Abstract
Purpose
We report a case of acute macular neuroretinopathy (AMN) following routine annual inactivated influenza vaccination. Projection-resolved optical coherence tomography angiography (PR-OCTA) was used to analyze the retinal capillary flow within the AMN lesion.
Observations
Our patient reported visual symptoms of her right eye nine days after routine annual influenza vaccination. Multimodal imaging revealed small vessel peripheral vasculitis and AMN in the affected eye. Infectious, immunologic, and hypercoagulable etiologies were investigated and excluded. PR-OCTA B-scans within the AMN lesion demonstrated reduced flow in the deep capillary plexus (DCP) at baseline with relatively improved flow signal in the DCP on follow up, 3 weeks later.
Conclusions and importance
We report a new association of AMN following routine inactivated influenza immunization. Recent influenza vaccination should be included in the differential diagnosis for patients presenting with AMN. PR-OCTA demonstrated compromised DCP flow in the AMN lesion which has not been previously described.
Keywords: Influenza vaccine, Vasculitis, Acute macular neuroretinopathy, AMN, Optical coherence tomography angiography, OCTA
1. Introduction
Influenza immunization has been shown to be one of the most effective public health measures in reducing morbidity and mortality from seasonal influenza. Vaccination is generally recommended for all health-care personnel and individuals six months of age and older.12,14 Many large studies have shown it to be safe and effective with an estimated 147.8 million vaccinations distributed in the US during the 2014-15 season according to the Centers for Disease Control and Prevention [CDC, 2015].3,4,14,15 Common side-effects of the influenza vaccine include soreness and/or swelling at the sight of injection, headache, fever, nausea, and muscle aches which are generally mild and self-limiting.16 However, sporadic cases of more serious post influenza vaccine related complications such as systemic vasculitis and Guillain-Barre syndrome have been reported.2,8,11,12,17 Ocular involvement is extremely rare and to our knowledge there are only 2 reported cases of isolated ocular complications following influenza vaccination.5,6
Acute macular neuroretinopathy (AMN) is a rare though increasingly diagnosed disorder of the outer retinal layers that can affect one or both eyes with symptoms ranging from paracentral scotomas to impaired visual acuity. Originally it was described in otherwise healthy, young to middle-aged females on oral contraceptives.1 Although the exact pathophysiology remains unknown, various vascular pathologies have been associated with AMN including post-viral illness, use of vasoconstrictive agents, trauma, and hypovolemic shock.13 More recently, anemia, thrombocytopenia and conditions causing hypovolemia and hyperviscosity have been associated with AMN.7
We present herein a case of a 47-year-old Caucasian female with post-influenza vaccine related ocular small vessel vasculitis and AMN.
2. Case report
A 47-year-old Caucasian female presented to the Northwestern University retina service with a 15-day history of a “greyish” central spot in her right eye. Her medical history was significant for Raynaud's phenomenon, Barrett's esophagus and herpes labialis. She was taking vitamin D supplements, omeprazole daily and valacyclovir 1000 mg as needed. She denied any recent travel. She had received a routine annual intramuscular influenza vaccine (FLUVIRIN® PF, 0.5 ml suspension for intramuscular injection, 2016–2017 formula) approximately 3 weeks prior to presentation and reported onset of visual symptoms 9 days following vaccination. She denied use of over-the-counter flu medications or decongestants.
