Highlights
-
•
In middle Gangetic plains, high arsenic concentration is present in water, which causes a significant health risk.
-
•
Two bacterial isolates, AK1 (KY569423) and AK9 (KY569424) were isolated and characterised for Arsenic detoxification.
-
•
aoxR, aoxB and aoxC genes were also observed in the isolated starin which help in arsenic detoxification by oxidation method.
Keyword: Arsenic, Bacteria, Bioremediation, Middle Gangetic plain, Oxidation
Abstract
In middle Gangetic plain, high arsenic concentration is present in water, which causes a significant health risk. Total 48 morphologically distinct arsenite resistant bacteria were isolated from middle Gangetic plain. The minimum inhibitory concentration (MIC) values of arsenite varied widely in the range 1–15 mM of the isolates. On the basis of their MIC, two isolates, AK1 (KY569423) and AK9 (KY569424) were selected. The analysis of the 16S rRNA gene sequence of selected isolates revealed that they are belong to the genus Pseudomonas. The AgNO3 test based microplate method revealed that isolates, AK1 and AK9, have potential in transformation of arsenic species. Further, the presence of aoxR, aoxB and aoxC genes in the both isolated strain AK1 and AK9 was confirmed, which play an important role in arsenic bioremediation by arsenite oxidation. Isolated strains also showed heavy metal resistance against Cr(IV), Ni(II), Co(II), Pb(II), Cu(II), Hg(II), Ag(I) and Cd(II).
1. Introduction
Arsenic is a toxic metalloid, widely distributed due to natural and anthropogenic activities in the environment. It occurs in four oxidation states (+5, +3, 0, and −3) although the arsenate (AsV) and arsenite (AsIII) are the most common forms and AsIII is more toxic than AsV [[1], [2]]. Drinking water and food are the main sources of exposure of arsenic to the consumers, including animals and humans. Arsenic species are deposited in the skin, lungs, kidney, liver, etc. and cause several severe diseases by oxidative stress, altered DNA methylation, altered DNA repair, mitochondrial damage, proliferation of cell, tumour promotion and co-carcinogenesis [[1], [3]]. It is reported that conventional methods such as oxidation or reduction, chemical precipitation, filtration, ion exchange, reverse osmosis and evaporation recovery of cleaning contaminated water are too much expensive and laborious [4]. So, there is need to develop eco-friendly and low cost technique to mitigate the arsenic contamination.
It is well documented that bioremediation is a cost-effective and a comparatively innocuous alternative to physical methods for heavy-metal remediation [[3], [4], [5], [6]]. Bioremediation of arsenic species by microbial community involves their reduction, oxidation, intracellular bioaccumulation and methylation [6]. The arsenite-oxidising ability to the bacteria is provided by aox operon. The expression of the aox operon is controlled by AoxR, after expression of the aox operon AoxAB complex is synthesized and exported to the periplasm. The AoxAB complex, an arsenite oxidase is involve in the oxidation of the AsIII into AsV [[6], [7], [8]].
Ganga river, originating from the Himalaya, is one of the natural source of arsenic in the Gangetic plain of Bihar. But there is no proof regarding the natural emission of arsenic in the Ganga plain so far [9]. However, the arsenic is released in the Gangetic plain of Bihar by the natural processes in groundwater from holocene sediments containing clay and silt [[10], [11]]. The concentration of arsenic in Gangetic plain of Bihar found above the permissible limit of 10 ppb [12]. So, there is an urgent need of remediation of these contaminated areas.
As far as our knowledge, study related to bioremediation of arsenic toxicity by employing arsenite-oxidising bacteria from arsenic contaminated groundwater of the middle Gangetic plain, Bihar, India is not available. So considering the importance of work in present study, we describe the stimulation of the indigenous bacteria for bioremediation of arsenic toxicity. The bacterial isolates were also evaluated for other heavy metal resistance such as Cr(IV), Ni(II), Co(II), Pb(II), Cu(II), Hg(II), Ag(I) and Cd(II).
2. Material and methods
2.1. Sample collection
The water samples were collected before the rainy season from handpumps of 12 different arsenic contaminated sites of Gangetic plain in the Bihar region. Water samples were collected in two different storage bottles, one sample was treated with 2–3 drops of nitric acid to prevent the metal from precipitation, adsorption and microbial degradation. Another water sample was used for isolation of bacteria hence kept as it is at ice. The selected sites were namely Karja (25°39′09.2″N 84°42′27.7″E), Pararia (25°24′05.9″N 84°34′41.8″E), Bhakhura (25°38′21.1″N 84°09′00.2″E), Semaria (25°40′27.8″N 84°43′26.6″E), Keshopur (25°39′54.7″N 84°43′06.6″E), Maner Thana (25°38′51.6″N 84°53′07.6″E), Danapur (25°34′56.4″N 85°02′36.9″E), Badhora (25°22′08.0″N 84°59′58.1″E), Danapur (Auto-stand) (25°38′14.1″N 85°02′42.4″E), Maner Thana (Lodipur) (25°38′52.0″N 84°53′06.8″E), Akauna (25°07′18.5″N 85°24′16.6″E), Science College (25°37′05.6″N 85°10′10.2″E) (Fig. 1). The samples were kept in sterile sample collection plastic bottles and preserved at 4 °C for further use.
