However, HbA1c can be affected by various coexisting conditions, such as conditions that influence the erythrocyte lifespan (2). Accordingly, HbA1c can be an unreliable marker in some patients in whom the average glucose level may be an alternative for evaluating glycemic control.
We herein report a patient with diabetes mellitus complicated by dehydrated hereditary stomatocytosis (DHS, also known as hereditary xerocytosis) due to a mutation of the PIEZO-type mechanosensitive ion channel component 1 (PIEZO1) gene (3). DHS is typically associated with mild to moderate hemolysis (4).
This patient presented with hemolytic anemia and had diabetes mellitus and liver cirrhosis due to hemochromatosis. HbA1c was affected by a reduced erythrocyte lifespan, and glycated albumin (GA) was also unreliable due to the coexisting conditions. In this patient, we evaluated the average glucose level measured by continuous glucose monitoring (CGM) and self-monitoring of blood glucose (SMBG). We estimated the HbA1c using the A1c-Derived Average Glucose (ADAG) Study Groups formula (5):
Average glucose level (mg/dL)=28.7×HbA1c (%)-46.7
We then compared the measured HbA1c and the estimated HbA1c for two years in this patient.
Case Report
A 41-year-old man had been diagnosed with hemolytic anemia as a neonate, after which he was lost to follow-up for almost 30 years. He began to experience thirst, malaise, and weight loss approximately six months prior to emergency admission for diabetic ketoacidosis. He was first treated at a regional hospital, where admission laboratory data showed that HbA1c was 7.6% and blood glucose level was 670 mg/dL. He presented with systemic jaundice, increased indirect bilirubin, hemolytic anemia, liver cirrhosis, and poorly controlled diabetes mellitus. He had a history of gallstones and splenomegaly. Multiple daily injections of insulin were initiated, and he was referred to our hospital to consult an endocrinologist.
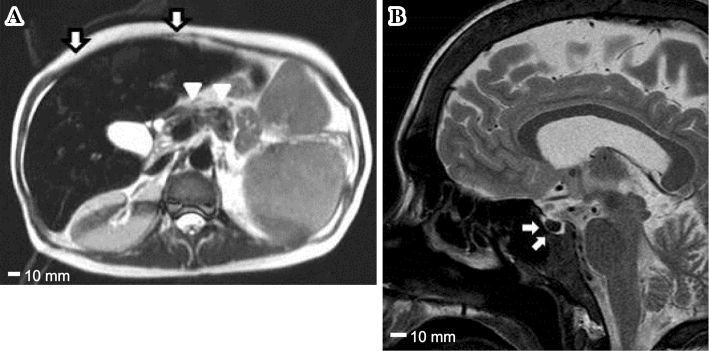
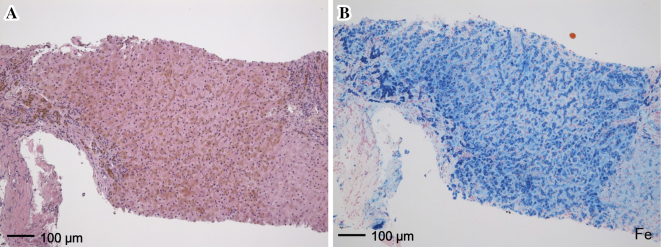
On a physical examination at our hospital, his height was 166 cm, weight was 54.6 kg, and body mass index was 19.8 kg/m2. The serum ferritin level was markedly high at 3,642 ng/mL (Table 1). Magnetic resonance imaging (MRI) revealed findings compatible with hemochromatosis, showing iron deposits in organs such as the liver, pancreas, and pituitary (Fig. 1). The Child-Pugh score was 8 (class B). A percutaneous liver biopsy was performed and histologically confirmed prominent iron deposition, compatible with hemochromatosis as well as liver fibrosis (Fig. 2). The patient showed evidence of multiple organ failure, including hypogonadotropic hypogonadism (luteinizing hormone and follicle-stimulating hormone did not respond in the luteinizing hormone-releasing hormone test), osteoporosis (the bone mineral density T score was -3.2 at L2-4), and cardiac dysfunction (transthoracic echocardiography revealed an ejection fraction of 52% with base/mid left ventricular wall hypokinesis). The further evaluation of hemolytic anemia showed that the direct Coombs test was negative, while stomatocytes were seen in the blood smear. However, these results could not distinguish among various hemolytic diseases.