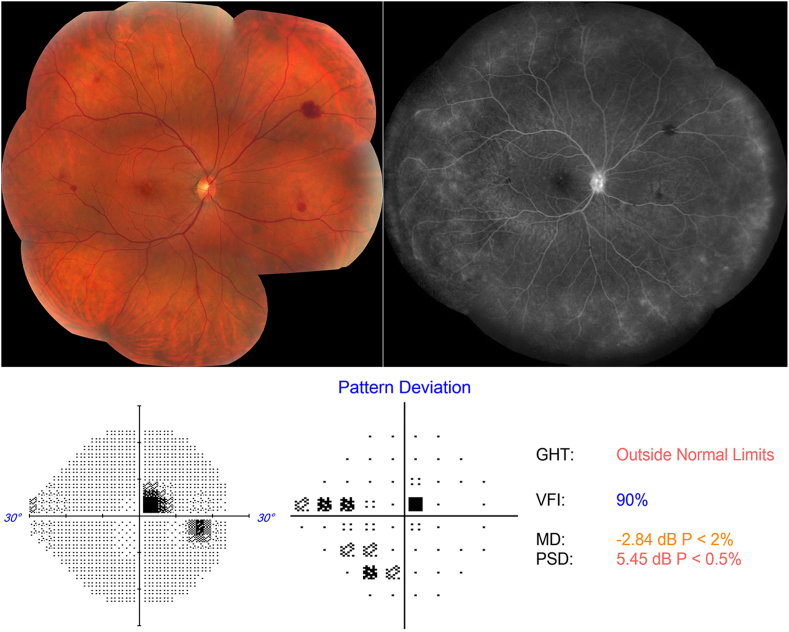
On examination, her best-corrected visual acuity (BCVA) was 20/20 Snellen in both eyes. The anterior segment exam was unremarkable in either eye. Fundus examination of her right eye revealed a few intra-retinal blot hemorrhages along with pigment mottling and few drusen in the macula (Fig. 1). The left eye was unremarkable except for a few macular drusen. A 24-2 Humphrey visual field (HVF) demonstrated a right superotemporal paracentral scotoma (Fig. 1).
Fig. 1.
Color fundus photo, fluorescein angiogram (FA) and Humphrey 24–2 Threshold perimetry on presentation.
Left: Color fundus photo montage of the right eye shows macular pigment mottling and mid-peripheral intra-retinal blot hemorrhages. Right: late FA shows staining of the disc and diffuse leakage and staining of the peripheral capillary vasculature. Bottom: Demonstrates a right superotemporal paracentral scotoma. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Chest X-ray and initial laboratory work-up including complete blood count, comprehensive metabolic panel, lipids and glycated hemoglobin (HbA1c) were normal. Hyperviscosity and hypercoaguability studies including protein electrophoresis, homocysteine, protein C/S, Factor V Leiden, and serum viscosity were also unremarkable. Inflammatory markers (ESR, CRP, anticardiolipin Ab, antiphospholipid III Ab, C and P ANCA) were within normal limits. Testing for syphilis (RPR), Bartonella, tuberculosis (QuantiFERON® Gold), and HIV were negative.
Fluorescein angiogram demonstrated normal transit times and changes consistent with mild vasculitis with late optic nerve staining and peripheral perivascular leakage in the right eye (Fig. 1). Indocyanine green angiography and B-scan ultrasonography were normal.
