Abstract
Azolla spp., a water fern often used for phytoremediation, is a strong phosphorus (P) accumulator due to its high growth rate and N2 fixing symbionts (diazotrophs). It is known that plant growth is stimulated by P, but the nature of the interactive response of both symbionts along a P gradient, and related changes in growth-limiting factors, are unclear. We determined growth, and N and P sequestration rates of Azolla filiculoides in N-free water at different P concentrations. The growth response appeared to be biphasic and highest at levels ≥10 P µmol l−1. Diazotrophic N sequestration increased upon P addition, and rates were three times higher at high P than at low P. At 10 µmol P l−1, N sequestration rates reached its maximum and A. filiculoides growth became saturated. Due to luxury consumption, P sequestration rates increased until 50 µmol P l−1. At higher P concentrations (≥50 µmol l−1), however, chlorosis occurred that seems to be caused by iron- (Fe-), and not by N-deficiency. We demonstrate that traits of the complete symbiosis in relation to P and Fe availability determine plant performance, stressing the role of nutrient stoichiometry. The results are discussed regarding Azolla’s potential use in a bio-based economy.
Introduction
The genus Azolla comprises a broad range of floating aquatic fern species growing in tropical, subtropical and temperate freshwater ecosystems1,2. Azolla spp. are able to reach high growth rates by asexual reproduction and are very strong phosphorus (P) and nitrogen (N) accumulators3, which makes them very suitable for phytoremediation, bio-gas production, animal food and crop fertilization1,4,5. Due to their symbiosis with atmospheric nitrogen (N2) fixing microorganisms (diazotrophs), the primary production of the plants is hardly ever N-limited under natural conditions. The diazotrophs live inside Azolla’s leaf cavities (Supplementary Fig. S1) and include the cyanobacteria Nostoc/Anabaena azollae6,7 that forms unbranched, multi-cellular chains that contain both photosynthetic, vegetative cells and N2 fixing heterocysts8. Using nitrogenase enzymes, the diazotrophs reduce atmospheric N2 to ammonium (NH4+), which is then excreted into the Azolla leaf cavity and taken up by the fern9. In response to N-limitation, when there is no exogenous N available to Azolla, the heterocyst fraction increases in diazotroph chains10,11.
Given its high potential P uptake rates, luxury accumulation of P12,13 and high growth rates, Azolla may well be used for phytoremediation with respect to P4,12,14–17. The cultivation of Azolla spp. in P-enriched water could therefore offer a sustainable way of P recovery and recycling from eutrophic water12,18–22. Sustainable practices of P recycling are important, as global reserves of phosphate rock are becoming increasingly depleted23,24. Next to P recycling, Azolla cultivation may yield annually up to 35 t dry N-rich biomass, independent of N-fertilizer25. Although nutrient supply can easily be managed in hydro-cultures, nutrient management is more challenging when growing Azolla in flooded fields, due to biogeochemical interactions in the flooded soil and surface water26. The supply of macronutrients such as P and iron (Fe) and micronutrients by inflowing water, or by mobilization from the sediment to the overlying water should be sufficient to support Azolla’s growth26,27. Natural waters may well show non-optimal nutrient stoichiometry resulting in nutrient deficiency, impeded Azolla growth or even chlorosis1,28 due to P or Fe limitation28,29.
High P concentrations in surface waters are well known to stimulate the growth of Azolla spp.30,31 and to increase N2 fixation rates of their symbionts31–33. For efficient and broad application of A. filiculoides in a biobased economy, however, a profound understanding of the interactive responses of this species and its diazotroph microbiome to different P loadings is required, which is currently lacking. To test the type of growth response to P and find an explanation for this, A. filiculoides was grown in a greenhouse experiment in N-free surface water at different P concentrations. We hypothesized that both N and P sequestration rates are positively related to exogenous P concentrations, but level off because endogenous N provided by the diazotrophs becomes limited. We further hypothesized that at high P levels, Fe deficiency may occur which may induce chlorosis and limit Azolla growth, as this element is not only essential for Azolla but also for the production of the nitrogenase enzyme1,28.
Results
Growth response to P availability
RGRs of A. filiculoides growing in the 0.5 and 2 µmol P l−1 treatments were significantly lower than those of the higher P treatments (Fig. 1). Azolla’s growth response to increasing P showed a biphasic response (Michaelis-Menten fit: R2 = 0.62, p < 0.001). During 21 days, A. filiculoides growing in 0.5 and 2 µmol P l−1 treatments increased their biomasses 2.2 times, whereas in 10, 50 and 100 µmol P l−1 treatments, biomasses increased 3.4 times. During the experiment Azolla’s biomass was lower than the self-crowding threshold of 2 kg FW m−2 (data not shown).
Figure 1.