Table 1.
Laboratory Findings on Admission.
Figure 1.
Findings of a magnetic resonance imaging study. (A) A horizontal image of the abdomen. The arrows indicate the liver, and the arrowheads indicate the pancreas. (B) A sagittal image of the head. The arrows indicate the pituitary gland. T2-weighted magnetic resonance images show a markedly decreased signal intensity due to iron deposition in the liver, pancreas and pituitary gland (scale bar, 10 mm).
Figure 2.
Histopathological findings of the liver. (A) Hematoxylin and Eosin staining and (B) iron staining show prominent iron deposits compatible with hemochromatosis and liver fibrosis (scale bar, 100 μm). Fe: iron staining
The patient's father and sibling also had hemolytic anemia, hemochromatosis, and diabetes mellitus, suggesting that the family had an inherited disease. After obtaining written informed consent from the patient and his family, a chromosome analysis and genetic testing were performed. Although an analysis of five candidate genes for hereditary hemochromatosis (HFE, HJV, HAMP, TFR2, and SLC40A1) showed no mutations, he was found to have DHS by targeted sequencing of PIEZO1. The details of the mutation found in this patient have been described elsewhere (3). In brief, DHS is a dominantly inherited pleiotropic syndrome characterized by increased permeability of the red cell membrane to cations and is frequently associated with idiopathic iron overload. The response to transfusion and splenectomy is not always satisfactory (6).
Treatment for hemochromatosis was initiated with an oral iron-chelating agent (deferasirox 20 mg/kg/day). The serum ferritin level decreased to 1,640 ng/mL after 3 months. Pancreatic iron overload can cause impairment of the β-cell function, resulting in decreased insulin secretion and the development of diabetes mellitus. In this patient, the fasting serum C-peptide immunoreactivity (CPR) was 0.68 ng/mL, and the serum CPR was 1.02 ng/mL after the intravenous glucagon stimulatory test (Table 1). He was negative for anti-GAD and anti-insulin autoantibodies. The adrenal function and thyroid function were normal. Therefore, his diabetes mellitus was considered to be caused by liver cirrhosis and pancreatic iron deposition. The patient had no diabetic retinopathy, nephropathy or neuropathy during hospitalization or the follow-up period.
Treatment of diabetes
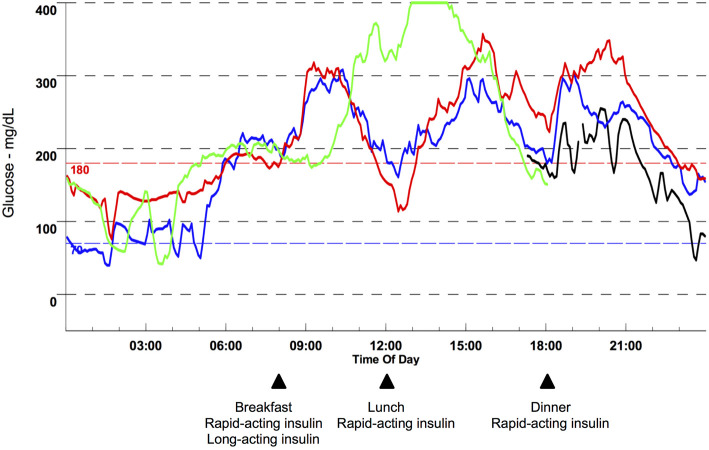
The patient subcutaneously injected 14 units of insulin aspart before breakfast, 5 units each before lunch and dinner, and 20 units of insulin glargine before breakfast, for a total daily dose of 44 units (0.81 units/kg). A 1,840-kcal diet containing 260 g of carbohydrate, 80 g of protein and 55 g of fat was prescribed. We evaluated his glucose excursion and the average glucose level by CGM (Fig. 3), and the patient performed 4-point (before meals and bedtime) SMBG during the CGM study. An analysis of the CGM data revealed that the 48-h average glucose level for days 2 and 3 was 205 mg/dL (Table 2), which theoretically corresponded to an estimated HbA1c of 8.8% using the previously mentioned formula. However, his measured HbA1c level was 6.1%, which corresponded to an average glucose level of 128 mg/dL. This patient's average glucose level estimated by 4-point SMBG was close to that obtained by CGM (195.3 vs. 192 on day 2, and 218.5 vs. 218 on day 3). During follow-up, he continued to perform SMBG four times a day.
