Abstract
Background
Mobile health technologies have been found to improve the self-management of chronic diseases. However, there is limited research regarding their feasibility in supporting recovery from substance use disorders (SUDs) in China.
Objective
The objective of this study was to examine the feasibility of a mobile phone-based ecological momentary assessment (EMA) app by testing the concordance of drug use assessed by the EMA, urine testing, and a life experience timeline (LET) assessment.
Methods
A total of 75 participants dependent on heroin or amphetamine-type stimulant (ATS) in Shanghai were recruited to participate in a 4-week pilot study. Of the participants, 50 (67% [50/75]) were randomly assigned to the experimental group and 25 (33% [25/75]) were assigned to the control group. The experimental group used mobile health (mHealth) based EMA technology to assess their daily drug use in natural environments and received 2 short health messages each day, whereas the control group only received 2 short health messages each day from the app. Urine tests and LET assessments were conducted each week and a post-intervention survey was administered to both groups. The correlations among the EMA, the LET assessment, and the urine test were investigated.
Results
The mean age of the participants was 41.6 (SD 8.0) years, and 71% (53/75) were male. During the 4 weeks of observation, 690 daily EMA survey data were recorded, with a response rate of 49.29% (690/1400). With respect to drug use, the percent of agreement between the EMA and the LET was 66.7%, 79.2%, 72.4%, and 85.8%, respectively, for each of the 4 weeks, whereas the percent of agreement between the EMA and the urine test was 51.2%, 65.1%, 61.9%, and 71.5%, respectively. The post-intervention survey indicated that 46% (32/70) of the participants preferred face-to-face interviews rather than the mHealth app.
Conclusions
This study demonstrated poor agreement between the EMA data and the LET and found that the acceptance of mHealth among individuals with SUDs in China was not positive. Hence, greater efforts are needed to improve the feasibility of mHealth in China.
Keywords: mHealth, substance use, heroin dependence, amphetamine-type stimulant (ATS) dependence, mobile app, China
Introduction
Substance use disorder (SUD) is a major public health issue, not only in China but worldwide as well [1,2]. Based on the report from the United Nations Office on Drugs and Crime (UNODC), approximately 290 million people worldwide use illicit drugs, and marijuana heads the list as the most popular drug used throughout the world [3]. China, the largest developing country, faces serious drug problems. Between 2000 and 2016, the number of registered people in China with a drug addiction increased sharply from 0.86 to 2.50 million [4]. Although the number of users of club drugs, such as methamphetamine, ecstasy, and ketamine, has increased since the last decade, heroin remains a major drug problem, with 49.3% of people registered with a drug addiction being addicted to it [5]. To control the problems of heroin abuse and HIV and AIDS infection, harm-reduction programs, including methadone maintenance treatment (MMT) clinics, have been established throughout China beginning in 2004 [6,7]. By the end of 2014, there were more than 300 MMT clinics providing services to 344,254 addicts [8]. Although the effectiveness of MMT has been demonstrated, its implementation still faces certain problems, including the high cost of MMT, a lack of psychotherapy, and the low dosage of methadone, which contributes to high rates of drop-out and relapse [9,10]. Together, these conditions suggest the need to develop new intervention strategies that meet the needs of people with drug addiction in China [11,12].
A large body of studies has demonstrated that drug addiction is a chronic relapsing brain disease that features cycles of relapse and remission [13]. A chronic condition means that those with SUDs require a chronic care model that can provide an integrated care system that includes services and self-management tools designed to prevent relapse [14,15].
Mobile health (mHealth) service is a new technology that has been widely used in the health care service field as well as in substance abuse treatment programs in western countries [16]. Service providers integrate a tailored mHealth self-management app for patients and collect data from the app to help them monitor their patients’ behaviors [17]. For example, a mobile phone can be programmed to send a message when people with a drug addiction enter a high-risk area that can lead to relapse. Other functions include improving disease management, delivering therapeutic interventions, and increasing healthy behavior [18].
