Abstract
Long-term methotrexate (MTX) treatment can cause MTX-related lymphoproliferative disorder (MTX-LPD). We experienced a case of MTX-LPD that was associated with severe osteonecrosis of the jaw mimicking medication-related osteonecrosis of the jaw. The patient was an 81-year-old woman with rheumatoid arthritis (RA) who was treated with MTX and bisphosphonate. After 7 years, she was referred to our department for the assessment of giant ulcer and exposure of the alveolar bone of the left maxilla. Histopathological and immunological analyses confirmed a diagnosis of MTX-LPD. At seven months after the cessation of MTX treatment, the ulcerative and necrotic lesions had markedly decreased in size. A 1-year follow-up examination showed no evidence of recurrence and good RA control.
Keywords: methotrexate-related lymphoproliferative disorder, medication-related osteonecrosis of the jaw, rheumatoid arthritis, lymphoma, Epstein-Barr virus
Introduction
Methotrexate (MTX) is the current first-line treatment for rheumatoid arthritis (RA) (1). However, many recent case studies have reported that long-term MTX treatment resulted in MTX-related lymphoproliferative disorder (MTX-LPD) in RA patients (2,3). The recent World Health Organization classification of lymphoid neoplasms categorizes MTX-LPD as an “other iatrogenic immunodeficiency-associated LPD” (4). MTX-LPD may resolve several weeks after the withdrawal of MTX therapy; however, additional treatments, such as chemotherapy and radiotherapy, should be considered for patients with persistent LPD after MTX withdrawal (5-7). Regarding the mechanism underlying the onset of MTX-LPD, it is considered that immunosuppression by MTX reduces the host immunosurveillance of Epstein-Barr virus (EBV)-infected B cells, because approximately 50% of patients with MTX-LPD are EBV-positive (8).
Bisphosphonates (BPs) are the primary treatment for osteoporosis, bone metastasis, hypercalcemia caused by malignancies, Paget disease of bone, and osteolytic lesions of multiple myeloma. Although BP treatment has many benefits for patients with skeletal complications, it is associated with a number of adverse effects, the most important of which is BP-related osteonecrosis of the jaw (BRONJ) (9,10). Since BRONJ was first reported in 2003, many additional cases have been reported (11,12). Because osteonecrosis of the jaw can also be caused by other drugs, the American Association of Oral and Maxillofacial Surgery updated their position paper on BRONJ in 2014. The term BRONJ was replaced with medication-related osteonecrosis of the jaw (MRONJ) (13). The clinical manifestations of MRONJ include soft tissue swelling, fistula, abscess, pain, and bone exposure (14). There is currently no gold standard for the treatment of MRONJ or MTX-LPD. MTX-LPD generally develops at extra-nodal sites (8). To the best of our knowledge MTX-LPD of the oral cavity is rare, and no previous studies have reported the serial treatment of MTX-LPD with MRONJ.
We herein describe a case of MTX-LPD with MRONJ. In addition, we performed a systematic literature review to identify all cases of MTX-LPD of the oral cavity, to investigate the characteristics of oral MTX-LPD.
Case Report
In July 2015, an 81-year-old Japanese woman was referred to the Department of Oral Surgery at Kyushu University Hospital for the evaluation of upper left mandible pain. In 2009, the patient was diagnosed with RA based on the findings of bone destruction and swelling at her wrists and a positive serum test for rheumatoid factor. She had been taking BP (BonalonⓇ; 35 mg/week) and prednisolone (PrednisolonⓇ; 5 mg/day) for 7 years and MTX (MetolateⓇ; 8 mg/week) for 4 years. In April 2015, she fell while walking on stairs and noticed slight bleeding near her left first molar. Two weeks earlier she visited another dentist due to continuous pain of the left maxillary gingiva that had persisted for 2 months. The dentist suspected MRONJ based on the exposure of the maxillary bone.