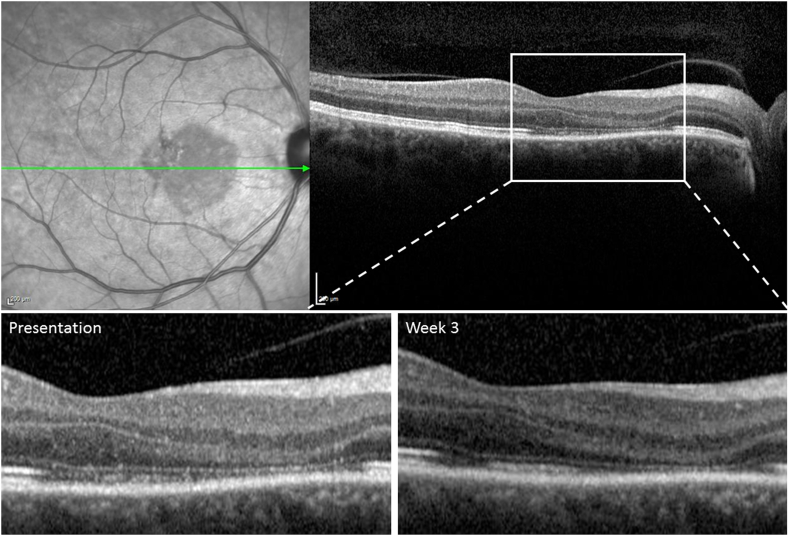
Spectral-domain optical coherence tomography (SD-OCT) taken at another institution, 4 days after the onset of symptoms, showed diffuse hyper-reflectivity of the outer plexiform layer (OPL), Henle's layer and the outer nuclear layer (ONL) at and nasal to the fovea. Near-infrared imaging of the right eye on presentation to our service showed a well demarcated wedge-shaped hypo-reflective macular lesion involving the fovea (Fig. 2, Fig. 3). SD-OCT through the affected area revealed thinning of the ONL and disruption of the ellipsoid zone (EZ) and interdigitation zone (IZ) (Fig. 2, Fig. 3). The findings of early hyper-reflectivity of the OPL and ONL followed later by thinning of the ONL and disruption of the EZ/IZ are consistent with AMN.10
Fig. 2.
Spectral Domain (SD-OCT) within the acute macular neuroretinopathy (AMN) lesion on presentation and week 3.
Top Left: On presentation, near-Infrared imaging shows wedge-shaped hypo-reflective macular lesion. Top Right, Bottom Left: SD-OCT shows thinning of the outer nuclear layer and disruption of the ellipsoid and interdigitation zones. Bottom Right: 3 weeks later, SD-OCT shows persistent thinning of outer nuclear layer with some restoration of the ellipsoid and interdigitation zones.
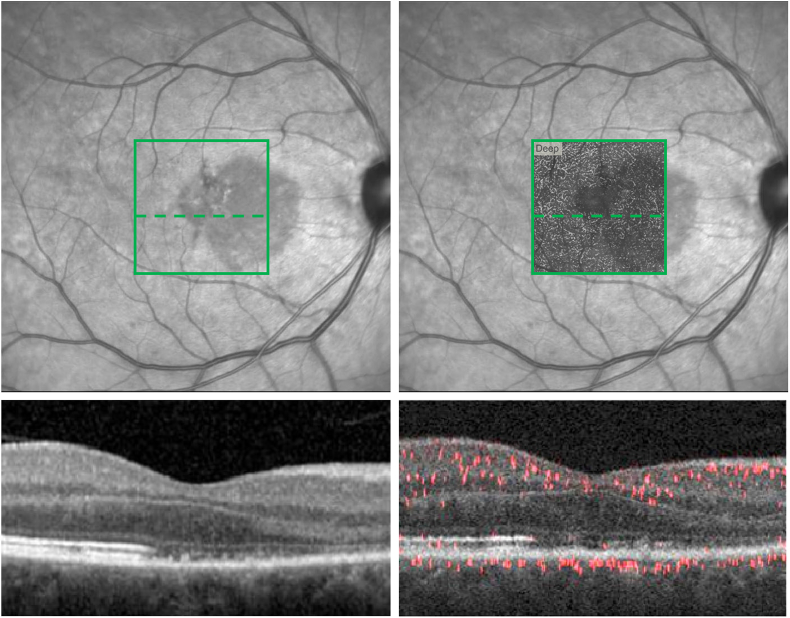
Fig. 3.
Spectral Domain (SD-OCT) and Projection-Resolved (PR-OCTA) overlay within the acute macular neuroretinopathy (AMN) lesion at presentation.