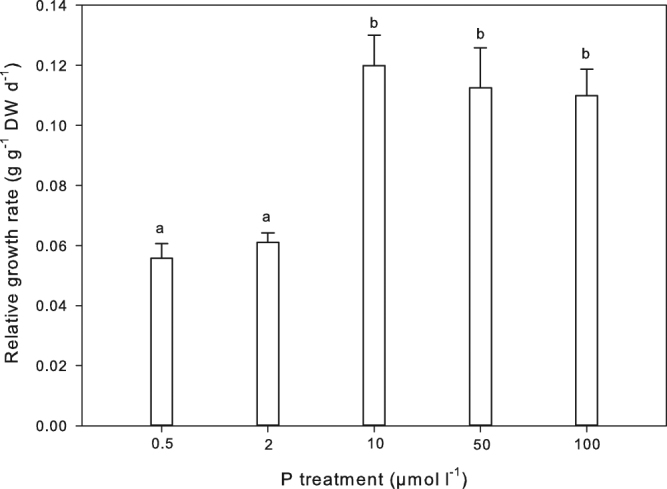
Relative growth rates (RGRs, g g−1 DW d−1) ± SE of A. filiculoides after 21 days of growth at 0.5, 2, 10, 50 and 100 µmol P l−1 (n = 5). Significant differences are indicated by different letters (Tukey HSD post hoc test). Additional results of the statistical analysis are presented in Supplementary Table S2.
Morphology of A. filiculoides also changed in response to different P treatments. Plants from the 0.5 and 2 µmol P l−1 treatments were smaller and more fragile than those from the 10, 50 and 100 µmol P l−1 treatments. Furthermore, plants from the lowest two concentrations turned reddish, while plants from the 10 µmol P l−1 treatment stayed green. Plants from the 50 and 100 µmol P l−1 treatments turned chlorotic (yellowish) after 21 days of growth.
Plant nutrient composition
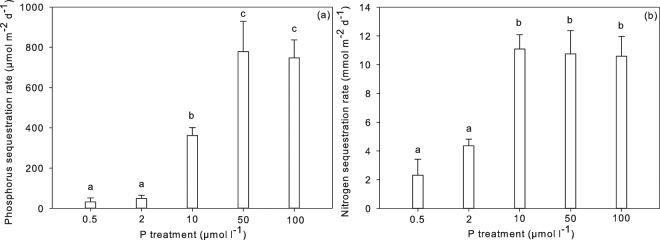
Plant P contents increased when P concentrations in the nutrient solution increased, but declined over time (Fig. 2a). P sequestration rates also increased, until the 50 µmol P l−1 treatment (Fig. 3a). Plant N content was stable over time, and highest at 50 µmol P l−1. Lowest N content was found for the 0.5 and 2 µmol P l−1 treatments (Fig. 2b). P concentrations ≥ 10 µmol l−1 in the nutrient solution significantly increased N sequestration rates of Azolla and its symbionts (Fig. 3b). N sequestration rates were approximately 4.5 and 2.5 times higher in the higher P treatments (≥10 µmol l−1) compared to the 0.5 and 2 µmol P l−1 treatments, respectively. Plant N: P ratios showed an extensive range, and decreased with increasing P availability (Fig. 2c). At day 21, the highest value (55 ± 1.7 mol mol−1) was reached in the 0.5 µmol P L−1 treatment, and the lowest in the 50 and 100 µmol P l−1 treatments (12.4 ± 0.3 and 10.9 ± 0.3 mol mol−1, respectively). Plant K, P, N, Na and S contents were significantly higher in the >10 µmol P l−1 treatments, whereas Zn and Ca contents were significantly lower and Fe and Si were similar (Supplementary Table S3). Further elemental composition of the plants is shown in Supplementary Table S3.
Figure 2.
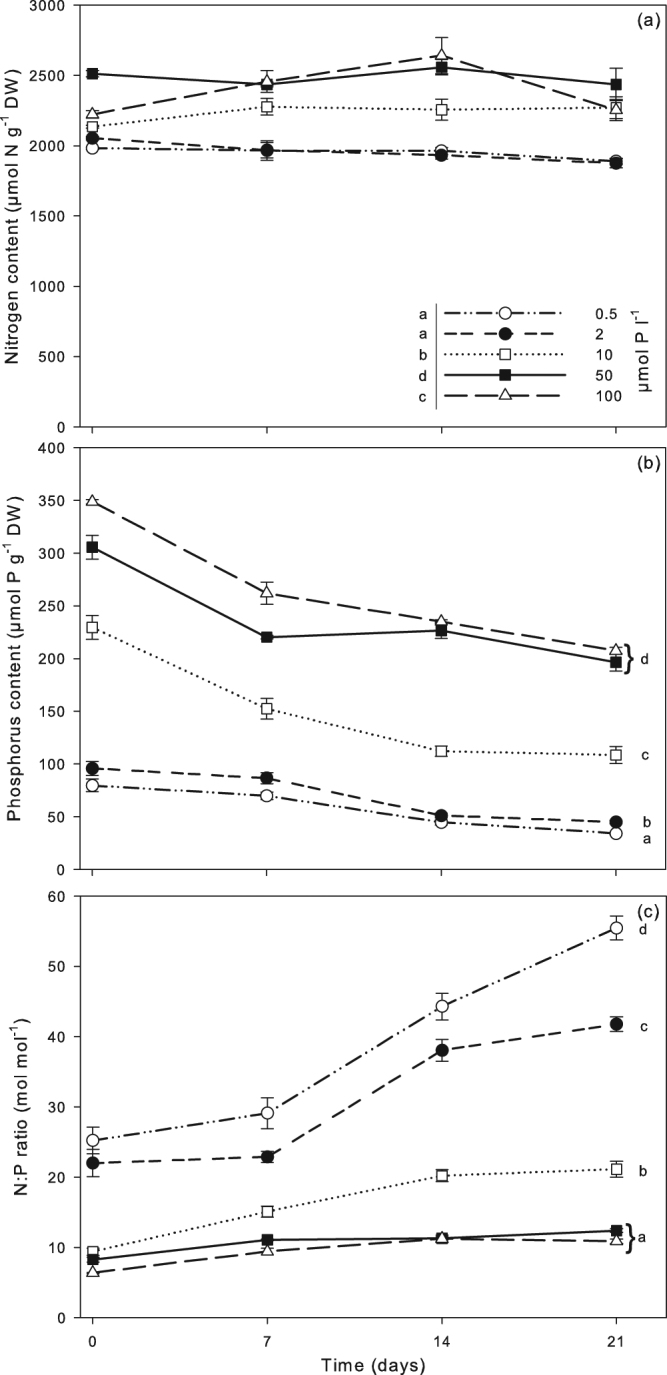
P content (µmol P g−1 DW; (a) N content (µmol N g−1 DW; (b) and N: P ratios (mol mol−1; (c) ±SE of A. filiculoides during 21 days of growth at 0.5, 2, 10, 50 and 100 µmol P l−1 (n = 5). Different letters indicate significant differences (Bonferroni post hoc test). Additional results of the statistical analyses are presented in Supplementary Table S2.
