Abstract
There is an urgent need to develop vaccines against pathogenic bacteria. However, this is often hindered by antigenic diversity and difficulties encountered manufacturing membrane proteins. Here we show how to use structure-based design to develop chimeric antigens (ChAs) for subunit vaccines. ChAs are generated against serogroup B Neisseria meningitidis (MenB), the predominant cause of meningococcal disease in wealthy countries. MenB ChAs exploit factor H binding protein (fHbp) as a molecular scaffold to display the immunogenic VR2 epitope from the integral membrane protein PorA. Structural analyses demonstrate fHbp is correctly folded and the PorA VR2 epitope adopts an immunogenic conformation. In mice, immunisation with ChAs generates fHbp and PorA antibodies that recognise the antigens expressed by clinical MenB isolates; these antibody responses correlate with protection against meningococcal disease. Application of ChAs is therefore a potentially powerful approach to develop multivalent subunit vaccines, which can be tailored to circumvent pathogen diversity.
Factor H binding protein (fHbp) and PorA are components of experimental serogroup B N. meningitidis vaccines. Here the authors graft the VR2 loop of PorA onto an fHBp-based scaffold to demonstrate proof-of-principle of a chimeric antigen strategy and vaccination against meningococcal disease.
Introduction
Toxoid and capsule-based vaccines have prevented millions of deaths caused by bacterial pathogens. Toxoid-based vaccines have almost eliminated diphtheria and tetanus in wealthy countries1, while capsule-based vaccines have substantially reduced disease caused by Haemophilus influenzae2, Streptococcus pneumoniae3, and some strains of Neisseria meningitidis4. However, challenges remain in developing vaccines against pathogens for which toxoid and capsule-based vaccines are not feasible. These pathogens include non-typeable strains of H. influenzae and S. pneumoniae2,3, un-encapsulated pathogens such as Neisseria gonorrhoeae and Moraxella catarrhalis5,6 and encapsulated serogroup B N. meningitidis, for which a capsule-based vaccine is not feasible7. Given the rise in the emergence of multi-drug resistant bacteria8, new approaches for vaccine development are required. However, strategies for generating successful vaccines are hampered by pathogen diversity9 and the difficulties associated with presenting epitopes from membrane-embedded surface proteins to the immune system10.
Two main approaches have been used to develop vaccines against serogroup B N. meningitidis; outer membrane vesicle vaccines (OMVV) and recombinant protein subunit vaccines. OMVVs were first developed in the 1980s11–13. The immunodominant antigen in meningococcal OMVVs is PorA14, an abundant outer-membrane porin with eight surface-exposed loops15. Loops one and four are termed variable region 1 and 2 (VR1 and VR2), respectively, as they generate immune responses and are subject to antigenic variation16. The VR2 loop dominates PorA-specific immunity elicited by OMVVs, which offer limited or no cross-protection against strains expressing PorA with a different VR217,18. To broaden coverage, OMVVs containing multiple PorAs have been developed19–21 and selected for the prevalence of PorA sequences in circulating strains21,22. However, OMVVs present complex manufacturing and regulatory issues23. The recombinant subunit vaccines Bexsero and Trumenba contain an important meningococcal antigen, factor H binding protein (fHbp), which is a lipoprotein composed of two β-barrels that tightly bind domains 6 and 7 of human complement factor H (CFH)24–27. fHbp is antigenically variable; databases of genome sequences contain more than 900 different fHbp peptides28, which fall into three variant groups or two subfamilies: V1 (subfamily B), V2 and V3 (both subfamily A)29,30. In general, immunisation with a particular fHbp induces cross-protection against strains that express fHbp belonging to the same, but not a different, variant group, although there can be cross-protection between fHbp variant groups 2 and 3 (subfamily A)31,32. Bexsero contains a single fHbp peptide (V1.1), with two other recombinant antigens as well as an OMV33, while Trumenba is composed solely of two fHbp peptides (V1.55 and V3.45)34. Antigens in Bexsero and Trumenba have exact sequence matches to 36 and 4.8% of serogroup B N. meningitidis disease isolates currently circulating in the UK, respectively28, leading to concerns about their ability to provide broad coverage against an antigenically diverse pathogen.
Here we use structure-based design to generate chimeric antigens (ChAs) against serogroup B N. meningitidis. ChAs exploit fHbp as a molecular scaffold to present the surface-exposed PorA VR2 loop, which is achieved by inserting the VR2 loop into a β-turn region in fHbp. ChAs retain epitopes from both fHbp and PorA, and can elicit functional immune responses against both antigens. We demonstrate that integration of a VR2 loop does not alter the overall architecture of fHbp and that the VR2 loop folds into a conformation recognised by a bactericidal mAb. We provide proof-in-principle that ChAs can be used to display selected epitopes from integral membrane proteins, such as PorA.
Results
Design and construction of ChAs
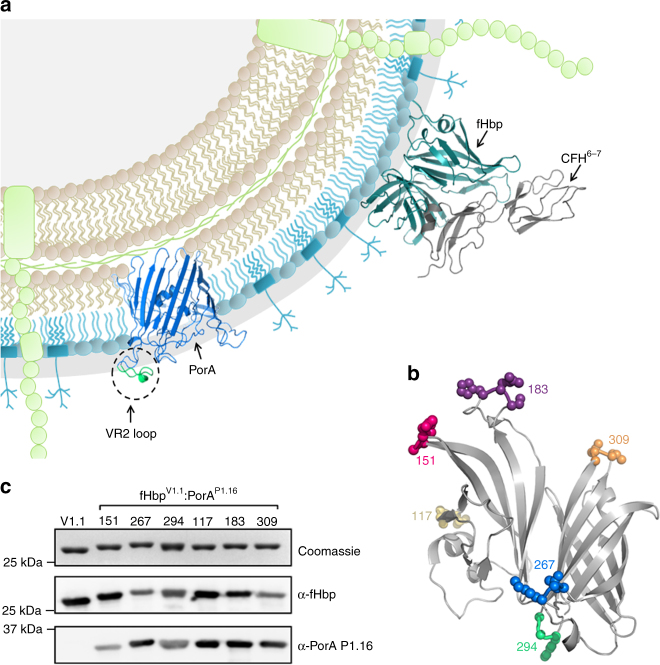
The surface-exposed proteins fHbp and PorA (Fig. 1a) elicit bactericidal antibody responses, which are a correlate of protection against meningococcal disease34. fHbp is a lipoprotein that can be expressed as a soluble protein in Escherichia coli following removal of the N-terminal lipobox motif32. As an isolated extracellular loop, the PorA VR2 is likely soluble when expressed separately from the hydrophobic β-barrel of PorA. We exploited soluble fHbp peptide V1.1 as a molecular scaffold to display the PorA VR2 loop, P1.16 (VR2 P1.16). VR2 P1.16 (YYTKDTNNNLTLVP) was inserted into six different β-turn regions in fHbp. At each VR2 P1.16 insertion site, a single amino acid was deleted from the fHbp scaffold (Fig. 1b). The resulting ChAs were named according to a scheme whereby fHbpV1.1:PorA151/P1.16 denotes fHbp V1.1 with VR2 P1.16 inserted in the place of fHbp residue 151 (Supplementary Table 1). ChAs all express to high levels in E. coli and are purified by nickel affinity chromatography. Western blot analyses confirm all ChAs retain epitopes recognised by an α-P1.16 mAb and α-fHbp pAbs (Fig. 1c).