Recently, the mobile phone-based ecological momentary assessment (EMA) began to be used in the field of medical treatment. The EMA is a mHealth technology that is capable of collecting individuals’ data in real time. Compared to other retrospective surveys, the EMA is thought to substantially improve the accuracy of reporting since self-reporting sometimes requires participants to recall events over long periods, a situation that may introduce a systematic bias [19,20].
A few studies have compared the accuracy of the EMA with other measures to assess drug use behaviors. Kranzler [21] assessed the amount of drinking SUDs using a timeline follow-back method (TLFB) and a daily interactive voice recording (IVR) and found poor agreement between the two approaches. Similarly, Searles and Lincoln’s results revealed poor agreement between daily drinking recall and IVR measures [22,23]. Conversely, other researchers [24-26] conducted a set of outcome-based analyses using both the TLFB and the IVR drinking outcomes and found no differences between the two measures.
To our knowledge, there are no published studies that address the feasibility and acceptability of this novel technology among individuals with SUDs in China. This lack of evidence hinders the implementation of mHealth in China. To address this knowledge gap, we conducted secondary analyses based on a pilot study of a mobile phone-based intervention designed to support recovery from addiction. The aims of the present study were to test the concordance of drug use collected via the EMA, urine testing, and a retrospective self-report survey instrument, and to investigate the acceptability of mHealth in China. The overall goal was to provide evidence for the future use of mHealth technology in drug relapse prevention.
Methods
Sample Recruitment and Study Procedures
Participants were recruited from 3 MMT clinics and the Zi-Qiang social work consortium. The social work consortium is a community-based social work service network with approximately 500 social workers hired from eligible individuals in the community to help clients living within the same community. All participants were recruited through advertisements in the MMT clinics and the social work consortium. Inclusion criteria were (1) aged 18 to 65 years; (2) above primary education; (3) able to use a mobile phone with app capabilities; (4) a newly enrolled patient in an MMT clinic or social work consortium or relapsed (positive urine test) within the past 30 days; (5) met the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for heroin dependence or amphetamine-type stimulant (ATS) dependence; and (6) consent to participate in the study. Exclusion criteria were (1) a main substance that was not heroin or ATS; and (2) diagnosis of serious mental illness and inability to complete the evaluation. Protocols for this study were approved by the institutional review board (IRB) of Shanghai Mental Health Center (No 2012-56C2) and the University of California, Los Angeles (12-000809).
From June 2015 to April 2016, 89 clients were screened at baseline. Of the clients, 7 (8% [7/89]) reported that they had no mobile phone, and 5 (6% [5/89]) refused to participate in the study. Thus, 75 participants were recruited and assigned to either the experimental group (n=50) or the control group (n=25), resulting in a 2:1 ratio.
All participants were required to install the app and were given instructions on how to use it. The app for the experimental group included 6 functions: surveys (daily survey and self-initiated survey), messages, settings, profile, craving, and help. The app for the control group included messages, settings, profile, and help. Participants in the experimental group were asked to complete a daily survey on the app and they received 2 health messages every day for 4 weeks. Participants in the control group only received 2 health messages from the app. Demographics, substance abuse histories, and clinical scale data were collected at baseline. Whether participants engaged in drug use was assessed using the EMA app, the urine test, and the LET once a week during the study period.
Description of the Mobile Health-Based App
The mHealth was developed specifically to help individuals with SUDs achieve and maintain recovery. Thus far, it is the first mobile app designed for people with SUDs in China. The mechanism of the mHealth app is based on cognitive behavior therapy (CBT) and self-determination theory (SDT). CBT emphasizes triggers and coping strategies for relapse prevention, whereas SDT is a theory that motivates people to change and act for themselves. mHealth is combined with CBT and SDT to create 3 main tools: surveys, messages, and cravings, to foster positive behavior and manage behavior. These tools provide timely assessment and intervention and help individuals with SUDs to control their cravings and prevent relapses.