Figure 3.
P (panel a) and N (panel b) sequestration rates (µmol m−2 d−1; n = 5) ± SE by A. filiculoides for the 0.5, 2, 10, 50 and 100 µmol P l−1 treatments. Different letters indicate significant differences (ANOVA Tukey post hoc test). Additional results of the statistical analyses are presented in Supplementary Table S2.
Deficiency testing
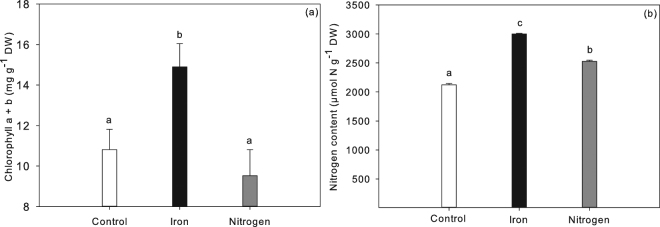
To test the cause of the observed chlorosis an additional, but similar, experiment was carried out, in which plants also turned chlorotic after 5 weeks of 100 µmol P l−1 treatment. They were subsequently treated with Fe or NH4+-NO3−. After two weeks, only the plants treated with Fe became green again and had a significantly higher chlorophyll a + b content (15.2 ± 2.3 mg g−1 DW) than the control (10.8 ± 2 mg g−1 DW) or the N treatment (9.5 ± 2.7 mg g−1 DW, Fig. 4a). In addition, highest N contents were found in plants treated with Fe (3000 ± 11 µmol N g−1 DW) and lowest in the control (2125 ± 20 µmol N g−1 DW; Fig. 4b). Plants treated with N showed an intermediate N content of 2529 ± 18 µmol N g−1 DW (Fig. 4b and Supplementary Table S4). Compared to both the control and the N treatment, the Fe content (55.8 ± 8 µmol Fe g−1 DW) in the Fe treatment was 1.6 and 1.5 times as high (Supplementary Table S4). Additional elemental composition of the plants is shown in Supplementary Table S4.
Figure 4.
Chlorophyll a + b (mg g−1 DW, (a) and plant N content (µmol N g−1 DW, (b) of A. filiculoides treated with 100 µmol P l−1 (Control), after Fe (Iron) or nitrogen addition (limitation experiment, n = 4) ± SE. Significant differences are indicated by different letters (ANOVA Tukey HSD pairwise comparison). Additional statistical results are presented in Supplementary Table S2.
Discussion
Both Azolla filiculoides growth and the diazotrophic activity of its microbiome (resulting in N sequestration) showed a biphasic response to P availability, when grown without external N supply. For high P levels, after maximum fixation rates by diazotrophic symbionts had been reached, the plants turned chlorotic. Additional external N supply did not increase plant health, but additional Fe supply did increase chlorophyll a + b and plant N contents. This not only proves that Fe was limiting at high P availability, but also means that traits of the complete symbiosis in relation to P and Fe availability determine Azolla’s performance, stressing the role of nutrient stoichiometry.
We found that A. filiculoides is able to double its biomass in one week in an N-free nutrient solution growing in a P-rich environment, entirely relying on the symbiosis with diazotrophs for its N supply. The growth rates measured are in the range reported in literature32,34–37. However, higher RGRs may be obtained when growing Azolla under optimal conditions using synthetic media in aquaculture1,38. Self-crowding may also result in lower RGRs of the fern, however, during the experiment the biomass was lower than the self-crowding threshold of 2 kg FW m−2 and was therefore not responsible for the biphasic growth response39. Here we show that the RGR of A. filiculoides shows a biphasic response to P availability, and becomes saturated at 10 µmol P l−1. The lack of differences between RGRs of plants grown with 10, 50 and 100 µmol P l−1, together with the substantially higher P content in plants grown at high P, either suggests that the maximum growth of this strain of A. filiculoides and its diazotrops were reached, or that a factor other than P became limiting above 10 µmol P l−1.
In aquatic plants, N: P ratios >17 (mol mol−1) generally indicate P deficiency, whereas a N: P ratios < 10 (mol mol−1) indicate N deficiency40–42. Based on their N: P ratios, A. filiculoides grown at P levels ≤ 2 µmol l−1 were severely P-limited43,44, which was also indicated by their red color1. Addition of P shifted the limitation through N and P co-limitation (in plants receiving 10 µmol P l−1) towards N-limitation (in plants receiving 50 and 100 µmol P l−1), based on N:P ratios and color.
