Abstract
No valid treatment for isolated myeloid sarcoma (IMS) has yet been established, and no thorough genetic examinations have been performed because of its low incidence and unique manner of development. We herein report a 34-year-old man with pancreatic IMS with t(8;21)/RUNX1-RUNX1T1 rearrangement. He was treated with high-dose cytarabine followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). This is the first report of pancreatic IMS with t(8;21). Positron emission tomography/computed tomography and genetic study are useful for the diagnosis, and allo-HSCT achieved complete remission in this patient.
Keywords: isolated myeloid sarcoma, t(8;21), pancreas
Introduction
Myeloid sarcoma (MS) is a characteristic disease entity in myeloid neoplasms. In most MS cases, leukemic blasts in the peripheral blood and bone marrow (BM) are found at the diagnosis (1). MS is recognized in approximately 5% of acute myeloid leukemia (AML) cases (2). However, AML cells are not detected in the peripheral blood or BM of isolated MS (IMS) cases. Given that IMS is found in 25% of MS cases (1), IMS is assumed to be present in 1% of AML cases.
MS, including IMS, consists of immature myeloid cells and may develop in lesions throughout the body. The skin, lymph node, testis and digestive tract are common sites of MS. IMS, which is not concomitant with leukemic blasts in the peripheral blood or BM, often lacks specific symptoms and is therefore difficult to diagnose properly. In many cases of IMS, it is difficult to access and obtain sufficient specimens for a diagnosis because of the anatomical sites of the tumors. Occasionally, a needle biopsy is clinically useful. However, needle biopsy samples are not adequate for immunochemistry and genetic analyses.
The low incidence, varied and non-specific clinical symptoms, difficulty in obtaining diagnostic specimens and variety of histological appearance of IMS make its diagnosis and treatment challenging (2,3). No valid treatment has been established, and how to treat the disease is a matter of concern in clinical hematology.
Case Report
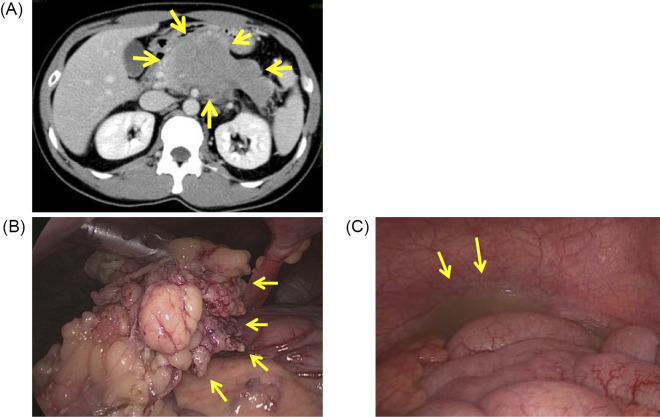
A 34-year-old man complained of a 3-month history of upper abdominal pain. Abdominal echography and computed tomography (CT) images revealed a tumor in the pancreas. Thereafter, he was referred to our hospital (Kumamoto University Hospital, Kumamoto, Japan). The peripheral blood cell counts were all within normal ranges: white blood cell count 4.4×109/L, hemoglobin 133 g/L and platelets 2.37×1011/L with no evidence of leukemic blasts in the peripheral blood. Serum lactate dehydrogenase and C-reactive protein levels were elevated at 218 U/L (upper limit of normal, 213) and 10.7 mg/L (upper limit of normal, 3.0), respectively, although levels of other serum liver enzymes, amylase and creatinine were not markedly increased. Contrast-enhanced CT showed a 10×6-cm ischemic bulky tumor in the pancreas body and tail with a thickened greater omentum (Fig. 1A).
Figure 1.
Bulky tumor of the pancreas found with enhanced-contrast computed tomography (CT) and laparoscopy. (A) Contrast-enhanced CT revealed ischemic bulky tumor of the pancreas (arrows). (B) A laparoscopic examination revealed a pancreatic tumor and thick omentum (arrows). (C) Turbid ascites (arrows).