Fig. 1.
Water sample collected from twelve different arsenic contaminated sites of Gangetic planes in Bihar region before rainy season.
2.2. Evaluation of total arsenic in water samples
The collected water samples were analysed for total arsenic present by using MQuant Arsenic Test kit (Merk). Total arsenic concentration was measured semiquantitatively by visual comparison of the reaction zone of the test strip with the fields of a colour scale. The measuring range of the test strip was varied from 5 ppb to 500 ppb.
2.3. Isolation of arsenic resistant bacteria
For isolation of arsenic resistant bacteria, 100 μl of water samples were inoculated in 10 ml of Luria-Bertani (LB) media and incubated at 30 °C for overnight [[3], [4], [18]]. The 100 μl of revived culture was spread onto LB-agar plates containing 1.33 mM of AsIII. The plates were incubated at 30 °C for 72 h. The colonies, which showed resistance to AsIII and were morphologically different, were picked up and isolated after successful purification by growing repeatedly on LB medium and stored at 4 °C.
2.4. Evaluation of the MIC value
The minimum inhibitory concentration of AsIII for all the 48 isolates was evaluated by growing them on AsIII supplemented medium [[18], [21]]. 2 μl of the freshly revived cultures were spotted onto LB-agar plates supplemented with increasing concentration (1.33–20 mM) of AsIII. The plates were then incubated at 30 °C for 72 h and colonial growth was observed, and two isolates (AK1 and AK9) were selected for further work.
2.5. Cellular and morphological characterization
Gram’s staining was done using Gram stains-Kit (Himedia) and cellular structure was observed under a bright field microscope. For morphological study, streaking of the fresh culture was done by using inoculation loop on solidified LB-agar plates and incubated at 30 °C, and colony morphology was recorded after 72 h of incubation [18]. The colony morphology of AK1 and AK9 isolates was observed in terms of their general surface form, elevation, margin, surface texture, colony size, appearance, pigmentation and optical property.
2.6. Biochemical analysis of the isolated strains
Biochemical characterization of AK1 and AK9 isolates was done in terms of production of enzymes such as β-galactosidase, urease, nitrate reductase and carbohydrate utilization. The biochemical tests were done by using Biochemical test kit (Himedia) and analysed by using its result analysis index provided with kit. The experiments were performed in triplicates with freshly revived culture.
2.7. Physiological characterization of the isolated strains
For optimum growth of the bacterial isolates, physiological characterization was carried out on the basis of optimum pH and temperature.
2.8. pH and temperature optimisation
To determine the optimum pH for growth of isolates, 0.4% (v/v) of freshly revived culture was inoculated in 10 ml of LB media adjusted to pH 5–10 and incubated at 30 °C on 180 rpm for 12 h [[3], [4], [18]]. After incubation, the optical density was measured at 600 nm by UV–visible spectrophotometer (Evolution 201, ThermoScientific). All the experiments were performed in triplicates.
To optimise the temperature for optimum growth of isolates, 10 ml of LB media was inoculated with 0.4% (v/v) of fresh culture and incubated at 20, 25, 30, 35 and 40 °C. After 12 h of incubation period, absorbance was measured at 600 nm using UV–vis spectrophotometer. The experiments were performed in triplicates.
2.9. Screening of arsenic transforming bacteria by microplate screening assay
Microplate screening assay was used to estimate the efficiency of arsenic transformation by the arsenic-resistant strains [15]. The method is based on the reaction between AgNO3 and AsIII and/or AsV in Tris-HCl which results in colour precipitate ranging from light brownish-red with AsV to light yellow with AsIII. The different concentration of AsV/AsIII was defined as a colour scale which gives a correlation between the colour intensity and the ratio of AsV and AsIII species. To estimate the arsenic transforming efficiency of isolates, AK1 and AK9, to transform the arsenic species, isolates were grown overnight at 30 °C at 180 rpm to reach an optical density of 0.4-0.6. Further, 2 ml of the culture was centrifuged at 2,152g for 15 min. Pellet was washed twice with sterile Milli-Q water. Pellets were further suspended in 1.2 ml of Milli-Q water to prepare a cell suspension. In the microtiter plates, 20 μl cell suspensions and 80 μl of 0.2 M Tris-HCl buffer (pH 7.4) supplemented with AsIII/AsV to reach a final concentration 1.33 mM of AsIII/AsV. Microtiter plates were further incubated at 30 °C for 24, 48 & 72 h. Adding 100 μl of 0.1 M of AgNO3 to the wells after the incubation period and left for 15 min for colour precipitate formation and compared with the colour scale.