Top Left: Near-Infrared image with represented 3 × 3mm2en face PR-OCTA area (green square) and corresponding cross-sectional segment (dashed green line). Bottom left: Cross-sectional SD-OCT within the AMN lesion in the represented 3 × 3mm2 area. Top Right: Near-Infrared image with 3 × 3mm2en face PR-OCTA deep capillary plexus (DCP) slab overlay and corresponding cross-sectional segment (dashed green line). Bottom Right: Cross-sectional PR-OCTA within the AMN lesion. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Optical coherence tomography angiography (OCTA) using RTVue-XR Avanti system (Optovue Inc., Fremont, California) was performed. En face imaging of the capillary layers was limited by projection and segmentation artifacts in the automated software due to the irregularity of the OPL, ONL and EZ of the right eye. Therefore, we applied projection-resolved (PR-OCTA) post-processing to reduce artifacts from superficial vessels projected onto the deeper capillary layers and to facilitate accurate localization of the three capillary plexuses: superficial (SCP), middle (MCP), and deep capillary plexus (DCP) (Fig. 3, Fig. 4). We implemented a version of the PR-OCTA algorithm developed by Zhang et al.18 This algorithm has been used to study the three capillary plexuses of the inner retina in the macula.22 The authors noted that the OCTA projection tail artifacts have lower decorrelation values than real vessels. Taking advantage of this observation, the algorithm searches each A-scan for successive, high-valued peaks, which correspond to real vessels. The OCTA values at the peak positions are kept, while the remaining pixels in the A-scan are set to zero, resulting in the removal of projection artifacts.
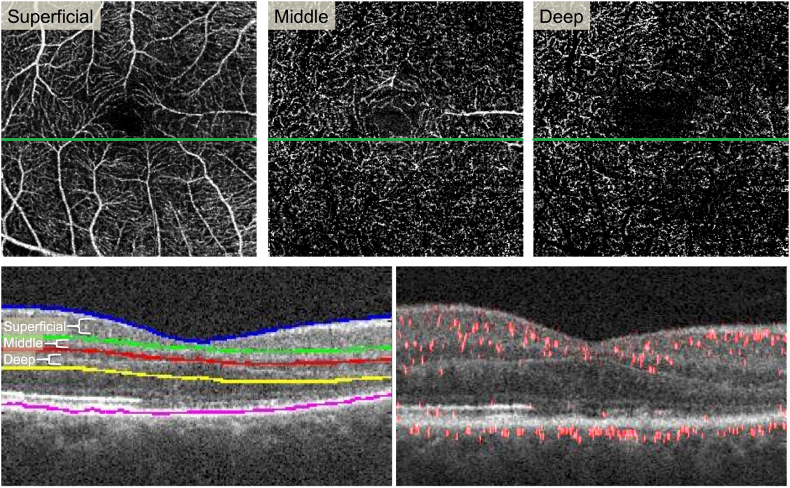
Fig. 4.
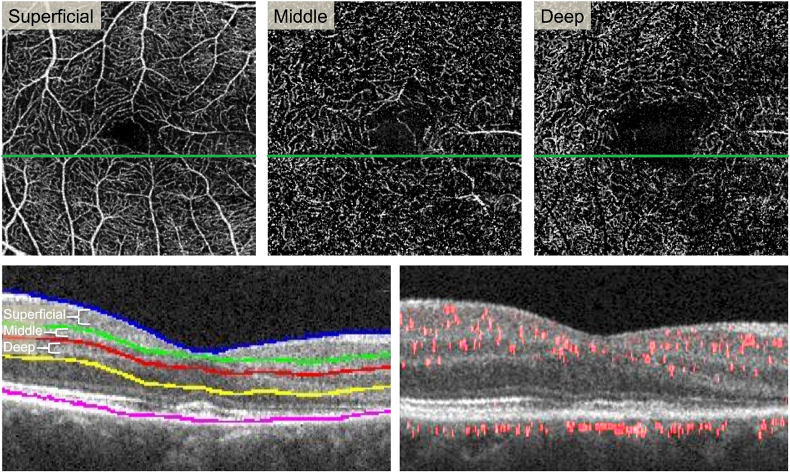
Projection-resolved (PR-OCTA) reveals attenuated flow signal in the deep capillary plexus.