Fig. 1.
Structure-based design of ChAs. a Schematic of meningococcal cell surface, depicting the key surface-exposed antigens, fHbp and PorA. b Location of fHbp residues replaced with VR2 P1.16. c Analysis of recombinant ChAs by SDS-PAGE and western blot. Immunoblots are probed with α-V1.1 fHbp pAb and α-PorA P1.16 mAb. Complete gel and western blots are shown in Supplementary Figure 5
ChAs are stable and can bind CFH
Stability of an antigen is an important property of a vaccine, and insertion of PorA epitopes might disrupt the overall structure of the ChA scaffold. Therefore, we determined the thermal stability of ChAs by differential scanning calorimetry (DSC, Table 1 and Supplementary Figure 1A). Insertion of VR2 P1.16 into the N- or C-terminal β-barrel of fHbp decreased the thermal stability of that β-barrel by 1.0–15.5 °C, with little or no effect on the other β-barrel. Overall, the lowest measured melting temperature (Tm) of any β-barrel was 60.5 °C, which is considerably higher than the N-terminal Tm of V3.45 (41 °C), one of the fHbp peptides in Trumenba® (Table 1).
Table 1.
ChA stability and affinity for CFH6/7
| Melting temperature (°C) | Affinity for CFH6/7 | ||
|---|---|---|---|
| Protein | N-terminal | C-Terminal | Kd (nM) |
| fHbp V1.1 | 69.8 | 87.9 | 3.6 ± 0.4 |
| fHbpV1.1:PorA151/P1.16 | 60.5 | 87.3 | 2.3 ± 0.2 |
| fHbpV1.1:PorA267/P1.16 | 68.4 | 75.7 | 28 ± 3.0 |
| fHbpV1.1:PorA294/P1.16 | 61.7 | 72.4 | 2.7 ± 0.4 |
| fHbpV1.1:PorA117/P1.16 | 65.0 | 87.9 | 2.3 ± 0.8 |
| fHbpV1.1:PorA183/P1.16 | 68.8 | 88.0 | NBD |
| fHbpV1.1:PorA309/P1.16 | 69.0 | 76.9 | 5.5 ± 0.6 |
| fHbp V1.4 | 64.4 | 89.4 | – |
| fHbp V3.45 | 41.0 | 83.0 | – |
| fHbpV1.4:PorA151/P1.1.10_1 | 54.0 | 89.0 | – |
| fHbpV1.4:PorA151/P1.14 | 55.0 | 88.0 | – |
| fHbpV1.4:PorA151/P1.15 | 55.0 | 89.0 | – |
| fHbpV3.45:PorA158/P1.4 | 40.0 | 81.0 | – |
| fHbpV3.45:PorA158/P1.9 | 39.0 | 80.0 | – |
Kd dissociation constant of binding to CFH, NDB no binding detected
A key property of fHbp is its ability to bind CFH24–26 (Supplementary Figure 2A). Therefore, surface plasmon resonance (SPR) was used to determine the affinity of each ChA for domains 6 and 7 of CFH (CFH6/7, Table 1 and Supplementary Figure 3). Similar to wild-type fHbp, most ChAs bind CFH6/7 at high affinity, indicating the fHbp scaffold retains its function. The exceptions were fHbpV1.1:PorA183/P1.16, to which there was no detectable CFH6/7 binding, and fHbpV1.1:PorA267/P1.16, to which CFH6/7 binding was reduced by approximately eight-fold. In these two ChAs, VR2 P1.16 is situated in the fHbp/CFH interface (Supplementary Figure 2B), potentially inhibiting CFH6/7 binding.
fHbp:PorA ChAs generate protective immune responses
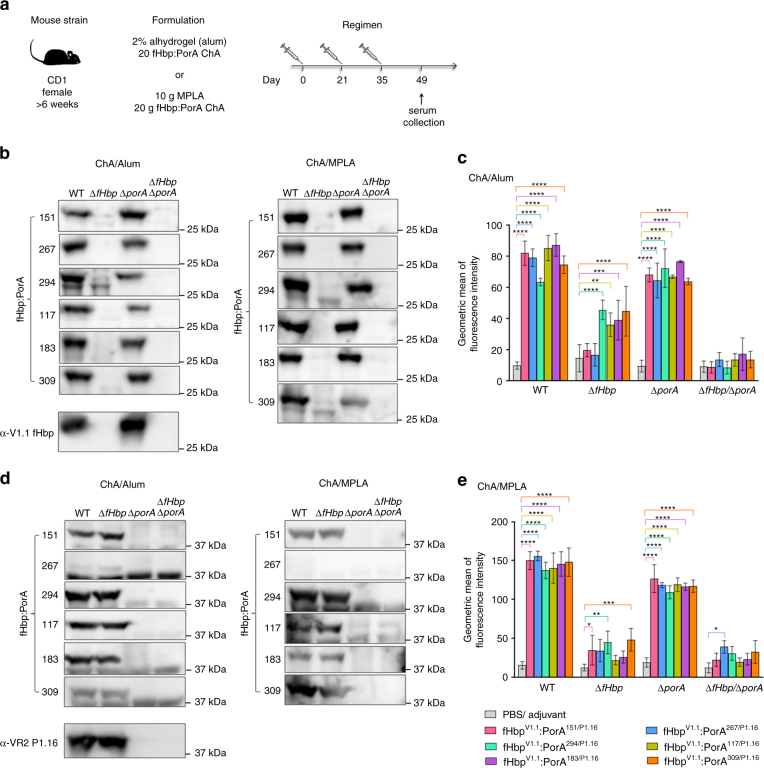
To examine the ability of ChAs to elicit immune responses, we immunised groups of CD1 mice with each ChA using alum or monophospholipid A (MPLA) as the adjuvant (Fig. 2a). Post immunisation, sera were obtained from mice and pooled. Immune responses were assessed against N. meningitidis H44/76, a serogroup B strain which expresses fHbp V1.1 and PorA VR2 P1.16. We constructed isogenic strains to define immune responses directed against fHbp (H44/76∆porA) and PorA (H44/76∆fHbp), as well as a H44/76∆fHbp∆porA control. Western blot analyses of lysates from these strains demonstrate that all ChAs elicited antibodies that recognise both fHbp and PorA (Fig. 2b, d), with the exception of fHbpV1.1:PorA267/P1.16/MPLA, which generated sera that only recognised fHbp (Fig. 2d).
Fig. 2.
