Abstract
The period 1800 to 800 Ma (“Boring Billion”) is believed to mark a delay in the evolution of complex life, primarily due to low levels of oxygen in the atmosphere. Earlier studies highlight the remarkably flat C, Cr isotopes and low trace element trends during the so-called stasis, caused by prolonged nutrient, climatic, atmospheric and tectonic stability. In contrast, we suggest a first-order variability of bio-essential trace element availability in the oceans by combining systematic sampling of the Proterozoic rock record with sensitive geochemical analyses of marine pyrite by LA-ICP-MS technique. We also recall that several critical biological evolutionary events, such as the appearance of eukaryotes, origin of multicellularity & sexual reproduction, and the first major diversification of eukaryotes (crown group) occurred during this period. Therefore, it appears possible that the period of low nutrient trace elements (1800–1400 Ma) caused evolutionary pressures which became an essential trigger for promoting biological innovations in the eukaryotic domain. Later periods of stress-free conditions, with relatively high nutrient trace element concentration, facilitated diversification. We propose that the “Boring Billion” was a period of sequential stepwise evolution and diversification of complex eukaryotes, triggering evolutionary pathways that made possible the later rise of micro-metazoans and their macroscopic counterparts.
Introduction
The “Boring Billion” (period between 1800 and 800 Ma) in Earth’s history, also referred to as the “dullest period in Earth’s history”1 is believed to represent a period of geobiological stasis2. The so-called stasis is manifested as a remarkably stable and flat carbon isotope trend due to prolonged lack of nutrient supply and tectonic stability2. Other stable and undisturbed trends are depicted by S, Mo, Cr, Sr isotopes, and more particularly, by low values of trace element concentrations and P in marine black shales3–10. Outcomes of previous studies point towards a delay in the evolution of complex life primarily due to lack of oxygen in the atmosphere11. It is believed it was not until the Neoproterozoic (towards the end of the Boring Billion), that a great diversity in complex life (both microscopic and macroscopic) developed. Absence of banded iron formations, evaporites, phosphorites, glaciation events and major ore deposits related to convergent plate margins (Orogenic Au, porphyry, VHMS and MVT deposits) also correlate with the period of stasis12. Plate motions at that time were suppressed, possibly owing to stagnant lid tectonics, with modern plate tectonics not operative until towards the end of the Neoproterozoic13. General consensus therefore is that the 1800–800 Ma period represents a billion years of geological standstill that stalled evolution of complex life.
Contrary to above, we have identified certain gaps in the understanding of the “Boring Billion” period. First, even though geochemical proxies indicate “stasis”, certain key evolutionary breakthroughs occurred during this time span. These include the appearance of eukaryotes (including its cell organelles) possibly via endosymbiosis, multicellularity and origin of sexual reproduction14–18, and the evolution of precursors of metazoans (Urmetazoa)19. Without these developments, any subsequent metazoan and macroevolution would be impossible. Second, we observe the first major diversification of eukaryotes (Crown group)20–22, the appearance of metaphytes15,18 and metazoans15,23, between the Great Oxidation Event (GOE) and the Neoproterozoic Oxidation Event indicating that oxygen may not have been the only driver of these developments. Experiments also support low oxygen requirements of modern analogues of primitive metazoans24. The sudden appearance of metazoans with no obvious prior evolutionary developments is hard to comprehend. That is because metazoan evolution requires multicellularity, development of scaled epithilea and extracellular digestion, a nervous system, mesoderm, bilaterity and establishment of a tubular gut25. Also, any one element (e.g. O) cannot account for the course of biologic evolution26. A suite of bio-essential elements including H, C, N, O, P, S, Cl, Na, Mg, K, Ca, Al, Si (macro elements) and Fe, V, Cr, Mn, Co, Ni, Cu, Zn, Mo, B, F, As, Se (trace elements) are required by all kinds of life forms26.
Interestingly, bio-essential trace element abundance, unlike major elements, does not necessarily signify its importance in an organism as it is utilized only if it has functional roles26. Scarcity of an element plays an equally important role as it encourages organisms to adapt to an alternative element that has similar functional roles. This results in the selection of new protein molecules with advanced roles27,28. Thus, knowledge of multi-trace element variability in the ocean, particularly multi-trace element minima, is an essential aspect in understanding biologic evolution that has not been previously addressed. It has been noted by Sterner29 and Morel et al.30 that the major elements have received far more attention than the trace elements as potential limiting nutrients related to biological evolution.
