Abstract
A 63-year-old man developed vomiting, paraparesis, dysuria, bulbar palsy, and orthostatic hypotension over a period of 5 months. Neuroradiological examinations showed a swollen lower brainstem with a dural arteriovenous fistula at the craniocervical junction (DAVF-CCJ). A steroid was administered intravenously in the hospital to relieve brainstem edema. A few hours later, however, the patient developed acute tetraparesis with respiratory failure. Recently, there have been several reports describing the acute worsening of paraparesis in patients with a spinal dural arteriovenous fistula after steroid treatment. In addition to these reports, the present case suggests the risk of administering steroids to patients with DAVF-CCJ, especially those with brainstem dysfunction.
Keywords: dural arteriovenous fistula at the craniocervical junction, steroid, brainstem dysfunction
Introduction
A dural arteriovenous fistula (DAVF) is an abnormal connection between arteries and the venous system; they can arise at various sites in the central nervous system. A spinal DAVF is a subtype of DAVF that causes subacute or chronic myelopathy. Recently, the worsening of paraparesis after steroid therapy has been reported in spinal DAVF patients (1,2).
A dural arteriovenous fistula at the craniocervical junction (DAVF-CCJ) is another subtype of DAVF that rarely causes brainstem dysfunction (3). In the present study, we describe the case of a patient with a DAVF-CCJ who developed tetraparesis with respiratory failure immediately after the intravenous administration of a steroid.
Case Report
Five months prior to admission, a 63-year-old, right-handed man started experiencing repetitive vomiting. Two months prior to admission, he developed sudden weakness in both legs and difficulty urinating. One month later, he developed swallowing difficulty and dimmed vision when standing. The severity of these symptoms fluctuated even within a day. One day before admission, he vomited repeatedly, and finally became unable to stand and was transferred to our hospital. A neurological examination showed bilateral miosis, hoarseness, bilateral lower extremity spasticity with Babinski sign, brisk tendon reflexes of the four limbs, dysuria, and orthostatic hypotension. Weakness of the extremities was not notable on the initial examination. Magnetic resonance imaging (MRI) showed lower brainstem swelling with an abnormally high intensity on T2-weighted imaging (Fig. 1a and b). An abnormal flow void was also detected in the subdural space of the ventral medulla oblongata. Computed tomography angiography showed abnormal vessels at the ventral lower brainstem with a draining anterior spinal vein.
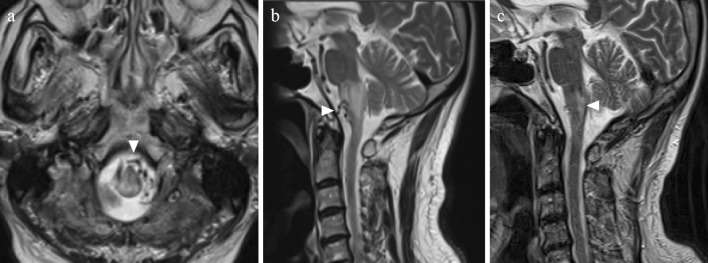
Figure 1.
Magnetic resonance imaging (MRI). (a) (b) Edematous changes of the lower brainstem are shown on axial and sagittal T2-weighted images. Abnormal flow voids in the subdural space are also seen (arrowheads). (c) A follow-up MRI study shows the resolution of brainstem congestion, while an infarction was observed to have developed at the dorsal medulla oblongata (arrowhead).