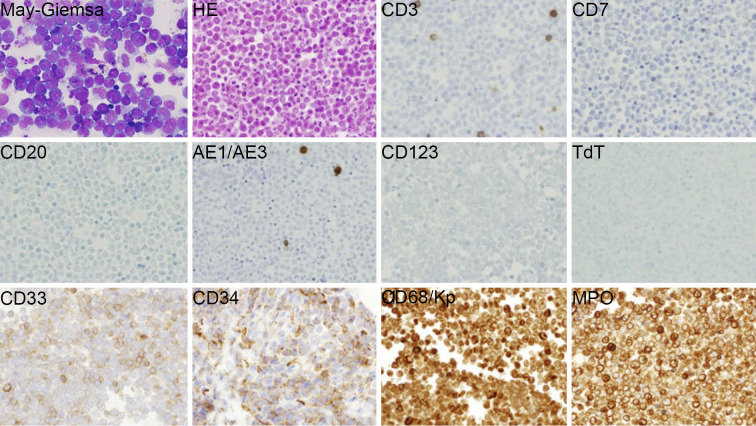
A tumor biopsy was performed with laparoscopy, and a few milliliters of turbid ascites was obtained (Fig. 1B and C). Cytologically, the tumor cells were immature leukemic blast-like cells with blue-gray cytoplasm and an indented nucleus with fine chromatin without adepithelial connections (Fig. 2, May-Giemsa staining). A histopathological study revealed that the tumor cells were negative for lymphoid and epithelial markers, CD3, CD7, CD20, terminal deoxynucleotidyl transferase (TdT) and cytokeratin AE1/AE3. A flow cytometric analysis and immunohistochemical study revealed that the cells in ascites were positive for myeloid and monocyte antigens, CD4, CD15, CD33, CD34, CD56, CD68/Kp-1 and myeloperoxidase (MPO) (Fig. 2). Therefore, the pancreatic tumor was diagnosed as MS. Positron emission tomography (PET)/CT showed that 18F-fluorodeoxyglucose (FDG) was taken up only by the pancreatic tumor and thickened greater omentum (maximum standard uptake value [SUVmax]: 8.8). Leukemic blasts were not found in the BM by histological or cytological analyses. The patient was diagnosed with pancreatic IMS with invasion to the greater omemtum and ascites. He was treated with the AML regimen of idarubicin for three days and cytarabine for seven days.
Figure 2.
Cytology and histology of tumor cells. A biopsy of the pancreatic tumor revealed myeloid sarcoma. May-Giemsa staining revealed that the tumor cells with blue-gray cytoplasm were leukemic blast-like cells. An immunohistochemical analysis showed that the tumor cells were negative for lymphoid and epithelial markers, CD3, CD7, CD20, TdT or cytokeratin AE1/AE3, and positive for myeloid and monocyte antigens, CD33, CD34, CD68/Kp-1 and MPO. HE: Hematoxylin and Eosin staining, MPO: myeloperoxidase, TdT: terminal deoxynucleotidyl transferase. Magnification: all photomicrographs ×400
The WT1 mRNA level in the peripheral blood increased to 1,000 copies/μgRNA at the diagnosis. The tumor cells obtained by a biopsy were not enough for a G-banding analysis. To predict the prognosis, fluorescence in situ hybridization (FISH) for RUNX1-RUNX1T1 rearrangement was additionally ordered based on the knowledge that such a rearrangement is sometimes found in MS cases. RUNX1-RUNX1T1 fusion signals were detected in 17.6% of the tumor cells by FISH using the Vysis LSI RUNX1/RUNX1T1 Dual Color Dual Fusion Probes (Abbott Molecular, Des Plaines, USA). In contrast, the fusion signal was not detected in BM cells at the diagnosis. The patient was ultimately diagnosed with pancreatic IMS with t(8;21)(q22;q22)/RUNX1-RUNX1T1 rearrangement. cKIT exon 8 and 17 mutations were not found by sequencing cDNA extracted from the tumor cells. Reverse transcription polymerase chain reaction (RT-PCR) revealed that RUNX1-RUNX1T1 fusion mRNA was detected in the tumor cells and the BM mononuclear cells (Fig. 3). These data suggest the presence of a small number of leukemic cells or non-leukemic cells with the RUNX1-RUNX1T1 fusion gene in the BM.