The present study focuses on using a technique where trace element concentrations in sedimentary pyrite, determined using LA-ICP-MS, provide useful first order insights on trace element availability (abundance and scarcity) in the ocean with time8. Without undermining the role of macronutrients (such as P, N, O, C etc.) we aim to establish the link between trace element availability and biologic evolution through the Boring Billion period.
Method and Materials
Our method involves measuring trace element concentrations in sedimentary pyrite in black shales using Laser Ablation- Inductively Coupled- Mass Spectrometry (LA-ICP-MS) as proxies for atmospheric oxygenation and nutrient trace element availability8 (supplementary information: SI). It is based on the premise that the availability of most trace elements in seawater is controlled by a combination of oxidative weathering on land, and erosion/run-off to the ocean related to active tectonics. Subsequently, the trace elements become absorbed into sedimentary pyrite forming in organic matter-bearing muds depositing in anoxic portions of the ocean. Sedimentary pyrite analyses are preferred to whole rock shale analyses as the method is more sensitive to trace element concentrations, and trace elements are preferentially partitioned and concentrated into pyrite compared to whole rock31,32. The validity of the technique has been the subject of several publications33–36 which includes the proof of concept paper8. Additional information is also provided in the SI.
The study provides new analyses from the Riversleigh Siltstone in the Mt. Isa Basin in Australia and collates data from ~40 marine, least metamorphosed (lower greenschist and below), undeformed organic matter rich-sedimentary black shale formations from various sedimentary basins around the world (Table SI1a,b)8,36,37. Samples were mainly collected in the form of drill cores in order to ensure the pyrite was well preserved and not oxidised. Polished laser mounts were prepared for petrological analysis using reflected light microscope followed by LA-ICP-MS pyrite analyses for trace elements at CODES, University of Tasmania8. Polished mounts were studied under reflected light in order to select samples that contain fine grained early-formed sedimentary pyrite and discard coarse, recrystallised and diagenetically altered pyrite. Most samples comprised sedimentary pyrite in the form of individual microcrystals (5 to 10 u), aggregates of microcrystals, framboids and nodular concretions in black shales. Samples containing coarser euhedral pyrites were discarded as trace element budget is affected due to recrystallization8,31,32,34. Analyses were carried out using CompexPro 110 ArF Excimer laser microprobe equipped with S155 cell and coupled to an Agilent 7700 ICP-MS. A total of ~ 1400 robust analyses were obtained after exclusion of analyses that did not meet the criteria outlined for sedimentary pyrite (done on the basis of trace element ratios such as Ni/Co >1 in pyrite)34. Standards (or reference materials) were run at the start, about after every two samples (about every 1.5 hours) and at the end, in order to calculate the drift of the instrument (see SI)8. The signal from the mass spectrometer was generated in counts per second (cps) and was processed by CODES in-house software designed according to standard methods38 (Longerich et al.), using Fe as the internal standard element. Additional information on LA-ICP-MS data processing is provided in the SI.
Statistical methods
The trace element data were standardised by subtracting the mean and dividing by the standard deviation and then analysed using principal components analysis (PCA); (Table SI2) (PCA)39. The standardisation was to ensure that the results were not influenced unduely by variables of large magnitude. The PCA was calculated using PROC PRINCOMP in the SAS System version 9.3. The scores from the first component of the PCA were used to construct boxplots that were plotted against the corresponding geologic age. The PCA scores of the first component were plotted against geologic age for all trace element data in Fig. 1 and for selected variables such as Ni, Co, Se, Zn, Mo, Cd in Fig. 2. In both figures, the scores are presented as box plots along with the median trend (solid line) and the overall median score (dashed line). The use of boxplots allowed the spread of scores at each age to be represented graphically. Boxplots were calculated using R version 3.3.3.
Figure 1.
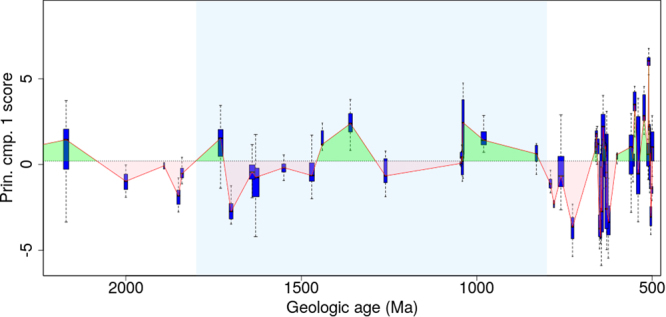
First principal component scores using all trace element variables against geologic age. Shown are the PCA scores for the first dimension as boxplots and also the median trend as a solid line. The overall median score is shown as a dotted line with areas above it coloured green, and below coloured pink.
Figure 2.