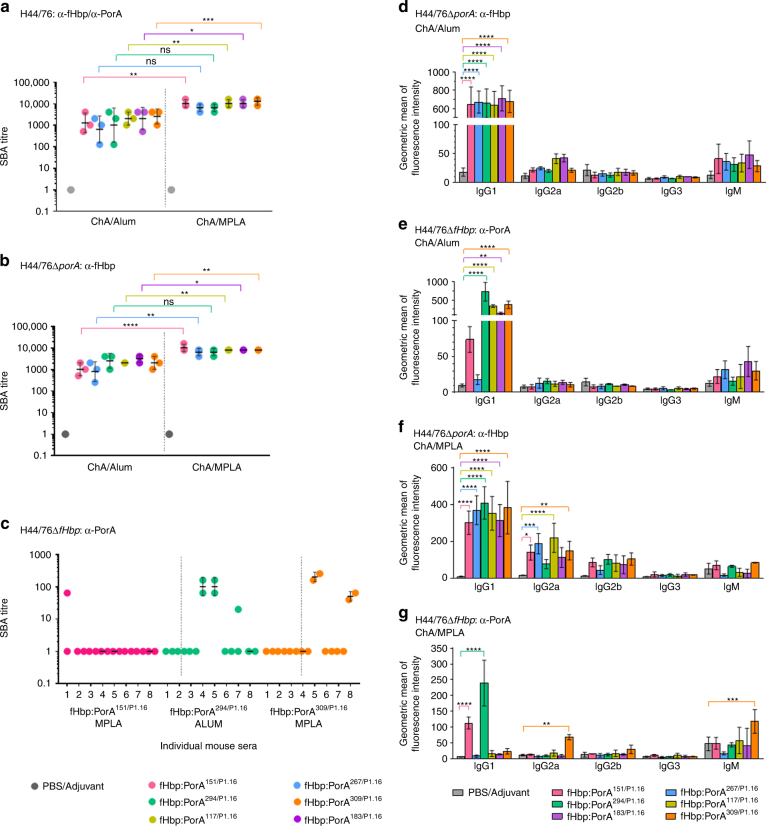
Detection of fHbp and PorA by ChA antisera. a Immunisation strategy. Mice (n = 8 per group) were subcutaneously immunised three times with each combination of ChA/Adjuvant. b, d Western blots of N. meningitidis whole-cell lysates probed with each set of ChA/adjuvant antisera: b detection of fHbp, d detection of PorA, complete western blots are in Supplementary Figures 6 and 7. c, e Flow cytometry analysis showing binding of each set of ChA/adjuvant antisera to N. meningitidis H44/76 strains WT, ∆fHbp, ∆porA and ∆fHbp∆porA. s.d. of independent assays (n = 3) is indicated. Two-way ANOVA and Dunnett’s method of multiple comparison were used to compare the fluorescence intensity of ChA antisera with PBS control sera (c, e *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001)
Next, we used flow cytometry to assess the ability of antibodies raised by immunisation with ChAs to recognise fHbp and PorA on the surface of N. meningitidis. We detected antibody binding to fHbp and PorA by flow cytometry, using N. meningitidis strains H44/76∆porA and H44/76∆fHbp, respectively. Results were compared with findings obtained with control sera from mice immunised with phosphate-buffered saline (PBS) and adjuvant alone. Antisera raised against each ChA detected fHbp on the bacterial surface (p ≤ 0.0001, Figs. 2c, e), and certain antisera also demonstrated significant binding to PorA: antisera from mice immunised with alum and ChAs fHbpV1.1:PorA294/P1.16, fHbpV1.1:PorA117/P1.16, fHbpV1.1:PorA183/P1.16 or fHbpV1.1:PorA309/P1.16 (p ≤ 0.01 by two-way ANOVA, Fig. 2c), and from mice immunised with MPLA and ChAs fHbpV1.1:PorA151/P1.16, fHbpV1.1:PorA294/P1.16 or fHbpV1.1:PorA309/P1.16 (Fig. 2e, p ≤ 0.05 by two-way ANOVA). Significant binding (p ≤ 0.05 by two-way ANOVA) is observed with fHbpV1.1:PorA267/P1.16/MPLA antisera to the H44/76∆fHbp∆porA negative control strain, which is due to non-specific binding (Supplementary Figure 4).
The serum bactericidal assay (SBA) assesses the ability of antibodies to initiate complement-mediated lysis of N. meningitidis. When using baby rabbit complement, an SBA titre of ≥ 8 is an accepted correlate of protective immunity against N. meningitidis35. SBAs conducted with each set of pooled ChA/adjuvant antisera and wild-type N. meningitidis H44/76 all had titres of ≥ 128 (Fig. 3a). Significantly higher titres (p ≤ 0.05 by two-way ANOVA) were observed for antisera raised against fHbpV1.1:PorA151/P1.16, fHbpV1.1:PorA117/P1.16, fHbpV1.1:PorA183/P1.16 and fHbpV1.1:PorA309/P1.16 when MPLA, rather than alum, was used as the adjuvant.
Fig. 3.
Immunogenicity of ChAs. Titres from SBAs performed with N. meningitidis strains H44/76 (a, n = 3), H44/76∆porA (b, n = 3), and H44/76∆fHbp (c, n = 2) and ChA/adjuvant antisera. SBAs with H44/76 and H44/76∆porA were conducted using pooled ChA/adjuvant antisera, and SBAs with H44/76∆fHbp were conducted with ChA/adjuvant antisera from individual mice. Geometric mean and s.d. of independent assays (n > 2) are indicated. Flow cytometry was used to detect binding of mouse isotypes IgG1, IgG2a, IgG2b, IgG3 and IgM in ChA antisera to N. meningitidis strains H44/76∆porA (d, f) and H44/76∆fHbp (e, g). Mouse isotypes binding to N. meningitidis were detected with isotype specific secondary antibodies; s.d. of independent assays (n = 3) is indicated. Two-way ANOVA and Dunnett’s method of multiple comparison were used to compare SBA titres from pooled antisera (a, b) and ChA antisera to PBS control sera (d–g) (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001)
To measure fHbp-specific responses, we performed SBAs with N. meningitidis H44/76∆porA; all sets of ChA/adjuvant antisera generated protective SBA titres ≥ 256 (Fig. 3b). To evaluate α-PorA-specific responses we initially performed SBAs with pooled antisera; however, no PorA-dependent serum bactericidal activity was detected. Therefore, we examined PorA-dependent responses in individual mice. SBA titres were detected with antisera raised against fHbpV1.1:PorA294/P1.16 when alum was used as the adjuvant, and with antisera raised against fHbpV1.1:PorA151/P1.16 (a low level of SBA from a single mouse), or fHbpV1.1:PorA309/P1.16 when MPLA was the adjuvant (Fig. 3c). Although PorA was detected on the surface of N. meningitidis by antisera from mice immunised with alum and fHbpV1.1:PorA117/P1.16 or fHbpV1.1:PorA183/P1.16, or with MPLA and fHbpV1.1:PorA294/P1.16 (Fig. 2c, e), these antigens/adjuvants did not elicit PorA-dependent SBA titres.
