Abstract
Objectives
Though sub-concussive impacts are common during contact sports, there is little consensus whether repeat blows affect brain function. Using a “lifetime exposure” rather than acute exposure approach, we examined oculomotor performance and brain activation among collegiate football players and two control groups. Our analysis examined whether there are group differences in eye movement behavioral performance and in brain activation during smooth pursuit.
Methods
Data from 21 off-season Division I football “starters” were compared with a) 19 collegiate cross-country runners, and b) 11 non-athlete college students who were SES matched to the football player group (total N = 51). Visual smooth pursuit was performed while undergoing fMRI imaging via a 3 Tesla scanner. Smooth pursuit eye movements to three stimulus difficulty levels were measured with regard to RMS error, gain, and lag.
Results
No meaningful differences were found for any of the standard analyses used to assess smooth pursuit eye movements. For fMRI, greater activation was seen in the oculomotor region of the cerebellar vermis and areas of the FEF for football players as compared to either control group, who did not differ on any measure.
Conclusion
Greater cerebellar activity among football players while performing an oculomotor task could indicate that they are working harder to compensate for some subtle, long-term subconcussive deficits. Alternatively, top athletes in a sport requiring high visual motor skill could have more of their cerebellum and FEF devoted to oculomotor task performance regardless of subconcussive history. Overall, these results provide little firm support for an effect of accumulated subconcussion exposure on brain function.
Keywords: Smooth pursuit, fMRI, Collegiate athletics
Highlights
-
•
Elite collegiate football players have identical smooth eye movements in comparison to cross country runners and controls.
-
•
There is more activation in ocularmotor structures in football players in comparison to cross country runners or controls.
1. Introduction
The public and research emphasis on concussions can sometimes obscure the fact that athletes in a variety of sports experience hundreds of sub-concussive impacts each year (McAllister et al., 2012a). Estimates for sub-concussive impact exposure in football players at the high school and collegiate level range from 244 to 1444 per season (Broglio et al., 2010; Crisco et al., 2010; Greenwald et al., 2008). In contrast, the per season concussion risk is 7.25% for high school and 5.52% for collegiate football athletes (Dompier et al., 2015). Beginning with the documentation of “punch-drunk” dementia in the 1920's (Carroll, 1936; Martland, 1928; Parker, 1934) and the coining of the term Dementia Pugilistica in the 1930's (Millspaugh, 1937), scientists and clinicians have been concerned about the cumulative dose effects of impacts to the skull, including those that do not produce acute concussion but nevertheless result in clinical signs and symptoms. The animal model literature began documenting the cumulative effects of repeated “subconcussive” impacts to the skull in the 1940's (Tedeschi, 1944). Research on this topic burgeoned further with heightened public awareness of the issue and with the development of key research tools such as cognitive testing, brain imaging and wearable accelerometers. Despite the availability of these tools, however, there remains a striking lack of agreement within the literature as to whether repeat subconcussive blows has a measurable effect on the brain and in what areas.
Studies on cognition examine a variety of neurocognitive functions, including: verbal learning, verbal recognition, spatial recognition, visual working memory, visual-motor speed, impulse inhibition, visual attention, and concentration. Using athletes from a variety of sports but primarily soccer, some studies find an effect on cognition (Downs and Abwender, 2002; Ellemberg et al., 2007; Killam et al., 2005; Matser et al., 1999, Matser et al., 2001, Matser et al., 1998; McAllister et al., 2012a; Miyashita et al., 2017; Straume-Naesheim et al., 2009; Talavage et al., 2014; Tsushima et al., 2016; Tysvaer and Løchen, 1991; Witol and Webbe, 2003; Zhang et al., 2013), while others find no effect (Abreau et al., 1990; Guskiewicz et al., 2002; Janda et al., 2002; Kaminski et al., 2008; Kemp et al., 2016; Miller et al., 2007; Putukian et al., 2000; Rutherford et al., 2009; Salinas et al., 2009; Stephens et al., 2010; Straume-Naesheim, 2005; Vann Jones et al., 2014). The subconcussive effect of hits on balance is also inconclusive with some reporting a positive effect (Haran et al., 2013; Miyashita et al., 2017), and others reporting no effect (Broglio et al., 2004; Gysland et al., 2012; Mangus et al., 2004; Schmitt et al., 2004). A meta-analysis of 30 studies on the subconcussive effects on cognition of soccer players concluded that there are too many shortcomings of the current research to draw conclusions. Common shortcomings include small sample sizes, inappropriate selection of control groups, low quality assessment of head impact frequency, and inappropriate control for type 1 errors (Tarnutzer et al., 2016). Examining the potential effects on cognition of subconcussive hits is important from a symptom and quality of life standpoint, but from a scientific standpoint, it is also challenging. Learning and memory skills are easily affected by a large number of relatively difficult to control factors, such as motivation, sleep, age, drug use, blood sugar levels, hydration, etc. Combining neurocognitive testing with neuroimaging may be more promising (Tarnutzer et al., 2016).