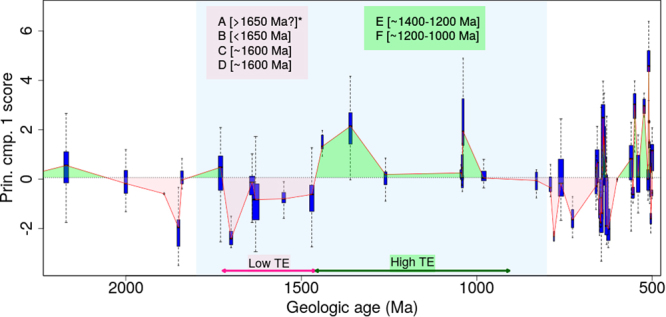
First principal component scores for selected trace element (TE) variables (Se, Ni, Co, Zn, Mo, Cd) against geologic age. Shown are the PCA scores of the first dimension as boxplots and also the median trend as a solid line. The overall median score is shown as a dotted line with areas above it coloured green and below coloured pink. (A) Endosymbiosis; (B) First eukaryotes and its cell organelles14–16; (C) Multicellularity18; (D) Sexual reproduction18,41; (E) Diversification and radiation of eukaryotes20–22,53–59; (F) Crown group eukaryotes15. *Exact timing of endosymbiosis is unknown but must have occurred prior to the appearance of first eukaryotes.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files)
Results
The pyrite-LA-ICP-MS analyses for a suite of trace element (Co, Ni, Zn, Se, Mo, Cd, Cu, Mn, As, Ag, Sb, Tl and Pb) are presented in Table SI1(Supplementary Information). The data are a compilation from Large et al.8,36,37 along with additional new analyses from this study. The first component of the PCA accounted for 31% variation in the data using all trace element variables, and 38% when using the selected variables (Ni, Co, Se, Mo, Zn and Cd) (Table 1). The eigenvector for the first component of the PCA using all trace element variables and for selected variables, had positive coefficients (Table 1).
Table 1.
Shown are the PCA eigenvector coefficients and eigenvalues when using all variables or using the selected variables.
| Analysis | Variable | Prin1 | Prin2 | Prin3 |
|---|---|---|---|---|
| Co | 0.087 | 0.560 | 0.015 | |
| Ni | 0.247 | 0.498 | −0.037 | |
| Zn | 0.294 | −0.202 | 0.396 | |
| Se | 0.304 | 0.056 | −0.102 | |
| Mo | 0.330 | −0.218 | −0.045 | |
| Cd | 0.297 | −0.146 | 0.235 | |
| Cu | 0.294 | 0.228 | 0.266 | |
| All variables | Mn | 0.209 | 0.008 | 0.495 |
| As | 0.270 | −0.063 | −0.531 | |
| Ag | 0.371 | −0.031 | −0.073 | |
| Sb | 0.349 | 0.095 | −0.394 | |
| Tl | 0.135 | −0.505 | −0.119 | |
| Pb | 0.273 | −0.073 | 0.035 | |
| Eigenvalue | 4.07 | 1.94 | 1.53 | |
| % explained | 31 | 15 | 12 | |
| Co | 0.070 | 0.702 | 0.388 | |
| Ni | 0.337 | 0.621 | −0.131 | |
| Selected variables | Zn | 0.466 | −0.227 | 0.569 |
| Se | 0.466 | 0.039 | −0.651 | |
| Mo | 0.451 | −0.132 | −0.152 | |
| Cd | 0.494 | −0.227 | 0.250 | |
| Eigenvalue | 2.25 | 1.54 | 0.861 | |
| % explained | 38 | 26 | 14 |
The interpretation of the first component is that it corresponds to a weighted average of the trace element variables. In other words, the first component of the PCAs provided a proxy for the trace element concentrations. In Fig. 1, the trace element trend is mostly below the median value between 2500–1400 Ma (except at 2500, ~1900 and 1730 Ma). Between 1400–800 Ma, the trend is above the median value except at ~1260 Ma. In Fig. 2, where only selected trace elements have been used to derive the principal component score, we observe a similar trend as Fig. 1. The trace element trend is initially above the median value but drops below it and remains so until 1400 Ma (Fig. 2). Between 1400–800 Ma the trace element trend is above the median value.
During the period 2000–1400 Ma there were 4 biological innovations (Eukarya, organelles, multicellularity, sex) discussed below. We can calculate the probability of the 4 events occurring in this period by assuming that these 4 events could occur independently and with uniform probability anytime between 2000 and 800 Ma. Since the periods 2000–1400 Ma and 1400–800 Ma are equal length intervals and, by our assumption, the events could occur with the same probability in each, then the probability of them co-occurring only between 2000–1400 Ma would be (1/2)4 = 0.0625.