Figure 3.
Glucose profile obtained by continuous glucose monitoring (CGM). CGM measured glucose every 5 min for 4 days, and data were processed by the CGMS SolutionsTM software program (Medtronic MiniMed, Northridge, USA). The black, blue, red and green lines represent days 1, 2, 3 and 4, respectively. Glucose levels ranging from 40 to 400 mg/dL were measured. Glucose levels below or above this range were expressed as 40 or 400 mg/dL.
Table 2.
Results of Continuous Glucose Monitoring.
| Day 1 | Day 2 | Day 3 | Day 4 | Total | |
|---|---|---|---|---|---|
| Number of sensor values | 81 | 288 | 288 | 217 | 874 |
| Average glucose level (mg/dL) | 175 | 192 | 218 | 222 | 207 |
| Minimum-Maximum glucose levels (mg/dL) | 47-255 | 40-308 | 76-357 | 42-400 | 40-400 |
| Standard deviation (mg/dL) | 49 | 73 | 71 | 101 | 80 |
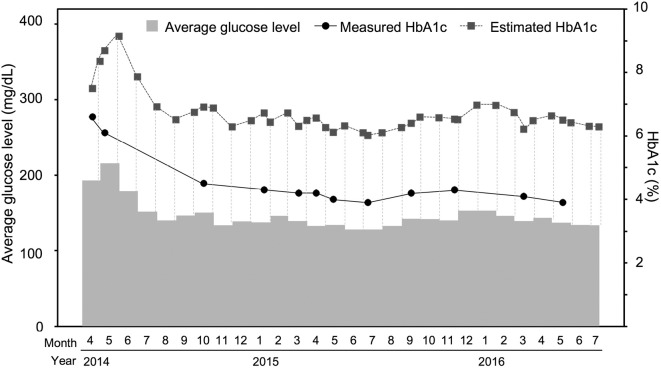
The average glucose level over 30 days, measured HbA1c level and estimated HbA1c level are shown in Fig. 4. The estimated HbA1c was calculated from the average glucose level using SMBG, and the measured HbA1c was determined by our laboratory. The measured HbA1c was significantly lower than the estimated HbA1c according to the two-tailed paired t-test (p<0.001). The GA, measured HbA1c, GA-to-HbA1c ratio and hemoglobin levels during the follow-up period are shown in Table 3.
Figure 4.
Average glucose level, measured HbA1c and estimated HbA1c. The measured HbA1c was significantly lower than the estimated HbA1c (p<0.001).
Table 3.