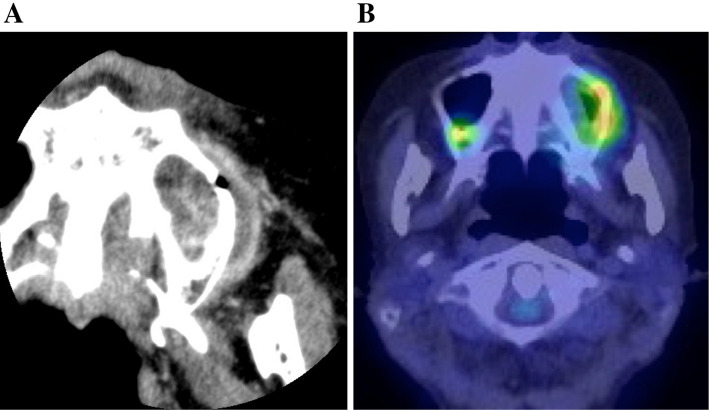
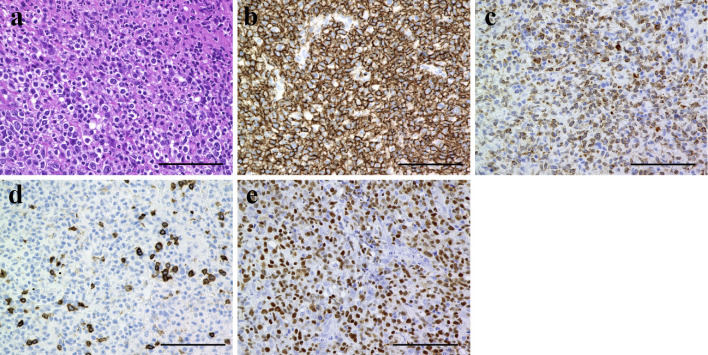
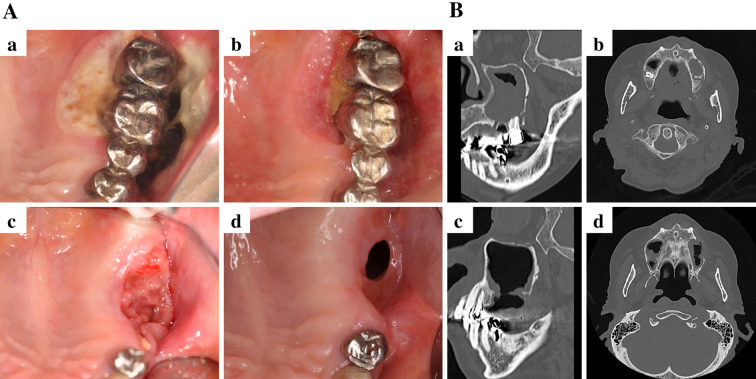
Our initial examination revealed an ulcer with induration on the buccal gingiva, near the upper left molar, which was mobile (Fig. 1A). Radiography showed left sinusitis and the loss of vertical bone near the upper left molars (Fig. 1B). These findings suggested gingival carcinoma or lymphoma. A cytological analysis of the ulcerative gingiva revealed class III cytology accompanied by numerous atypical lymphocytes; however, these findings were not conclusive for malignancy. The results of serological tests were unremarkable; however, the patient's C-reactive protein concentration was slightly elevated (0.64 mg/dL). Coronal computed tomography revealed a lesion arising from the upper left second premolar to the left sinus and the destruction of the buccal cortical bone in the left posterior maxilla (Fig. 2A). 18Fluoro-2-deoxyglucose positron emission tomography (FDG-PET) was performed to evaluate the extent of the primary lesion, the status of the regional lymph nodes, and the possibility of distant metastasis. The images showed the abnormal accumulation of FDG in the left upper maxilla and sinus (maximum standardized uptake value, 4.94) and no accumulation in several systemic lymph nodes or other lesions (Fig. 2B). These findings indicated that the lesion was malignant or another type of tumor. She was immediately referred to the hematology department of our hospital and an incisional biopsy of the upper left region was performed. Immunophenotyping of the upper left gingiva gated by CD45+ cells yielded the following findings: CD3+ CD20-, 48.3%; CD3- CD20+, 14.6%; natural killer cells, 30.5%; CD19+, 17.7%; κ chain, 4.25%; and λ chain, 3.72%. These findings were not conclusive for a diagnosis of any type of malignant lymphoma. A pathological analysis showed the infiltration of numerous lymphocytes and the diffuse proliferation of medium- to large-sized atypical lymphoid cells with large nuclei and clumped chromatin. Moreover, an immunohistochemical analysis revealed extensive lymphocytic infiltration. The cells were mostly B cells (positive for CD20 and CD79a); however a few T cells were found to be CD3-positive. The additional performance of in situ hybridization to detect EBV-encoded RNA (15) showed the strong infiltration of EBV-positive atypical lymphoid cells (Fig. 3). In sum, these