Discussion
Here, we interpret the trace element trend in light of biologic evolution observed in the paleontological rock record in the Boring Billion period. Figures 1 and 2 demonstrate that trace element concentrations were mostly below the median value between 2000 and 1400 Ma. This is in agreement with previous research where low trace element concentrations have been proposed for this particular time span3,4,6,9. This can be attributed to the drop in oxygen levels after the GOE event. As evident from various geochemical proxies in the rock record, the GOE was transient in nature and oxygen dropped to lower levels at ~2000 Ma8,11,40. This is also clear from the Se/Co in pyrite (oxygenation proxy; see SI for more information) trend through time in this study (Fig. SI1). The decrease in bio-essential trace element concentrations (Ni, Co, Se, Zn, Mo, Cd) is possibly due to a decreased trace element flux in the ocean as a result of decrease in oxidative weathering (Figs 1, 2). Coinciding with this drop in oxygen and trace element concentrations, a number of key evolutionary breakthroughs are believed to have occurred, including: appearance of first eukaryotes, acquisition of certain cell organelles (or cell components such as plastids, mitochondria, nucleus, endoplasmic reticulum, cytoskeleton etc.) and multicellularity between 2000 and 1400 Ma14–16. Based on the paleontological record, the origin of sexual reproduction is also believed to have evolved between 2000–1400 Ma18,41. The micro-paleontological record confirms that the phenomena were global in nature as proposed by previous studies; for instance, in the Paleoproterozoic Changcheng and Ruyang Groups, China42–44, Vindhyan Supergroup, India45, and Roper Group, Australia46, and Mesoproterozoic of Jixian Group (1.56 Ga), China47; Belt Group (1.5 Ga), USA48; Kotuikan Fm (~1.5 Ga) Russia49; Roper Group (1.5 Ga), Australia22. Interestingly, most of the key biological innovations took place in a period where bio-essential trace element concentrations are consistently low between 2000 to 1400 Ma. These particular events were not all independent of each other in that Eukarya had to evolve before organelles (although some prokaryotes possess analogous structures)50. However, the 4 events, Eukarya, acquisition of organelles, sex, and multicellularity, could be considered plausibly to have happened in any order and time, although the latter two may not be independent17. If they are independent events then the likelihood of their co-occurrence during the low trace element period has been demonstrated to be quite small (~0.0625).
Unlike past studies, the trace element trend observed in the total Boring Billion period is not entirely low and flat, but after 1400 Ma, is, followed by a long period of relatively higher trace element concentrations (1400–800 Ma); i.e., higher than the median value for the Proterozoic. We observe an increase in trace element concentrations at around 1400 Ma which also coincides with an oxygenation event recognized using independent geochemical proxies (pyrite redox-sensitive trace element chemistry in black shales31, Mo isotopes in black shales51 and U isotopes52). Interestingly, this event is coeval with a major diversification of eukaryotes20–22. Complex cellular morphological capacities (cytoskeletal architecture) were observed and eukaryote habitation spread to a wider range of environments20,21. Diversification of eukaryotes is also noted in the Kamo Gp (1.3 Ga)53; Kaltasy Fm (1.4–1.45 Ga)54; Sarda Fm (~1.3 Ga), India55; Arctic Canada (~1.3 Ga)56,57; in the 1.1–0.9 Ga Mbuyi-Mayi Supergroup, DR Congo58; in the 1.1 Ga Atar/El Mreiti Gp, Mauritania59. This was followed by the diversification of crown group eukaryotes between 1200–1100 Ma15 and the emergence of a gene (1000–800 Ma), recently identified, that is linked with protein and choline kinases responsible for cell adhesion and transfer of signals within cells efficiently60,61. The period ended with the appearance of metazoans at ~750 Ma15,23 and the origin of fungi between 760 and 1000 Ma62.
In summary, key biological innovations in eukaryotes seemed to have co-occurred during the low trace element period, followed by a broad-scale diversification of eukaryotes, in the relatively high trace element period. We attribute this trend from a prokaryotic community (>1800 Ma) to the formation of the first eukaryotes (1800–1500 Ma) and their diversification (1400–800 Ma) to nutrient trace element availability as shown in Figs 1 and 2. Of course, macronutrient availability too may have played a critical role. However, previous work suggests a low and stable P and N through most of Earth’s history until towards the end of the Boring Billion (~800 Ma)10. This further supports the putative role of trace elements in shaping the course of evolution.