Glycated Albumin, Glycated Hemoglobin and Hemoglobin Levels.
| 2014/07 | 2014/10 | 2015/01 | 2015/03 | 2015/05 | 2015/07 | 2015/09 | 2015/11 | |
|---|---|---|---|---|---|---|---|---|
| GA (%) | 21.7 | 20.4 | 17.6 | 15.4 | 16.2 | 15.5 | 17.1 | 15.0 |
| HbA1c (%) | NA | 4.5 | 4.2 | 4.2 | 4.0 | 3.9 | 4.2 | 3.9 |
| GA / HbA1c | NA | 4.53 | 4.19 | 3.67 | 4.05 | 3.97 | 4.07 | 3.85 |
| Hgb (g/dL) | 8.3 | 9.5 | 10.6 | 10.0 | 11.1 | 9.6 | 9.7 | 9.4 |
GA: glycated albumin, HbA1c: glycated hemoglobin, Hgb: hemoglobin, NA: not available
Continuous glucose monitoring
A CGMSⓇ Gold (Medtronic MiniMed, Northridge, USA) and a SofsensorⓇ (Medtronic MiniMed, Northridge, USA) were used for CGM. Conventional finger-stick measurements of the blood glucose level were performed using a GLUCOCARD G+ MeterⓇ (Arkray, Kyoto, Japan), and the CGM data were calibrated four times a day with this meter.
Measurement of hemoglobin A1c
An Adams A1c HA-8180 automatic glycohemoglobin analyzer (Arkray, Kyoto, Japan) was used to measure the HbA1c levels. The detection range was 3-20%.
Self-monitoring of blood glucose
A GLUCOCARD G+ meterⓇ was used for SMBG.
Discussion
We encountered a patient with diabetes mellitus and DHS who had a PIEZO1 gene mutation (3). He initially presented with congenital hemolytic anemia and was later found to have hemochromatosis. DHS is frequently associated with iron progressive overload, leading to organ failure, and the course of hemochromatosis depends on the severity of hemolysis (4). Untreated iron overload can cause various complications, including heart failure, liver cirrhosis, hypogonadism and diabetes mellitus (20-50%) (7). This patient showed slight CPR increment to glucagon stimulation, suggesting he was insulin-dependent due to damage to the beta cells by hemochromatosis. His relatively high total daily dose of insulin might be due to the insulin resistance accompanying liver cirrhosis. Patients with a serum ferritin level over 1,000 ng/mL are at an extremely high risk of developing complications (8). The early diagnosis will lead to better treatment to improve the prognosis. In patients with hemolytic anemia, the HbA1c is falsely low because of the shortened erythrocyte survival (9). Chronic liver disease and chronic renal failure also affect the HbA1c level (2). The average glucose level might therefore be more useful for evaluating glycemic control than HbA1c if the latter is affected by coexisting conditions that shorten or prolong the mean erythrocyte lifespan (2,9).
GA is affected in patients with liver cirrhosis as the albumin turnover is prolonged (10). In this patient, although the serum albumin level and platelet level were within the normal range, the cholinesterase level was low and the prothrombin time prolonged, both signs of liver insufficiency, and the patient had histological findings supporting a diagnosis of liver cirrhosis. Thus, there is a possibility that the albumin turnover was not normal in this patient. Albumin has two major binding sites and several minor sites for small ligands, to which endogenous substances such as hormones, metabolites and various drugs bind (11). There is also the possibility that the deferasirox administered in this patient might have affected the GA levels, as this compound strongly binds to albumin, potentially interfering with the glycation of albumin (12). However, due to the lack of a conversion formula between GA and average glucose, it was impossible to directly assess the accuracy of the GA measurement in this patient. Determining the GA-to-HbA1c ratio in this patient was difficult, as not only was HbA1c falsely low but also GA might have been unreliable due to coexisting conditions interfering with albumin turnover and glycation.
One advantage associated with using CGM instead of conventional SMBG to calculate the average glucose level is that CGM can record the glycemic excursion 24 h a day. However, one limitation of using CGM is the relatively narrow range of measurement (e.g., 40-400 mg/dL in CGMSⓇ Gold) compared with SMBG. In this patient, hyperglycemia over 400 mg/dL and hypoglycemia less than 40 mg/dL were observed, as shown in Fig. 3, so the estimated HbA1c might be less accurate due to the compromised calculations using 400 or 40 mg/dL during the periods when the patient had hyperglycemia or hypoglycemia.