findings confirmed a diagnosis of EBV-positive diffuse large B-cell lymphoma (DLBCL). Based on the pathological and clinical findings, the patient was diagnosed with MTX-LPD, and MTX therapy was immediately stopped (Fig. 4A-a). To control RA, the patient was treated with prednisolone (3 mg/day). We continued to monitor the patient carefully during the initial 2-week cessation of MTX. The exposed bone was rinsed three times a week with 0.05% glucuronic acid chlorhexidine solution, and the necrotic gingiva and induration gradually disappeared (Fig. 4A-b). At four months after the withdrawal of MTX and rinsing with glucuronic acid chlorhexidine solution, the upper left molars were easily extracted, along with the surrounding floating bone (Fig. 4A-c). The necrotic and indurated tissue had disappeared, and no other adverse events were noted (Fig. 4A-d). CT images obtained at 4 and 7 months after the withdrawal of MTX showed the improvement of the left sinusitis and the absence of inflammation or floating bone in the maxilla (Fig. 4B). No inflammation was observed near the upper left gingiva during this period, and the patient did not require antibiotic treatment. There was no recurrence of MTX-LPD or extra-nodal lymphoma, and her RA remained well controlled at 1 year after the discontinuation of MTX treatment.
Figure 1.
A photograph and photoradiograph obtained at the first examination. (A) The exposure of the buccal and palate bone, and the buccal root of the molars were noted (mirror reflection). The upper-left first and second molars showed deflection. (B) The left sinus had a cloudy appearance (yellow arrowhead).
Figure 2.
Computed tomography (CT) and fluorodeoxyglucose positron emission tomography (FDG-PET). (A) CT revealed a mass around the upper-left maxilla. (B) FDG-PET indicated abnormal accumulation in the upper-left gingiva.
Figure 3.
The histological and immunohistochemical findings of the upper-left gingiva. Hematoxylin and Eosin staining (a). Immunohistochemical staining for CD20 (b), CD79a (c), CD3 (d), and Epstein-Barr encoding region (EBER) (e). Scale bars: 100 μm.
Figure 4.
Photographs and photoradiographs obtained after the withdrawal of methotrexate (MTX). (A) Photographs of the upper-left maxilla at 0 days (a), 2 weeks (b), 4 months (c), and 7 months (d). All of the photographs show mirror reflections. (B) CT images obtained after the withdrawal of MTX for 4 months (a, b) and 7 months (c, d).