A. filiculoides from the 50 and 100 µmol P l−1 treatment had a similar N: P ratio around 10 mol mol−1, which may indicate that the plant’s N-requirement and N-supply by the diazotrophs were in equilibrium to sustain Azolla’s growth. Apparently, the diazotrophs were not able to fix additional N that could be utilized to increase biomass growth. Interestingly, plants supplied with additional Fe had significantly higher N content compared to plants that were only supplied with N and plants that did not receive additional Fe or N, indicating Fe limitation for both plant growth and N2 fixation by the diazotrophs1,25. Luxury consumption of N did not seem to occur, probably because N2 fixation rates reached their maximum due to Fe limitation. The ferns, however, continued to accumulate P up to average rates of 778 µmol m−2 d−1, indicating luxury consumption (Fig. 5). N and P sequestration rates were indeed positively related to exogenous P concentrations. For N the maximum was reached at 10 µmol P l−1, whereas for P this was at 50 µmol P l−1. Our results clearly show that the diazotrophic community and A. filiculoides are able to respond dynamically to P availability in the aquatic environment, but become saturated or limited at a certain level.
Figure 5.
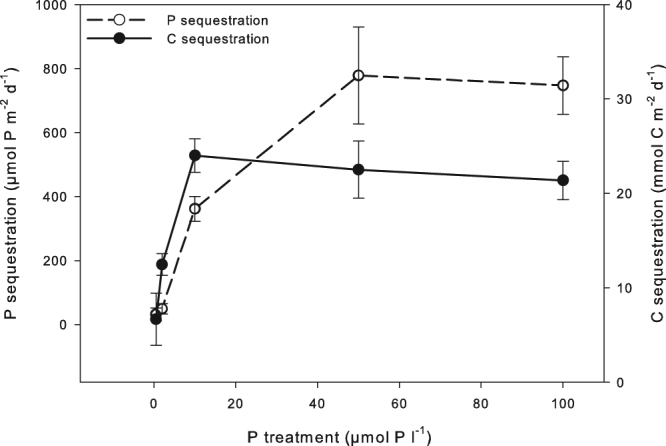
P (µmol P m−2 d−1; left y-axis) and carbon (C; mmol C m−2 d−1; right y-axis) sequestration rates in A. filiculoides under different P loadings (n = 15; ± SE).
In natural environments, eutrophication usually includes increased loading of both N and P. Presence of exogenous N influences both N2 fixation and growth of A. filiculoides. Availability of N, has been shown to inhibit nitrogenase activity in Azolla11,15,45,46, while not lowering plant growth47. In temperate regions, under natural conditions, surface water N concentrations rapidly decrease in spring as a result of high denitrification rates due to increased microbial activity, but surface water P availability remains high, especially if sediment P gets mobilized due to anoxia in the water layer27,48. Under these environmental conditions, Azolla can become highly competitive, using N produced by the diazotrophs combined with enhanced P availability.
At high P levels, plants may turn chlorotic, which has been attributed to absolute N-limitation in literature1. Here, we show that chlorosis and lower chlorophyll contents are caused by Fe deficiency at high P levels. Fe limitation can be expected to have resulted in lower growth and N sequestration rates in the high P treatments. The fact that Azolla’s condition (chlorophyll a + b) did improve after supplying exogenous Fe is in agreement with the hypothesis that Fe was deficient for Azolla grown at high P levels. In addition, it has been shown that Azolla is capable of higher growth rates when grown at >100 µmol P l−1 provided Fe is sufficiently available2,32. Fe availability in the environment or total Fe content of the plant are not the critical processes leading to chlorosis; multiple mechanisms can be responsible for Fe induced chlorosis including Fe precipitation in the apoplast, which is physiologically unavailable49–51. The presence of P can also affect plant Fe availability44,52,53. Interaction of P and Fe leading to Fe chlorosis can be caused by an internal immobilization of Fe probably due to formation of Fe-PO454–57.
Our results indicate that nutrients in the water should be balanced when growing Azolla to optimize plant health and maximize yields. The use of A. filiculoides (and its diazotrophic community) as a tool to recover and recycle P in a bio-based economy12,25 is also dependent on Fe availability. When Azolla is grown on inundated agricultural lands (Azolla farming) for example, not only P re-mobilization from the sediment27,48, but also Fe mobilization should be considered.