A limitation associated with this study is that we used 4-point SMBG during the follow-up period rather than 7-point SMBG. As the current health insurance reimbursement program in Japan makes performing daily 7-point SMBG financially difficult for non-type 1 patients or non-pregnant patients, we averaged the 4-point SMBG to determine the average glucose levels during the follow-up period. As periodic 7-point SMBG includes three post-prandial measurements, for example once a week, it would have provided a more accurate assessment of the average glucose level during the follow-up period in this patient.
Nathan et al. and the A1c-Derived Average Glucose (ADAG) Study Group investigated the relationship between HbA1c and the average glucose level using a linear regression analysis, leading to the development of the ADAG study formula (5). The ADAG study was a multicenter observational study of CGM and SMBG in patients with type 1 or type 2 diabetes and nondiabetic volunteers 18-70 years of age. In their study, the linear regression equations did not differ significantly across subgroups based on age, sex, or type of diabetes. Accordingly, we used this formula to evaluate the estimated HbA1c in the present patient in order to achieve more stable and better glycemic control.
The usefulness of SMBG was established in the Diabetes Control and Complications Trial (DCCT). In Japan, the health insurance system allows for more than 60 SMBG tests per month for patients with non-type 1 diabetes. CGM enables adjustments for individualized treatment (13). In recent guidelines, the American Diabetes Association, the European Association for the Study of Diabetes, the American Association of Clinical Endocrinologists and the International Diabetes Federation have recommended setting an SMBG target in order to achieve the recommended HbA1c goal (13-15) and mention the utility of the average glucose level.
In 2016, the Japan Diabetes Society and the Japan Geriatrics Society released a consensus statement regarding the lower limit HbA1c as the objective for managing diabetes in elderly patients (>65 years of age) to prevent severe hypoglycemia (16). However, this consensus statement does not account for the fact that the HbA1c level is not always accurate due to various conditions, especially in elderly patients. We would like to propose that, when setting a lower limit of HbA1c, a footnote should be added recommending that the actual glucose level be used instead of the HbA1c level in patients with coexisting conditions that might affect HbA1c, such as anemia. Otherwise, a falsely low HbA1c level might result in the undertreatment of diabetes and a risk of hyperglycemia complications, dehydration and severe infection. Even in such situations, calculating the average glucose level will help to evaluate the glycemic control. It is also important to pay attention to day-to-day differences in SMBG measurements.
To our knowledge, this is the first report regarding the detailed glucose profile obtained using CGM in a diabetic patient with DHS and liver cirrhosis due to hemochromatosis. We noted the benefits of using the average glucose level and estimated HbA1c in this patient. DHS is a rare disease and is often confused with other red cell membrane disorders, such as spherocytosis or hereditary high phosphatidylcholine hemolytic anemia (17). Identifying the causative genes will greatly aid in the differential diagnosis of these hematological disorders and help improve the understanding of the clinical characteristics of congenital hemolytic anemia (18).
An observational study in Japan suggested that high bilirubin levels may protect against the development of diabetic retinopathy (19). However, this hypothesis does not justify poor glycemic control in patients with high bilirubin levels, as chronic hyperglycemia is a major risk factor for dehydration and severe infection.
Even in patients with concomitant conditions that might affect HbA1c or GA, the principles of managing diabetes are the same, with the aim of reducing the development and progression of complications while also minimizing hypoglycemia. Determining the average glucose level and estimated HbA1c may be useful for evaluating glycemic control in such patients. Furthermore, CGM provides a glycemic profile for 24 h a day and more accurately reflects the average glucose level than occasional SMBG. Wider usage of CGM might help improve the quality of diabetes care, especially in patients with coexisting conditions that affect HbA1c and GA.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank all of their colleagues who were involved in caring for patients at the National Hospital Organization Kyoto Medical Center. The authors wish to acknowledge the assistance of Hajime Yamakage with the statistical analysis.