Discussion
Evidence from numerous reports indicates that the risk of LPD in RA (RA-LPD) patients is 2.0-5.5 times that of the general population (16). The underlying mechanism is unclear; however, patients with RA have persistent immunological abnormalities that may lead to clonal selection, which can result in the malignant transformation of B cells, the decreased apoptosis of infected B cells, reduced natural killer cell activity, the proliferation of latent EBV infection, and direct oncogenic action. All of these conditions may be potentiated by immunomodulation therapies. The number of reported LPD cases has been increasing among patients with RA receiving MTX, a condition referred to as MTX-LPD (8). The characteristics of MTX-LPD are as follows: (1) the most frequent subtype of MTX-LPD is DLBCL rather than Hodgkin lymphoma or Hodgkin disease-like lymphoma, (2) the frequency of EBV-positive case is higher in MTX-LPD than in RA-LPD or age-related LPD, (3) the possibility of improvement is high (20-60%), but only when MTX is withdrawn for several weeks, (4) the frequency of extra-nodal lymphoma is higher in MTX-LPD cases than in RA-LPD or age-related LPD cases, and (5) the duration to the outcome is shorter in patients with MTX-LPD than in those with RA-LPD (17,18). In approximately 40-50% of cases, MTX-LPD develops at extra-nodal sites, such as the skin, lung, liver, gastrointestinal tract, and kidney (8).
A review of the literature revealed only 19 cases of oral MTX-LPD, including our 3 cases (Table 1) (7,19-30). At the time of the diagnosis of MTX-LPD, the mean age of the patients was 71.1 years (range, 44-87 years) and the male-to-female ratio was 4:15 (the male-to-female ratio in RA was 1:4). The mean duration of MTX treatment was 6.6 years (range, 0.1-20 years). The frequency of bone exposure and DLBCL was 56.2% (9/16), the frequency of EBV positivity was 100.0% (13/13), and MTX-LPD resolved in 80.0% (12/15) of patients after the discontinuation of MTX. The data on the mean age, sex ratio, and the duration of MTX treatment were similar to those of previous reports on oral MTX-LPD. In contrast, the frequency of DLBCL and EBV-positivity in the patients with oral MTX-LPD were higher in comparison to previous reports on non-oral MTX-LPD, as summarized in Table 2 (6,21,24,31). Some reports have suggested that spontaneous remission occurs due to the withdrawal of MTX and noted an association between EBV positivity and the spontaneous remission of MTX-LPD (32). The initial symptoms of oral MTX-LPD included ulcer (80.0%, 12/15) and swelling (20.0%, 3/15), and the main sites affected included the gingiva (66.7%, 12/18), the hard palate (11.1%, 2/18) and the buccal mucosa (11.1%, 2/18). Other regions of the oral cavity, including the tongue, soft palate, sinus, and lip, can also be target organs because EBV in the head and neck region, including the pharynx and the salivary glands, generally remains dormant. Dentists and clinicians should bear in mind that MTX-LPD can be observed in any lesion.
Table 1.
Clinicopathological Findings of 19 Cases with Oral Methotrexate-related Lymphoproligeractive Disorders (MTX-LPD).
