Abstract
After treatment for a non-functioning pituitary adenoma (NFA) health-related quality of life (HR-QoL) improves considerably. However, the literature about the normalization of HR-QoL after treatment is inconclusive. Some researchers described a persistently decreased HR-QoL compared to reference data, while others did not. Considering this variety in observed HR-QoL outcomes, the aim of the present review was to provide a literature overview of health outcomes in patients with a NFA, using a conceptual HR-QoL model. A concrete conceptualization of the health outcomes of patients with a NFA can be helpful to understand the observed variety in HR-QoL outcomes and to improve clinical care and guidance of these patients. For this conceptualization, the Wilson and Cleary model was used. This model has a biopsychosocial character and has been validated in several patient populations. In the present review, health outcomes of patients with a NFA were described at each stage of the model e.g. biological and physiological variables, symptom status, functional status, general health perceptions and overall HR-QoL. The Wilson–Cleary model elucidates that elements at each stage of the model can contribute to the impairment in HR-QoL of patients with a NFA, which explains the reported variety in the literature. Furthermore, by applying the model, potential interventions targeting these elements can be identified. While optimal biomedical treatment has always been the focus, it is clearly not sufficient for good HR-QoL in patients with a NFA. Further improvement of HR-QoL should be supported by a pituitary specific care trajectory, including psychosocial care (e.g. self-management training), to beneficially affect characteristics of the patient and the (healthcare) environment, with the utmost goal to optimize HR-QoL in patients after treatment.
Keywords: Quality of life, QoL, Health-related quality of life, HR-QoL, Well-being, Patient reported outcome, Pituitary adenoma, Non-functioning pituitary adenoma, Wilson–Cleary model
Background
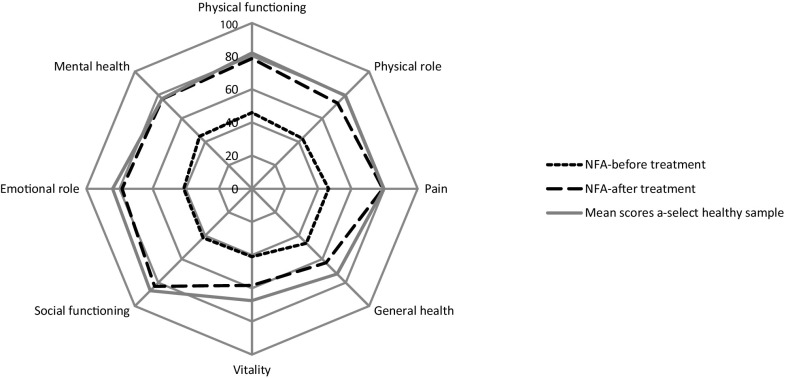
Pituitary adenomas are benign tumours, with an estimated prevalence of 78–94 cases per 100,000 individuals, and an incidence of four cases per 100,000 individuals [1]. Ten percent of all pituitary adenomas are non-functional adenomas (NFAs) [2]. NFAs commonly occur during adulthood with a median age at diagnosis of 51.5 years (range 19–79 years) [3]. At time of diagnosis, tumour size is relatively large compared to functioning tumours, since hormone excesses are absent, and therefore mainly manifest via compression of surrounding tissues, predominantly compression on the optic chiasm. Primary treatment consists of surgical resection of the tumour to relieve mass effects. Conventional radiotherapy may be used in case of tumour growth or when surgical resection is not an option due to the localization [2]. After treatment, patient reported health-related quality of life (HR-QoL) improves considerably (Fig. 1) [4], however, the evidence about normalization of HR-QoL is inconclusive. While some researchers described a persistent decreased HR-QoL compared to healthy controls and reference data [5, 6], others did not [7, 8].
Fig. 1.
HR-QoL scores of patients with a NFA (Short Form 36 scores), figure derived from [4]. Higher scores indicate better HR-QoL
Furthermore, the cause of the persistent impairments in HR-QoL seems to be multifactorial and several contributing factors have been reported, including visual function, type of surgery (craniotomy vs. transsphenoidal), hypopituitarism, and the need for hormone replacement therapy [4].
The aim of the present review was to provide an overview of health outcomes of patients with a NFA using a conceptual HR-QoL model i.e. the Wilson and Cleary model [9]. A concrete conceptualization of the health outcomes of patients with a NFA will be helpful in the understanding of the observed variety in HR-QoL outcomes, the identification of potential interventions, and can be used for further improvement of the clinical care trajectory and somatic and psychosocial guidance of these patients.
Health-related quality of life
Over the past decade, alongside the improved treatment options, the scope of relevant outcomes has expanded from primary outcomes, such as mortality and morbidity, towards the evaluation of functional status and HR-QoL. Although it is established that HR-QoL should cover physical-, psychological-, and social well-being (in accordance with the biopsychosocial model) [10], a single concrete definition of HR-QoL is lacking, which results in major challenges for the evaluation and interpretation of HR-QoL [11]. A commonly used definition is that HR-QoL is “the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient” [12]. For the assessment of HR-QoL several measures have been developed and validated, and it is recommended that a generic measure (covering general HR-QoL domains) is combined with a disease-specific measure (covering HR-QoL aspects relevant for a specific disease) [11]. Unfortunately, a disease-specific HR-QoL questionnaire for NFA is currently lacking.