Materials and Methods
Experimental set-up
Before the start of the experiment, A. filiculoides was acclimatized to experimental conditions in 15 l tanks containing an N-free nutrient solution to which 0.5, 2, 10, 50 or 100 µmol P l−1 was added (as NaH2PO4 * 2H2O). The general composition of the solution was similar to the quality of natural surface waters where Azolla is abundant (Supplementary Table S1). After sufficient Azolla biomass had been produced in the greenhouse for 30 days37, the plants were pretreated for 10 days in larger basins without harvesting to minimize P history effects13. Nutrient solutions were replaced 3 times a week. After this period13, 7.5 g (surface cover of 90%) of carefully dry-blotted A. filiculoides was transferred to a 1 l glass aquarium (10 cm × 10 cm × 12 cm; L × W × H; 750 g FW m−2), which was continuously fed by the same nutrient solution (pH = 7.3) as during the acclimatization period, at a rate of 1 l d−1 using peristaltic pumps (Masterflex L/S; Cole-Palmer, Chicago, IL, USA). Nutrient concentrations were checked biweekly. Each P treatment had 5 replicates. The water level was kept constant by an overflow outlet. Aquaria were randomly placed in a temperature controlled water bath at 18 °C situated in the greenhouse facilities, at a mean day temperature of 21.2 °C (SE ± 0.007) and night temperature of 18.6 °C (SE ± 0.007) during the experimental period of 21 days. Light was mostly natural, but an artificial light regime of 16 h: 8 h (light: dark) was maintained by four 400 W high-pressure sodium lamps (Hortilux-Schréder, Monster, The Netherlands) to illuminate the experiment whenever light intensity fell below 250 W m−2 (300 µmol m−2 s−1 PAR; Quantum sensor, Skye Instruments LTD, Wales, England). Sides of the aquaria were covered with black plastic foil to avoid light penetration from the sides.
Growth measurement
Total plant biomass in each aquarium was determined at 0, 7, 14 and 21 days and subsamples were taken to determine fresh weight (FW) to dry weight (DW) ratios. Samples were rinsed, carefully blotted dry and weighed. After FW determination, 7.5 g (750 g FW m−2) of A. filiculoides was returned into its original aquarium to prevent crowding effects. The rest of the biomass was dried for 48 h at 60 °C, ground and homogenized using a ball mill (type Mixer Mill 301, Retsch GmbH, Haan, Germany) for further analyses. From the biomass increase, relative growth rates were calculated (RGR; g g−1 DW d−1).
Plant nutrient analyses
200 mg dried plant material was digested using 4 ml HNO3 (65%) and 1 ml H2O2 (35%) in Teflon vessels using an Ethos D microwave (1200 MLS, Milestone, Sorisole, Italy), after which digestates were analyzed for P, Fe, aluminum (Al), manganese (Mn), sodium (Na), sulfur (S), silicon (Si), zinc (Zn) using an inductively coupled plasma emission spectrophotometer (ICP-OES; model IRIS Intrepid II XDL, Thermo Fisher Scientific, Franklin, USA). N contents of 3 mg homogenized dried plant material were determined by an elemental CNS analyzer (model NA 1500, Carlo Erba; Thermo Fisher Scientific, Franklin, USA). Plant N and P sequestration rates were calculated by combining plant biomass production and N and P contents for every harvest. As A. filiculoides was grown in an N-free medium, the increase in plant N could fully be attributed to N2 fixation by the diazotrophs.
Iron versus nitrogen limitation experiment
To determine which element caused the chlorotic appearance of A. filiculoides found at 100 µmol P l−1, the original experiment was repeated for this treatment. Azolla may turn yellow/chlorotic due to the depletion of chlorophyll1. Also, Fe is often a limiting element for Azolla because it is an essential component of nitrogenase1,28. Chlorosis induced by iron deficiency, however, does not always implicate low total Fe contents in plant tissue58–62, because Fe may be immobilized in the apoplast and not be physiologically available63,64. Iron deficiency can be identified optimally when spraying plants with Fe, which causes regreening and an increase of chlorophyll a + b content57,59,61,65. To test N limitation, increasing both NO3− and NH4+ in the medium is the optimal approach45. When A. filiculoides became chlorotic after 5 weeks, plants were daily treated with iron (sprayed with Fe-EDTA [Pro Analysis quality]; 10 µmol l−1) or nitrogen (NH4-NO3 [Pro Analysis quality]; 100 µmol l−1) for 2 weeks. Both methods were used to ensure optimal uptake of either Fe or N. To ensure that there would not be a time effect on the chlorotic appearance of A. filiculoides, the original treatment was continued (control, 100 µmol P l−1). Treatments were replicated 4 times. Nutrient content and chlorophyll a + b content66 were determined in all treatments at the end of the experiment, after 2 weeks of Fe and N application.
Statistical analyses
Homogeneity of variance and normality of the residuals were tested, and Analyses of Variances (ANOVAs) were used to analyze potential differences in RGR, N and P sequestration rates and plant chlorophyll a + b in response to different P concentrations or to element addition (response chlorophyll a + b). Differences between treatments were determined using Tukey HSD post hoc tests. Data of P sequestration were square root transformed to authorize the use of parametric analyses. Linear mixed models (LMMs) were used to analyze differences in plant P and N content and N: P ratio over time at different P concentrations. Time was nested in aquaria and the covariance type was selected based on the smallest Hurvich and Tsai’s Criterion (AICC). P content or N: P ratio of the plants was set as a dependent variable while the P treatment was set as a fixed factor. Overall effects and P treatments were displayed in Estimated Marginal Means of Fitted Models and the main effects were compared using Bonferroni pairwise comparisons. Plant P content was log transformed and N: P ratios were square root transformed. All statistical analyses were carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, U.S.A.).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to thank Jelle Eygensteyn, Paul van der Ven and Sebastian Krosse for their help with chemical analyses. Furthermore, we thank Gerard van der Weerden for the use of the experimental greenhouse facilities. RJMT, SFH and MMLvK were supported by the European Union GO ERDF 2007–2013 (Water-Rijk, Rich Water World). RJMT was also supported by TTW-Open Technology Program grant “Bridging Thresholds” (#14424).