| No. | Age | Sex | Lesion | Complaining | MTX intake (y) | Histology | EBV | BP intake (y) | Bone Exposure | MTX withdrawal | Recurrence | Chemotherapy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 72 | F | gingiva | ulcer | NA | poly-B cell lymphoma | + | NA | + | + | - | - | (19) |
| 2. | 69 | F | gingiva | ulcer | NA | Wegener’s lymphoma | NA | NA | + | + | - | - | (19) |
| 3. | 73 | F | oral cavity | NA | 2 | peripheral T cell lymphoma | NA | - | - | + | - | - | (21) |
| 4. | 73 | F | oral cavity | NA | 2 | DLBCL | LMP-1 | - | + | + | - | - | (20) |
| 5. | 70 | F | palate | ulcer | 6 | DLBCL | EBNA2 | - | - | + | - | - | (7) |
| 6. | 69 | F | gingiva | NA | NA | Hodgkin | LMP-1 | - | NA | + | NA | NA | (22) |
| 7. | 80 | M | tongue | ulcer | NA | NA | NA | NA | - | NA | NA | NA | (30) |
| 8. | 76 | F | gingiva | ulcer, bleeding | 10 | DLBCL | EBER | - | - | + | + | R-THP-COP | (24) |
| 9. | 67 | F | palate | ulcer | 9 | DLBCL | EBER | 9 | + | + | - | - | (25) |
| 10. | 75 | F | gingiva | swelling | 5 | DLBCL | EBER | NA | + | + | + | R-CHOP | (26) |
| 11. | 60 | M | gingiva | swelling | 20 | DLBCL | EBER | - | + | + | - | - | (27) |
| 12. | 71 | F | buccal mucosa | ulcer | 0.1 | NA | NA | + | - | + | - | - | (28) |
| 13. | 87 | F | buccal mucosa | ulcer | 2 | NA | NA | + | - | + | - | - | (28) |
| 14. | 66 | F | gingiva | ulcer | 3 | DLBCL | NA | 8M | + | + | - | - | (29) |
| 15. | 76 | F | gingiva | NA | NA | Hodgkin | LMP-1 | NA | NA | + | NA | NA | (23) |
| 16. | 67 | M | palate | NA | NA | NA | LMP-1 | NA | NA | + | NA | NA | (23) |
| 17. | 81 | F | gingiva | ulcer | 4 | DLBCL | EBER | 7 | + | + | - | - | This case |
| 18. | 71 | F | gingiva | ulcer | NA | BL | EBER | + | + | + | - | - | our cases |
| 19. | 77 | F | gingiva | ulcer | 12 | DLBCL | EBER | + | + | + | + | R-CHOP |
MTX: methotrexate, EBV: Epstein-Barr Virus, DLBCL: diffuse large B-cell lymphoma, LMP: latent infection membrane protein, EBNA: EBV nuclear antigen, EBER: Epstein-Barr encording region, R-THP-COP: rituximab, pinorubin, oncovin, endoxan, and prednisolone, R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, +: positive, −: negative, NA: not available
Table 2.
Comparison of Clinicopathological Findings between Oral and Non-oral MTX-LPD.
| Oral MTX (n=17) | Non-oral MTX (n=52) | |
|---|---|---|
| Mean age (years) | 71.1 | 63.0-69.0 |
| Sex (male : female) | 4:13 | 20:32 |
| Affected regions | gingiva, palate, buccal mucosa, tongue | lymph node, spleen, lung, skin, liver, pleura, kidney, small bowel, breast, neck, thymus, stomach, heart |
| Duration of MTX treatment (years) | 6.6 | 3.1-5.8 |
| DLBCL (%) | 56.2 | 30.8-55.3 |
| EBV-positive (%) | 100 | 50.0-62.8 |
| Reference | Table 1 | (7), (21), (32) |
MTX-LPD: methotrexate- related lymphoproliferative disorder, DLBCL: diffuse large B-cell lymphoma, EBV: Epstein-Barr virus
Our patient had been treated with BPs for 9 years and MTX for 4 years. A diminished CTL function caused by the immunosuppressive effect of MTX permits the reactivation of EBV and the monoclonal proliferation of EBV-infected B cells in the buccal gingiva. Since the gingiva has a unique environment, characterized by its direct contact with the alveolar bone through the periodontal pocket, patients with LPD affecting the cervical gingiva are prone to develop bone exposure, and those who are treated with BPs may subsequently develop necrosis of the alveolar bone.
Recently, EBV-positive mucocutaneous ulcer (EBV-MCU) was proposed as a novel clinical entity that presents as mucocutaneous ulcers of the oropharynx, gastrointestinal tract, or skin, which are caused by latent EBV infection. Iatrogenic immunosuppression for autoimmune disease and age-related immunosenescence have been implicated as risk factors (30).The clinical conditions of this case might be close to those of EBV-MCU.