The Wilson–Cleary model of HR-QoL
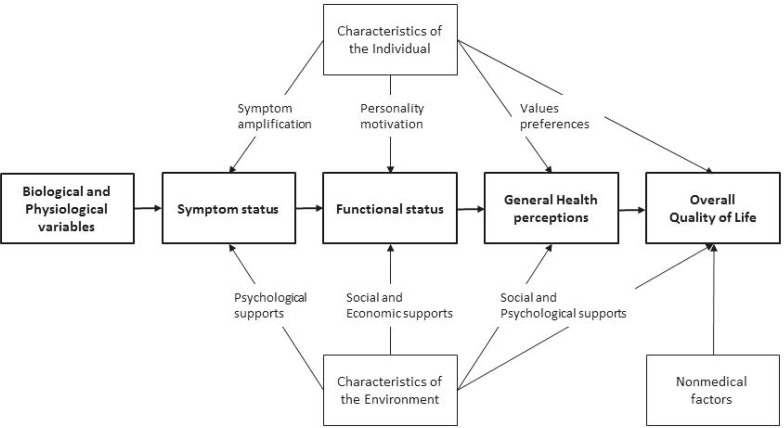
A model that is frequently used to conceptualize HR-QoL, which validity is supported by empirical evidence over the years [13], and has been widely applied to different patient populations [14–16], is the conceptual model proposed by Wilson and Cleary (1995) [9]. This model establishes the biopsychosocial model [10] by integrating the clinical paradigm (i.e. the biomedical paradigm), and the quality of life model (i.e. social science paradigm). Where the biomedical paradigm focusses on biological, physiological, and clinical outcomes, the social science paradigm focusses on dimensions of functioning and overall well-being. The Wilson and Cleary model states that health can be considered as a continuum of increasing biological, psychological and social complexity, with pure biological measures on the left side of the model, and measures of general health perceptions on the right (Fig. 2). It clarifies the proposed dominant causal relationships (bold) and mediating factors. From left to right, it goes from cell-level to the individual, to the interaction of the individual in its social context. The arrows used in Fig. 2 do not imply that there are no reciprocal relations, just as the absence of arrows does not imply that there are no such relationships. Furthermore, it should be noted that the relation between symptom status and biological and physiological variables is rather complex. In other words, biological and physiological variables can be profoundly abnormal without the patient perceiving symptoms, or the other way around. In the next paragraphs the Wilson and Cleary model will be elaborated for patients with a NFA.
Fig. 2.
Wilson–Cleary model of HR-QoL [9]. Biological and physiological variables: function of cell, organs, and organ systems e.g. diagnoses, laboratory values, measures of physiological function, and physical examination findings. Symptom status: a patient’s perception of an abnormal physical, emotional or cognitive state. Functional status: ability of the individual to perform particular tasks. The main domains of functioning are physical functioning, social functioning, role function, and psychological function. General health perceptions: subjective rating of health, and represent and integrates all the previous health concepts
Biological and physiological variables
Pituitary dysfunction may occur in all pituitary adenomas due to a variety of causes e.g. mass effect of the tumour, surgical treatment, or radiotherapy. Severe hormone deficits, (pan)hypopituitarism, is diagnosed by blood sampling for gonadotropin, thyroid stimulating hormone, and prolactin, and dynamic stimulation tests for adrenocorticotrope hormone (ACTH), cortisol and growth hormone, and measurement of urine production for vasopressin deficiency [17]. Mild hypopituitarism can be difficult to diagnose, due to individual set-points, hormone sensitivity, and circadian variability. Nevertheless, also mild hypopituitarism may affect end organ function. Therefore, the majority of the patients with hypopituitarism need lifelong hormone replacement therapy, aiming to mimic the physiology of end organ hormones as good as possible. Replacement therapy for adrenal insufficiency is of particular relevance, since too low cortisol levels can lead to a potentially life threatening acute adrenal crisis (i.e. Addison’s crisis). Contrary to this, when replacement therapy exceeds supra-physiological cortisol levels, it can result in Cushing’s syndrome like symptoms. Therefore, adequate replacement therapy in adrenal insufficiency as well as, adaptation of the dose during stress, is crucial [18]. In clinical practice, endocrine diseases are followed by evaluating clinical signs and hormone measurements. Serum, plasma, salivary, or urinary hormone concentrations are currently the best tools for clinicians to classify disease status in (chronic) care. It has been acknowledged that the currently available physiological measures do not always reliably represent the clinical situation. The assessment of cortisol levels in scalp hair is a relatively new method to assess cortisol exposure over longer time periods and has been evaluated in patients with primary and secondary adrenal insufficiency [19]. Furthermore, it was examined whether hair cortisol levels correlated with patient reported HR-QoL, and it appeared that HR-QoL correlates slightly with hair cortisol levels [20]. These results are not surprising, considering the Wilson–Cleary model with biological and physiological variables on the one end, and HR-QoL on the other end with patient- and environmental characteristics influencing this continuum. These observations support the idea that HR-QoL is not only determined by biological disease status, but by a multidimensional underlying mechanism.