Author Contributions
R.J.M.T., A.J.P.S., L.P.M.L. and M.M.L.v.K. designed the experiments. R.J.M.T., S.F.H., R.C.J.H.P. and G.v.D. performed the experiments. R.J.M.T. analyzed the data. R.J.M.T., S.F.H., A.J.P.S., G.v.D., L.P.M.L. and M.M.L.v.K. interpreted the results and co-wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22760-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wagner G. Azolla: A review of its biology and utilization. The Botanical Review. 1997;63:1–26. doi: 10.1007/BF02857915. [DOI] [Google Scholar]
- 2.Sadeghi R, Zarkami R, Sabetraftar K, Van Damme P. A review of some ecological factors affecting the growth of Azolla spp. Caspian Journal of Environmental Sciences. 2013;11:65–76. [Google Scholar]
- 3.Lumpkin T, Plucknett D. Azolla: Botany, physiology, and use as a green manure. Economic Botany. 1980;34:111–153. doi: 10.1007/BF02858627. [DOI] [Google Scholar]
- 4.Valderrama A, Tapia J, Peñailillo P, Carvajal DE. Water phytoremediation of cadmium and copper using Azolla filiculoides Lam. in a hydroponic system. Water and Environment Journal. 2013;27:293–300. [Google Scholar]
- 5.van Hove, C. & Lejeune, A. In Cyanobacteria in Symbiosis (Eds N. Rai, B. Bergman & U. Rasmussen) (Kluwer Academic Press, 2002).
- 6.Baker JA, Entsch B, McKay DB. The cyanobiont in an Azolla fern is neither Anabaena nor Nostoc. FEMS Microbiology Letters. 2003;229:43–47. doi: 10.1016/S0378-1097(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 7.Ran L, et al. Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLoS ONE. 2010;5:e11486. doi: 10.1371/journal.pone.0011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore AW. Azolla: Biology and agronomic significance. The Botanical Review. 1969;35:17–34. doi: 10.1007/BF02859886. [DOI] [Google Scholar]
- 9.Peters GA, Meeks JC. The Azolla-anabaena symbiosis: basic biology. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:193–210. doi: 10.1146/annurev.pp.40.060189.001205. [DOI] [Google Scholar]
- 10.Singh RP, Singh PK. Effect of nitrogen fertilizers on nitrogen fixation and heterocyst frequency of cyanobacterium Anabaena azollae in 7 species of Azolla. Biochemie und Physiologie der Pflanzen. 1989;185:429–433. doi: 10.1016/S0015-3796(89)80068-X. [DOI] [Google Scholar]
- 11.Hechler WD, Dawson JO. Factors affecting nitrogen fixation in Azolla caroliniana. Transactions of the Illinois State Academy of Science. 1995;88:97–107. [Google Scholar]
- 12.Shilton AN, Powell N, Guieysse B. Plant based phosphorus recovery from wastewater via algae and macrophytes. Curr Opin Biotechnol. 2012;23:884–889. doi: 10.1016/j.copbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Peeters ETHM, Neefjes REM, Zuidam BGv. Competition between Free-Floating Plants Is Strongly Driven by Previously Experienced Phosphorus Concentrations in the Water Column. PLOS ONE. 2016;11:e0162780. doi: 10.1371/journal.pone.0162780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forni C, Chen J, Tancioni L, Grilli Caiola M. Evaluation of the fern Azolla for growth, nitrogen and phosphorus removal from wastewater. Water Research. 2001;35:1592–1598. doi: 10.1016/S0043-1354(00)00396-1. [DOI] [PubMed] [Google Scholar]
- 15.Costa, M. L., Santos, M. C. R., Carrapio, F., and Pereira, A. L. Azolla-anabaena’s behaviour in urban wastewater and articial media influence of combined nitrogen. Water Research43 (2009). [DOI] [PubMed]
- 16.Song U, Park H, Lee E. Ecological responses and remediation ability of water fern (Azolla japonica) to water pollution. J. Plant Biol. 2012;55:381–389. doi: 10.1007/s12374-012-0010-5. [DOI] [Google Scholar]
- 17.Tang Y, et al. Aquatic macrophytes can be used for wastewater polishing but not for purification in constructed wetlands. Biogeosciences. 2017;14:755–766. doi: 10.5194/bg-14-755-2017. [DOI] [Google Scholar]
- 18.Peñuelas, J. et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun4, 10.1038/ncomms3934 (2013). [DOI] [PubMed]
- 19.Brouwer P, et al. Azolla domestication towards a biobased economy? New Phytologist. 2014;202:1069–1082. doi: 10.1111/nph.12708. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury ATMA, Kennedy IR. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biology and Fertility of Soils. 2004;39:219–227. doi: 10.1007/s00374-003-0706-2. [DOI] [Google Scholar]
- 21.de Macale M, Vlek PG. The role of Azolla cover in improving the nitrogen use efficiency of lowland rice. Plant and Soil. 2004;263:311–321. doi: 10.1023/B:PLSO.0000047742.67467.50. [DOI] [Google Scholar]
- 22.Bhuvaneshwari K, Singh PK. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. Biotech. 2015;5:523–529. doi: 10.1007/s13205-014-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Vuuren DP, Bouwman AF, Beusen AHW. Phosphorus demand for the 1970-2100 period: A scenario analysis of resource depletion. Global Environmental Change-Human and Policy Dimensions. 2010;20:428–439. doi: 10.1016/j.gloenvcha.2010.04.004. [DOI] [Google Scholar]
- 24.Carpenter RS, Bennett EM. Reconsideration of the planetary boundary for phosphorus. Environmental Research Letters. 2011;6:014009–014009. doi: 10.1088/1748-9326/6/1/014009. [DOI] [Google Scholar]
- 25.Brouwer, P. et al. Metabolic Adaptation, a Specialized Leaf Organ Structure and Vascular Responses to Diurnal N2 Fixation by Nostoc azollae Sustain the Astonishing Productivity of Azolla Ferns without Nitrogen Fertilizer. Frontiers in Plant Science8, 10.3389/fpls.2017.00442 (2017). [DOI] [PMC free article] [PubMed]
- 26.van Diggelen JMH, et al. New Insights into Phosphorus Mobilisation from Sulphur-Rich Sediments: Time-Dependent Effects of Salinisation. PLoS ONE. 2014;9:e111106. doi: 10.1371/journal.pone.0111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zak D, Wagner C, Payer B, Augustin J, Gelbrecht J. Phosphorus mobilization in rewetted fens: the effect of altered peat properties and implications for their restoration. Ecological Applications. 2010;20:1336–1349. doi: 10.1890/08-2053.1. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe, I. In Microbiology of Tropical Soils and Plant Productivity. Developments in Plant and Soil Sciences Vol. 5 (eds Y. R. Dommergues & H. G. Diem) 169–185 (Springer Netherlands, 1982).