Though imaging is not a viable method for diagnosing concussion, the sheer number of subconcussive hits to the skull during contact sports could conceivably result in visible damage to brain matter. Both EEG (Tysvaer and Storli, 1989), and CT (Sortland and Tysvaer, 1989) have been utilized in this effort. More recently, numerous MRI studies have been conducted on subconcussion, including using diffusion imaging and the diffusion tensor model (DTI), which looks for damage in the white matter (Bahrami et al., 2016; Bazarian et al., 2014; Chappell et al., 2008, Chappell et al., 2006; Davenport et al., 2016, Davenport et al., 2014; Helmer et al., 2014; Koerte et al., 2017, Koerte et al., 2012; Lipton et al., 2013; Mayinger et al., 2017; McAllister et al., 2014; Shin et al., 2014). A smaller number of studies have addressed anatomical defects (grey matter changes) (Adams et al., 2007; Davenport et al., 2014; Koerte et al., 2016). Once again, however, these studies do not yield a clear consensus (for review see (Bailes et al., 2013; Belanger et al., 2016; Koerte et al., 2015; Maher et al., 2014)).
To more accurately identify athletes who experience the largest and/or greatest number of subconcussive blows during a season, a small number of researchers have begun using head-mounted accelerometers. So far, the use of this technique has resulted in positive findings. Both McAllister et al. (2014) and Bazarian et al. (2014) reported significant correlations between total accelerometer-based hit exposure and white matter measures of the brain using the DTI model of diffusion imaging. One study used helmet accelerometers and neurocognitive measures among football and hockey players and found a weak effect (McAllister et al., 2012b). Talavage et al. (2014) found a significant correlation with functional MRI (fMRI) activation while athletes performed the short-term memory n-back task. The use of accelerometers in research is limited by cost (of equipment, installation and maintenance), hassle (some players find them cumbersome), and concerns over accuracy. When tested against biomechanical sensors in a laboratory setting, the accuracy of helmet-based accelerometers must be interpreted cautiously (O'Connor et al., 2017; Siegmund et al., 2016).
The study by Talavage and colleagues highlights yet another promising but thus far minimally explored approach: the use of fMRI in combination with behaviorally relevant neurocognitive testing. Research involving resting-state fMRI conducted while subjects relax in an MRI scanner has not shown consistent results (Abbas et al., 2015; Johnson et al., 2014; Slobounov et al., 2017), but the study by Talavage et al. evaluated memory, a neurocognitive function known to be affected by head trauma generally and concussion specifically. Another known sequelae of concussion is sensory motor deficits (Howell et al., 2017; Kontos et al., 2017). Sensory motor testing holds promise for the detection of subconcussive damage in part because it is multimodal. To the best of our knowledge, there is one study series examining fMRI activation during a oculomotor sensorimotor task (Johnson et al., 2015a, Johnson et al., 2015b). We chose to examine eye movement behavior due to the solid body of literature showing ocular motor performance to be one of the most robust indicators of concussion (Balaban et al., 2016; Brahm et al., 2009; Capó-Aponte et al., 2012a, Capó-Aponte et al., 2012b; Cifu et al., 2015; DeHaan et al., 2007; Drew et al., 2007; Heitger et al., 2009, Heitger et al., 2007a, Heitger et al., 2007b, Heitger et al., 2006, Heitger et al., 2004; Hoffer et al., 2017; Kraus et al., 2007; Liston et al., 2017; Maruta et al., 2017; Master et al., 2016; Mucha et al., 2014; Pearce et al., 2015; Samadani et al., 2015; Storey et al., 2017; Thiagarajan et al., 2011). Oculomotor control has been strongly linked to neural integrity (John Leigh and Zee, 2015; Pierrot-Deseilligny et al., 2004) and tasks that assess oculomotor function are linked to a number of cognitive functions including attention, visuospatial processing, working memory, processing speed and predictive behavior (Barnes, 2008; Hutton, 2008; John Leigh and Zee, 2015; Pierrot-Deseilligny et al., 2004; Schütz et al., 2011). In addition, unlike other sensory motor behaviors (e.g., balance and gait), eye movements lend themselves well to fMRI neuroimaging.