The presence of ulcer and adjacent bone exposure in the gingiva complicated the initial diagnosis because the common manifestations of MRONJ include ulcer, swelling, fistula, abscess, and bone exposure. However, our patient also exhibited the progression of ulceration and induration. We therefore performed an incisional biopsy as soon as we suspected malignancy or another tumor. After the diagnosis was confirmed, MRONJ was treated by daily cleaning around the site of bone necrosis with glucuronic acid chlorhexidine solution. After this, the floating necrotic bone and molars gradually resolved. The gingival ulceration and induration resolved in association with bone removal. No guidelines have been established for the treatment of MTX-LPD; however, pathological and immunological examinations are necessary in the selection of treatment.
We described a case of MTX-LPD with severe MRONJ and the effects of treatment after MTX withdrawal. Many previous case reports have described gingival ulcers or swelling near the sites of osteonecrosis; however, no previous reports have described the treatment of MRONJ or differentiation between MRONJ and MTX-LPD with MRONJ. The present case and the associated review of the literature on oral MTX-LPD suggest that oral MTX-LPD will continue to be a concern because RA patients are increasingly treated with MTX and BPs. Despite the risk of severe adverse effects, MTX is the treatment of choice for RA. Clinicians should bear in mind that oral MTX-LPD is occasionally complicated by MRONJ, and that a pathological examination (e.g., an incisional biopsy and immunohistological examination) is required for the final diagnosis. Additionally, close collaboration with internists or hematologists should be considered when developing a treatment plan.
Written informed consent was obtained from the patient for publication of this report and the use of any accompanying images.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by grants from the “Shiinoki Foundation (Kyushu University)”.
References
- 1. Liote F, Pertuiset E, Cochand-Priollet B, et al. . Methotrexate related B lymphoproliferative disease in a patient with rheumatoid arthritis. Role of Epstein-Barr virus infection. J Rheumatol 22: 1174-1178, 1995. [PubMed] [Google Scholar]
- 2. Kamel OW, van de Rijn M, LeBrun DP, Weiss LM, Warnke RA, Dorfman RF. Lymphoid neoplasms in patients with rheumatoid arthritis and dermatomyositis: frequency of Epstein-Barr virus and other features associated with immunosuppression. Hum Pathol 25: 638-643, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Hoshida Y, Xu JX, Fujita S, et al. . Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 34: 322-331, 2007. [PubMed] [Google Scholar]
- 4. Swerdlow SCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. IARC, Lyon, 2008. [Google Scholar]
- 5. Rizzi R, Curci P, Delia M, et al. . Spontaneous remission of “methotrexate-associated lymphoproliferative disorders” after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol 26: 1-9, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Ichikawa A, Arakawa F, Kiyasu J, et al. . Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 91: 20-28, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Uneda S, Sonoki T, Nakamura Y, Matsuoka H, Nakakuma H. Rapid vanishing of tumors by withdrawal of methotrexate in Epstein-Barr virus-related B cell lymphoproliferative disorder. Intern Med 47: 1445-1446, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Salloum E, Cooper DL, Howe G, et al. . Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clinl Oncol 14: 1943-1949, 1996. [DOI] [PubMed] [Google Scholar]
- 9. Yamachika E, Matsubara M, Ikeda A, Matsumura T, Moritani N, Iida S. Treatment of osteonecrosis of the jaw. J Craniofac Surg 26: e575-e577, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Preidl RH, Ebker T, Raithel M, Wehrhan F, Neukam FW, Stockmann P. Osteonecrosis of the jaw in a Crohn's disease patient following a course of Bisphosphonate and Adalimumab therapy: a case report. BMC Gastroenterol 14: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gabbert TI, Hoffmeister B, Felsenberg D. Risk factors influencing the duration of treatment with bisphosphonates until occurrence of an osteonecrosis of the jaw in 963 cancer patients. J Cancer Res Clin Oncol 141: 749-758, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Assoc 139: 23-30, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Ruggiero SL, Dodson TB, Fantasia J, et al. . American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 72: 1938-1956, 2014. [DOI] [PubMed] [Google Scholar]