Surveys
There are 2 types of surveys built into the app: the daily survey and the self-initiated survey. Participants were alerted to complete the daily survey every 24 hours. This survey required them to report their alcohol, tobacco, and drug use as well as their cravings, triggers, emotions, coping strategies, and daily goal progress for the last 24-hour period. Once participants completed the daily survey, it was no longer available to the participant until the next day at the scheduled time, which was set by the participant. It took 1 to 2 minutes to complete the daily survey. The self-initiated survey was available to participants at any time. The questions on this survey addressed cravings, triggers, and drug use. Once the participant indicated a craving for a drug, he or she could complete the survey and received messages to help control the craving, to a certain extent. A screenshot of the mHealth app home screen is shown in Figure 1.
Figure 1.
Home screen of the mobile health (mHealth) app.
Data Collection
Ecological Momentary Assessment
The EMA data were collected from the experimental group using the survey function in the app. The daily situation (eg, drug use, craving, coping) was collected by the daily survey, which was conducted every day at a scheduled time. Before the scheduled time, a reminder message was sent to the participants to remind them to complete the daily survey. Craving status was collected by the self-initiated survey, which could be completed any time the participant experienced a craving for a drug. A guide message was then sent to the participant to help control the craving.
Life Experience Timeline Assessment
The LET was developed by the University of California, Los Angeles (UCLA) and exhibited good reliability and validity [27,28]. The questionnaire consists of 20 items that record 20 events (eg, substance use, emotion, coping, and craving) over the past week. Participants were requested to complete the LET survey at baseline and each week during the study period. The outcomes collected by the LET indicated whether the individual used any of the identified substances in the last week and, if so, how many days they used drugs in last week.
Urine Test
The urine test board was used to test drug use at baseline and each week during the study period. The test identified heroin, ATS, marijuana, cocaine, and ketamine use.
Post-Intervention Survey
To measure the acceptability of the electronic mobile (e-mobile) method among individuals with SUDs, the participants were asked to respond “agree” or “disagree” for each of the following items:
It is easy for me to understand these questions.
I feel okay when I answer questions about drug use.
I can recall the days I used drugs during the last week.
It is fine for me to answer the same questions each year.
This mHealth app is easy to use.
Compared to a face-to-face interview, I prefer to use the mHealth app.
An informal group discussion was conducted with 7 participants who voluntarily attended the discussion. The main purpose of the group discussion was to collect supplementary information as to why individuals with SUDs did not want to use the mHealth app.
Data Analysis
All statistical analyses were conducted using the Statistical Package for Social Science (SPSS, version 22.0). The differences in characteristics between the experimental group and the control group were analyzed using the t test for continuous measures and the chi-square test for categorical measures. Statistical significance was set at alpha=.05. Cohen kappa was conducted to measure the correlations among the 3 measures. Concordance correlation coefficients less than 0.20 were regarded as poor, 0.21 to 0.40 as fair, 0.41 to 0.60 as moderate, 0.61 to 0.80 as good, and 0.81 to1.00 as very good. The chi-square test was used to compare the consistency of the EMA and the LET data. A logistic regression was conducted to explore the factors that influenced the correspondence between the EMA and the urine test during the study period. Only non-missing data during the whole week were included in the analysis.
Results
Baseline characteristics of the participants of the experimental and control groups are shown in Table 1. The mean age of the participants was 41.6 (SD 8.0) years, and 71% (53/75) of the participants were male. Among the experimental group, 76% (38/50) were heroin users and 24% (12/50) were ATS users, whereas among the control group, 80% (20/25) were heroin users and 20% (5/25) were ATS users. There were no significant differences between the groups in terms of the type of drug, age, gender, education, marriage, employment status, initial age of use drug, or length of main substance used. Drug use test results are summarized in Table 2.
Table 1.