The aim of this study is to measure differences in the oculomotor control network in athletes playing in concussion-prone sports as compared to two control groups (non-concussion-prone sport, cross country runners, and socioeconomically matched, SES, non-athletes college students). To do so we used a smooth pursuit task, which has been demonstrated to show reduced performance in concussion patients. The smooth pursuit task in particular has been thoroughly studied in both primates and humans and is known to engage many regions of the brain, both cortical and subcortical (Fukushima et al., 2013). FMRI studies have revealed a network of brain regions linked to the task, including the frontal eye fields (FEF) and supplementary eye fields (SEF) in the frontal lobe, the intraparietal cortex, the occipital cortex, and the cerebellum (Lencer and Trillenberg, 2008; Petit and Haxby, 1999). Smooth pursuit has the additional advantages of being an almost autonomic process and being shown in concussion and post-concussion studies to be impaired in concussed patients (Cifu et al., 2015; Heitger et al., 2009, Heitger et al., 2006; Hoffer et al., 2017).
We compared athletes from an NCAA Football Bowl Subdivision team to both athlete and non-athlete control groups. A pattern in the research design of much of the existing literature is the use of either a single athlete control group from non-contact sports or a single control group drawn from the general student body. Using a non-athlete control group only does not take into account any effects on the brain of years of athletic training and competition. Another pattern within the literature is the lack of matching of either athlete or non-athlete control groups on socioeconomic (SES) status, despite the fact that SES status is known to be related to cognitive abilities (Ursache et al., 2015) and health (Meyer et al., 2014). Depending on the study setting, the socioeconomic backgrounds of even athlete control group participants may be significantly different from that of the concussion prone sport athletes. We therefore included both a non-contact sport control group (drawn from the cross country team) and a non-athlete group matched to the football players on age, gender and SES.
If repetitive sub-concussive impacts have a deleterious effect on neural processing, collegiate football players should show performance decrements on the smooth pursuit task and differences in brain activation when compared to both control groups. At the present time there is insufficient literature to attempt to predict which regions of the network implicated in oculomotor processing (i.e., cerebellum, parietal cortex, or frontal regions) might be affected, but greater activation within the smooth pursuit network, along with possible recruitment of additional regions to handle the increased processing load, would be suggestive of an effect of subconcussion.