We propose that co-limitation of nutrient trace elements may have caused an environmental stress, a plausible driver of the biological innovations in the early part of the Boring Billion including endosymbiosis63, whereby unicellular prokaryotes are forced into a symbiotic relationship, due to nutrient limitation, conceivably resulting in Eukarya. Hoffmann and Hercus64 have previously proposed environmental stress as a major driver for evolutionary changes (DNA/genotypic/phenotypic variations, adaptation capacities). A decrease in the population (clearing of the ecological space), as a result of stressful conditions, causes renewed predation and competition that promotes subsequent evolutionary radiations in the newly reconstructed ecological space64,65. Another possible mechanism for evolutionary change are major population bottleneck events, such as may be expected during low trace element stress periods, which were then fixed as a result of small population sizes50. Experiments have demonstrated that stress can double rates of stress-induced genetic mutations in bacteria whereby new genes are created with different, and often advanced, functions66. We propose that a prolonged period of low nutrient trace element conditions may have contributed to an environmental stress that triggered the key biological innovations we observe in the rock record.
On the other hand, an increase in nutrient trace elements (after a prolonged nutrient crisis) may have facilitated diversification of the eukaryotes between 1400 and 800 Ma. Once the diversification process commenced, the eukaryotic community expanded in the marine realm. One could pose the question that if nutrient conditions did in fact improve, what delayed the rise of animals until ~750 Ma? It is quite possible that the evolution and expansion of complex eukaryotic communities played a significant role in fostering ocean oxygenation (as proposed by Lenton et al.)67 and bring about major changes in P and N cycles making the conditions conducive for the subsequent evolution of metazoans and their macroscopic counterparts. Therefore, the Boring Billion period is a critical time in Earth’s evolutionary history when the prerequisites for macroevolution were established.
Conclusions
Our primary conclusions lead to a paradigm shift in understanding evolution during the Proterozoic. First, the Proterozoic ocean witnessed periods of both low and high trace element availability; trace element trends are not flat and uniform as previously assumed. Second, we emphasize that the low nutrient trace element periods were possibly essential triggers in the course of evolution. Previous studies claim low concentrations of trace element may have stalled evolution3. However, we argue that these periods of trace element crisis forced organisms to explore their options to adapt to stressful conditions and promoted mechanisms to cope and evolve. On the upside of nutrient trace element cycles, when conditions improved in terms of high trace element availability from 1400 Ma to 800 Ma, organisms diversified. Thus, the need for both unfavorable and favorable nutrient conditions may have been required to generate the necessary evolutionary pressure and diversification respectively through time. Third, we propose that the high and low trace element periods in the “Boring Billion” may have played a critical role in establishing the prerequisites for metazoan evolution. It is unlikely that metazoans appeared all at once without prior evolutionary achievements. Therefore, sequential stepwise evolution and diversification of complex eukaryotes was likely a result of fluctuating nutrient trace element conditions (stress/stress-free) through the “Boring Billion”.
Electronic supplementary material
Acknowledgements
The project was funded by the Australian Research Council (DP150102578). We would like to thank Prof. Malcolm Walter, Prof. Mihir Deb, Dr. Alexander Stepanov, Chris Large and Dr. Jacob Mulder for their comments and suggestions.
Author Contributions
I.M. wrote the main manuscript text and R.C. assisted with the statistics and prepared Figures 1–2 and Table 1. All authors reviewed and contributed to the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22695-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buick R, Des Marais DJ, Knoll AH. Stable isotopic compositions of carbonates from the Mesoproterozoic Bangemall Group, northwestern Australia: Chemical Geology. 1995;123(1–4):153–171. doi: 10.1016/0009-2541(95)00049-r. [DOI] [PubMed] [Google Scholar]
- 2.Brasier MD, Lindsay JF. A billion years of environmental stability and the emergence of eukaryotes: new data from northern Australia: Geology. 1998;26:555–558. doi: 10.1130/0091-7613(1998)026<0555:abyoes>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Anbar AD, Knoll AH. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science. 2002;297:1137–1142. doi: 10.1126/science.1069651. [DOI] [PubMed] [Google Scholar]
- 4.Scott C, et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature. 2008;452:456–459. doi: 10.1038/nature06811. [DOI] [PubMed] [Google Scholar]
- 5.Kendall B, Creaser RA, Gordon GW, Anbar AD. Re-Os and Mo isotope systematics of black shales from the Middle Proterozoic Velkerri and Wollogorang Formations, McArthur Basin, northern Australia. Geochim Cosmochim Acta. 2009;73:2534–2558. doi: 10.1016/j.gca.2009.02.013. [DOI] [Google Scholar]
- 6.Partin, C. A. et al. Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales: Earth Planetary Science Letters, 369–370 (2013).