Baseline characteristics of the participants (N=75).
| Characteristic | Total | Experimental group (N=50) | Control group (N=25) | t/F/Z | P* | |
| Age, mean (SD) | 41.6 (8.0) | 41.7 (8.7) | 41.3 (6.8) | 0.235 | .815 | |
| Male, n (%) | 53 (71) | 35 (70) | 18 (72) | 0.138 | .858 | |
| Education, n (%) | 0.538 | .463 | ||||
| ≤Middle school | 32 (43) | 23 (46) | 9 (36) | |||
| ≥High school | 43 (57) | 27 (54) | 16 (64) | |||
| Marital status, n (%) | 0.001 | .972 | ||||
| Married | 32 (43) | 20 (40) | 12 (48) | |||
| Single | 43 (57) | 30 (60) | 13 (52) | |||
| Employment, n (%) | 2.600 | .107 | ||||
| Employed | 45 (61) | 33 (67) | 12 (48) | |||
| Initial age of use drug, mean (SD) | 26.1 (8.9) | 25.9 (8.8) | 26.4 (9.2) | -0.211 | .834 | |
| Length of main drug used (months), mean (SD) | 15.5 (6.0) | 15.8 (6.1) | 14.9 (6.0) | 0.615 | .541 | |
*Significant at P<.05.
Table 2.
Drug use test results by week.
| Test | First week (n) | Second week (n) | Third week (n) | Fourth week (n) | |
| Urine test | |||||
| Use | 24 | 21 | 15 | 11 | |
| No use | 19 | 22 | 27 | 31 | |
| Total | 43 | 43 | 42 | 42 | |
| Self-report LETa | |||||
| Use | 15 | 12 | 10 | 7 | |
| No use | 33 | 36 | 38 | 41 | |
| Total | 48 | 48 | 48 | 48 | |
| EMAb | |||||
| Use | 12 | 10 | 6 | 5 | |
| No use | 28 | 25 | 26 | 25 | |
| Total | 40 | 35 | 32 | 30 | |
aLET: life experience timeline.
bEMA: ecological momentary assessment.
Ecological Momentary Assessment Data Description
The EMA data were collected from the experiment group. A total of 350 EMA daily surveys were expected each week (50 times 7). The daily survey data received was 59.1% (207/350) the first week; 48.9% (171/350) the second week, 48.0% (168/350) the third week, and 41.1% (144/350) the fourth week. The average response rate was 49.29% (690/1400). These results indicated that the daily survey response rates were generally low (Figure 2).
Figure 2.
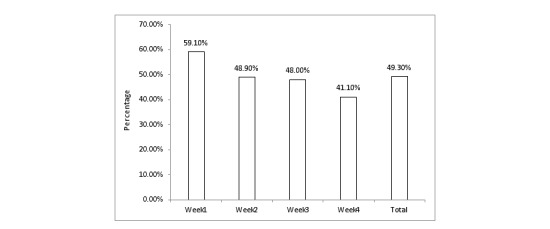
The weekly ecological momentary assessment (EMA) data in the experimental group.
Methods of Measuring Drug Use
Three methods (ie, EMA, LET, and urine test) were used to assess drug use each week. The correspondence between the EMA and the LET over the 4 weeks was 67% (32/48), 79% (38/48), 72% (35/48), and 86% (41/48), respectively. The correspondence between the EMA and the urine test was 51% (22/43), 65% (28/43), 62% (26/42), 72% (30/42), respectively (Figure 3). The agreement between the EMA and the LET was not good, with Cohen kappas of 0.128, 0.412, 0.111, and 0.241, respectively. With respect to the agreement between the EMA and the urine test, the results were not optimistic according to Cohen kappas, which were 0.076, 0.292, 0.017, and 0.103, respectively. The logistic regression analysis revealed that work and age were correlated with correspondence between the EMA and the urine test during the study period (odds ratio [OR]=0.167, 1.137, 0.195; P<.05), as shown in Table 3 and Figure 3.
Figure 3.
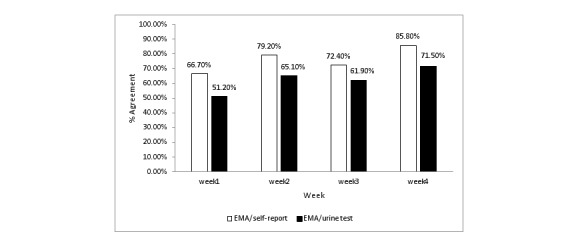
The concordance of test results.
Table 3.