Top Row:En face PR-OCTA projections of the superficial, middle, and deep capillary plexuses. Some large superficial vessels are seen in the middle plexus due to segmentation error. The deep plexus shows signal attenuation in the areas of the lesion (far right). Bottom Left: Cross-sectional SD-OCT showing segmentation lines for the three plexuses. Bottom Right: Cross-sectional PR-OCTA with red flow overlay reveals reduced red flow signal in the inner nuclear layer and outer plexiform layer in the area of the lesion (right half). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We used a previously described method to manually adjust the segmentation based on known anatomical locations of the three plexuses.23 The SCP was segmented to include the nerve fiber layer and ganglion cell layer. The MCP encompassed the outer border of the inner plexiform layer and superficial boundary of the inner nuclear layer (50 μm thick). The DCP included the boundary between the deep inner nuclear layer and outer plexiform layer (40 μm thick). The locations of the 3 plexuses have been established through immunohistochemical methods.24 We then verified that there were no segmentation errors in the area of AMN. We assessed the blood flow within each plexus using the PR-OCTA B-scans. By directly evaluating the layers that were affected using structural OCT included in the PR-OCTA B-scans and knowledge of the anatomical location of each plexus, we eliminated any potential segmentation issues. The post-processed PR-OCTA B-scans with flow overlay through the AMN lesion demonstrated attenuation of flow signal at the level of the DCP, which is located at the junction between the inner nuclear layer and the OPL (Fig. 4).
At her 3-week follow-up, BCVA remained 20/20 Snellen in both eyes but she continued to complain of a right paracentral scotoma. Fundus examination showed near resolution of the few scattered intra-retinal blot hemorrhages and repeat SD-OCT demonstrated similar thinning of the ONL but with some restoration of the EZ/IZ (Fig. 2). OCTA B-scans, as well as PR-OCTA, revealed persistent decrease but relatively improved flow signal in the DCP compared to the initial scan suggesting partial reperfusion of the DCP (Fig. 5).
Fig. 5.
Projection-resolved (PR-OCTA) on follow-up shows increased flow signal in the deep capillary plexus.
Top Row:En face PR-OCTA projections of the superficial, middle, and deep capillary plexuses. Some large superficial vessels are seen in the middle plexus due to segmentation error (center). The deep plexus shows some signal attenuation in the areas of the lesion (far right), but more flow compared to presentation (Fig. 4). Bottom Left: Cross-sectional SD-OCT showing segmentation lines for the three plexuses. Bottom Right: Cross-sectional PR-OCTA with red flow overlay reveals some recovery of flow signal in the inner nuclear layer and outer plexiform layer in the area of the lesion (right half).
3. Discussion
Post-influenza vaccine related vasculitis has been described as an infrequent though severe complication in otherwise healthy individuals.8,11,12,17 There are only 2 previously reported cases of ocular complications following influenza vaccination. In 1980 Blumberg et al. was the first to report a possible association of influenza vaccination with uveitis and optic neuritis.6 In this case, the patient received a split-product of inactivated A/New Jersey swine and A/Victoria influenza immunization with onset of symptoms 11 days after immunization. Of note, the A/New Jersey swine vaccine used in 1976 was later linked to increased incidence of Guillain-Barré syndrome (GBS) indicating a higher risk for auto-immune related complications leading to its improved safety over the following years.2,17 More recently Williams described a case of anterior uveitis and retinal arterial vasculitis 8 weeks following routine annual inactivated influenza immunization.5 The influenza vaccine reported in this instance was similar to the one our patient received. Both cases from Blumberg et al. and Williams et al. reported no recurrences of uveitis or retinal vasculitis following topical 1% prednisolone treatment.