Symptom status
When changes in biological and physiological variables occur, an individual might perceive this via symptoms. Symptom status is defined by Wilson and Cleary as a patient’s perception of an abnormal physical, emotional, or cognitive state [9]. As was mentioned previously, NFAs are usually relatively large at time of diagnosis, giving either compression on the pituitary or the optic chiasm, resulting in headaches, hypopituitarism, visual loss, third nerve palsy, pituitary apoplexy, tiredness, decreased libido, and sometimes even galactorrhoea [3]. These symptoms tend to improve after surgery, however, extensive longitudinal literature of perioperative HR-QoL is limited. Wolf et al. demonstrated that headache severity and vision related HR-QoL improved significantly up to 6 months after transsphenoidal surgery [21]. Furthermore, patients may suffer from impaired olfactory function as a complication of the transsphenoidal surgery. Little et al. showed an initial decrease of sinonasal HR-QoL after (both microscopic and endoscopic) surgery, which improved at later follow-up [22]. Wang et al. demonstrated a decrease in the ability to detect odours up to 4 months after surgery [23]. Although symptoms improve after biomedical treatment, persistent symptoms are reported after long-term remission. During focus group conversations with patients in a chronic state of their disease, patients reported physical pain, sleeping problems, changes in physical appearance (i.e. weight changes), cognitive problems (i.e. problems in concentration, short-term memory, and executive functioning), decreased libido, physical sexual dysfunction, depressive symptoms, melancholy, mood swings, worries, increased sensitivity to stress, fear of tumour recurrence, decreased self-esteem, loneliness, anger, difficulties in communication about the disease, and a lack of empathy from the environment. The reported sleep problems were characterized by sleeping in blocks of 2–3 h [24]. Sleep characteristics have also been quantitatively examined, showing sleep alterations in patients treated for a NFA, including decreased subjective sleep quality, disturbed distribution of sleep stages and disturbances in diurnal rhythmicity [6, 25]. Although it can be postulated that these sleeping problems can be explained by imperfections in hormone replacement therapy (i.e. hydrocortisone replacement) [26], there is increasing evidence that these problems are caused by hypothalamic dysfunction [27]. Joustra et al. examined sleep characteristics in patients treated for a NFA and patients with primary adrenal insufficiency treated with hydrocortisone replacement therapy and demonstrated that patients with primary adrenal insufficiency have normal sleep characteristics in contrast to patients with a NFA. These results provided evidence that sleeping problems might be caused by hypothalamic dysfunction [28]. Furthermore, sleep disturbances and daytime sleepiness were also associated with increased impairment in HR-QoL [6, 29].
Functional status
This refers to the ability of the patient to perform particular defined tasks [9]. The symptom status largely determines whether patients perceive issues in their functioning. In accordance, the previously described symptoms result into impairments in several functional domains. During the focus group conversations patients reported problems in physical functioning, cognitive functioning, sexual functioning, psychological functioning, and social functioning. For instance, work related problems, such as diminished ability to function, to cooperate and to concentrate. As a result patients lost their job or were (partly) rejected [24]. The cognitive complaints reported by patients have also been examined through neuropsychological tests. Previous studies demonstrated that patients treated for NFA had a worse performance on verbal memory and executive functioning compared to healthy matched controls and references values [30, 31]. Interestingly, some reported the negative effect of additional radiotherapy on cognitive functioning [17, 30, 32], while others did not [33, 34].
Characteristics of the individual
These individual characteristics (or patient characteristics) as formulated in the Wilson–Cleary model cover factors such as personality, motivation, values, and preferences. Patients’ preferences or values refer to the value patients attach to a particular consequence of their disease. For instance, a patient can experience a symptom as a burden, while the same symptom does not bother another patient. The way patients perceive their illness and its treatment are also known as ‘illness perceptions’ and ‘beliefs about medication’.
Illness perceptions and beliefs about medication
Illness perceptions and beliefs about medication are formulated by the extended Common-Sense Model of Self-Regulation (CSM) and can be categorised into values and preference in the Wilson–Cleary model. These preferences and values play an important role at several points of the Wilson–Cleary model and are particularly important in the understanding of general health perceptions and overall HR-QoL, which is in accordance with the extended CSM, since this model also states that illness perceptions and beliefs about medication correlate with HR-QoL [35]. During the focus group conversations patients reported negative illness perceptions, such as the chronic time course of their disease, and concerns about potential side effects of their medication (i.e. hydrocortisone) [24].
Coping strategies
Furthermore, following the extended CSM, illness perceptions and beliefs about medicines influence coping behaviour. During the focus group conversations patients reported less efficient coping strategies, such as withdrawal, and overdoing activities [24]. Coping strategies were also quantitatively assessed by Tiemensma et al. as they demonstrated that patients with pituitary disease use less effective coping strategies compared to an a-select sample of the Dutch population, including performing less active coping, seeking less social support, and using more avoidant coping strategies [36].
Personality
A changed personality, another characteristic of the individual, is considered a problem by patients. Sievers et al. quantitatively examined personality traits in patients with a NFA, and demonstrated that compared to a normal population control group, patients with a NFA reported more neuroticism, social desirability, anticipatory worries, pessimism, fear of uncertainty, fatigability, and asthenia [37]. Individual demographic characteristics have also been found to play a role in HR-QoL in patients with a NFA, since female sex and older age were found to negatively influence HR-QoL [5, 7, 33].
Characteristics of the environment
Economical-, psychological-, and social support, are environmental characteristics. The latter two play an important role in general health perception and overall quality of life.