Logistic regression of concordance between the ecological momentary assessment and the urine test.
| Week | Factor | β | SE | Chi-square | P | ORa | 95% CI |
| 1 | Job | -1.792 | 0.755 | 5.625 | .018 | 0.167 | 0.038-0.733 |
| 2 | Age | 0.129 | 0.048 | 7.074 | .008 | 1.137 | 1.034-1.251 |
| 3 | Job | -1.636 | 0.854 | 3.670 | .034 | 0.195 | 0.037-1.038 |
aOR: odds ratio.
Post-Intervention Survey
The post-intervention survey was conducted with all participants to evaluate their attitude toward the mHealth-based app (Table 4). Even though most of the participants (72% [51/71]) admitted that the mHealth app was easy to use, almost half of them (46% [32/70]) stated that compared to the mHealth data collection method, they preferred a face-to-face interview.
Table 4.
Post-intervention survey results.
| Item | Agree, n (%) | Disagree, n (%) |
| 1. It is easy for me to understand these questionsa. | 44 (67) | 22 (33) |
| 2. I feel comfortable when I answer these questionsb. | 49 (69) | 22 (31) |
| 3. I can recall the days I used drugs during the last weekb. | 42 (59) | 29 (41) |
| 4. I prefer to answer these questions each yearb. | 51 (72) | 20 (28) |
| 5. The mHealth app is easy to useb. | 51 (72) | 20 (28) |
| 6.Compared to a face-to-face interview, I prefer to use the mHealth appc. | 38 (54) | 32 (46) |
aN=66.
bN=71.
cN=70.
Discussion
Principal Findings
The purpose of this study was to investigate the feasibility and acceptability of mHealth among individuals with SUDs in China. The findings from our study demonstrated that the veracity and acceptability of the EMA was not optimistically received among those with SUDs in China.
In China, people with drug addiction are treated as immoral. Poor adherence to treatment is the primary problem when dealing with substance-related issues [29] because it leads to relapse. Schomerus [30] found that the stigma associated with alcohol dependence was higher than it was for other mental disorders in general population studies and created a barrier for people to routinely enter treatment [31,32]. Thus, mHealth-based data collection methods hold a unique place among people with drug addiction. Nonetheless, our findings indicated that the response rate for EMA data collection was low, with an average response rate of 49.29% (690/1400), and half of the participants preferred face-to-face interviews. The reasons for the low response rate were drawn from the post-intervention survey. Patients who were unwilling or hesitant to use mHealth expressed concerns with privacy. Some mentioned that the mHealth-based app was not safe and they were afraid of being arrested because they thought the police could obtain information about their relapse from the app. They also expressed concerns about the Global Positioning System (GPS) function of the app, claiming that it made them uncomfortable. Moreover, because most of the individuals with SUDs were isolated from society and their relatives, they preferred the face-to-face interviews because they perceived them as a way to communicate with the public. Another factor that affected the response rate is age [33]. Compared to older patients, young patients are more inclined to accept novel technologies, such as mHealth. Conversely, older patients are more conservative and tend to be more concerned about their privacy. Although age was not mentioned as a barrier in this study, it should be considered in future research and in clinical applications.
Another aim of the study was to explore the veracity of the EMA data. The EMA aims to minimize recall bias, maximize ecological validity, and enable the study of behavior in the real world. Our study results indicated that the correlations between the EMA and the LET and the EMA and the urine test were poor. These results are similar to those of previous studies. For example, Pearson [34] compared the accuracy of self-reported electronic cigarette (e-cigarette) puff counts using the EMA to objective puff count data collected by a Bluetooth-enabled e-cigarette device and found poor agreement between the device data and the self-reported data. Solhan [35] examined the discrepancies among trait questionnaires, retrospective reports, and EMA measures of affective instability in psychiatric outpatients and found poor agreement between recalled mood changes and the EMA. Griffith [36] also found poor agreement between the TLFB and the EMA with respect to heavy smokers.
Regarding the impact factors, Patrick [37] found that gender moderated the 3 methods of data collection. Our work indicated that job and age were predictors of the correspondence between the EMA and the urine test. With respect to the influence of employment, people with a job were found to exhibit better correspondence between the EMA and the urine test. This may be because people who have jobs are better able to cooperate with others and are more likely to engage in honest communications and receive assistance from others.