2.10. Molecular characterization
2.10.1. Genomic DNA isolation
Genomic DNA of isolates was extracted by modified cetyl trimethyl ammonium bromide (CTAB) method [16]. The bacterial strains were grown overnight in 10 ml LB medium at 30 °C with shaking at 180 rpm 2 ml of the culture was centrifuged at 8,609g for 30 s and then the pellet was suspended in 567 μl of TE buffer (10 mM Tris, 1 mM EDTA), 30 μl of 10% SDS and 3 μl of 20 mg/ml proteinase K and mixed well followed by incubation at 37 °C for 1 h. After incubation, 100 μl of 5 M NaCl and 80 μl of CTAB/NaCl (10% CTAB and 5 M NaCl, 1:1 (v/v)) was gently mixed by inverting the tube and incubated at 65 °C for 10 min and further an equal volume of Phenol:Chloroform:Isoamyl Alcohol (PCI) (25:24:1, v/v) was added, mixed to emulsify and spin for 5 min at 5510 g. The supernatant was transferred into a fresh tube then PCI treatment was repeated as earlier. The supernatant was incubated with RNase (50 μg/ml) for 30 min at 37 °C followed by PCI treatment as earlier. Further, sodium acetate (0.1 time of the total volume) and isopropanol (0.6 vols of the total volume) was added to precipitate DNA and centrifuged at 12,396g for 20 min, and supernatant was drained. 70% ethanol was used to clean the DNA followed by centrifugation at 12,396g for 20 min. Pellet was dried completely and dissolved in 50 μl of TE buffer for storage at −20 °C.
2.10.2. PCR amplification of 16S rDNA
The isolated genomic DNA of the isolates was used as template DNA for PCR amplification of 16S rRNA gene. The 16S rRNA gene fragment was amplified by using 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) primers. The PCR reaction mixtures contains 1x PCR buffer (G-biosciences), 2.5 mM MgCl2, 0.2 mM dNTPs, 1 μM each primer, approximately 50 ng DNA templates, 1 unit Taq DNA polymerase (G-biosciences) and molecular grade water to a final volume of 50 μl. PCR condition was; initial denaturation at 95 °C for 5 min, 30 cycles of denaturation (94 °C, 1 min), annealing (56 °C, 1 min), extension (72 °C, 10 min), and the final extension at 72 °C for 7 min and storing was done at 4 °C. All the PCR products were separated on 1.5% agarose gel and further eluted by using QIAGEN- QIAquick Gel Extraction Kit for DNA sequencing.
2.10.3. DNA sequencing and phylogenetical analysis
The ∼1.5 kb PCR amplified products 16S rDNA was used for DNA sequencing (Eurofins Genomics India Pvt Ltd., Bangalore, India) with the same primers: 27F and 1492R. The NCBI nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to search similar nucleotide sequences of 16S rRNA gene. The 16S rRNA genes of the isolates and similar sequences retrieved from the NCBI database were aligned using CLUSTAL W and the phylogenetical analysis was made using the neighbour-joining method. The sequence analysis was carried out by using the software MEGA 6.
2.10.4. PCR amplification of aox genes
The primers sets used for PCR amplification of aox genes are listed in Table 1 [16]. The PCR reaction mixtures were composed of 1x PCR buffer (G-biosciences), 2.5 mM MgCl2, 0.2 mM dNTPs, 1 μM each primer, approximately 50 ng of DNA templates, 1 units Taq DNA polymerase (G-biosciences) and molecular grade water to a final volume of 50 μl. The PCR conditions consisted of 5 min of initial denaturation at 95 °C, 30 cycles of 1 min at denaturation at 94 °C, 1 min annealing at as mentioned in Table 1, 1.5 min extension at 72 °C, and the final extension at 72 °C for 7 min. The PCR was conducted in a ProFlex PCR system (Life Technologies, USA) and the PCR product was identified by gel electrophoresis using 1.5% agarose gels.
Table 1.