During the focus group conversations patients reported unmet needs regarding care and guidance they had perceived. For instance, they would have received more information about adverse effects of medication, physical-, psychological-, and cognitive complaints and issues regarding sexual functioning. Furthermore, they would have preferred more recognition for certain complaints. These unmet needs can be categorised into characteristics of the individual (i.e. patient characteristics), since they can be influenced by personal factors. On the other hand, unmet needs can also be influenced by characteristics of the environment (e.g. availability of healthcare facilities). For example, patients reported dissatisfaction with other aspects of medical care i.e. stress-management training, lifestyle recommendations, physiotherapists, dietitians, medical sports experts, and psychologists. For instance, these unmet needs can be caused by limitations in economic support or inadequate referrals. Some types of support (e.g. psychological-, social support) are less well developed for a rare disease such as pituitary disease compared to more prevalent (chronic) diseases.
Tools to meet unmet healthcare needs
Recently, a disease-specific patient reported outcome measure (PROM) was developed and validated by our research group, that assesses to which extent patients with pituitary disease are bothered by certain complaints, as well as their needs for support from healthcare professionals, i.e. the Leiden Bother and Needs Questionnaire for Pituitary disease (LBNQ-Pituitary)). This PROM covers five subscales i.e. mood problems, negative illness perceptions, issues in sexual functioning, physical and cognitive complaints, issues in social functioning [38]. This PROM can help healthcare professionals to address the unmet needs experienced by patients.
Besides professional environmental factors (i.e. healthcare facilities), there are also personal environmental factors. Often the single most important person in a patient’s social network is their spouse or partner. Focus group conversations with partners of patients with a pituitary condition revealed that partners were worried about the complaints of the pituitary disease, had negative beliefs about medication, and perceived coping challenges, relationship issues, social issues, and unmet needs regarding care [39]. These observations clearly demonstrate that chronic care for patients with pituitary disease is not limited to just the patient. In order to support patients and their partners in coping with the consequences of the pituitary disease, a self-management program was developed for patients with pituitary disease and their potential partners i.e. Patient and Partner Education Program for Pituitary disease (PPEP-Pituitary). This program aims to (at least partly) fulfil the unmet needs regarding support for psychological and social issues. PPEP-Pituitary was based on a standardized Patient and Partner Education Program initially developed for patients (and partners) with Parkinson’s disease [40]. A multicenter randomized-controlled trial revealed that patients reported more self-efficacy after PPEP-Pituitary which was still present after 6 months. Self-efficacy is described in the ‘Social Cognitive Theory’ of Bandura [41] and is defined as the person’s beliefs in his or her own capabilities and skills to perform a certain action, in a certain situation. Following this theory, behavior is directly influenced by goals and self-efficacy beliefs. In accordance, several studies showed that self-efficacy beliefs influences self-management behavior [42, 43]. Patients also reported to be less bothered by mood problems directly after PPEP-Pituitary, however this returned to baseline levels 6 months later. Partners reported an increase in vitality, a decrease in depressive symptoms and an increase in treatment control after PPEP-Pituitary. This persisted at follow up after 6 months [44]. It can be postulated that offering this program as standard clinical care will improve the quality of the (healthcare) environment, and ultimately the patient and partner reported HR-QoL.
General health perceptions and HR-QoL
In accordance to the Wilson–Cleary model, the domains described in the preceding paragraphs all contribute to patient perceived HR-QoL. This increase in interest in HR-QoL has led to an increase in the number of HR-QoL studies in patients with a NFA and these studies show some diversity regarding HR-QoL outcomes. Johnson and colleagues reported HR-QoL impairments in patients with an untreated NFA, especially in physical and mental functioning during active disease [45]. Some confirmed this decreased HR-QoL in patients treated for a NFA compared to reference values and healthy controls [5, 6], however others did not report any differences [7, 8, 46]. Furthermore, some studies demonstrated the negative effect of tumour recurrence [7], hypopituitarism [5, 47] and radiotherapy [48] on HR-QoL, while other did not (pituitary deficiency [46], radiotherapy [33, 46]). In addition, no differences in HR-QoL were found between patients surgically treated for a NFA and patients treated with mastoid surgery [48]. No differences were found while comparing patients with growth hormone deficiency (GHD) due to a NFA compared to traumatic brain injury [49, 50]. Male patients with GHD due to a NFA, compared to patients with GHD due to a craniopharyngioma, reported a better HR-QoL, whereas female patients with a NFA reported a worse HR-QoL [51]. Intervention studies reported that HR-QoL of patients with a NFA improved after transsphenoidal surgery [47, 52]. Furthermore, patients treated with craniotomy reported more HR-QoL impairments compared to patients treated with transsphenoidal surgery [8]. There have been several systematic reviews on endoscopic and microscopic transsphenoidal surgery, describing comparable or better clinical results after endocopic surgery [53]. Furthermore, a qualitative study performed by Lwu et al. described that patients perceived less burden after endoscopic surgery compared to microscopic surgery [54]. However, there is limited knowledge about the long-term outcomes of endoscopic vs. microscopic surgery in terms of HR-QoL. Intervention studies about the effect of growth hormone replacement therapy in patients treated for NFA with GHD all reported a positive effect on HR-QoL [49–51, 55–57]. On the other hand, a cross-sectional study of Capatina et al. demonstrated that non-replaced GHD was an independent predictor of a better score in bodily pain, general health perception and energy/vitality [7].