- 14. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 65: 369-376, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Biehs B, Hu JK, Strauli NB, et al. . BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nature Cell Biol 15: 846-852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer 88: 497-502, 2000. [PubMed] [Google Scholar]
- 17. Kamel OW. Iatrogenic lymphoproliferative disorders in nontransplantation settings. Semin Diagn Pathol 14: 27-34, 1997. [PubMed] [Google Scholar]
- 18. Kamel OW, Holly EA, van de Rijn M, Lele C, Sah A. A population based, case control study of non-Hodgkin's lymphoma in patients with rheumatoid arthritis. J Rheumatol 26: 1676-1680, 1999. [PubMed] [Google Scholar]
- 19. Kalantzis A, Marshman Z, Falconer DT, Morgan PR, Odell EW. Oral effects of low-dose methotrexate treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100: 52-62, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka A, Shigematsu H, Kojima M, Sakashita H, Kusama K. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult Still's disease. J Oral Maxillofac Surg 66: 1492-1495, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Kojima M, Itoh H, Hirabayashi K, et al. . Methtrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract 202: 679-685, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Kikuchi K, Miyazaki Y, Tanaka A, et al. . Methotrexate-related Epstein-Barr Virus (EBV)-associated lymphoproliferative disorder--so-called “Hodgkin-like lesion”--of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol 4: 305-311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kikuchi K, Ishige T, Ide F, et al. . Overexpression of activation-induced cytidine deaminase in MTX- and age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders of the head and neck. J Oncol 2015: 605750, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishida M, Hodohara K, Yoshii M, et al. . Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol 6: 2237-2241, 2013. [PMC free article] [PubMed] [Google Scholar]
- 25. Tokuyama R, Sato T, Tatehara S, et al. . Methotrexate-associated lymphoproliferative disorder complicated by bisphosphonate-related osteonecrosis of the jaw arising in a female rheumatoid arthritis patient: Report of a case. J Oral Maxillofac Surg Med Pathol 26: 374-378, 2014. [Google Scholar]
- 26. Kudoh M, Harada H, Matsumoto K, Sato Y, Omura K, Ishii Y. Methotrexate-associated lymphoproliferative disorder arising in the retromolar triangle and lung of a patient with rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol 118: e105-e110, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Horie N, Kawano R, Kaneko T, Shimoyama T. Methotrexate-related lymphoproliferative disorder arising in the gingiva of a patient with rheumatoid arthritis. Aust Dent J 60: 408-411, 2015. [DOI] [PubMed] [Google Scholar]
- 28. Jinbu Y. Bilateral oral lichenoid lesions on the buccal mucosa due to methotrexate: Report of two cases. J Oral Maxillofac Surg Med Pathol 27: 102-105, 2015. [Google Scholar]
- 29. Mishima S, Takahashi K, Tomioka T, Bessho K. Numb chin syndrome as initial manifestation of bisphosphonate-related osteomyelitis of the jaw and methotrexate-associated lymphoproliferative disorders: a rare case. Br J Oral Maxillofac Surg 54: 114-115, 2016. [DOI] [PubMed] [Google Scholar]
- 30. Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 34: 405-417, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshida Y, Takahashi Y, Yamashita H, Kano T, Kaneko H, Mimori A. Clinical characteristics and incidence of methotrexate-related lymphoproliferative disorders of patients with rheumatoid arthritis. Mod Rheumatol 24: 763-765, 2014. [DOI] [PubMed] [Google Scholar]
- 32. Weiss LM, Chen YY, Liu XF, Shibata D. Epstein-Barr virus and Hodgkin's disease. A correlative in situ hybridization and polymerase chain reaction study. Am J Pathol 139: 1259-1265, 1991. [PMC free article] [PubMed] [Google Scholar]