Primers used for PCR amplification of aox genes.
| Primer | Primer sequence | Taa(°C) |
|---|---|---|
| aoxRF | 5′-AATCGCTCATCCAGCGACTTTCGC-3′ | 59 |
| aoxRR | 5′-TTGCGTCCTCGCCAAGCGTACTGA-3′ | |
| aoxBF | 5′-TGCGGCTACCACGCCTACACC-3′ | 59 |
| aoxBR | 5′-TGCCCCAGGTGTTTTCGTAAC-3′ | |
| aoxCF | 5′-TGGCATCGGGAGGAGGAT-3′ | 53 |
| aoxCR | 5′-TAGCCTGGGAAGTATGGC-3′ |
Annealing temperature used in this study.
2.10.5. Effect of AsIII on the growth of isolates
The effect of AsIII on the growth of isolates was determined in the presence of AsIII and without AsIII act as control. 0.4% (v/v) of the fresh culture was added to 10 ml of LB media supplemented with 1.33 mM of AsIII and incubated at 30 °C on 180 rpm [[3], [4]]. The optical density was measured at 600 nm using UV–vis spectrophotometer at a regular interval of 1 h. The experiments were performed in triplicates.
2.10.6. Heavy metal tests
The ability of isolates, AK1 and AK9, to tolerate the different heavy metal salts, like Ag (as AgNO3), Hg(II) (as HgCl2), Co(II) (as CoCl2·6H2O), Pb(II) (as Pb(NO3)2), Cd(II) (as Cd(NO3)2), Cu(II) (as CuSO4·5H2O), Ni(II) (as Ni(NO3)2·6H2O) and Cr(IV) (as K2Cr2O7), was evaluated at different concentrations [[3], [4]]. 10 μl of the fresh culture was spread over LB-agar plates supplemented with increasing concentration from 0.5–20 mM of heavy metal salts. The plates were incubated at 30 °C for a period of 72 h. Further, the colony growth was observed and MIC of the heavy metals was recorded. All the experiments were performed in triplicates.
3. Results
3.1. Arsenic analysis of the water sample
Water samples were collected from the middle Gangetic plain of Bihar and analysed for the presence of arsenic. The results show that total arsenic in the water sample varied from 0 to 100 ppb (Table 2).
Table 2.
Total arsenic concentration in water samples.
| Sl. No. | Sample | Total arsenic (ppb) |
|---|---|---|
| 01. | Karja | 100 |
| 02. | Pararia | 100 |
| 03. | Bhakhura | 0 |
| 04. | Semaria | 25 |
| 05. | Keshopur | 50 |
| 06. | Maner Thana | 5 |
| 07. | Danapur | 5 |
| 08. | Badhora | 5 |
| 09. | Danapur (auto stand) | 0 |
| 10. | Maner Thana (Lodipur) | 5 |
| 11. | Akauna | 25 |
| 12. | Science College | 10 |
3.2. Isolation of arsenic resistant bacterial strains
A total of 48 morphologically distinct arsenic transforming bacterial strains were isolated. They have potential to tolerate 1.33 mM of AsIII stress. It was observed that the MIC value of AsIII for the isolated strains varied from 3 to 15 mM. Out of 48 isolates, two isolates namely AK1 and AK9 showed highest tolerance value for the AsIII and having different colony morphology. The observed MIC value of AsIII for AK1 and AK9 was 13 mM and 15 mM respectively. So, for further study AK1 and AK9 were selected.
3.3. Cellular and colony morphology
The cellular morphology of isolates was observed under a bright field microscope. AK1 was a gram negative coccus in nature, whereas AK9 was a gram negative bacillus. The colony morphology of AK1 was flat, filamentous margin, rugose surface texture with white colour shiny appearance and AK9 was convex, entire margin, smooth surface texture with a tan colour shiny appearance.
3.4. Biochemical analysis of isolated strains
Biochemical characterization of isolates, AK1 and AK9, was done in terms of production of enzymes; e.g. β-galactosidase, urease, nitrate reductase, and carbohydrate utilization (Table 3).
Table 3.
Biochemical characterization of the strains AK1 and AK9.
| Sl. No. | Tests | AK1 | AK9 |
|---|---|---|---|
| 01. | Indole | − | − |
| 02. | Voges- Proskauer | − | − |
| 03. | Citrate utilization | − | − |
| 04. | Lysine utilization | − | − |
| 05. | Ornithine utilization | − | − |
| 06. | Arginine utilization | − | − |
| 07. | Nitrate reduction | + | + |
| 08. | Malonate utilization | + | + |
| 09. | Urease | + | + |
| 10. | Phenylalanine deanimation | − | − |
| 11. | H2S production | − | − |
| 12. | ONPG | − | − |
| 13. | Glucose utilization | + | + |
| 14. | Mannitol utilization | + | + |
| 15. | Xylose utilization | − | − |
| 16. | Inositol utilization | − | − |
| 17. | Sorbitol utilization | − | − |
| 18. | Rhamnose utilization | − | − |
| 19. | Sucrose utilization | + | + |
| 20. | Lactose utilization | − | + |
| 21. | Arabinose utilization | − | − |
| 22. | Adonitol utilization | − | − |
| 23. | Raffinose utilization | − | − |
| 24. | Salicin utilization | − | − |
3.5. Physiological characterization of isolated strains
3.5.1. pH and temperature optimization for growth
The strains, AK1 and AK9 were grow at pH range of 6 to 9 but the optimal growth was observed at pH 7.0 (Fig. 2). The AK1 strain was growing at 20, 25 and 30 °C but the highest growth was observed at 30 °C. AK1 was unable to grow at 35 and 40 °C. Whereas AK9 can grow on 20, 25, 30, 35 and 40 °C but the optimum temperature for it was 30 °C (Fig. 3).