Conclusion
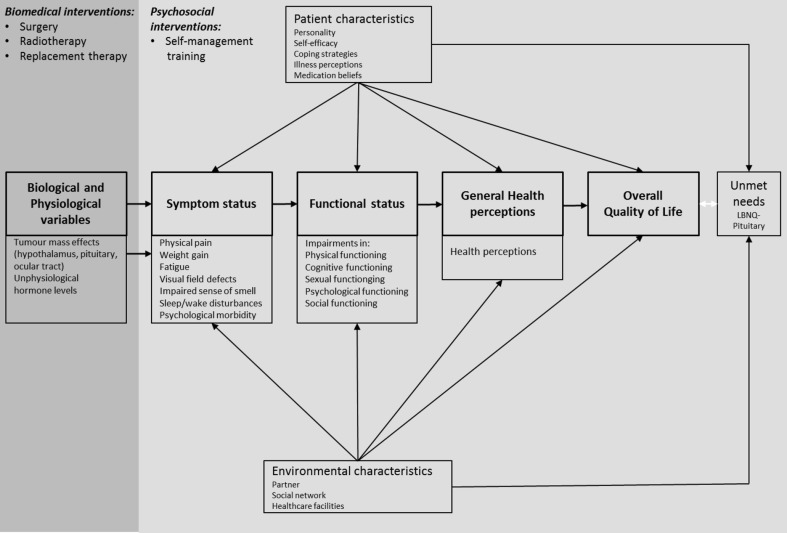
The present review emphasizes that although patients may be in a stable medical condition, health issues are present at each level of the Wilson–Cleary model (Fig. 3). Application of the Wilson–Cleary model to patients with a NFA enables to observe that persistent impairments in HR-QoL might be explained by issues at each stage of this model. This also provides further insight into why there is such a variety in clinical outcomes, and why some patients experience severe problems, while others experience either no or only mild problems. This emphasises that improvement in overall HR-QoL in patients with pituitary disease requires optimal biomedical treatment initiating a cascade of improvement in health outcomes starting with a better symptom status. Nevertheless, this model also clarifies that besides the currently available biomedical interventions (i.e. surgery, radiotherapy, hormone replacement therapy) targeting biological and physiological variables, interventions are needed that pay attention to other (psychosocial) elements of the model e.g. cognitive functioning, sexuality/intimacy, psychological well-being, social functioning, coping behaviour, self-efficacy beliefs, illness perceptions, medication beliefs, quality of the partner relationship, and the social network/support. Therefore, further improvement of HR-QoL should be supported by a pituitary specific care trajectory, including psychosocial care (e.g. self-management training), in order to beneficially affect characteristics of the patient and the (healthcare) environment, with the utmost goal to optimize HR-QoL in patients after treatment for a NFA.
Fig. 3.
Wilson–Cleary model of HR-QoL elaborated for NFA
Acknowledgements
We thank Jan W. Schoones for his contribution to the literature search.
Compliance with ethical standards
Disclosure
The authors have nothing to disclose.
References
- 1.Karavitaki N. Prevalence and incidence of pituitary adenomas. Ann Endocrinol (Paris) 2012;73(2):79–80. doi: 10.1016/j.ando.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Javorsky BR, Aron DC, Findling JW, Tyrrell JB. Hypothalamus & pituitary gland. In: Gardner DG, Shoback D, editors. Greenspan’s basic & clinical endocrinology. Singapore: McGraw-Hill Companies; 2011. pp. 65–114. [Google Scholar]
- 3.Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire UK) Clin Endocrinol (Oxf) 2010;72(3):377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 4.Andela CD, Scharloo M, Pereira AM, Kaptein AA, Biermasz NR. Quality of life (QoL) impairments in patients with a pituitary adenoma: a systematic review of QoL studies. Pituitary. 2015;18(5):752–776. doi: 10.1007/s11102-015-0636-7. [DOI] [PubMed] [Google Scholar]
- 5.Dekkers OM, van der Klaauw AA, Pereira AM, Biermasz NR, Honkoop PJ, Roelfsema F, Smit JW, Romijn JA. Quality of life is decreased after treatment for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2006;91(9):3364–3369. doi: 10.1210/jc.2006-0003. [DOI] [PubMed] [Google Scholar]
- 6.Biermasz NR, Joustra SD, Donga E, van PereiraDuinen AMN, van Dijk M, van der Klaauw AA, Corssmit EPM, Lammers GJ, van Kralingen KW, van Dijk JG, Romijn JA. Patients previously treated for nonfunctioning pituitary macroadenomas have disturbed sleep characteristics, circadian movement rhythm, and subjective sleep quality. J Clin Endocrinol Metab. 2011;96(5):1524–1532. doi: 10.1210/jc.2010-2742. [DOI] [PubMed] [Google Scholar]
- 7.Capatina C, Christodoulides C, Fernandez A, Cudlip S, Grossman AB, Wass JA, Karavitaki N. Current treatment protocols can offer a normal or near-normal quality of life in the majority of patients with non-functioning pituitary adenomas. Clin Endocrinol (Oxf) 2013;78(1):86–93. doi: 10.1111/j.1365-2265.2012.04449.x. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen EH, Lindholm J, Laurberg P, Bjerre P, Christiansen JS, Hagen C, Juul S, Jorgensen J, Kruse A, Stochholm K. Nonfunctioning pituitary adenoma: incidence, causes of death and quality of life in relation to pituitary function. Pituitary. 2007;10(1):67–73. doi: 10.1007/s11102-007-0018-x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- 10.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 11.McGee H, Ring L. Quality of Life. In: French D, Vedhara K, Kaptein AA, Weinman J, editors. Health psychology. Chichester: BPS Blackwell; 2010. pp. 329–344. [Google Scholar]