- 29.Rains, D. W. & Talley, S. N. In Nitrogen and rice symposium proceedings. (International Rice Research Institute, Los Baons, Philippines).
- 30.Cheng W, Sakai H, Matsushima M, Yagi K, Hasegawa T. Response of the floating aquatic fern Azolla filiculoides to elevated CO2, temperature, and phosphorus levels. Hydrobiologia. 2010;656:5–14. doi: 10.1007/s10750-010-0441-2. [DOI] [Google Scholar]
- 31.Singh RP, Singh PK. Symbiotic algal nitrogenase activity and heterocyst frequency in seven Azolla species after phosphorus fertilization. Hydrobiologia. 1988;169:313–318. doi: 10.1007/BF00007554. [DOI] [Google Scholar]
- 32.Cary PR, Weerts PGJ. Growth and nutrient composition of Azolla pinnata R. Brown and Azolla filiculoides Lamarck as affected by water temperature, nitrogen and phosphorus supply, light intensity and pH. Aquatic Botany. 1992;43:163–180. doi: 10.1016/0304-3770(92)90041-G. [DOI] [Google Scholar]
- 33.Kushari DP, Watanabe I. Differential responses of azolla to phosphorus deficiency. Soil Science and Plant Nutrition. 1991;37:271–282. doi: 10.1080/00380768.1991.10415037. [DOI] [Google Scholar]
- 34.Subudhi BPR, Watanabe I. Differential phosphorus requirements of Azolla species and strains in phosphorus-limited continuous culture. Soil Science and Plant Nutrition. 1981;27:237–247. doi: 10.1080/00380768.1981.10431275. [DOI] [Google Scholar]
- 35.Moretti A, Siniscalco Gigliano G. Influence of light and pH on growth and nitrogenase activity on temperate-grown Azolla. Biology and Fertility of Soils. 1988;6:131–136. doi: 10.1007/BF00257662. [DOI] [Google Scholar]
- 36.van Kempen MML, Smolders AJP, Bögemann GM, Lamers LPM, Roelofs JGM. Interacting effects of atmospheric CO2 enrichment and solar radiation on growth of the aquatic fern Azolla filiculoides. Freshwater Biology. 2016;61:596–606. doi: 10.1111/fwb.12724. [DOI] [Google Scholar]
- 37.van Kempen MML, et al. Responses of the Azolla filiculoides Stras.–Anabaena azollae Lam. association to elevated sodium chloride concentrations: Amino acids as indicators for salt stress and tipping point. Aquatic Botany. 2013;106:20–28. doi: 10.1016/j.aquabot.2012.12.006. [DOI] [Google Scholar]
- 38.Peters GA, et al. Characterization and comparison of five N2-fixing Azolla-Anabaena associations, I. Optimization of growth conditions for biomass increase and N content in a controlled environment. Plant Cell & Environment. 1980;3:261–269. [Google Scholar]
- 39.van Hove, C. Azolla and its multiple uses with emphasis on africa. Technical report, Food and Agricultural Organization of the United Nations (FAO), Rome, Italy. (1989).
- 40.Atkinson MJ, Smith SV. C:N:P ratios of benthic marine plants. Limnology and Oceanography. 1983;28:568–574. doi: 10.4319/lo.1983.28.3.0568. [DOI] [Google Scholar]
- 41.Hellström T. An empirical study of nitrogen dynamics in lakes. Water Environment Research. 1996;68:55–65. doi: 10.2175/106143096X127208. [DOI] [Google Scholar]
- 42.Ekholm, P. N:P ratios in estimating nutrient limitation in aquatic systems. Finnish Environment Institute (2008).
- 43.Epstein, E. Plant Biochemistry. (Academic Press, 1965).
- 44.Marschner, H. Mineral nutrietion of higher plants. (Academic, 2nd edition edition, 1995).