Fig. 2.
Effect of pH on the growth of arsenic oxidising bacterial strains (a) AK1 and (b) AK9.
Fig. 3.
Effect of temperature on the growth of arsenic oxidising bacterial strains (a) AK1 and (b) AK9.
3.5.2. Screening for arsenic transforming bacteria
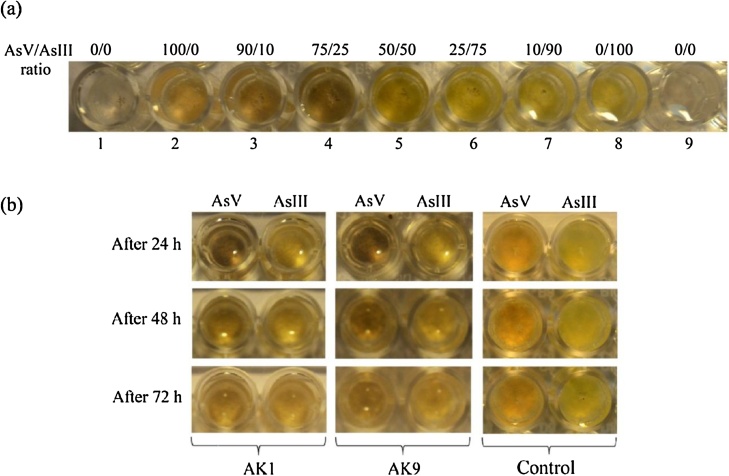
The ability of bacterial isolates to oxidise AsIII or reduce AsV was observed by AgNO3 test based microplate method. The interaction of AgNO3 with Tris-HCl results in precipitate formation. The precipitates containing arsenic were coloured from light yellow colour in the presence of AsIII to light brown-red in the presence of AsV. According to AgNO3 test, both AK1 and AK9 strains, have potential to transform arsenic from AsIII to AsV and AsV to AsIII. The colour scale which, can be easily distinguished by eye, was developed to show different ratios of AsV/AsIII in the solution can be easily distinguished by naked eye (Fig. 4(a)). The arsenic transforming ability of both the strains was observed after 24, 48 and 72 h of incubation at 30 °C (Fig. 4(b)). Both AK1 and AK9 reduced 1.33 mM of AsV to AsIII completely and oxidised the 1.33 mM AsIII to AsV upto 25% after the incubation of 72 h.
Fig. 4.
(a) Determination of precipitate colour as a function of AsV/AsIII ratio. Each well contained: 100 μl of 0.2 M Tris-HCl buffer and 100 μl of 0.1 M AgNO3 in the absence (wells 1 and 9) or in the presence of arsenic (wells 2–8). (b) Microplate screening assay of the arsenic transforming AK1 and AK9. Each well contained 20 μl of bacterial cell suspension of strains AK1 and AK9 and 80 μl of 0.2 M Tris-HCl buffer (pH 7.4). Initial concentration was 1.33 mM of AsIII and AsV in the respective wells. In control no bacterial suspension was added.
3.6. 16S rDNA amplification and phylogenetic analysis
3.6.1. Genomic DNA isolation and PCR amplificationof 16S rRNA gene
Genomic DNA isolation of isolates, AK1 and AK9, was done by the cetyltrimethyl ammonium bromide (CTAB) method. The nucleic acid concentration of AK1 was 60 ng/μl and that of AK9 was 160 ng/μl. The genomic DNA of isolates was visualised on agarose gel (Fig. 5(a)). The 16S rRNA genes of both the strains were PCR amplified with 27F-1492R primers and found to provide PCR product of 1500 bp (Fig. 5(b)).
Fig. 5.
(a) Genomic DNA of the arsenic oxidising bacterial strain AK1 and AK9. (b) PCR amplification of 16S rRNA gene of the arsenic transforming bacterial strains AK1 and AK9. Lane M- 100 bp ladder.