- 12.Schipper H, Clinch JJ, Olweny CLM. Quality of life studies: definitions and conceptual issues. In: Spilker B, editor. Quality of life assessments in clinical trials. New York: Raven Press, Ltd; 1990. p. 16. [Google Scholar]
- 13.Sousa KH, Kwok OM. Putting Wilson and Cleary to the test: analysis of a HRQOL conceptual model using structural equation modeling. Qual Life Res. 2006;15(4):725–737. doi: 10.1007/s11136-005-3975-4. [DOI] [PubMed] [Google Scholar]
- 14.Kanters TA, Redekop WK, Rutten-Van Molken MP, Kruijshaar ME, Gungor D, van der Ploeg AT, Hakkaart L. A conceptual disease model for adult Pompe disease. Orphanet J Rare Dis. 2015;10:112. doi: 10.1186/s13023-015-0334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahrbanian S, Duquette P, Ahmed S, Mayo NE. Pain acts through fatigue to affect participation in individuals with multiple sclerosis. Qual Life Res. 2016;25(2):477–491. doi: 10.1007/s11136-015-1098-0. [DOI] [PubMed] [Google Scholar]
- 16.Mayo NE, Scott SC, Bayley M, Cheung A, Garland J, Jutai J, Wood-Dauphinee S. Modeling health-related quality of life in people recovering from stroke. Qual Life Res. 2015;24(1):41–53. doi: 10.1007/s11136-013-0605-4. [DOI] [PubMed] [Google Scholar]
- 17.Lecumberri B, Estrada J, Garcia-Uria J, Millan I, Pallardo LF, Caballero L, Lucas T. Neurocognitive long-term impact of two-field conventional radiotherapy in adult patients with operated pituitary adenomas. Pituitary. 2015;18(6):782–795. doi: 10.1007/s11102-015-0653-6. [DOI] [PubMed] [Google Scholar]
- 18.BijnierNET (2016) Stressinstructies. http://www.bijniernet.nl/
- 19.Staufenbiel SM, Andela CD, Manenschijn L, Pereira AM, van Rossum EF, Biermasz NR. Increased hair cortisol concentrations and BMI in patients with pituitary-adrenal disease on hydrocortisone replacement. J Clin Endocrinol Metab. 2015;100(6):2456–2462. doi: 10.1210/jc.2014-4328. [DOI] [PubMed] [Google Scholar]
- 20.Andela CD, Staufenbiel SM, Joustra SD, Pereira AM, van Rossum EF, Biermasz NR. Quality of life in patients with adrenal insufficiency correlates stronger with hydrocortisone dosage, than with long-term systemic cortisol levels. Psychoneuroendocrinology. 2016;72:80–86. doi: 10.1016/j.psyneuen.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Wolf A, Goncalves S, Salehi F, Bird J, Cooper P, Van Uum S, Lee DH, Rotenberg BW, Duggal N. Quantitative evaluation of headache severity before and after endoscopic transsphenoidal surgery for pituitary adenoma. J Neurosurg. 2016;124(6):1627–1633. doi: 10.3171/2015.5.JNS1576. [DOI] [PubMed] [Google Scholar]
- 22.Little AS, Kelly DF, Milligan J, Griffiths C, Prevedello DM, Carrau RL, Rosseau G, Barkhoudarian G, Jahnke H, Chaloner C, Jelinek KL, Chapple K, White WL. Comparison of sinonasal quality of life and health status in patients undergoing microscopic and endoscopic transsphenoidal surgery for pituitary lesions: a prospective cohort study. J Neurosurg. 2015;123(3):799–807. doi: 10.3171/2014.10.JNS14921. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Chen Y, Li J, Wei L, Wang R. Olfactory function and quality of life following microscopic endonasal transsphenoidal pituitary surgery. Medicine. 2015;94(4):e465. doi: 10.1097/MD.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andela CD, Niemeijer ND, Scharloo M, Tiemensma J, Kanagasabapathy S, Pereira AM, Kamminga NG, Kaptein AA, Biermasz NR. Towards a better quality of life (QoL) for patients with pituitary diseases: results from a focus group study exploring QoL. Pituitary. 2014;18(1):86–100. doi: 10.1007/s11102-014-0561-1. [DOI] [PubMed] [Google Scholar]
- 25.Joustra SD, Thijs RD, van den Berg R, van Dijk M, Pereira AM, Lammers GJ, van Someren EJ, Romijn JA, Biermasz NR. Alterations in diurnal rhythmicity in patients treated for nonfunctioning pituitary macroadenoma: a controlled study and literature review. Eur J Endocrinol. 2014;171(2):217–228. doi: 10.1530/EJE-14-0172. [DOI] [PubMed] [Google Scholar]
- 26.Romijn JA, Smit JW, Lamberts SW. Intrinsic imperfections of endocrine replacement therapy. Eur J Endocrinol. 2003;149(2):91–97. doi: 10.1530/eje.0.1490091. [DOI] [PubMed] [Google Scholar]
- 27.Romijn JA. Pituitary diseases and sleep disorders. Current opinion in endocrinology. diabetes obesity. 2016;23(4):345–351. doi: 10.1097/MED.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 28.Joustra SD, Kruijssen E, Verstegen MJ, Pereira AM, Biermasz NR. Determinants of altered sleep-wake rhythmicity in patients treated for nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2014;99(12):4497–4505. doi: 10.1210/jc.2014-2602. [DOI] [PubMed] [Google Scholar]