2. Methods
2.1. Participants
A total of 51 male subjects participated in the study. Of these 21 were 4th and 5th year undergraduates (many were red-shirts) who were considered ‘starters’ on the Indiana University (IU) varsity football team (age 21.1 ± 1.5), 19 were on the IU cross country team (age: 20.2 ± 2.5), and 11 were non-collegiate athletes from IU that were socioeconomically matched to the football players (age 19.9 ± 3). One football player did not complete the study and his fMRI data was discarded. One cross country runner's fMRI data was unusable and was discarded. None of the athletes had been diagnosed with a concussion in the preceding year. The only exclusion criterion was for MRI safety at 3 Tesla (e.g. no implanted metal). In terms of concussion history, two football players had been diagnosed with a concussion approximately 3 years before the study and one player had been diagnosed with a concussion approximately 2 years before the study. No cross-country runners were diagnosed with a concussion while at IU. In general sport related head impacts in cross-country are extremely rare and the lowest of all the NCAA sanctioned sports (Zuckerman et al., 2015). It should also be noted some football players may have received a diagnosed concussion in high school, and others may have successfully hidden prior concussions from the medical staff of either high school, college, or both. This information was unfortunately not available to us but we can estimate 7.25% players to have received concussion previous to college given published data (Dompier et al., 2015). Football players were given a socioeconomic status questionnaire after completing the scanning session. The socioeconomic match group filled out the same questionnaire and were selectively chosen to match the football players based on sex, age, estimated family income, and the area in which they were raised (urban, small town, suburbs, or urban). Participants gave informed written consent that was approved by the Indiana University Institutional Review Board. All participants were recruited through flyers handed out by the athletic trainers of each team or posted around the campus. Beyond repeated hit exposure, there are differences in the work out schedules/team practices of IU football players and cross-country runners. IU football players have a morning practice 6 days a week lasting approximately 1.5 h, with 2 days of contact practice and 4 days of non-contact practice per week. Six days a week, in the afternoon, football players participate in 30 min of ‘muscle-building’ strength and condition workouts. IU cross-country runners have morning runs lasting approximately 30 min and afternoon runs lasting approximately 1.0–1.5 h, 6 days a week. Twice a week the runners participate in ‘toning’ strength and conditioning workouts. We have no data on the exercise habits of the control subjects.
2.2. Procedures and stimuli
Participants performed a visual smooth pursuit task while undergoing imaging. The movements of participants' right eyes were tracked using an MRI compatible SR Research EyeLink 1000 running monocular at 1000 Hz with an accuracy of roughly 0.2°. Data were recorded and analyzed to ensure that participants were performing the task correctly and to assess performance based on mean gain, lag, and root-mean-square (RMS) error. The stimuli were projected onto a screen located behind the subject and viewed through a mirror attached to the head coil. The task consisted of 20 blocks, each of which started with a 12 s rest followed by three smooth pursuit conditions presented in randomized order (sinusoidal frequency = 0.25 [slow], 0.5 [medium], and 1.0 Hz [fast]), each of which was 11 s in duration. The total time for each block was 45 s and total task time was 15 min. The sinusoidal stimuli traveled ± 5° of amplitude (10° total) from the center of the screen. They traveled along the horizontal meridian and the peak velocity of the stimuli were 8, 15, and 30°/s for the slow, medium, and fast stimuli, respectively. Many of the classes of eye movements have been used in concussion research and shown to have impairment (e.g. saccade, anti-saccades, and binocular eye movements) (Johnson et al., 2015a). Each class of eye movement involves different brain networks (John Leigh and Zee, 2015)and probably reveals a different aspect of the concussion and subconcussion sequelae (Collins et al., 2014). We only had time for one eye movement class in this study and chose smooth pursuit due to documented dysfunction after a concussion (Cifu et al., 2015; Heitger et al., 2009, Heitger et al., 2006; Hoffer et al., 2017).
2.3. Data acquisition
All participants were scanned using a 3 Tesla TIM Trio scanner located in the Imaging Research Facility at Indiana University using a 12-channel head coil. The 32 channel head coil did not fit our larger subjects, so all subjects were scanned with the 12-channel. Functional scans were obtained with the following sequence: TR/TE = 2240/30 ms, TI = 900 ms, flip angle = 70°, FoV = 240, measurements = 480, bandwidth = 1860 Hz/pixel, iPAT factor = 2, 36 axial slices, matrix = 96 × 256, slice thickness = 3.5 mm, 0 gap, 2.5 mm × 2.5 mm × 3.5 mm voxels, total acquisition time = 18:03. Additionally, high resolution anatomical scans were acquired using the following sequence: TR/TE = 1800/2.67 ms, TI = 900 ms, flip angle = 9°, bandwidth = 150 Hz/pixel, 160 sagittal slices, FOV = 256 mm, matrix = 256 × 256, slice thickness = 1 mm, resulting in 1 mm × 1 mm × 1 mm voxels, total acquisition time = 7:42. In order for the eye tracker to have a good view of the right of our large subjects, the subject's head was often tilted.