To activate the classical pathway, bound immunoglobulin (Ig) must recruit the C1q subunit of C136. The ability of Ig classes to bind C1q varies; a single IgM can be sufficient for C1q recruitment37, while several IgGs must be bound in close proximity and in a particular conformation38. Therefore, we examined which Ig isotypes are elicited by ChAs. Flow cytometry demonstrated that IgG1 was the predominant Ig bound to the surface of N. meningitidis (Fig. 3d–g). When compared with sera from mice immunised with PBS/adjuvant alone, all ChAs elicited significant α-fHbp-specific IgG1 responses (Fig. 3d, f, p ≤ 0.0001 by two-way ANOVA). However, significant IgG1 binding to PorA on the surface of H44/76ΔfHbp was observed only with antisera raised against fHbpV1.1:PorA294/P1.16, fHbpV1.1:PorA117/P1.16, fHbpV1.1:PorA183/P1.16 or fHbpV1.1:PorA309/P1.16 with alum (p ≤ 0.01 by two-way ANOVA; Fig. 3e), and fHbpV1.1:PorA151/P1.16 or fHbpV1.1:PorA294/P1.16 with MPLA (p ≤ 0.0001 by two-way ANOVA; Fig. 3g). Interestingly, there was no detectable IgG1 binding to PorA using sera raised against fHbpV1.1:PorA309/P1.16/MPLA, against which two mice had α-PorA SBA titres (Fig. 3c); instead this antisera contained significant levels of α-PorA-specific IgG2a and IgM (p ≤ 0.01 by two-way ANOVA; Fig. 3g).
ChAs retain the architecture of fHbp and the PorA loop
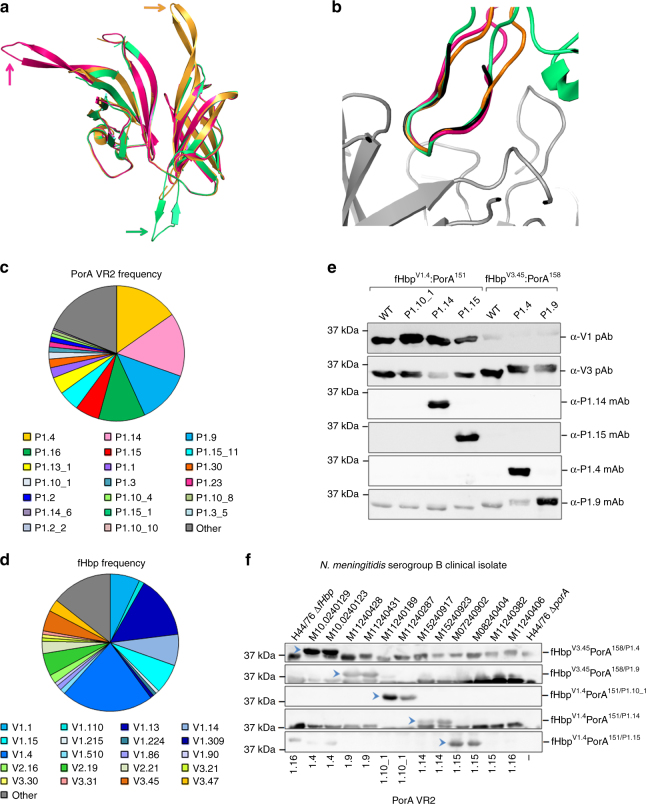
We determined the atomic structures of ChAs with VR2 P1.16 inserted into position 151, 294, or 309, all of which generated PorA-dependent SBA titres. These structures were solved using molecular replacement to resolutions of 2.9 Å for fHbpV1.4:PorA151/P1.16, 3.7 Å for fHbpV1.1:PorA294/P1.16 and 2.6 Å for fHbpV1.4:PorA309/P1.16. All ChA scaffolds align with good agreement (route-mean-square deviations (RMSDs) range between 0.447 and 0.614) to fHbp V1.1 (Supplementary Figure 2C), demonstrating that the fHbp scaffold is not perturbed by insertion of VR2 P1.16. In each structure VR2 P1.16 adopts a β-turn conformation that extends away from the fHbp scaffold (Fig. 4a). Importantly, these VR2 P1.16 conformations all mimic a VR2 P1.16 peptide in a complex with a bactericidal Fab fragment39 (Fig. 4b, RMSDs of atomic positions range between 0.346 and 0.970), demonstrating that in ChAs VR2 P1.16 adopts a conformation that can induce bactericidal antibody responses40.
Fig. 4.
Structure of ChAs and frequency of fHbp/PorA alleles in the UK. a Alignment of fHbp scaffolds from fHbpV1.4:PorA151/P1.16 (pink), fHbpV1.1:PorA294/P1.16 (green) and fHbpV1.4:PorA309/P1.16 (orange), VR2 P1.16 is indicated for each ChA by the correspondingly coloured arrow. b Alignment of VR2 P1.16 region 'KDTNNNL' from fHbpV1.4:PorA151/P1.16 (pink), fHbpV1.1:PorA294/P1.16 (green) and fHbpV1.4:PorA309/P1.16 (orange) with the P1.16 peptide 'KDTNNNL' (black) in a complex with a bactericidal Fab fragment (grey) from mAb MN12H2 (PDB ID: 2MPA). Frequency of PorA VR2 (c) and fHbp peptides (d) in N. meningitidis serogroup B strains (n = 243) isolated in 2016 in the UK. Data downloaded from the Meningococcal Research Foundation, 27 June 2017 (ref. 41). Other: remaining alleles that occur in <4 isolates. e Analysis of recombinant ChAs by SDS-PAGE and western blot. Immunoblots are probed with α-PorA VR2 mAbs: P1.4, P1.9, P1.14 and P1.15. f Detection of PorA (indicated by blue arrows) in a panel of N. meningitidis serogroup B isolates by mouse polyclonal antisera from ChAs fHbpV1.4:PorA151/P1.1.10_1, fHbpV1.4:PorA151/P1.14, fHbpV1.4:PorA151/P1.15, fHbpV3.45:PorA158/P1.4 and fHbpV3.45:PorA158/P1.9. Complete western blots are shown in Supplementary Figures 8 and 9
ChAs with different PorA VR2 loops generate protective responses
To test the adaptability of ChAs, we generated several ChAs composed from different combinations of fHbp and PorA VR2. The comprehensive meningococcal genome data available for strains isolated in the UK enable design of ChAs that have exact sequence matches to the most common antigens in a given region41. In 2016, the most prevalent PorA VR2 peptides in serogroup B N. meningitidis isolates were P1.4 (15.2%), P1.14 (15.2%), P1.9 (12.8%), P1.16 (11.1%) and P1.15 (5.8%, Fig. 4c). While the most prevalent fHbp peptides belonging to variant groups 1 and 3 were V1.4 and V3.45, present in 21.8% and 4.9% of serogroup B N. meningitidis isolates, respectively (Fig. 4d). We constructed five different ChAs, in which a VR2 peptide was inserted into position 151 in V1.4 or position 158 in V3.45 (Fig. 1b). Following ChA expression and purification, western blot analyses confirmed these ChAs, all retained epitopes recognised by their cognate α-VR2 mAb and α-fHbp pAbs (Fig. 4e).