- 29.van der Klaauw AA, Dekkers OM, Pereira AM, van Kralingen KW, Romijn JA. Increased daytime somnolence despite normal sleep patterns in patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab. 2007;92(10):3898–3903. doi: 10.1210/jc.2007-0944. [DOI] [PubMed] [Google Scholar]
- 30.Brummelman P, Elderson MF, Dullaart RP, van den Bergh AC, Timmer CA, van den Berg G, Koerts J, Tucha O, Wolffenbuttel BH, van Beek AP. Cognitive functioning in patients treated for nonfunctioning pituitary macroadenoma and the effects of pituitary radiotherapy. Clin Endocrinol (Oxf) 2011;74(4):481–487. doi: 10.1111/j.1365-2265.2010.03947.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiemensma J, Kokshoorn NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA, Pereira AM, Romijn JA. Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J Clin Endocrinol Metab. 2010;95(6):2699–2714. doi: 10.1210/jc.2009-2032. [DOI] [PubMed] [Google Scholar]
- 32.Noad R, Narayanan KR, Howlett T, Lincoln NB, Page RC. Evaluation of the effect of radiotherapy for pituitary tumours on cognitive function and quality of life. Clin Oncol (R Coll Radiol) 2004;16(4):233–237. doi: 10.1016/j.clon.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 33.van Beek AP, van den Bergh AC, van den Berg LM, van den Berg G, Keers JC, Langendijk JA, Wolffenbuttel BH. Radiotherapy is not associated with reduced quality of life and cognitive function in patients treated for nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys. 2007;68(4):986–991. doi: 10.1016/j.ijrobp.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Peace KA, Orme SM, Thompson AR, Padayatty S, Ellis AW, Belchetz PE. Cognitive dysfunction in patients treated for pituitary tumours. J Clin Exp Neuropsychol. 1997;19(1):1–6. doi: 10.1080/01688639708403831. [DOI] [PubMed] [Google Scholar]
- 35.Horne R. Treatment perceptions and self-regulation. In: Cameron LD, Leventhal H, editors. The self-regulation of health and illness behaviour. London: Routledge; 2003. pp. 138–183. [Google Scholar]
- 36.Tiemensma J, Kaptein AA, Pereira AM, Smit JW, Romijn JA, Biermasz NR. Coping strategies in patients after treatment for functioning or nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2011;96(4):964–971. doi: 10.1210/jc.2010-2490. [DOI] [PubMed] [Google Scholar]
- 37.Sievers C, Ising M, Pfister H, Dimopoulou C, Schneider HJ, Roemmler J, Schopohl J, Stalla GK. Personality in patients with pituitary adenomas is characterized by increased anxiety-related traits: comparison of 70 acromegalic patients with patients with non-functioning pituitary adenomas and age- and gender-matched controls. Eur J Endocrinol. 2009;160(3):367–373. doi: 10.1530/EJE-08-0896. [DOI] [PubMed] [Google Scholar]
- 38.Andela CD, Scharloo M, Ramondt S, Tiemensma J, Husson O, Llahana S, Pereira AM, Kaptein AA, Kamminga NG, Biermasz NR. The development and validation of the Leiden Bother and Needs Questionnaire for patients with pituitary disease: the LBNQ-Pituitary. Pituitary. 2016;19(3):293–302. doi: 10.1007/s11102-016-0707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andela CD, Tiemensma J, Kaptein AA, Scharloo M, Pereira AM, Kamminga NGA, Biermasz NR. The partner’s perspective of the impact of pituitary disease: looking beyond the patient. J Health Psychol. 2017 doi: 10.1177/1359105317695427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macht M, Gerlich C, Ellgring H, Schradi M, Rusinol AB, Crespo M, Prats A, Viemero V, Lankinen A, Bitti PE, Candini L, Spliethoff-Kamminga N, de Vreugd J, Simons G, Pasqualini MS, Thompson SB, Taba P, Krikmann U, Kanarik E. Patient education in Parkinson’s disease: Formative evaluation of a standardized programme in seven European countries. Patient Educ Couns. 2007;65(2):245–252. doi: 10.1016/j.pec.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice-Hall, Inc.; 1986. [Google Scholar]
- 42.Abubakari AR, Cousins R, Thomas C, Sharma D, Naderali EK. Sociodemographic and clinical predictors of self-management among people with poorly controlled type 1 and type 2 diabetes: the role of illness perceptions and self-efficacy. J Diabetes Res. 2016 doi: 10.1155/2016/6708164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilski M, Tasiemski T. Illness perception, treatment beliefs, self-esteem, and self-efficacy as correlates of self-management in multiple sclerosis. Acta Neurol Scand. 2015;133(5):338–345. doi: 10.1111/ane.12465. [DOI] [PubMed] [Google Scholar]