Figure 3.
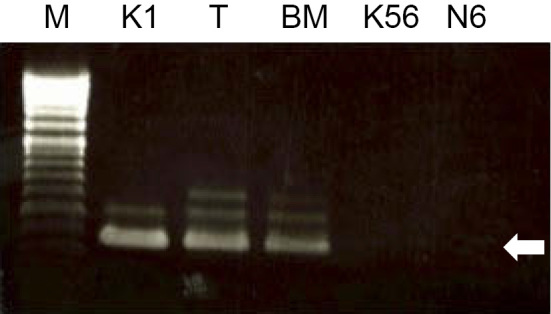
A genetic analysis with RT-PCR. RUNX1-RUNX1T1 fusion mRNA was detected by reverse transcription polymerase chain reaction in tumor cells and BM cells at the diagnosis. The Kasumi-1 acute myeloid leukemia cell line was utilized as a positive control representing cells with the RUNX1-RUNX1T1 gene. The K562 and Nalm6 cell lines were negative controls. The fusion mRNA-specific PCR product length was 395 base pairs (pointed by the white arrow). Larger sized bands are non-specific. BM: bone marrow mononuclear cell, K1: Kasumi1, K56: K562, M: marker, N6: Nalm6, T: tumor cell
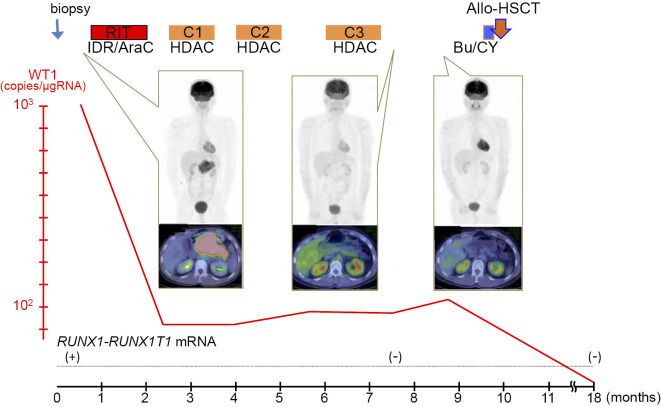
The idarubicin and cytarabine regimen was followed by three cycles of high-dose cytarabine regimens. The WT1 mRNA level was reduced to 93 copies/μgRNA. In addition, PET/CT revealed no 18F-FDG-positive lesion in the pancreas, and RT-PCR showed no RUNX1-RUNX1T1-positive cells in the BM. Therefore, we concluded that our patient had achieved complete remission (CR). He was treated with allogeneic hematopoietic stem cell transplantation (allo-HSCT) from an human leukocyte antigen (HLA)-matched unrelated donor with busulfan and cyclophosphamide as the conditioning regimen using cyclosporine and short-course methotrexate as graft-versus-host disease prophylaxis. He has been in CR for 1.5 years since the diagnosis (Fig. 4). The RUNX1-RUNX1T1 fusion gene has not been detected by PCR, and the WT1 mRNA count has dropped below 50 copies/μgRNA (lower limit of normal) since HSCT.
Figure 4.