3.6.2. 16S rRNA gene sequencing
The partial sequencing of the gel eluted PCR product of 16S rRNA gene was done. The size of the 16S rRNA gene of AK1 and AK9 was 1430 bp and 1429 bp respectively. The gene sequences of AK1 and AK9 were submitted to Genebank (NCBI) with the accession numbers KY569423 and KY569424, respectively.
3.6.3. Phylogenetic analysis
The 16S rRNA gene sequence of isolates, AK1 and AK9, were aligned using the Basic Local Alignment Tool (BLAST). The BLASTN search results of the 16S rRNA gene sequence showed that they were novel strains belong to the genus Pseudomonas of γ- proteobacteria with a sequence similarity of 99% to Pseudomonas extremorientalis strain (Fig. 6).
Fig. 6.
Phylogenetic tree based on 16S rRNA gene sequences, showing the relationship between strains AK1 (KY569423), AK9 (KY569424) and related bacterial species. The branching pattern was generated by the neighbour-joining method. Based on 500 replicons, bootstrap percentages above 50% are shown.
3.6.4. PCR amplification of aox genes
The presence of the aox genes were evaluated using PCR by specific primers from the genomes of the bacterial isolates. Both the strains, AK1 and AK9, showed the presence of the aox operon genes. The PCR amplified products show aoxR (two bands, 750 bp and 670 bp), aoxB (∼1600 bp) and aoxC (∼550 bp) (Fig. 7). The agarose gel image was processed in Bio-Rad's ChemiDoc™ MP and analysed with ImageLab Version 4.1.
Fig. 7.
Amplification of aoxR, B, & C genes of the strains AK1 and AK9. Lanes M: DNAmark™ 100 bp Plus Ladder (G Biosciences, USA).
3.6.5. Effect of AsIII on bacterial growth
Both isolates follow a sigmoid pattern of growth (Fig. 8). It was observed that strain AK1 having growth rate constant (k) calculated in the absence of AsIII was 0.641 h−1 (a doubling time of 1.56 h), and in the presence of AsIII it was 0.495 h−1 (a doubling time of 2.02). Strain AK9 having growth rate constant (k) calculated in the absence of AsIII was 0.850 h−1 (a doubling time of 1.176 h), and in the presence of AsIII it was 0.691 h−1 (a doubling time of 1.447). The results showed that in the presence of AsIII, 23% and 19% reduction in cellular growth of AK1 and AK9 respectively. The doubling time of both isolates, increases in the presence of AsIII.
Fig. 8.
Effect of AsIII on the growth of the arsenic oxidising bacterial strains (a) AK1 and (b) AK9.
3.6.6. Evaluation of metal tolerance
The ability of isolates, AK1 and AK9, to tolerate other toxic metal salts, like Ag(I), Hg(II), Pb(II), Ni(II), Cu(II), Cd(II), Co(II) and Cr(IV) was observed at different concentrations on LB-agar medium. AK1 and AK9, both have potential to tolerate other heavy metals and the MIC value of heavy metals was recorded (Table 4). The ability of isolates to tolerate the different heavy metal gives an opportunity to use them in metal contaminated water, soil and effluents for its bioremediation.
Table 4.
MIC of the isolates for different heavy metals.
| Heavy Metals | AK1 | AK9 |
|---|---|---|
| Arsenic (AsIII) | 13 mM | 15 mM |
| Silver Nitrate | 0.5 mM | 0.5 mM |
| Mercury (II) Chloride | 0.5 mM | 0.5 mM |
| Lead (II) Nitrate | 8 mM | 9 mM |
| Cadmium (II) Nitrate | 0.5 mM | 0.5 mM |
| Copper (II) Sulphate | 4 mM | 3 mM |
| Nickel (II) Nitrate | 4 mM | 3 mM |
| Cobalt (II) Chloride | 0.5 mM | 1 mM |
| Potassium Dichromate (VI) | 4 mM | 3 mM |
4. Discussion
Microbial transformation, i.e. oxidation and reduction of toxic AsIII and AsV has evident potential in the treatment process of arsenic from contaminated water. It is emerging as superior to conventional chemical and physical methods. River originating from the Himalaya, like the Ganga river, is the natural source of release of arsenic into the environment [7]. Thus, microorganisms present in this aquatic system have developed some arsenic detoxification mechanisms for their survival. It is well documented that the bacteria have potential for survival in metal stress condition [[17], [18]]. This suggests a wide distribution of arsenic transforming bacteria may be present in Ganga river that has the potential for arsenic detoxification.
In the present study, two prominent arsenic oxidising bacterial strains AK1 and AK9 belong to genus Pseudomonas have been reported and isolated from middle Gangetic plain and both isolates AK1 and AK9 were able to transform AsV to AsIII and AsIII to AsV.