Limitations
Limitations to our study included a convenience sample that may not be representative of other areas in China or of foreign countries. However, our findings may still be beneficial to those with similar situations. Second, self-reporting and the response to the text message could have been inaccurate due to a number of factors. Third, the low response rate to the EMA daily diaries indicated poor acceptance of mHealth among individuals with SUDs. A future study could combine pre-intervention education and EMA technology to improve the response rate. Finally, because the technology was limited, we were unable to provide accurate feedback or interventions to participants when a craving arose. Despite these limitations, the study presents valuable information regarding the feasibility of electronic health (e-health) among individuals with SUDs.
Conclusions
Mobile phones have been widely used among the Chinese population, with 1.3 billion mobile phone users in 2016. Thus, mobile phones provide a potential opportunity for the development of mHealth in China. The current study demonstrated poor agreement among the data from the EMA, the LET assessment, and urine testing. Hence, the acceptance of mHealth among individuals with SUDs in China was not enthusiastic. This indicates that more work is needed to optimize the mHealth app and improve its acceptance among individuals with SUDs in China as well as in other countries that are experiencing similar issues.
Acknowledgments
This study was supported by grants from National Key R&D Program of China (2017YFC1310400), National Natural Science Foundation of China (U1502228,81771436),Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20152235), Shanghai Key Laboratory of Psychotic Disorders (13DZ2260500), Program of Shanghai Academic Research Leader (17XD1403300), key subject of Shanghai Municipal Health and Family Planning Commission (Psychiatry; 2017ZZ02021), Shanghai Mental Health Center Qihang Program (2017-QH-07), and Shanghai Mental Health Center Clinical Research Project (CRC2017YB04).
Abbreviations
- ATS
amphetamine-type stimulant
- CBT
cognitive behavior therapy
- e-cigarette
electronic cigarette
- EMA
ecological momentary assessment
- IVR
interactive voice recording
- LET
life experience timeline
- mHealth
mobile health
- MMT
methadone maintenance treatment
- SDT
self-determination theory
- SUD
substance use disorder
- TLFB
timeline follow-back method
Footnotes
Conflicts of Interest: None declared.
References
- 1.Lord S, Moore SK, Ramsey A, Dinauer S, Johnson K. Implementation of a substance use recovery support mobile phone app in community settings: qualitative study of clinician and staff perspectives of facilitators and barriers. JMIR Ment Health. 2016 Jun 28;3(2):e24. doi: 10.2196/mental.4927. http://mental.jmir.org/2016/2/e24/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Li W, Zhi-Min L. Similarity and difference in drug addiction process between heroin- and methamphetamine-dependent users. Subst Use Misuse. 2017 Mar 21;52(4):459–467. doi: 10.1080/10826084.2016.1245331. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Office on Drugs and Crime . Executive Summary 2016 World Drug Report. Vienna, Austria: United Nations Dffice on Drugs and Crime; 2016. http://www.unodc.org/doc/wdr2016/WDR_2016_ExSum_english.pdf . [Google Scholar]
- 4.National Drug Control Committee . 2017 China’s Anti-Drug Report. Beijing, China: National Drug Control Committee; 2017. http://www.nncc626.com/2017-03/23/c_129516372.htm . [Google Scholar]
- 5.Jia Z, Liu Z, Chu P, McGoogan JM, Cong M, Shi J, Lu L. Tracking the evolution of drug abuse in China, 2003-10: a retrospective, self-controlled study. Addiction. 2015 Jan;110 Suppl 1:4–10. doi: 10.1111/add.12769. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Wang Z, Liu Z. Factors associated with illicit opioid use in methadone maintenance treatment clients in 5 provinces, China. Environ Health Prev Med. 2016 Nov;21(6):480–486. doi: 10.1007/s12199-016-0570-y. http://europepmc.org/abstract/MED/27699691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Yan S, Bao Y, Lian Z, Qu Z, Liu Z. Cross-sectional study of the severity of self-reported depressive symptoms in heroin users who participate in a methadone maintenance treatment program. Shanghai Arch Psychiatry. 2016 Feb 25;28(1):35–41. doi: 10.11919/j.issn.1002-0829.215127. http://europepmc.org/abstract/MED/27688642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health and Family Planning Commission of the People’s Republic of China 2015 China AIDS Response Progress Report. 2015. May, [2018-01-29]. http://www.unaids.org/sites/default/files/country/documents/CHN_narrative_report_2015.pdf .