He was diagnosed as having a DAVF-CCJ. On the 4th hospital day, he complained of numbness of all four limbs and mild weakness of the bilateral arms. Intravenous betamethasone (4 mg) and 400 mL of 10% glycerin were administered. A few hours later, however, he developed a sudden exacerbation of weakness in all limbs, which was associated with respiratory failure and was mechanically ventilated. On the same day, cerebral angiography identified a DAVF-CCJ supplied by the ascending pharyngeal and occipital arteries; the DAVF drained into the anterior spinal vein (Fig. 2). Endovascular surgery interrupted the abnormal shunt flow. His extremity weakness and dyspnea gradually improved after surgery. He was weaned from ventilator support on the 8th hospital day. He became ambulatory and was discharged 36 days after surgery. Follow-up MRI, which was performed 2 months after discharge, showed the resolution of brainstem edema and the abnormal flow voids, while an old ischemic infarction at the dorsal medulla oblongata was newly detected (Fig. 1c). His symptoms gradually improved, and he eventually made an almost full recovery. Mild dysesthesia affecting the four extremities remained as a sequela.
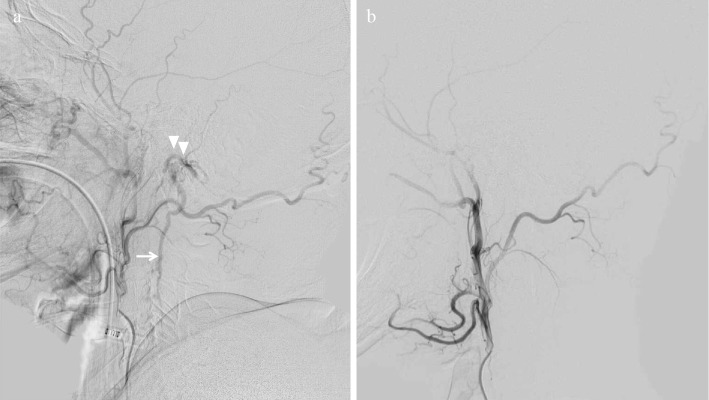
Figure 2.
Cerebral angiography. (a) The left ascending pharyngeal artery and occipital artery fed the abnormal fistula (arrowheads), and arterial blood entered the enlarged anterior spinal vein (arrow). (b) Abnormal shunt flow and the anterior spinal vein were not detected in a follow-up study after embolization therapy.
Discussion
Generally, patients with DAVF-CCJ develop a subarachnoid hemorrhage or myelopathy; brainstem dysfunction is relatively rare in such patients (3). The previously reported cases of DAVF-CCJ with brainstem dysfunction, including the present case, are summarized in Table (4-8). In these cases, the diagnosis was often challenging, and some patients were initially misdiagnosed with atypical brainstem infarction. As for the present case, the history of a stepwise course, the fluctuating nature of each symptom, and the abnormal flow void on MRI were helpful in confirming the diagnosis of DAVF-CCJ. Although some cases developed serious conditions, such as disturbed consciousness and respiratory failure, most patients made a partial or complete recovery after surgery. Thus, we should pay careful attention to DAVF-CCJ with brainstem dysfunction, which is a rare and harmful - but treatable - disease.
Table.
DAVF-CCJ Patients with Brainstem Dysfunction.