Many arsenic transforming bacteria were widely reported from different aquifers throughout the world [[16], [19], [20]], but upto our knowledge no such studies have been done earlier from arsenic prominent middle Gangetic Plain of Bihar.
Isolates AK1 and AK9 in this study were resistant of AsIII upto 13 mM and 15 mM respectively. Both show complete aerobic reduction of AsV after 48 h of incubation. Bacteria belongs to genus Pseudomonas can reduce AsV aerobically upto 200 mM [21]. AK1 and AK9 were oxidised 25% of AsIII after 72 h of incubation. Some genus including Pseudomonas can oxidise 1 mM of AsIII within 25–30 h [21]. This property permits these bacteria to survive with the elevated arsenic concentrations in native groundwater.
The detection of aox genes in the two bacterial isolates, AK1 and AK9, show their potential to oxidise AsIII to AsV. The genes, aoxR, aoxB and aoxC are involved arsenite oxidation [[6], [8]]. Chang et al. also showed the aoxR and aoxB genes amplified from the genomes of Damyang and Woopo wetlands bacteria [16]. Further growth and physiology of isolated strains were carried out. pH and temperature are the major environmental factors that have a great importance in the growth and metal accrual properties of the bacterial strains [18]. It was observed that both the arsenic resistant isolates, AK1 and AK9, show optimum growth at pH 7.0 and temperature 30 °C. Similarly, it was reported that arsenic oxidising bacteria can grow at 40 °C and 50 °C [19]. The doubling time of the AK1 decreases while it increases in case of AK9 in the presence of AsIII which indicates that AK9 was more resistant to AsIII than AK1. Further, the growth rate of both, AK1 and AK9, decreases in the presence of AsIII. Subsequently, the doubling time of several bacterial strains increases in the presence of arsenic and growth rate decreases [22]. In addition to arsenic resistance, both isolate can grow in presence of other toxic heavy metals. Both, AK1 and AK9, were also resistance to Pb(II), Cu(II), Ni(II) and Cr(IV) with MIC in the range of 4–8 mM (Table 4). Both isolates were showing less tolerance for Ag, Hg(II), Cd(II) and Co(II) upto the concentration 0.5 mM. The heavy metal tolerance properties of these two isolates make them to be a good candidate for bioremediation of toxic heavy metals.
The present work described the first evidence of arsenic transformation from genus Pseudomonas from the middle Gangetic plain of Bihar, India. The strains were able to oxidise AsIII and reduce AsV at high rate. Thus, these new strains could be an excellent candidates for application in arsenic remediation processes. This study provides the first look for the relevant microorganism that exists in the middle Gangetic plain of Bihar, India. Our observations showed that isolates, AK1 and AK9, capable in biotransformation of arsenic were naturally present in the water sample. The identified strains, seem to be very much familiar to arsenic concentrations present in the water samples. This information is important since, the effectiveness of in situ bioremediation technology depends on the activities of the native microbial communities present in polluted sites.
Conflict of interest
No
Acknowledgements
The authors are thankful to Prof. H.C.S. Rathore, Hon’ble Vice Chancellor, Central University of South Bihar, Patna for providing infrastructure and Dr. Shyam for helping in the collection of water samples. This work was supported by the financial grant of Start-up grant from University Grant Commission (UGC), New Delhi. Ghanshyam Kumar Satyapal thanks Rajiv Gandhi National Fellowship from UGC, New Delhi, India.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.btre.2018.02.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Obinaju B.E. Mechanisms of arsenic toxicity and carcinogenesis. Afr. J. Biochem. Res. 2009;3(5):232–237. [Google Scholar]
- 2.Mateos L.M., Ordóñez E., Letek M., Gil J.A. Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int. Microbiol. 2010;9(February 24 (3)):207–215. [PubMed] [Google Scholar]
- 3.Butt A.S., Rehman A. Isolation of arsenite-oxidizing bacteria from industrial effluents and their potential use in wastewater treatment. World J. Microbiol. Biotechnol. 2011;27(10):2435–2441. [Google Scholar]
- 4.Rehman A., Butt S.A., Hasnain S. Isolation and characterization of arsenite oxidizing Pseudomonas lubricans and its potential use in bioremediation of wastewater. Afr. J. Biotechnol. 2010;9(March 8 (10)):1493–1498. [Google Scholar]