- 44.Andela CD, Repping-Wuts H, Stikkelbroeck NMML, Pronk MC, Tiemensma J, Hermus AR, Kaptein AA, Pereira AM, Kamminga NGA, Biermasz NR. Enhanced self-efficacy after a self-management programme in pituitary disease: a randomized controlled trial. Eur J Endocrinol. 2017;177(1):59–72. doi: 10.1530/EJE-16-1015. [DOI] [PubMed] [Google Scholar]
- 45.Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary. 2003;6(2):81–87. doi: 10.1023/B:PITU.0000004798.27230.ed. [DOI] [PubMed] [Google Scholar]
- 46.Raappana A, Pirila T, Ebeling T, Salmela P, Sintonen H, Koivukangas J. Long-term health-related quality of life of surgically treated pituitary adenoma patients: a descriptive study. ISRN Endocrinol. 2012 doi: 10.5402/2012/675310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanemura E, Nagatani T, Aimi Y, Kishida Y, Takeuchi K, Wakabayashi T. Quality of life in nonfunctioning pituitary macroadenoma patients before and after surgical treatment. Acta Neurochir (Wien) 2012;154(10):1895–1902. doi: 10.1007/s00701-012-1473-3. [DOI] [PubMed] [Google Scholar]
- 48.Page RC, Hammersley MS, Burke CW, Wass JA. An account of the quality of life of patients after treatment for non-functioning pituitary tumours. Clin Endocrinol (Oxf) 1997;46(4):401–406. doi: 10.1046/j.1365-2265.1997.1400957.x. [DOI] [PubMed] [Google Scholar]
- 49.Casanueva FF, Leal A, Koltowska-Haggstrom M, Jonsson P, Goth MI. Traumatic brain injury as a relevant cause of growth hormone deficiency in adults: a KIMS-based study 54. Arch Phys Med Rehabil. 2005;86(3):463–468. doi: 10.1016/j.apmr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Kreitschmann-Andermahr I, Poll EM, Reineke A, Gilsbach JM, Brabant G, Buchfelder M, Fassbender W, Faust M, Kann PH, Wallaschofski H. Growth hormone deficient patients after traumatic brain injury–baseline characteristics and benefits after growth hormone replacement–an analysis of the German KIMS database. Growth Hormon IGF Res. 2008;18(6):472–478. doi: 10.1016/j.ghir.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Verhelst J, Kendall-Taylor P, Erfurth EM, Price DA, Geffner M, Koltowska-Haggstrom M, Jonsson PJ, Wilton P, Abs R. Baseline characteristics and response to 2 years of growth hormone (GH) replacement of hypopituitary patients with GH deficiency due to adult-onset craniopharyngioma in comparison with patients with nonfunctioning pituitary adenoma: data from KIMS (Pfizer International Metabolic Database) J Clin Endocrinol Metab. 2005;90(8):4636–4643. doi: 10.1210/jc.2005-0185. [DOI] [PubMed] [Google Scholar]
- 52.Milian M, Honegger J, Gerlach C, Psaras T. Health-related quality of life and psychiatric symptoms improve effectively within a short time in patients surgically treated for pituitary tumors–a longitudinal study of 106 patients. Acta Neurochir (Wien) 2013;155(9):1637–1645. doi: 10.1007/s00701-013-1809-7. [DOI] [PubMed] [Google Scholar]
- 53.Li A, Liu W, Cao P, Zheng Y, Bu Z, Zhou T. Endoscopic versus microscopic transsphenoidal surgery in the treatment of pituitary adenoma: a systematic review and meta-analysis. World Neurosurg. 2017;101:236–246. doi: 10.1016/j.wneu.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Lwu S, Edem I, Banton B, Bernstein M, Vescan A, Gentili F, Zadeh G. Quality of life after transsphenoidal pituitary surgery: a qualitative study. Acta Neurochir. 2012;154(10):1917–1922. doi: 10.1007/s00701-012-1455-5. [DOI] [PubMed] [Google Scholar]
- 55.Svensson J, Finer N, Bouloux P, Bevan J, Jonsson B, Mattsson AF, Lundberg M, Harris PE, Koltowska-Haggstrom M, Monson JP. Growth hormone (GH) replacement therapy in GH deficient adults: predictors of one-year metabolic and clinical response. Growth Hormon IGF Res. 2007;17(1):67–76. doi: 10.1016/j.ghir.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Giavoli C, Profka E, Verrua E, Ronchi CL, Ferrante E, Bergamaschi S, Sala E, Malchiodi E, Lania AG, Arosio M, Ambrosi B, Spada A, Beck-Peccoz P. GH replacement improves quality of life and metabolic parameters in cured acromegalic patients with growth hormone deficiency. J Clin Endocrinol Metab. 2012;97(11):3983–3988. doi: 10.1210/jc.2012-2477. [DOI] [PubMed] [Google Scholar]
- 57.Hoybye C, Ragnarsson O, Jonsson PJ, Koltowska-Haggstrom M, Trainer P, Feldt-Rasmussen U, Biller BM. Clinical features of GH deficiency and effects of 3years of GH replacement in adults with controlled Cushing’s disease. Eur J Endocrinol. 2010;162(4):677–684. doi: 10.1530/EJE-09-0836. [DOI] [PubMed] [Google Scholar]