To examine the ability of these ChAs to elicit immune responses, groups of CD1 mice were immunised with each ChA and alum (Fig. 2a); antisera obtained post immunisation were pooled. To assess the resulting PorA immune responses, western blots were conducted with pooled antisera and cell lysates from a panel of serogroup B N. meningitidis disease isolates. Figure 4f demonstrates that all ChAs elicited PorA-specific antibodies that recognised their cognate PorA VR2. To evaluate PorA-mediated SBA responses, we performed SBAs with pooled ChA/alum antisera and serogroup B N. meningitidis strains with mismatched fHbp variants to negate fHbp cross-protection. Titres range between ≥20 and ≥1280 and are above the ≥8 threshold for an accepted correlate of protective immunity against N. meningitidis35 (Table 2).
Table 2.
Serum bactericidal assay titres
| Pooled antisera | Serogroup B isolate | fHbp peptide | PorA VR2 | SBA titre |
|---|---|---|---|---|
| fHbpV3.45:PorA158/P1.4 | M10240123 | V1.92a | P1.4 | 1/160 |
| fHbpV3.45:PorA158/P1.9 | M11240431 | V2.19 | P1.9 | 1/1280 |
| fHbpV1.4:PorA151/P1.1.10_1 | M11240189 | V3.84 | P1.10_1 | 1/20 |
| fHbpV1.4:PorA151/P1.14 | M15240853 | V3.45 | P1.14 | 1/640 |
α-PorA SBA titres generated using pooled ChA/alum antisera and serogroup B N. meningitidis isolates with mismatched fHbp variants
afHbp truncated at residue 242
Discussion
During infection pathogens present our immune system with an assortment of surface-exposed, lipid anchored, and integral membrane proteins, all of which can be used as components in subunit vaccines. While lipoproteins are easily engineered for recombinant expression (by removal of their lipid anchor), integral membrane proteins present several challenges for vaccine development. Recombinant forms of integral membrane proteins are often poorly expressed and their native conformations may be compromised during purification, potentially reducing their ability to elicit immune responses against conformational epitopes, such as those found in surface loops10,42. Furthermore, immunisation with integral membrane proteins can generate irrelevant immune responses, which are directed towards epitopes that are masked by the bacterial outer membrane43. To circumvent these issues, structure-based design was used to develop ChAs. We selected a key surface-exposed epitope (VR2) from the integral membrane protein PorA and inserted it into the immunogenic scaffold of the lipoprotein fHbp. We show that multivalent ChAs generate immune responses against two key surface antigens that can elicit protective immunity, providing proof-in principle that immunogenic epitopes from integral membrane proteins can be introduced into a soluble molecular scaffold to create functional antigens, an approach that could be applied to other antigens.
Combining two antigens within a single recombinant ChA could diminish the immunogenicity of one or both antigens. fHbp is highly immunogenic in all ChAs, inducing bactericidal antibody responses in excess of those correlated with protection35. We found PorA VR2 could induce antibody responses when displayed away from its native environment in the outer membrane. While all ChAs induced antibodies that detected PorA on the surface of N. meningitidis, not all induced bactericidal PorA antibodies. We observed a mixed bactericidal PorA response, which depended on the PorA VR2 peptide, the position of PorA VR2 in the fHbp scaffold and the adjuvant used for immunisation, indicating that several empirically determined parameters are crucial for obtaining bactericidal responses.
Previous studies found linear VR2 P1.16 peptides failed to elicit antibodies that recognised native PorA, while cyclic VR2 P1.16 peptides, with identical residues fixed in a β-turn, elicited PorA-specific bactericidal antibodies40,44–47. Structural observations using β-turn peptide mimics demonstrated that cyclised peptides, which mimic their native antigens, are able to induce functional immune responses40,45,48. In ChAs, the VR2 N- and C-termini are effectively locked into a 'biological' β-turn peptide mimic by neighbouring fHbp β-strands, as confirmed by our atomic structures. In previous work, cyclic peptides were coupled to carrier proteins and used with adjuvants not licensed for human use40,44–46. In the resulting SBAs, titres were only observed with antisera from some mice45, similar to our findings with ChAs and licensed adjuvants.
Alum allows extended antigen presentation and stimulates T-helper (Th)-2 responses, predominantly producing IgG1 and IgE49, while MPLA typically enhances Th1 responses, inducing IgG2a, IgG2b, and IgG350. Murine IgG2a has a greater ability than murine IgG1 to activate the classical pathway51. Consistent with this, ChAs administered with MPLA elicited significantly higher fHbp-specific IgG2a responses and thus higher α-fHbp SBA titres than ChAs administered with alum. Of note, antisera raised against fHbpV1.1:PorA309/P1.16/MPLA had PorA-dependent SBA titre and contained significant levels of α-PorA IgG2a and IgM.
fHbp and PorA are both antigenically variable. To improve vaccine coverage, we used the comprehensive epidemiology data available for UK meningococcal isolates41 to generate ChAs composed of the most prevalent fHbp and PorA antigens. This maximises vaccine coverage as ChA composition mirrors the prevalent fHbp and PorA antigens circulating within a given geographical area. For example, a vaccine composed of the three most common fHbp peptides from each variant group (fHbp peptides V1.4, V2.19 and V3.45) with a single PorA VR2 insertion (PorA VR2 P1.4, P1.9 and P1.14) would give exact sequence coverage against 57% of serogroup B strains circulating in the UK in 2016; this compares favourably with currently licensed meningococcal serogroup B vaccines Bexsero (36%) and Trumenba® (4.8%)33,34,41.
In summary, using structure-based design, we generated ChAs that retain epitopes of fHbp and PorA and generate immune responses against both antigens, demonstrating that a soluble antigen can be exploited as a scaffold to display epitopes from an integral membrane protein. Our work provides proof-in-principle for bacterial vaccine design employing structure-led protein engineering previously used in viral proteins to graft functional motifs onto unrelated protein scaffolds52–55, or β-hairpin peptide mimetics56,57 to develop novel conformationally restricted antigens.
Methods
Bacterial strains and growth
The bacterial strains used in this study are shown in Supplementary Table 2 and Supplementary Table 3. N. meningitidis was grown in the presence of 5% CO2 at 37 °C on Brain Heart Infusion (BHI, Oxoid, Basingstoke, United Kingdom) plates with 5% (v/v) horse serum (Oxoid) at 37 °C. E. coli was grown on Luria-Bertani (LB) agar plates or LB liquid at 37 °C supplemented with 100 μg μl−1 carbenicillin.