- 7.Planavsky NJ, et al. Low Mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals: Science. 2014;346:635–638. doi: 10.1126/science.1258410. [DOI] [PubMed] [Google Scholar]
- 8.Large RR, et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean- atmosphere evolution: Earth and Planetary Science Letters. 2014;389:209–220. [Google Scholar]
- 9.Robbins LJ, et al. Trace elements at the intersection of marine biological and geochemical evolution. Earth Science Reviews. 2016;163:323–348. doi: 10.1016/j.earscirev.2016.10.013. [DOI] [Google Scholar]
- 10.Reinhard CT, et al. Evolution of the global phosphorus cycle. Nature. 2017;541:386–389. doi: 10.1038/nature20772. [DOI] [PubMed] [Google Scholar]
- 11.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere: Nature, v. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 12.Holland, H. D. The oxygenation of the atmosphere and oceans Phil. Trans. R. Soc. 361 (2006). [DOI] [PMC free article] [PubMed]
- 13.Roberts NMW. The boring billion? – Lid tectonics, continental growth and environmental change associated with the Columbia supercontinent. Geoscience Frontiers. 2013;4:681–691. doi: 10.1016/j.gsf.2013.05.004. [DOI] [Google Scholar]
- 14.Butterfield NJ. Early evolution of the Eukaryota: Palaeontology. 2015;58:5–17. [Google Scholar]
- 15.Knoll, A. H. Paleobiological perspectives on early eukaryotic evolution: Cold Spring Harbor Perspectives in Biology, 10.1101/cshperspect.a01612 (2014). [DOI] [PMC free article] [PubMed]
- 16.Katz LA. Origin and diversification of eukaryotes: Annual Review of Microbiology. 2012;66:411–427. doi: 10.1146/annurev-micro-090110-102808. [DOI] [PubMed] [Google Scholar]
- 17.Porter SM. The fossil record of early eukaryotic diversification: Paleontological Society Papers. 2004;10:35–50. [Google Scholar]
- 18.Bengtson, et al. Three-dimensional preservation of cellular and subcellular structures suggests 1.6 billion-year-old crown-group red algae. PLoSBiol. 2017;15(3):e2000735. doi: 10.1371/journal.pbio.2000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller WEG. How was metazoan threshold crossed: the hypothetical Urmetazoa: Comparative Biochemistry and Physiology- Part A. 2001;129:433–460. doi: 10.1016/S1095-6433(00)00360-3. [DOI] [PubMed] [Google Scholar]
- 20.Javaux E, Knoll AH, Walter MR. 2003. Recognizing and interpreting the fossils of early eukaryotes: Origins of Life and Evolution of the Biosphere. 2003;33:75–94. doi: 10.1023/a:1023992712071. [DOI] [PubMed] [Google Scholar]
- 21.Javaux E, Knoll AH, Walter MR. TEM evidence for eukaryotic diversity in mid-Proterozoic oceans: Geobiology. 2004;2:121–132. [Google Scholar]
- 22.Javaux, E. J. and Knoll, A. H. Micropaleontology of the lower Mesoproterozoic Roper Group, Australia, and implications for early eukaryotic evolution: Journal of Paleontology, 10.1017/jpa.2016.124 (2017).