- 9.Li L, Comulada WS, Lin C, Hsieh J, Luo S, Wu Z. Factors related to client satisfaction with methadone maintenance treatment in China. J Subst Abuse Treat. 2017 Jun;77:201–206. doi: 10.1016/j.jsat.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou K, Li H, Wei X, Li X, Zhuang G. Medication adherence in patients undergoing methadone maintenance treatment in Xi'an, China. J Addict Med. 2017;11(1):28–33. doi: 10.1097/ADM.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 11.Tang YL, Zhao D, Zhao C, Cubells JF. Opiate addiction in China: current situation and treatments. Addiction. 2006 May;101(5):657–65. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Chow EP, Zhuang X, Liang Y, Wang Y, Tang C, Ling L, Tucker JD, Wilson DP. Methadone maintenance treatment participant retention and behavioural effectiveness in China: a systematic review and meta-analysis. PLoS One. 2013;8(7):e68906. doi: 10.1371/journal.pone.0068906. http://dx.plos.org/10.1371/journal.pone.0068906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai B, Volkow ND. Treatment for substance use disorder: opportunities and challenges under the affordable care act. Soc Work Public Health. 2013;28(3-4):165–74. doi: 10.1080/19371918.2013.758975. http://europepmc.org/abstract/MED/23731411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson K, Richards S, Chih M, Moon TJ, Curtis H, Gustafson DH. A pilot test of a mobile app for drug court participants. Subst Abuse. 2016;10:1–7. doi: 10.4137/SART.S33390. http://europepmc.org/abstract/MED/26917964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997 Oct 03;278(5335):45–7. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 16.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. http://dx.plos.org/10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford JH, Alagoz E, Dinauer S, Johnson KA, Pe-Romashko K, Gustafson DH. Successful organizational strategies to sustain use of A-CHESS: a mobile intervention for individuals with alcohol use disorders. J Med Internet Res. 2015 Aug 18;17(8):e201. doi: 10.2196/jmir.3965. http://www.jmir.org/2015/8/e201/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capon H, Hall W, Fry C, Carter A. Realising the technological promise of smartphones in addiction research and treatment: an ethical review. Int J Drug Policy. 2016 Dec;36:47–57. doi: 10.1016/j.drugpo.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009 Dec;21(4):486–97. doi: 10.1037/a0017074. http://europepmc.org/abstract/MED/19947783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smiley SL, Elmasry H, Webb HM, Niaura RS, Hamilton AB, Milburn NG. Feasibility of ecological momentary assessment of daily sexting and substance use among young adult African American gay and bisexual men: a pilot study. JMIR Res Protoc. 2017 Feb 02;6(2):e9. doi: 10.2196/resprot.6520. http://www.researchprotocols.org/2017/2/e9/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranzler HR, Abu-Hasaballah K, Tennen H, Feinn R, Young K. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004 Jul;28(7):1060–4. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- 22.Searles JS, Helzer JE, Walter DE. Comparison of drinking patterns measured by daily reports and timeline follow back. Psychol Addict Behav. 2000 Sep;14(3):277–86. doi: 10.1037//0893-164x.14.3.277. [DOI] [PubMed] [Google Scholar]