Clinical course of the case of pancreatic isolated myeloid sarcoma. Following chemotherapy, the bulky pancreatic tumor shrank and showed reduced uptake signals on positron emission tomography/computed tomography (PET/CT). WT1 mRNA was reduced to 10-1 copies after the consolidative therapies. Allogeneic hematopoietic stem cell transplantation was performed. The conditioning regimen was busulfan and cyclophosphamide. At 18 months after the diagnosis, the WT1 mRNA count dropped below 50 copies/μgRNA (lower limit of normal). RUNX1-RUNX1T1 mRNA RT-PCR was performed qualitatively. The black bars in the pictures of PET/CT are used to cover identifying patient information. Allo-HSCT: allogeneic hematopoietic stem cell transplantation, Bu/CY: busulfan and cyclophosphamide conditioning, C1-3: consolidation therapy cycles 1-3, HDAC: high dose cytarabine regimen, IDR/AraC: idarubicin and cytarabine regimen, RIT: remission induction therapy, RUNX1-RU NX1T1 mRNA: results of the qualitative RT-PCR for RUNX1-RUNX1T1 mRNA, WT1: Wilms’ tumor 1 mRNA
Discussion
A number of different cytogenetic abnormalities in MS have been reported, but which ones are specific to MS is not known (1,4). Translocation (8;21) is a common cytogenetic abnormalities. Avni et al. reported that the rate of t(8;21) in MS patients ranged from 3.3% to 43% (5). MS with t(8;21) tends to develop orbitally or peri-orbitally (6). Only 13 cases of pancreatic MS cases, including the present case, have been reported since 1987 (Table) (7-16). Our case is believed to be a rare report of genetically confirmed pancreatic IMS with t(8;21)(q22;q22)/RUNX1-RUNX1T1 rearrangement. A genetic analysis by FISH is useful for the diagnosis of MS.
Table.
Previously Reported Cases of Pancreatic MS.
| No. | Sex/age | Concomitant AML | Karyotype | Treatment | Clinical course | Reference |
|---|---|---|---|---|---|---|
| 1 | F/36 | No | NA | RT+ChT (CPA+VCR+AraC+PSL) | Relapse as M4Eo with diploidy after 7 months, RIT(DNR+AraC+thoguanine) CR with 7-months follow-up | 7 |
| 2 | M/32 | No | NA | Duodenopancreatectomy+ChT (IDR+HDAC)+ChT (amsacrine+ETP) | CR with 2-years follow-up | 8 |
| 3 | F/37 | Yes | NA | No | Died 45 days after tumor detection | 9 |
| 4 | M/31 | Yes | 46, XY | ChT (IDR+AraC+ATRA) | CR (follow-up unknown) | 10 |
| 5 | F/61 | Yes | Trisomy 8 and 13 | ChT (IDR+AraC) | Relapse after 10 cycles of ChT (IDR+AraC), died | |
| 6 | M/64 | In CR of M2 | NA | ChT (unknown) | CR, died from stroke | 11 |
| 7 | F/42 | Yes | 47,+mar | ChT (HDAC+IDR)+ChT (IDR+AraC+ETP)+CBT | CR at 49 months after CBT | 12 |
| 8 | F/75 | Yes | inv(16) | ChT (ETP+AraC+MIT) | Relapse after 7 months, died | 13 |
| 9 | M/40 | No | NA | Duodenopancreatectomy+ChT (AraC) | CR (follow-up unknown) | 14 |
| 10 | F/42 | No | NA | Distal pancreatectomy+splenectomy, ChT was refused | Relapse after 2months, died after 3 months | 15 |
| 11 | F/45 | No | NA | Duodenopancreatectomy+ChT (CDDP+AraC+DEX) | Early relapse, died | 16 |
| 12 | F/19 | No | NA | ChT (AraC-based chemotherapy) +3 cycles of consolidation | Relapse, ChT(amsacrine+AraC)+ChT (AraC) followed by BMT relapse, 6 months after BMT | |
| 13 | M/34 | No | t(8;21) | ChT (IDR+AraC)+ChT (HDAC, 3 cycles)+allo-BMT | CR with 1.5-year follow-up | Our case |