References
- 1. Practice Guideline for the Treatment for Diabetes in Japan. 2016. 5th ed. The Japan Diabetes Society, Eds. Q2-3 How glycemic goal should be determined? Nankodo, Tokyo, 2016: 27-29 (in Japanese). [Google Scholar]
- 2. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A1c in the management of diabetes. J Diabetes 1: 9-17, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Imashuku S, Muramatsu H, Sugihara T, et al. . PIEZO1 gene mutation in a Japanese family with hereditary high phosphatidylcholine hemolytic anemia and hemochromatosis-induced diabetes mellitus. Int J Hematol 104: 125-129, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Albuisson J, Jeunemaitre X, Patapoutian A, et al. . Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nature Commun 4: 1884, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group . Translation the A1c assay into estimated average glucose values. Diabetes Care 31: 1473-1478, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallagher PG, Glader B. Hereditary spherocytosis, hereditary elliptocytosis, and other disorders associated with abnormalities of the erythrocyte membrane. In: Wintrobe's Clinical Hematology. 13th ed. Greer JP, Arber DA, Glader B et al. , Eds. Wolters Kluwer, Philadelphia, 2014: 707-727. [Google Scholar]
- 7. Utzschneider KM, Kowdley KV. Hereditary hemochromatosis and diabetes mellitus: implications for clinical practice. Nat Rev Endocrinol 6: 26-33, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Powell LW. Hemochromatosis. In: Harrison's Principles of Internal Medicine. 19th ed. Kasper DL, Fauci AS, Longo DL et al. , Eds. McGraw-Hill, New York, 2015: 2514-2519. [Google Scholar]
- 9. O'keeffe DT, Maraka S, Robert A, Rizza RA. HbA1c in the evaluation of diabetes mellitus. JAMA 315: 605-606, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Enomoto H, Bando Y, Nakamura H, Nishiguchi S, Koga M. Liver fibrosis markers of nonalcoholic steatohepatitis. World J Gastroenterol 21: 7427-7435, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters TJ. Ligand binding by albumin. In: All about Albumin-Biochemistry, Genetics, and Medical Applications. Academic Press, San Diego, 1996: 76-132. [Google Scholar]
- 12. Weiss HM, Fresneau M, Camenisch GP, Kretz O, Gross G. In vitro blood distribution and plasma protein binding of the iron chelator deferasirox (ICL670) and its iron complex Fe-[ICL670]2 for rat, marmoset, rabbit, mouse, dog, and human. Drug Metab Dispos 34: 971-975, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Bailey TS, Grunberger G, Bode BW, Roberts VL, Rodbard D. American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glucose Monitoring Consensus Statement. Endocr Pract 22: 231-261, 2016. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association Glycemic targets. Diabetes Care 40 (Suppl 1): S48-S56, 2017. [DOI] [PubMed] [Google Scholar]
- 15. International Diabetes Federation Guideline Development Group Global guideline for type 2 diabetes. Diabetes Res Clin Pract 104: 1-52, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Japan Diabetes Society/Japan Geriatrics Society Joint Committee on Improving Care for Elderly Patients with Diabetes Committee Report: Glycemic targets for elderly patients with diabetes. J Diabetes Investig 8: 126-128, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa LD, Galimand J, Fenneteau O, Mohandas N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev 27: 167-178, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida K, Ogawa S. Gentetic analysis of hereditary hematological disorders: overview. Rinsho Ketsueki (Jpn J Clin Hematol) 56: 861-866, 2015. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 19. Yasuda M, Kiyohara Y, Wang JJ, et al. . High serum bilirubin levels and diabetic retinopathy: the Hisayama Study. Ophthalmology 118: 1423-1428, 2011. [DOI] [PubMed] [Google Scholar]