- 5.Shah D., Shen M.W., Chen W., Da Silva N.A. Enhanced arsenic accumulation in Saccharomyces cerevisiae overexpressing transporters Fps1p or Hxt7p. J. Biotechnol. 2010;150(October 1 (1)):101–107. doi: 10.1016/j.jbiotec.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Satyapal G.K., Rani S., Kumar M., Kumar N. Potential role of arsenic resistant bacteria in bioremediation: current status and future prospects. J Microb Biochem Technol. 2016;8:256–258. [Google Scholar]
- 7.Kruger M.C., Bertin P.N., Heipieper H.J., Arsène-Ploetze F. Bacterial metabolism of environmental arsenic – mechanisms and biotechnological applications. Appl. Microbiol. Biotechnol. 2013;97(9):3827–3841. doi: 10.1007/s00253-013-4838-5. [DOI] [PubMed] [Google Scholar]
- 8.Silver S., Phung L.T. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 2005;71(2):599–608. doi: 10.1128/AEM.71.2.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A.K. Chemistry of arsenic in groundwater of Ganges-Brahmaputra river basin. Curr. Sci. 2006;91(September 10 (5)):599–606. [Google Scholar]
- 10.Bhattacharya P., Chatterjee D., Jacks G. Occurrence of arsenic-contaminated groundwater in alluvial aquifers from delta plains, eastern India: options for safe drinking water supply. Int. J. Water Resour. Dev. 1997;13(1):79–92. [Google Scholar]
- 11.McArthur J.M., Banerjee D.M., Hudson-Edwards K.A., Mishra R., Purohit R., Ravenscroft P. Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl. Geochem. 2004;19(8):1255–1293. [Google Scholar]
- 12.Chakraborti D., Mukherjee S.C., Pati S., Sengupta M.K., Rahman M.M., Chowdhury U.K. Arsenic groundwater contamination in middle ganga plain, bihar, India : a future danger? Environ. Health Perspect. 2003;111(9):1194. doi: 10.1289/ehp.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S.K., Ghosh A.K., Kumar A., Kislay K., Kumar C., Tiwari R.R. Groundwater arsenic contamination and associated health risks in Bihar, India. Int. J. Environ. Res. 2014;8(January 1 (1)):49–60. [Google Scholar]
- 14.Nath A., Kumar S., Priyanka, Sinha P., Anshu A.K., Singh M. Arsenic in tube well water in six blocks of supaul district, bihar. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015;9(January (1)):05–08. [Google Scholar]
- 15.Simeonova D.D., Lièvremont D., Lagarde F., Muller D.A., Groudeva V.I., Lett M.C. Microplate screening assay for the detection of arsenite-oxidizing and arsenate-reducing bacteria. FEMS Microbiol. Lett. 2004;237(2):249–253. doi: 10.1016/j.femsle.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Chang J.S., Yoon I.H., Lee J.H., Kim K.R., An J., Kim K.W. Arsenic detoxification potential of aox genes in arsenite-oxidizing bacteria isolated from natural and constructed wetlands in the Republic of Korea. Environ. Geochem. Health. 2010;32(2):95–105. doi: 10.1007/s10653-009-9268-z. [DOI] [PubMed] [Google Scholar]
- 17.Rosen B.P. Biochemistry of arsenic detoxification. FEBS Lett. 2002;529(1):86–92. doi: 10.1016/s0014-5793(02)03186-1. [DOI] [PubMed] [Google Scholar]
- 18.Rahman A., Nahar N., Nawani N.N., Jass J., Desale P., Kapadnis B.P. Isolation and characterization of a Lysinibacillus strain B1-CDA showing potential for bioremediation of arsenics from contaminated water. J. Environ. Sci. Health. 2014;49(Part A (12)):1349–1360. doi: 10.1080/10934529.2014.928247. [DOI] [PubMed] [Google Scholar]
- 19.Kinegam S., Yingprasertchai T., Tanasupawat S., Leepipatpiboon N., Akaracharanya A., Kim K.W. Isolation and characterization of arsenite-oxidizing bacteria from arsenic-contaminated soils in Thailand. World J. Microbiol. Biotechnol. 2008;24(12):3091–3096. [Google Scholar]
- 20.Santini J.M., Sly L.I., Wen A., Comrie D., Wulf-Durand P.D., Macy J.M. New arsenite-oxidizing bacteria isolated from australian gold mining environments-phylogenetic relationships. Geomicrobiol. J. 2002;19(1):67–76. [Google Scholar]
- 21.Liao V.H.C., Chu Y.J., Su Y.C., Hsiao S.Y., Wei C.C., Liu C.W. Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J. Contam. Hydrol. 2011;123(1):20–29. doi: 10.1016/j.jconhyd.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee S., Datta S., Chattyopadhyay D., Sarkar P. Arsenic accumulating and transforming bacteria isolated from contaminated soil for potential use in bioremediation. J. Environ. Sci. Health. 2011;46(Part A (14)):1736–1747. doi: 10.1080/10934529.2011.623995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.