allo-BMT: allogeneic bone marrow transplantation, AraC: cytarabine, ATRA: all-trans retinoic acid, CBT: cord blood transplantation, CDDP: cisplatin, ChT: chemotherapy, CPA: cyclophosphamide, CR: complete remission, DEX: dexamethasone, DNR: daunorubicin, ETP: etoposide, F: female, HDAC: high-dose AraC therapy, IDR: idarubicin, M: male, MIT: mitoxantrone, M2: acute myeloid leukemia M2 by FAB classification, M4Eo: M4 with eosinophilia (FAB), NA: not applicable, PSL: prednisolone, RT: radiotherapy, VCR: vincristine
In our IMS case, only RT-PCR indicated the presence of cells harboring the RUNX1-RUNX1T1 gene in BM, which is similar to a previous report showing RUNX1-RUNX1T1 fusion mRNA in BM in a case of IMS (17). IMS patients subsequently develop AML after an average of 10 months (18,19). It is possible that there are a small number of leukemic cells in the BM at the diagnosis of IMS, leading to AML. Therefore, IMS should be treated by systemic chemotherapy. There have been no large prospective studies on a suitable therapy for IMS because of its low incidence. The efficacy of cytarabine-based regimens has been reported in small numbers of patients (20-22). In AML with t(8;21), MS is assumed to be a poor prognostic factor; the median survival time of patients with AML with t(8;21) is 59.5 months, compared to 5.4 months for patients with AML with t(8;21) and MS (23). Among 12 patients with pancreatic MS, CR of 2 years' duration was achieved in 2 patients treated with chemotherapy combined with surgical resection or HSCT (Cases 2 and 7 in Table). Given the poor prognosis of MS with t(8;21), the previous reports of pancreatic MS, and the existence of disseminative disease, we planned allo-HSCT for our case.
Allo-HSCT in addition to systemic chemotherapy may improve the prognosis of patients with IMS. The retrospective study by Antic et al. revealed that the 5-year overall survival (OS) of 12 IMS patients was 25%, and survival for >50 months was found in 2 of 3 patients treated with HSCT but only 1 of 9 patients without HSCT (24). Chevallier et al. reported that, in 30 patients with IMS who received allo-HSCT, the 5-year OS, leukemia-free survival rate and none-relapse mortality were 33%, 30% and 17%, respectively. In addition, the authors pointed out that having achieved CR at allo-HSCT and lacking a poor prognostic karyotype were indicators of a good prognosis (25). Lazzarotto et al. analyzed the clinical outcome of 48 MS patients, including 9 IMS patients, and similarly reported that allo-HSCT after intensive chemotherapy improved the prognosis (OS probability at 5 years for the whole population and for the 22 MS patients who received allo-HSCT: 33% and 53%, respectively). In addition, having achieved CR at allo-HSCT influenced a better prognosis (26). Allo-HSCT is assumed to improve the prognosis of patients with IMS. Allo-HSCT may be considered the primary treatment option for IMS, although the findings from these retrospective studies may include some selection bias. In addition, the evaluation of the treatment response and cytogenetic information in IMS cases are important.
MS is an extramedullary neoplastic tumor of immature myeloid cells, with various types and numbers of inflammatory cells. There are no known highly sensitive and specific antigens for the diagnosis of MS cells. It is usually difficult to obtain enough specimens for genetic examinations because of the site and size of IMS. However, genetic information is necessary to clarify the origin of IMS and plan treatment. Therefore, we performed a laparoscopic biopsy of the pancreas. Immunophenotyping with a histological analysis, flow cytometry and a genetic analysis of the biopsy samples resulted in a proper diagnosis of IMS.