- 23.Erwin DH, et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals: Science. 2011;334:1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 24.Danovaro R, et al. The first metazoa living in permanently anoxic conditions: BioMed Central. Biology. 2010;8:30. doi: 10.1186/1741-7007-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen C. Six major steps in animal evolution: are we derived sponge larvae? Evolution and Development. 2008;10:241–57. doi: 10.1111/j.1525-142X.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Ponnamperuma C. Trace elements in chemical evolution, I: Origins of Life and Evolution of the Biosphere. 1985;16(1):41–55. doi: 10.1007/BF01808048. [DOI] [PubMed] [Google Scholar]
- 27.Ochiai EI. The evolution of the environment and its influence on the evolution of life: Origins Life. 1978;9:81–91. doi: 10.1007/BF00931406. [DOI] [PubMed] [Google Scholar]
- 28.Ochiai EI. Principles in the selection of inorganic elements by organisms -Application to molybdenum enzymes: BioSystems. 1978;10:329–337. doi: 10.1016/0303-2647(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 29.Sterner RW. On the Phosphorus Limitation Paradigm for Lakes. International Review of Hydrobiology. 2008;93:433–445. doi: 10.1002/iroh.200811068. [DOI] [Google Scholar]
- 30.Morel. MMF, Husdon R, Price NM. Limitation of productivity by trace metals in the sea. Limnol. Oceanogr. 1991;36(8):1742–1755. doi: 10.4319/lo.1991.36.8.1742. [DOI] [Google Scholar]
- 31.Mukherjee I, Large R. Pyrite trace element chemistry of the Velkerri Formation, Roper Group, McArthur Basin: Evidence for atmospheric oxygenation during the Boring Billion: Precambrian Research. 2016;281:13–26. [Google Scholar]
- 32.Mukherjee I, Large RR. Application of Pyrite Trace Element Chemistry to Exploration for SEDEX Style Zn-Pb Deposits: McArthur Basin, Northern Territory Australia: Ore Geology Reviews. 2017;81:1249–1270. [Google Scholar]
- 33.Gregory D, Meffre S, Large R. Comparison of metal enrichment in pyrite framboids from a metal-enriched and metal-poor estuary: American Mineralogist. 2014;99:633–644. [Google Scholar]
- 34.Gregory DD, et al. Trace element content of sedimentary pyrite in black shales. Econ. Geol. 2015;110:1389–1410. doi: 10.2113/econgeo.110.6.1389. [DOI] [Google Scholar]
- 35.Gregory, D. D. et al. Whole rock and discrete pyrite geochemistry as complementary tracers of ancient ocean chemistry: An example from the Neoproterozoic Doushantuo Formation, China: Geochimica et Cosmochimica Acta. In press (2017).
- 36.Large RR, et al. Cycles of nutrient trace elements in the Phanerozoic ocean: Gondwana Research. 2015;28(4):1282–1293. [Google Scholar]
- 37.Large RR, et al. Ocean and Atmosphere Geochemical Proxies Derived from Trace Elements in Marine Pyrite: Implications for Ore Genesis in Sedimentary Basins. Economic Geology. 2017;112(2):423–450. doi: 10.2113/econgeo.112.2.423. [DOI] [Google Scholar]
- 38.Longerich HP, Jackson SE, Günther D. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. Journal of Analytical Atomic Spectrometry. 1996;11:899–904. doi: 10.1039/JA9961100899. [DOI] [Google Scholar]
- 39.Johnson, R. A. & Wichern, D. W. “Applied Multivariate Statistical Analysis”, 2nd Edition, Prentice-Hall: Englewood Cliffs (1998).
- 40.Canfield DE. A new model for Proterozoic ocean chemistry: Nature. 1998;396:450–453. [Google Scholar]
- 41.Kabnick KS, Peattie DA. Giardia: A Missing Link between Prokaryotes and Eukaryotes: American Scientist. 1991;79:34. [Google Scholar]
- 42.Lamb DM, Awramik SM, Chapman DJ, Zhu S. Evidence for eukaryotic diversification in the similar to 1800 million-year-old Changzhougou Formation, North China: Precambrian Research. 2009;173:93–104. [Google Scholar]
- 43.Xiao SH, Knoll AH, Kaufman AJ, Yin LM, Zhang Y. Neoproterozoic fossils in Mesoproterozoic rocks? Chemostratigraphic resolution of a biostratigraphic conundrum from the North China Platform: Precambrian Research. 1997;84:197–220. [Google Scholar]
- 44.Yin LM. 1997. Acanthomorphicacritarchs from Meso-Neoproterozoic shales of the Ruyang Group, Shanxi, China: Review of Palaeobotany and Palynology. 1997;98:15–25. [Google Scholar]
- 45.Singh VK, Sharma M. Morphologically complex organic-walled microfossils from the late Palaeoproterozoic–early Mesoproterozoic Chitrakut Formation, Vindhyan Supergroup, central India and their implications on the antiquity of eukaryotes: Palaeontological Society of India. Journal. 2014;59:89–102. [Google Scholar]
- 46.Javuax EJ, et al. Morphological and ecological complexity in early eukaryotic ecosystems. Nature. 2001;412:66–69. doi: 10.1038/35083562. [DOI] [PubMed] [Google Scholar]