AMN is a rare disorder that affects the outer retinal layers. SD-OCT demonstrates initial hyper-reflectivity of OPL/ONL followed by ONL thinning and EZ/IZ disruption similar to our case.10 Several associations have been described with AMN, including use of oral contraceptives, post-viral illness, vasoconstrictive agents, trauma, hypovolemic shock, anemia, and thrombocytopenia.1,7,9,10 Viral, immune-mediated, and ischemic vascular etiologies have been proposed but the cause of AMN is still under investigation. Ischemia of the DCP located in the outermost portion of the INL has been proposed as the pathophysiologic mechanism for AMN.10 In addition, DCP ischemia has been shown to be associated with disruption of the photoreceptor layers in patients with diabetic retinopathy, highlighting the importance of this capillary plexus for the metabolic demand of photoreceptors.20,21 In the current report, standard OCTA imaging was limited by segmentation errors due to irregularity of the outer retinal layers as well as the presence of projection artifacts.19 PR-OCTA18 facilitated the analysis of the vasculature of the DCP in the AMN lesion and demonstrated reduced flow of the DCP in the affected area at presentation, with subsequent partial restoration of flow signal at the level of the DCP, 3 weeks later.22 These findings support the original hypothesis that DCP ischemia occurs early on in AMN with apparent reperfusion in later stages.10,19 Further investigation using PR-OCTA in larger series of AMN is recommended to confirm these observations.
Extensive laboratory testing excluded other infectious, immunologic, or hypercoagulable etiologies, and the timing of complications 9 days following vaccination, point towards an association between influenza vaccination and the ocular findings in our patient. This is consistent with other reports of the onset of post-vaccination systemic vasculitis on day 12 following vaccination.12,17 The findings of AMN in our patient are similar to those cases often described in a post-viral setting. Although the pathogenesis of AMN is complex, a microvascular etiology affecting the DCP is consistent with our PR-OCTA findings.19 It is plausible that small capillary retinal vasculitis induced by vaccination resulted in the mid-peripheral intra-retinal hemorrhages and fluorescein angiography findings and also lead to ischemia of the DCP. We postulate that the vasculitic changes in our case may have contributed to ischemia of the DCP precipitating AMN.
4. Conclusion
We report a unique presentation of retinal small vessel vasculitis and AMN following influenza vaccination that has not been previously described. PR-OCTA confirmed the involvement of the DCP in this case of AMN, with partial restoration of flow over time. While influenza vaccination is clearly justified as an important public health measure, our case adds to those reports of potential serious post-immunization complications. Further study is needed to confirm this association; in the meantime, clinicians are encouraged to elicit a history of recent influenza vaccination from patients presenting with AMN.
Patient consent
Written consent to publish case details was obtained from the patient.
Acknowledgements and disclosures
Funding
Supported in part by an unrestricted grant from Research to Prevent Blindness.