There is no sensitive method for assessing deep CR, such as flow cytometry or RT-PCR, in IMS. PET/CT is effective for the evaluation of the residual tumor and treatment response. PET/CT can detect MS more sensitively than standard CT and magnetic resonance imaging (27,28). MS can develop at any site in the body, and PET/CT is useful for screening for MS, finding a biopsy site, and evaluating the treatment response (28,29). In the present case, 18F-FDG PET/CT were utilized for the assessment of the treatment response.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 21: 340-350, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol 13: 1800-1816, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood 118: 3785-3793, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Alexiev BA, Wang W, Ning Y, et al. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol 2: 42, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2: 309-316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonig H, Gobel U, Nurnberger W. Bilateral exopthalmus due to retro-orbital chloromas in a boy with t(8;21)-positive acute myeloblastic acute leukemia. Ped Hemat Oncol 19: 597-600, 2002. [DOI] [PubMed] [Google Scholar]
- 7.King DJ, Ewen SW, Sewell HF, Dawson AA. Obstructive jaundice. An unusual presentation of granulocytic sarcoma. Cancer 60: 114-117, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Moreau P, Milpied N, Thomas O, et al. Primary granulocytic sarcoma of the pancreas: efficacy of early treatment with intensive chemotherapy. Rev Méd Interne 17: 677-679, 1996. (in French, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 9.Marcos HB, Semelka RC, Woosley JT. Abdominal granulocytic sarcomas: demonstration by MRI. Magn Reson Imaging 15: 873-876, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi-Kashani F, Estey E, Cortes J, Medeiros LJ, Giles FJ. Granulocytic sarcoma of the pancreas: a report of two cases and literature review. Clin Lab Haematol 21: 219-224, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Servin-Abad L, Caldera H, Cardenas R, Casillas J. Granulocytic sarcoma of the pancreas. A report of one case and review of the literature. Acta Haematol 110: 188-192, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Breccia M, D'Andrea M, Mengarelli A, Morano SG, D'Elia GM, Alimena G. Granulocytic sarcoma of the pancreas successfully treated with intensive chemotherapy and stem cell transplantation. Eur J Haematol 70: 190-192, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Schafer HS, Becker H, Schmitt-Graff A, Lubbert M. Granulocytic sarcoma of Core-binding Factor (CBF) acute myeloid leukemia mimicking pancreatic cancer. Leuk Res 32: 1472-1475, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Rong Y, Wang D, Lou W, Kuang T, Jin D. Granulocytic sarcoma of the pancreas: a case report and review of the literatures. BMC Gastroenterol 10: 80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XP, Liu WF, Ji SR, Wu SH, Sun JJ, Fan YZ. Isolated pancreatic granulocytic sarcoma: a case report and review of the literature. World J Gastroenterol 17: 540-542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messager M, Amielh D, Chevallier C, Mariette C. Isolated granulocytic sarcoma of the pancreas: a tricky diagnostic for primary pancreatic extramedullary acute myeloid leukemia. World J Surg Oncol 10: 13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Kimura M, Satoh S, et al. Early detection of AML1/MTG8 fusion mRNA by RT-PCR in the bone marrow cells from a patient with isolated granulocytic sarcoma. Leukemia 12: 1501-1503, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Neiman RS, Barcos M, Berard C, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer 48: 1426-1437, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer 58: 2697-2709, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 94: 1739-1746, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Tsimberidou AM, Kantarjian HM, Estey E, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia 17: 1100-1103, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 113: 1370-1378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol 15: 466-475, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Antic D, Elezovic I, Milic N, et al. Is there a “gold” standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother 67: 72-77, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Chevallier P, Labopin M, Cornelissen J, Socie G, Rocha V, Mohty M. Allogeneic hematopoietic stem cell transplantation for isolated and leukemic myeloid sarcoma in adults: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Haematologica 96: 1391-1394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazzarotto D, Candoni A, Filì C, et al. Clinical outcome of myeloid sarcoma in adult patients and effect of allogeneic stem cell transplantation. Result from a multicenter survey. Leuk Res 53: 74-81, 2017. [DOI] [PubMed] [Google Scholar]
- 27.Ueda K, Ichikawa M, Takahashi M, Momose T, Ohtomo K, Kurokawa M. FDG-PET is effective in the detection of granulocytic sarcoma in patients with myeloid malignancy. Leuk Res 34: 1239-1241, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Stolzel F, Rollig C, Radke J, et al. 18F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica 96: 1552-1556, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantzarides M, Bonardel G, Fagot T, et al. Granulocytic sarcomas evaluated with F-18-fluorodeoxyglucose PET. Clin Nucl Med 33: 115-117, 2008. [DOI] [PubMed] [Google Scholar]