Expression and purification of ChAs
N-terminally truncated fHbp V1.1 was amplified from MC58 genomic DNA using primers fHbp F1 and fHbp R4 (Supplementary Table 4). PorA VR2 P1.16 (YYTKDTNNNLTLV) was introduced into one of six positions in fhbp by overlap PCR using the primers in Supplementary Table 4. PorA VR2 loops P1.4 (HVVVNNKVATHVP), P1.9 (YVDEQSKYHA), P1.10_1 (HFVQNKQNQPPTLVP), P1.14 (YVDEKKMVHA) and P1.15 (HYTRQNNADVFVP) were introduced into a single position in fHbp by overlap PCR using the primers in Supplementary Table 4. PCR products were digested with NdeI and XhoI (NEB) and then ligated into pET21b (Novagen); constructs were confirmed by sequencing. Protein expression was performed in E. coli strain B834. Expression cultures were incubated at 37 °C, upon reaching an OD600 of ~0.8, protein expression was induced with 1 mM IPTG. Cultures were harvested after overnight expression at 37 °C. Bacteria were resuspended in Buffer A (50 mM Na-phosphate pH 8.0, 300 mM NaCl, 30 mM Imidazole) and purified by Nickel affinity chromatography (His-trap FF Crude; GE Healthcare) at room temperature. Columns were washed with 25 column volumes (CV) of Buffer A, then 20 CV 80:20 Buffer A:Buffer B (50 mM Na-phosphate pH 8.0, 300 mM NaCl, 300 mM Imidazole); elution was performed with 10 CV 40:60 Buffer A:Buffer B. Proteins were dialysed overnight at 4 °C into 50 mM Na-Acetate pH 5.5 buffer and further purification was achieved by ion exchange chromatography (HiTrapSP HP; GE Healthcare) at room temperature with a 0–1 M NaCl gradient in 50 mM Na-Acetate pH 5.5 buffer, followed by gel filtration using a HiLoad 16/600 Superdex 75 pg (GE Healthcare) column equilibrated with PBS (Oxoid).
Generation of N. meningitidis strains
Deletion of porA was performed by replacing the open reading frame with a tetracyline resistance cassette. Briefly, ~500 bp of the up- and downstream regions of the porA locus flanking a tetracycline resistance cassette were generated by PCR using primers PorA KO: F1, R1, F2, R2 (Supplementary Table 4) and the mega-primer method58. The PCR product was transformed into N. meningitidis H44/76 and H44/76∆fHbp as described previously59. Genomic DNA was obtained (Wizard® genomic DNA purification kit; Promega) from the resulting strains and both mutations were backcrossed into the WT H44/76 background using genomic DNA59.
Western blot analyses
Western blots of purified proteins (0.5 μg) or 10 μl of N. meningitidis cell lysate60 were probed with one of the following monoclonal antibodies or polyclonal sera: PorA P1.4 mAb (NIBSC cat: 02/148, diluted 1 in 500), PorA P1.9 mAb (NIBSC cat: 05/190, diluted 1 in 250), PorA P1.14 mAb (NIBSC cat: 03/142, diluted 1 in 500), PorA P1.15 mAb (NIBSC cat: 02/144, diluted 1 in 1,000), PorA P1.16 mAb (NIBSC cat: 01/538, diluted 1 in 1,000), pAb to fHbp V1.160 (diluted 1 in 1000) or polyclonal sera from mice immunised with a ChA (diluted 1 in 500). Following incubation with anti-mouse HRP-conjugated secondary antibodies (diluted 1 in 10,000), membranes were visualised via ECL (GE Healthcare) on a LAS-4000 (FujiFilm).
Generation of immune sera
Immunisations were performed with each ChA and PBS controls, using alum or MPLA as the adjuvant. Alum immunisations were prepared by incubating 20 μg ChA or PBS, 2% Alhydrogel (Invivogen), 10 mM Histidine-HCl pH 6.5 and 155 mM NaCl overnight at 4 °C on an end-over-end rocker. For MPLA immunisations, lyophilised MPLA (Invivogen) was resuspended in sterile H2O by incubating for 5 min in a sonicating water bath. Ten micrograms of MPLA were mixed at room temperature with 20 μg ChA or PBS, 10 mM Histidine-HCl pH 6.5 and 155 mM NaCl. Groups of eight female CD1 mice (~6 weeks old, Charles Rivers, Margate) were immunised by the intraperitioneal route on days 0, 21 and 35. Four mice were kept in each cage, which were individually vented. Sera was obtained on day 49 following cardiac puncture under terminal anaesthesia. All procedures were conducted in accordance with UK Home Office guidelines. Ethical approval was obtained by the Central Committee on Animal Care and Ethical Review at the University of Oxford. All sera were stored at −80 °C until required; once defrosted, sera were stored at 4 °C.
Serum bactericidal assays
SBAs were performed as previously described60, with the following modifications. N. meningitidis was suspended in Dulbecco’s PBS with cations (Gibco) supplemented with 0.1% glucose (DPBS-G) to a final concentration of 1.25 × 104 CFU ml−1. Baby rabbit complement (Cedar lane, lot #15027680) was diluted with DPBS-G to a final dilution of 1/10. Serum, pooled or from individual mice, was heat inactivated for 1 h at 56 °C and added to the wells in a serial two-fold dilution, starting with a dilution of 1/5 or higher. Control wells contained no serum or no complement. Following static incubation for 1 h at 37 °C in the presence of 5% CO2, 10 µl from each well was plated onto BHI plates in triplicate and colonies from surviving bacteria counted. Bactericidal activity is expressed as the dilution of serum required to kill≥50% of bacteria in assays containing both complement and serum in comparison with control assays containing serum or complement alone. SBAs using pooled sera were repeated three times, and assays using sera from individual mice were repeated twice. SBA titres were input into GraphPad Prism and statistical analyses comparing titres obtained from alum immunisations with titres obtained from MPLA immunisations were performed using two-way ANOVA (statistical significance of p ≤ 0.05) and Dunnett’s method of multiple comparisons.
Surface plasmon resonance
SPR was performed using a Biacore 3000 (GE Healthcare). Recombinant ChAs were dissolved in 50 mM sodium acetate pH 4.5 and immobilised on a CM5 sensor chip (GE Healthcare). Increasing concentrations of CFH6/7 (1–16 nM) were injected over the flow channels at 40 µl min−1). Dissociation was allowed for 300 s. BIAevaluation software was used to calculate the Kd.
Structural biology
ChAs in PBS were concentrated to 7 mg ml−1 and screened for crystal formation via the sitting drop method at a 0.4:0.6 ratio of protein to mother liquor. Crystals of chimeras fHbpV1.4:PorA151/P1.16, fHbpV1.1:PorA294/P1.16, fHbpV1.1:PorA309/P1.16 and fHbpV1.4:PorA309/P1.16 grew under the respective conditions: 2.0 M ammonium sulphate, 0.15 M sodium citrate pH 5.5; 20% (w/v) PEG4000, 0.3 M ammonium sulphate; 0.01 M zinc chloride, 0.1 M sodium acetate, 20% (w/v) PEG6000; and 0.2 M potassium formate, 20% (w/v) PEG3350 respectively. Crystals were transferred to a cryoprotectant solution comprised of mother liquor and 30% ethylene glycol. Data were collected at Diamond light source and integrated/scaled using the Xia2 programme. Molecular replacement was carried out using Phaser from the CCP4 package61, with the fHbp V1.1 structure used as a search model (PDB 2W80). Refmac562, Coot63 and Phenix64–67 were utilised for rebuilding and refinement, with structural validation performed in Molprobity68. Refinement statistics for each structure are detailed in Supplementary Table 5.
Differential scanning calorimetry
Purified ChAs (20 μM) in PBS were subjected to a 20 to 120 °C temperature gradient on a Malvern VP Capillary DSC. Melting temperature (Tm) is recorded for the fHbp N-terminal and C-terminal β-barrels (Table 1, Supplementary Figure 1).