Conflicts of interest
All authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Bos P.J., Deutman A.F. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80(4):573–584. doi: 10.1016/0002-9394(75)90387-6. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz E.S., Schonberger L.B., Nelson D.B., Holman R.C. Guillain-Barré syndrome and the 1978-1979 influenza vaccine. N Engl J Med. 1981;304(26):1557–1561. doi: 10.1056/NEJM198106253042601. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo-santisteve P., Ciancio B.C., Nicoll A., Lopalco P.L. The importance of influenza prevention for public health. Hum Vaccin Immunother. 2012;8(1):89–95. doi: 10.4161/hv.8.1.19066. [DOI] [PubMed] [Google Scholar]
- 4.Grohskopf L.A., Olsen S.J., Sokolow L.Z. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) – United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- 5.Williams G.S., Evans S., Yeo D., Al-bermani A. Retinal artery vasculitis secondary to administration of influenza vaccine. BMJ Case Rep. 2015 doi: 10.1136/bcr-2015-211971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg S., Bienfang D., Kantrowitz F.G. A possible association between influenza vaccination and small-vessel vasculitis. Arch Intern Med. 1980;140(6):847–848. [PubMed] [Google Scholar]
- 7.Munk M.R., Jampol L.M., Cunha souza E. New associations of classic acute macular neuroretinopathy. Br J Ophthalmol. 2016;100(3):389–394. doi: 10.1136/bjophthalmol-2015-306845. [DOI] [PubMed] [Google Scholar]
- 8.Mader R., Narendran A., Lewtas J. Systemic vasculitis following influenza vaccination–report of 3 cases and literature review. J Rheumatol. 1993;20(8):1429–1431. [PubMed] [Google Scholar]
- 9.Garg A., Shah A.N., Richardson T., O'sullivan E., Eleftheriadis H. Early features in acute macular neuroretinopathy. Int Ophthalmol. 2014;34(3):685–688. doi: 10.1007/s10792-013-9850-3. [DOI] [PubMed] [Google Scholar]
- 10.Fawzi A.A., Pappuru R.R., Sarraf D. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina (Philadelphia, Pa) 2012;32(8):1500–1513. doi: 10.1097/IAE.0b013e318263d0c3. [DOI] [PubMed] [Google Scholar]
- 11.Gavaghan T., Webber C.K. Severe systemic vasculitic syndrome post influenza vaccination. Aust N Z J Med. 1993;23(2):220. doi: 10.1111/j.1445-5994.1993.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 12.Nichol K.L., Lind A., Margolis K.L. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333(14):889–893. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 13.Bhavsar K.V., Lin S., Rahimy E. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer D., Barr I., Hurt A. Seasonal influenza vaccine policies, recommendations and use in the World Health Organization's Western Pacific Region. Western Pac Surveill Response J. 2013;4(3):51–59. doi: 10.5365/WPSAR.2013.4.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Flu Vaccination Coverage, United States, 2014-15 Influenza Season. National Immunization Survey-Flu (NIS-Flu) and Behavioral Risk Factor Surveillance System (BRFSS). Retrieved from https://www.cdc.gov/flu/fluvaxview/coverage-1415estimates.htm (Accessed April 15, 2017).
- 16.CDC. Flu Vaccine Safety Information. National Center for Immunization and Respiratory Diseases (NCIRD). Retrieved from https://www.cdc.gov/flu/protect/vaccine/general.htm (Accessed April 15, 2017).
- 17.Zafrir Y., Agmon-levin N., Shoenfeld Y. Post-influenza vaccination vasculitides: a possible new entity. J Clin Rheumatol. 2009;15(6):269–270. doi: 10.1097/RHU.0b013e3181b56177. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M., Hwang T.S., Campbell J.P. Projection-resolved optical coherence tomography angiography. Biomed Opt Express. 2016;7(3):816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashraf M., Goldstein D., Fawzi A. Optical coherence tomography angiography: potential artifacts in acute macular neuroretinopathy. JAMA Ophthalmol. 2017;135(6):675–676. doi: 10.1001/jamaophthalmol.2017.0918. [DOI] [PubMed] [Google Scholar]
- 20.Scarinci F., Nesper P.L., Fawzi A.A. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016;168:129–138. doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesper P.L., Scarinci F., Fawzi A.A. Adaptive optics reveals photoreceptor abnormalities in diabetic macular ischemia. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang T.S.Z.M., Bhavsar K. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA ophthalmol. 2016;134(12):1411–1419. doi: 10.1001/jamaophthalmol.2016.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.J., Soetikno B.T., Fawzi A.A. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina. 2016;36(11):2039–2050. doi: 10.1097/IAE.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan P.E., Paula K.Y., Balaratnasingam C. Quantitative confocal imaging of the retinal microvasculature in the human retina. Invest Ophthalmol Vis Sci. 2012;53(9):5728–5736. doi: 10.1167/iovs.12-10017. [DOI] [PubMed] [Google Scholar]