| Reference | Sex and age (y) | Clinical features | Time from onset to surgical treatment | Primary clinical diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 4 | M, 73 | Dizziness, vomiting, tetraparesis, dyspnea, unconsciousness, bowel and bladder dysfunction | 1 year | Cerebral infarction | Embolization | Improved |
| 5 | M, 69 | Dizziness, orthostatic hypotension, dysuria, bulbar palsy, tetraparesis, respiratory failure, unconsciousness | 1 month | Brainstem infarction | Embolization, open surgical occlusion | Improved |
| 6 | F, 46 | Vertigo, nausea, gait disturbance, dysphagia, ataxia | 1 month | Brainstem infarction | Embolization | Recovered completely |
| 7 | F, 58 | Occipital neuralgia, tetraparesis, hiccups, bulbar palsy, dyspnea | 2 months | DAVF-CCJ | Embolization, suboccipital craniotomy | Improved |
| 8 | M, 43 | Double vision, hiccups, bulbar palsy, motor weakness, ataxia, dysuria | no data | DAVF-CCJ | Embolization | Recovered completely |
| Present case | M, 63 | Vomiting, dysuria, tetraparesis, bulbar palsy, orthostatic hypotension, respiratory failure | 6 months | DAVF-CCJ | Embolization | Improved |
DAVF-CCJ: dural arteriovenous fistula at the craniocervical junction, F: female, M: male
The present patient developed tetraparesis and respiratory failure immediately after the intravenous administration of a steroid. Whether steroids should be used in the treatment of DAVF patients is controversial. Steroid therapy might be useful for the relief of vasogenic edema, which seems to contribute to the pathogenesis of DAVF (9). Recently, however, the acute worsening of paraparesis after steroid therapy has been reported in cases of spinal DAVF (1,2). Furthermore, a patient with cervical DAVF who developed sudden brainstem dysfunction after steroid administration has also been reported (10). The pathogenic role of steroids in these patients is uncertain. Hypothetically, the parenchymal lesions of DAVF would be caused by venous hypertension and congestion due to the arterialized venous system of the abnormal arteriovenous shunt (11). Thus, it was hypothesized that the mineralocorticoid effects of steroids might cause fluid retention, leading to increased venous pressure and a worsening of the symptoms (1,10). Based on these previous reports, the use of steroids in the present case might have triggered the acute exacerbation. Thus, the use of steroids for DAVF-CCJ should be avoided because of the risk of an acute exacerbation, including tetraparesis and respiratory failure. In addition, the simultaneous use of glycerin might have played a harmful role in the transient venous congestion due to its osmotic effects. Brainstem edema and high intensity on T2-weighted imaging are seen in various diseases, including brainstem tumors and autoimmune diseases such as neuromyelitis optica spectrum disorders, and steroids and antiedema drugs are often effective for treating these conditions. However, before these therapies are considered, DAVF-CCJ should be carefully ruled out.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank the patient for his cooperation and consent in relation to this report.
References
- 1. Cabrera M, Paradas C, Márquez C, González A. Acute paraparesis following intravenous steroid therapy in a case of dural spinal arteriovenous fistula. J Neurol 255: 1432-1433, 2008. [DOI] [PubMed] [Google Scholar]
- 2. O'Keeffe DT, Mikhail MA, Lanzino G, et al. . Corticosteroid-induced paraplegia - a diagnostic clue for spinal dural arterial venous fistula. JAMA Neurol 72: 833-834, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Wang JY, Molenda J, Bydon A, et al. . Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci 22: 1701-1707, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Ezura M, Takahashi A, Yoshimoto T. Intracranial dural arteriovenous fistula with venous reflux to the brainstem and spinal cord mimicking brainstem infarction--case report. Neurol Med Chir (Tokyo) 44: 24-28, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Terao T, Taniguchi M, Ide K, et al. . Cervical dural arteriovenous fistula presenting with brainstem dysfunction: case report and review. Spine (Phila Pa 1976) 31: E722-E727, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Wu Q, Wang HD, Shin YS, Zhang X. Brainstem congestion due to dural ateriovenous fistula at the craniocervical junction. J Korean Neurosurg Soc 55: 152-155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peltier J, Baroncini M, Thines L, et al. . Subacute involvement of the medulla oblongata and occipital neuralgia revealing an intracranial dural arteriovenous fistula of the craniocervical junction. Neurol India 59: 285-288, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Salamon E, Patsalides A, Gobin YP, et al. . Dural arteriovenous fistula at the craniocervical junction mimicking acute brainstem and spinal cord infarction. JAMA Neurol 70: 796-797, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Kataoka H, Miyamoto S, Nagata I, et al. . Venous congestion as a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery 48: 1224-1230, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Rain S, Udding J, Broere D. Acute clinical worsening after steroid administration in cervical myelitis may reveal a subdural arteriovenous fistula. Case Rep Neurol 8: 234-242, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aminoff MJ, Barnard RO, Logue V. The pathophysiology of spinal vascular malformations. J Neurol Sci 23: 255-263, 1974. [DOI] [PubMed] [Google Scholar]