Flow cytometry
N. meningitidis strains H44/76, H44/76∆fHbp, H44/76∆porA and H44/76∆fHbp∆porA (1 × 109 cells) were fixed in 3% paraformaldehyde (PFA) for 1 h. PFA was removed by washing cells three times with PBS. To measure binding of serum antibodies to fHbp and PorA, 1 × 108 cells were incubated for 1 h at 4 °C, shaking at 1250 rpm, with sera diluted 1/50 in PBS. Cells were washed three times with PBS, then incubated for 30 min at 4 °C with shaking at 1250 rpm, with a secondary antibody (all ThermoFisher Scientific). Final dilutions of secondary antibodies were as follows: 5 μg ml−1 for Alexa-488 conjugated anti-mouse IgAGM, and 2.5 μg ml−1 for each of Alexa-488 conjugated anti-mouse IgM, IgG3 and IgG2b, and Alexa-647 conjugated anti-mouse IgG2a and IgG1. Cells were washed three times with PBS and then resuspended in PBS for analysis on a FACSCalibur (BD Biosciences). Data were imported into the FlowJoTM data analysis package and transformed to the Logicle scale. A uniform gate was applied to all data sets, based on bacteria visualised by forward and side-scatter, and the geometric mean of fluorescence calculated for FL1H (Alexa-488 signal) and FL4H (Alexa-647 signal). Each flow experiment was repeated three times, and the FL1H and FL4H geometric means were input into Graphpad Prism. Two-way ANOVA (statistical significance of p ≤ 0.05, using Dunnett’s method of multiple comparison) was used to compare the Alexa-488 or Alexa-647 geometric means with control Alexa-488 or Alexa-647 geometric means. Control results were obtained by incubating PFA fixed cells with sera from mice immunised with PBS and Alum or MPLA adjuvants, and the corresponding Alexa-fluor-labelled secondary Ig. Two-way ANOVA multiple comparisons were performed with geometric means from sets of experiments using the same secondary antibody, N. meningitidis strain and adjuvant; the only variable factor was the ChA used for immunisation.
Data availability
Structural data that support the findings of this study have been deposited in RCSB Protein Data Bank with the primary accession codes 5NQP for fHbpV1.4:PorA151/P1.16; 5NQX for fHbpV1.1:PorA294/P1.16; 5NQZ for fHbpV1.1:PorA309/P1.16; and 5NQY for fHbpV1.4:PorA309/P1.16.
Electronic supplementary material
Acknowledgements
We gratefully acknowledge financial support from Action Medical Research (Award number GN2205). We thank David Staunton for performing the DSC analysis, Sophie Andrews and Sasha Burgess for assisting with the production of expression vectors, Diamond and ESRF synchotrons for provision of beam time, and the Diamond-I02, Diamond-I04 and ESRF-ID29 beamline staff for help with beamline preparation and data collection. We gratefully thank the Meningococcal Reference Unit, Public Health England, for the meningococcal serogroup B disease isolates. Nicola Ruivo provided technical assistance.
Author contributions
S.H. and I.J. contributed by designing and generating constructs, protein purification, performing immunisations, analysing immune responses, and experimental design. S.H. also helped with setting up crystallisation and structural analyses. R.M.E. contributed to experimental design. S.J. and S.M.L. contributed to structural studies, SPR and design of constructs, C.M.T. contributed to project organisation and experimental design. S.H. prepared the figures. S.H., C.M.T., S.J., I.J., R.M.E. and S.M.L. wrote the manuscript.
Competing interests
The authors declare that they have filed a patent on Chimeric antigens (ref GB1614687, PCT filing date: 31st August 2017).
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03146-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arístegui J, et al. Facilitating the WHO expanded program of immunization: the clinical profile of a combined diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b vaccine. Int J. Infect. Dis. 2003;7:143–151. doi: 10.1016/S1201-9712(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 2.Murphy TF. Vaccines for nontypeable Haemophilus influenzae: the future is now. Clin. Vaccine Immunol. 2015;22:459–466. doi: 10.1128/CVI.00089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller LE, Robinson DA, McDaniel LS. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. MBio. 2016;7:e01792. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiden MC. The impact of protein-conjugate polysaccharide vaccines: an endgame for meningitis? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120147. doi: 10.1098/rstb.2012.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaller A, et al. Rapid typing of Moraxella catarrhalis subpopulations based on outer membrane proteins using mass spectrometry. Proteomics. 2006;6:172–180. doi: 10.1002/pmic.200500086. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance of antimicrobial resistance in Neisseria gonorrhoeae Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/567602/GRASP_Report_2016.pdf(Public Health England, 2016).
- 7.Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/S0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 8.Antimicrobial Resistance: Global Report on Surveillance 2014 (World Health Organisation, 2014).
- 9.Telford JL. Bacterial genome variability and its impact on vaccine design. Cell Host Microbe. 2008;3:408–416. doi: 10.1016/j.chom.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter EP, Beis K, Cameron AD, Iwata S. Overcoming the challenges of membrane protein crystallography. Curr. Opin. Struct. Biol. 2008;18:581–586. doi: 10.1016/j.sbi.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sierra GV, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. [PubMed] [Google Scholar]
- 12.O’Hallahan J, Lennon D, Oster P. The strategy to control New Zealand’s epidemic of group B meningococcal disease. Pediatr. Infect. Dis. J. 2004;23:S293–S298. [PubMed] [Google Scholar]
- 13.Bjune G, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 14.Saukkonen K, Leinonen M, Abdillahi H, Poolman JT. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989;7:325–328. doi: 10.1016/0264-410X(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 15.Blake, M. & Gotschilch, E. in Bacterial Outer Membranes as Model Systems (ed. Inouye, M.) 377–400 (John Wiley and Sons, New York, 1987).
- 16.van der Ley P, Heckels JE, Virji M, Hoogerhout P, Poolman JT. Topology of outer membrane porins in pathogenic Neisseria spp. Infect. Immun. 1991;59:2963–2971. doi: 10.1128/iai.59.9.2963-2971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin DR, Ruijne N, McCallum L, O’Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin. Vaccine Immunol. 2006;13:486–491. doi: 10.1128/CVI.13.4.486-491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Claassen I, et al. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410X(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Ley P, van der Biezen J, Poolman JT. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine. 1995;13:401–407. doi: 10.1016/0264-410X(95)98264-B. [DOI] [PubMed] [Google Scholar]
- 21.van den Dobbelsteen, G. et al. From HexaMen to NonaMen: expanding a multivalent PorA-based meningococcal outer membrane vesicle vaccine. In International Pathogenic Neisseria Conference, 153 (Milwaukee, WI, 2004).
- 22.Luijkx TA, et al. Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form. Infect. Immun. 2003;71:6367–6371. doi: 10.1128/IAI.71.11.6367-6371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 24.Madico G, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider MC, et al. Functional significance of factor H binding to Neisseria meningitidis. J. Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 26.Schneider MC, et al. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–893. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizza M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 28.Jolley, K. A. & Maiden, M. C. Neisseria Multi Locus Sequence Typing website.http://pubmlst.org/neisseria/ (Oxford University, 2005).