- 45.Ito O, Watanabe I. The relationship between combined nitrogen uptakes and nitrogen fixation in Azolla-anabaena symbiosis. New Phytologist. 1983;95:647–654. doi: 10.1111/j.1469-8137.1983.tb03528.x. [DOI] [Google Scholar]
- 46.Singh PK, Singh DP, Singh RP. Growth, acetylene reduction activity, nitrate uptake and nitrate reductase activity of Azolla caroliniana and Azolla pinnata at varying nitrate levels. Biochemie und Physiologie der Pflanzen. 1992;188:121–127. doi: 10.1016/S0015-3796(11)80019-3. [DOI] [Google Scholar]
- 47.Manna AB, Singh PK. Effects of nitrogen fertilizer application methods on growth and acetylene reduction activity of Azolla pinnata and yield of rice. Fertilizer research. 1991;28:25–30. doi: 10.1007/BF01048852. [DOI] [Google Scholar]
- 48.Zak D, Gelbrecht J. The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany) Biogeochemistry. 2007;85:141–151. doi: 10.1007/s10533-007-9122-2. [DOI] [Google Scholar]
- 49.Mengel K. Iron availability in plant tissues-iron chlorosis on calcareous soils. Plant and Soil. 1994;165:275–283. doi: 10.1007/BF00008070. [DOI] [Google Scholar]
- 50.Kosegarten H, Koyro H-W. Apoplastic accumulation of iron in the epidermis of maize (Zea mays) roots grown in calcareous soil. Physiologia Plantarum. 2001;113:515–522. doi: 10.1034/j.1399-3054.2001.1130410.x. [DOI] [Google Scholar]
- 51.Rediske JH, Biddulph O. The Absorption and Translocation of Iron. Plant Physiology. 1953;28:576. doi: 10.1104/pp.28.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrissey J, Guerinot ML. Iron uptake and transport in plants: The good, the bad, and the ionome. Chemical reviews. 2009;109:4553–4567. doi: 10.1021/cr900112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo S, Mikkelsen DS. Effect of P and Mn on growth response and uptake of Fe, Mn and P by sorghum. Plant and Soil. 1981;62:15–22. doi: 10.1007/BF02205021. [DOI] [Google Scholar]
- 54.Römheld V. The chlorosis paradox: Fe inactivation as a secondary event in chlorotic leaves of grapevine. Journal of Plant Nutrition. 2000;23:1629–1643. doi: 10.1080/01904160009382129. [DOI] [Google Scholar]
- 55.Fageria VD. Nutrient interactions in crop plants. Journal of Plant Nutrition. 2001;24:1269–1290. doi: 10.1081/PLN-100106981. [DOI] [Google Scholar]
- 56.Olsen C. Iron absorption and chlorosis in green plants. Compt. rend. trav. lab. Carlsberg, Sér. chim. 1935;21:15–52. [Google Scholar]
- 57.Rajaie, M. & Tavakoly, A. R. Iron and/or acid foliar spray versus soil application of Fe-EDDHA for prevention of iron deficiency in Valencia orange grown on a calcareous soil. Journal of Plant Nutrition, 1–9, 10.1080/01904167.2017.1382523 (2017).
- 58.De Kock PC, Wallace A. Excess Phosphorus and Iron Chlorosis. California Agriculture. 1965;19:3–4. [Google Scholar]
- 59.Bienfait HF. Prevention of stress in iron metabolism of plants. Acta Botanica Neerlandica. 1989;38:105–129. doi: 10.1111/j.1438-8677.1989.tb02035.x. [DOI] [Google Scholar]
- 60.Smolders AJP, Roelofs JGM. The roles of internal iron hydroxide precipitation, sulphide toxicity and oxidizing ability in the survival of Stratiotes aloides roots at different iron concentrations in sediment pore water. New Phytologist. 1996;133:253–260. doi: 10.1111/j.1469-8137.1996.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 61.Smolders AJP, Hendriks RJJ, Campschreur HM, Roelofs JGM. Nitrate induced iron deficiency chlorosis in Juncus acutiflorus. Plant and Soil. 1997;196:37–45. doi: 10.1023/A:1004293012462. [DOI] [Google Scholar]
- 62.Mengel K, Bubl W, Scherer HW. Iron distribution in vine leaves with HCO3− induced chlorosis. Journal of Plant Nutrition. 1984;7:715–724. doi: 10.1080/01904168409363236. [DOI] [Google Scholar]
- 63.Mengel K, Geurtzen G. Relationship between iron chlorosis and alkalinity in Zea mays. Physiologia Plantarum. 1988;72:460–465. doi: 10.1111/j.1399-3054.1988.tb09151.x. [DOI] [Google Scholar]
- 64.Sattelmacher B. The apoplast and its significance for plant mineral nutrition. New Phytologist. 2001;149:167–192. doi: 10.1046/j.1469-8137.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 65.Fernández V, Ebert G. Foliar Iron Fertilization: A Critical Review. Journal of Plant Nutrition. 2005;28:2113–2124. doi: 10.1080/01904160500320954. [DOI] [Google Scholar]
- 66.Harpenslager SF, Lamers LPM, van der Heide T, Roelofs JGM, Smolders AJP. Harnessing facilitation: Why successful re-introduction of Stratiotes aloides requires high densities under high nitrogen loading. Biological Conservation. 2016;195:17–23. doi: 10.1016/j.biocon.2015.12.031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.