- 23.Lincoln R, Rosenthal CF, Malte CA, Simpson T. A pilot study of memory impairment associated with discrepancies between retrospective and daily recall of alcohol consumption. Am J Addict. 2011;20(6):568–74. doi: 10.1111/j.1521-0391.2011.00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Toll BA, Cooney NL, McKee SA, O'Malley SS. Correspondence between interactive voice response (IVR) and timeline followback (TLFB) reports of drinking behavior. Addict Behav. 2006 Apr;31(4):726–31. doi: 10.1016/j.addbeh.2005.05.044. http://europepmc.org/abstract/MED/15975732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagman BT, Morgenstern J, Kuerbis A, Heidinger B. Correspondence between interactive voice response and time-line follow-back self-reports of alcohol consumption among problem drinking men-who-have-sex-with-men. 137st APHA Annual Meeting and Exposition; 2009 Nov 07-11; Philadelphia. 2009. Nov, [Google Scholar]
- 26.Tucker JA, Foushee HR, Black BC, Roth DL. Agreement between prospective interactive voice response self-monitoring and structured retrospective reports of drinking and contextual variables during natural resolution attempts. J Stud Alcohol Drugs. 2007 Jul;68(4):538–42. doi: 10.15288/jsad.2007.68.538. [DOI] [PubMed] [Google Scholar]
- 27.Linas BS, Genz A, Westergaard RP, Chang LW, Bollinger RC, Latkin C, Kirk GD. Ecological momentary assessment of illicit drug use compared to biological and self-reported methods. JMIR Mhealth Uhealth. 2016 Mar 15;4(1):e27. doi: 10.2196/mhealth.4470. http://mhealth.jmir.org/2016/1/e27/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. doi: 10.1037/0893-164X.12.2.101. [DOI] [Google Scholar]
- 29.Shen J, Wang M, Wang X, Zhang G, Guo J, Li X, Li J. Predictors of poor adherence to methadone maintenance treatment in Yunnan province, China. J Addict Med. 2016;10(1):40–5. doi: 10.1097/ADM.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 30.Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46(2):105–12. doi: 10.1093/alcalc/agq089. [DOI] [PubMed] [Google Scholar]
- 31.Stolzenburg S, Freitag S, Evans-Lacko S, Muehlan H, Schmidt S, Schomerus G. The stigma of mental illness as a barrier to self labeling as having a mental illness. J Nerv Ment Dis. 2017 Dec;205(12):903–909. doi: 10.1097/NMD.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 32.Chan RCH, Mak WWS. Common sense model of mental illness: understanding the impact of cognitive and emotional representations of mental illness on recovery through the mediation of self-stigma. Psychiatry Res. 2016 Dec 30;246:16–24. doi: 10.1016/j.psychres.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Dai M, Xu J, Lin J, Wang Z, Huang W, Huang J. Willingness to use mobile health in glaucoma patients. Telemed J E Health. 2017 Oct;23(10):822–827. doi: 10.1089/tmj.2016.0254. [DOI] [PubMed] [Google Scholar]
- 34.Pearson JL, Elmasry H, Das B, Smiley SL, Rubin LF, DeAtley T, Harvey E, Zhou Y, Niaura R, Abrams DB. Comparison of ecological momentary assessment versus direct measurement of e-cigarette use with a bluetooth-enabled e-cigarette: a pilot study. JMIR Res Protoc. 2017 May 29;6(5):e84. doi: 10.2196/resprot.6501. http://www.researchprotocols.org/2017/5/e84/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solhan MB, Trull TJ, Jahng S, Wood PK. Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychol Assess. 2009 Sep;21(3):425–36. doi: 10.1037/a0016869. http://europepmc.org/abstract/MED/19719353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffith SD, Shiffman S, Heitjan DF. A method comparison study of timeline followback and ecological momentary assessment of daily cigarette consumption. Nicotine Tob Res. 2009 Nov;11(11):1368–73. doi: 10.1093/ntr/ntp150. http://europepmc.org/abstract/MED/19808861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrick ME, Lee CM. Comparing numbers of drinks: college students' reports from retrospective summary, followback, and prospective daily diary measures. J Stud Alcohol Drugs. 2010 Jul;71(4):554–61. doi: 10.15288/jsad.2010.71.554. http://europepmc.org/abstract/MED/20553664. [DOI] [PMC free article] [PubMed] [Google Scholar]