- 47.Zhu, S. et al. Decimetre-scale multicellular eukaryotes from the 1.56-billion-year-old Gaoyuzhuang Formation in North China. Nature Communications, 10.1038/ncomms11500 (2016). [DOI] [PMC free article] [PubMed]
- 48.Adam ZR, Skidmore ML, Mogk DW. Paleoenvironmental implications of an expanded microfossil assemblage from the Chamberlain Formation, Belt Supergroup, Montana: The Geological Society of America Special Paper. 2016;522:20. [Google Scholar]
- 49.Vorob’eva NG, Sergeev VN, Yu P. Kotuikan Formation assemblage: a diverse organic-walled microbiota in the Mesoproterozoic Anabar succession, northern Siberia: Precambrian Research. 2015;256:201–222. [Google Scholar]
- 50.Diekmann Y, Pereira-Leal JB. Evolution of intracellular compartmentalization Biochemical Journal. 2013;449:319–331. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, et al. Sufficient oxygen for animal respiration 1,400 million years ago. Proc Natl AcadSci USA. 2016;113(7):1731–1736. doi: 10.1073/pnas.1523449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang S, Kendall B, Lu X, Zhang F, Zhang W. Uranium isotope compositions of mid-Proterozoic black shales: Evidence for an episode of increased ocean oxygenation at 1.36 Ga and evaluation of the effect of post-depositional hydrothermal fluid flow. Precambrian Research. 2017;298:187–201. doi: 10.1016/j.precamres.2017.06.016. [DOI] [Google Scholar]
- 53.Nagovitsin K. Tappania-bearing association of the Siberian platform: Biodiversity, stratigraphic position and geochronological constraints, Precambrian Research. 2009;173:137–145. [Google Scholar]
- 54.Sergeev VN, Knoll AH, Vorob’eva NG, Sergeeva ND. Microfossils from the lower Mesoproterozoic Kaltasy Formation, East European Platform. Precambrian Research. 2016;278:87–107. doi: 10.1016/j.precamres.2016.03.015. [DOI] [Google Scholar]
- 55.Prasad B, Asher R. Acritarch biostratigraphy and lithostratigraphic classification of Proterozoic and Lower Paleozoic sediments (Pre-unconformity sequence) of Ganga Basin, India. Precambrian Research. 2001;291:63–82. [Google Scholar]
- 56.Butterfield NJ. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology. 2000;26:386–404. doi: 10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. [DOI] [Google Scholar]
- 57.Loron, C. et al. Tonian (Neoproterozoic) eukaryotic and prokaryotic organic-walled microfossils from the upper Visingsö Group, Sweden, Palynology, 10.1080/01916122.2017.1335656 (2017).
- 58.Baludikay BK, Storme JY, François C, Baudet D, Javaux EJ. 2016. A diverse and exquisitely preserved organic-walled microfossil assemblage from the Meso- Neoproterozoic Mbuji-MayiSupergroup (Democratic Republic of Congo) and implications for Proterozoic biostratigraphy. Precambrian Research. 2016;281:166–184. doi: 10.1016/j.precamres.2016.05.017. [DOI] [Google Scholar]
- 59.Beghin J, et al. Microfossils from the late Mesoproterozoic – early Neoproterozoic Atar/El Mreïti Group, Taoudeni Basin, Mauritania, northwestern Africa. Precambrian Res. 2017;291:63–82. doi: 10.1016/j.precamres.2017.01.009. [DOI] [Google Scholar]
- 60.Amoutzias G, et al. One billion years of bZIP transcription factor evolution: conservation and change in dimerization and DNA- binding site specificity: Molecular Biology and Evolution. 2007;24:827–835. doi: 10.1093/molbev/msl211. [DOI] [PubMed] [Google Scholar]
- 61.Lai S, Safaei J, Pelech S. Evolutionary ancestry of eukaryotic protein kinases and choline kinases: The Journal of Biological Chemistry. 2016;291:5199–5205. doi: 10.1074/jbc.M115.691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lücking R, Huhndorf S, Pfister DH, Plata ER, Lumbsch HT. Fungi evolved right on track. Mycologia. 2009;101(6):810–822. doi: 10.3852/09-016. [DOI] [PubMed] [Google Scholar]
- 63.Sagan L. On the origin of mitosing cells: Journal of Theoretical Biology, v. 1967;14:225–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 64.Hoffmann AA, Hercus MJ. Environmental stress as an evolutionary force. BioScience. 2000;50:217–226. doi: 10.1641/0006-3568(2000)050[0217:ESAAEF]2.3.CO;2. [DOI] [Google Scholar]
- 65.Hoffmann A. A., Parsons P. A. Extreme Environmental Change and Evolution. Cambridge (UK): Cambridge University Press (1997).
- 66.Rosenberg SM, Shee C, Frisch RL, Hastings PJ. Stress-induced mutation via DNA breaks in Escherichia coli: A molecular mechanism with implications for evolution and medicine: Bioessays. 2012;34(10):885–892. doi: 10.1002/bies.201200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lenton TM, Boyle RA, Poulton SW, Shields-Zhou GA, Butterfield NJ. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era: Nature Geoscience. 2014;7:257–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files)