- 29.Brehony C, Wilson DJ, Maiden MC. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology. 2009;155:4155–4169. doi: 10.1099/mic.0.027995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy E, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J. Infect. Dis. 2009;200:379–389. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher LD, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect. Immun. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masignani V, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 2003;197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30:B87–B97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green LR, et al. Approach to the discovery, development, and evaluation of a novel Neisseria meningitidis serogroup B vaccine. Methods Mol. Biol. 2016;1403:445–469. doi: 10.1007/978-1-4939-3387-7_25. [DOI] [PubMed] [Google Scholar]
- 35.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab Immunol. 2003;10:780–786. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank MM, Joiner K, Hammer C. The function of antibody and complement in the lysis of bacteria. Rev. Infect. Dis. 1987;9:S537–S545. doi: 10.1093/clinids/9.Supplement_5.S537. [DOI] [PubMed] [Google Scholar]
- 37.Poon PH, Phillips ML, Schumaker VN. Immunoglobulin M possesses two binding sites for complement subcomponent C1q, and soluble 1:1 and 2:1 complexes are formed in solution at reduced ionic strength. J. Biol. Chem. 1985;260:9357–9365. [PubMed] [Google Scholar]
- 38.Hughes-Jones NC, Gardner B. Reaction between the isolated globular sub-units of the complement component C1q and IgG-complexes. Mol. Immunol. 1979;16:697–701. doi: 10.1016/0161-5890(79)90010-5. [DOI] [PubMed] [Google Scholar]
- 39.van den Elsen JM, et al. Bactericidal antibody recognition of a PorA epitope of Neisseria meningitidis: crystal structure of a Fab fragment in complex with a fluorescein-conjugated peptide. Proteins. 1997;29:113–125. doi: 10.1002/(SICI)1097-0134(199709)29:1<113::AID-PROT9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 40.Oomen CJ, et al. Immunogenicity of peptide-vaccine candidates predicted by molecular dynamics simulations. J. Mol. Biol. 2003;328:1083–1089. doi: 10.1016/S0022-2836(03)00377-2. [DOI] [PubMed] [Google Scholar]
- 41.Meningitis Research Foundation Meningococcus Genome Library. https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mrfgenomes (2011).
- 42.Bagal SK, et al. Ion channels as therapeutic targets: a drug discovery perspective. J. Med. Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 43.Zhu W, et al. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect. Immun. 2005;73:7558–7568. doi: 10.1128/IAI.73.11.7558-7568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christodoulides M, McGuinness BT, Heckels JE. Immunization with synthetic peptides containing epitopes of the class 1 outer-membrane protein of Neisseria meningitidis: production of bactericidal antibodies on immunization with a cyclic peptide. J. Gen. Microbiol. 1993;139:1729–1738. doi: 10.1099/00221287-139-8-1729. [DOI] [PubMed] [Google Scholar]
- 45.Oomen CJ, et al. Crystal structure of an Anti-meningococcal subtype P1.4 PorA antibody provides basis for peptide-vaccine design. J. Mol. Biol. 2005;351:1070–1080. doi: 10.1016/j.jmb.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 46.Christodoulides M, Rattue E, Heckels JE. Effect of adjuvant composition on immune response to a multiple antigen peptide (MAP) containing a protective epitope from Neisseria meningitidis class 1 porin. Vaccine. 1999;18:131–139. doi: 10.1016/S0264-410X(99)00190-5. [DOI] [PubMed] [Google Scholar]
- 47.Hoogerhout P, et al. Conjugates of synthetic cyclic peptides elicit bactericidal antibodies against a conformational epitope on a class 1 outer membrane protein of Neisseria meningitidis. Infect. Immun. 1995;63:3473–3478. doi: 10.1128/iai.63.9.3473-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson JA. Max Bergmann lecture protein epitope mimetics in the age of structural vaccinology. J. Pept. Sci. 2013;19:127–140. doi: 10.1002/psc.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 50.Baker PJ, et al. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect. Immun. 1988;56:3064–3066. doi: 10.1128/iai.56.12.3064-3066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michaelsen TE, Kolberg J, Aase A, Herstad TK, Høiby EA. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 2004;59:34–39. doi: 10.1111/j.0300-9475.2004.01362.x. [DOI] [PubMed] [Google Scholar]
- 52.Azoitei ML, et al. Computation-guided backbone grafting of a discontinuous motif onto a protein scaffold. Science. 2011;334:373–376. doi: 10.1126/science.1209368. [DOI] [PubMed] [Google Scholar]
- 53.Correia BE, et al. Computational design of epitope-scaffolds allows induction of antibodies specific for a poorly immunogenic HIV vaccine epitope. Structure. 2010;18:1116–1126. doi: 10.1016/j.str.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 54.McLellan JS, et al. Design and characterization of epitope-scaffold immunogens that present the motavizumab epitope from respiratory syncytial virus. J. Mol. Biol. 2011;409:853–866. doi: 10.1016/j.jmb.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ofek G, et al. Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. USA. 2010;107:17880–17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aebi JD, Guillaume D, Dunlap BE, Rich DH. Synthesis, conformation, and immunosuppressive activity of a conformationally restricted cyclosporine lactam analogue. J. Med Chem. 1988;31:1805–1815. doi: 10.1021/jm00117a022. [DOI] [PubMed] [Google Scholar]
- 57.Freidinger RM, Perlow DS, Veber DF. Protected lactam-bridged dipeptides for use as conformational constraints in peptides. J. Org. Chem. 1982;47:104–109. doi: 10.1021/jo00340a023. [DOI] [Google Scholar]
- 58.Ke SH, Madison EL. Rapid and efficient site-directed mutagenesis by single-tube ‘megaprimer’ PCR method. Nucleic Acids Res. 1997;25:3371–3372. doi: 10.1093/nar/25.16.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Exley RM, et al. Available carbon source influences the resistance of Neisseria meningitidis against complement. J. Exp. Med. 2005;201:1637–1645. doi: 10.1084/jem.20041548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jongerius I, et al. Distinct binding and immunogenic properties of the gonococcal homologue of meningococcal factor h binding protein. PLoS Pathog. 2013;9:e1003528. doi: 10.1371/journal.ppat.1003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 63.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 64.Afonine PV, Grosse-Kunstleve RW, Urzhumtsev A, Adams PD. Automatic multiple-zone rigid-body refinement with a large convergence radius. J. Appl. Crystallogr. 2009;42:607–615. doi: 10.1107/S0021889809023528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afonine PV, Grosse-Kunstleve RW, Adams PD, Urzhumtsev A. Bulk-solvent and overall scaling revisited: faster calculations, improved results. Acta Crystallogr. D Biol. Crystallogr. 2013;69:625–634. doi: 10.1107/S0907444913000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Headd JJ, et al. Use of knowledge-based restraints in phenix.refine to improve macromolecular refinement at low resolution. Acta Crystallogr. D Biol. Crystallogr. 2012;68:381–390. doi: 10.1107/S0907444911047834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structural data that support the findings of this study have been deposited in RCSB Protein Data Bank with the primary accession codes 5NQP for fHbpV1.4:PorA151/P1.16; 5NQX for fHbpV1.1:PorA294/P1.16; 5NQZ for fHbpV1.1:PorA309/P1.16; and 5NQY for fHbpV1.4:PorA309/P1.16.