2.4. Behavioral analysis
Eye tracking data were analyzed using custom written software in Matlab. Blinks were removed from the records and a spline interpolation was performed to recreate the eye movement missing during the blink. The eye records were filtered with a 50 coefficient FIR low pass filtered with a pass-band at 20 Hz and a stop-band at 90 Hz. Since we are interested in comparing total oculomotor tracking performance between the three subject groups, and not necessarily only strictly smooth pursuit eye movements, we did not “de-saccade” the data. RMS error is the euclidean distance between the stimulus and the eye every 1 ms sample. Gain and lag were calculated by fitting a sinusoid to the 10 s eye movement trial (the first second was skipped because of movement initiation) with free parameters for amplitude, lag, and frequency. Gain is defined as the ratio of the eye movement amplitude divided by the stimulus amplitude. Lag is defined as the difference in time between the fitted sinusoid and the stimulus sinusoid as measured with cross-correlation. One and two-way analysis of variance statistics were performed in Matlab with the ANOVAN function. Effect size was quantified using Cohen's d metric.
2.5. fMRI analysis
The functional data were analyzed using statistical parametric mapping, SPM 8. There were three subject groups: football players (experimental), cross country runners (control 1), and SES matches (control 2). fMRI data were preprocessed in several steps, including slice timing correction, motion correction by realignment, co-registration between functional and anatomical scans, spatial normalization, and smoothing. All functional data were resampled to 2mm3 isomorphic voxels normalized to the Montreal Neurological Institute (MNI) template. For spatial smoothing, an 8 mm FWHM Gaussian kernel was applied. On the preprocessed fMRI data of individual subjects, a canonical statistical analysis based on the general linear model (GLM) and Gaussian random field theory was performed (Friston et al., 1995). The hemodynamic response for the stimuli blocks were modeled with a canonical HRF built on the onsets of the blocks with the block duration included in the analysis. For each individual data analysis, regressors were built for the three levels of task difficulty, fixation blocks and 6 regressors from the realignment step were included in the model to remove unexpected effects from noise from head movement.
In order to examine the activation related to each level of difficulty of the pursuit task, each was compared to fixation for each group separately using a one-sample t-test. This was performed to allow for inspection of the results prior to group comparisons to ensure data quality. Two-sample t-tests were then performed to compare activation across groups (e.g., football vs. cross country; football vs. SES-matched non-athlete). For the contrasts examined, we applied a Monte Carlo simulation of the brain volume to establish an appropriate voxel contiguity threshold. The threshold obtained from the simulation has the advantage of higher sensitivity to smaller effect sizes (Slotnick and Schacter, 2004). The result of the Monte Carlo simulation indicated that a cluster size of 20 contiguous resampled voxels using an uncorrected threshold of p < 0.005 would be appropriate to control type I error (p < 0.05) corrected for the multiple comparisons in the whole brain volume analysis.
An ROI analysis was also performed. The mean bold signal for each contrast was also obtained using the SPM toolbox Marsbar and was analyzed in SPSS 24 and Matlab. Eight spherical regions of interest (ROIs) with a radius of 10 mm were defined around regions previously shown to be implicated in smooth pursuit tasks (talairach coordinance): bilateral FEF (−42, −12, 50; 42, −12, 50), SEF (−6, −4, 62; 6, −4, 62), cerebellar vermis (−12, −72 −22; 12, −72, −22), and the anterior lobe of the cerebellum (−11, −36, −14).
3. Results
3.1. Behavioral data
Fig. 1 shows the raw eye movement records for the first subject in the experiment (a football student athlete). The three stimulus frequencies (0.25, 0.5, and 1.0 Hz) are all in the range where human smooth pursuit performance is usually fairly good. We calculated the mean RMS (root mean squared) error, gain, and lag for every eye movement sample for every trial (20 trial per stimulus condition) as our metric of the subject's performance for each stimulus condition (slow, medium, and fast).
Fig. 1.
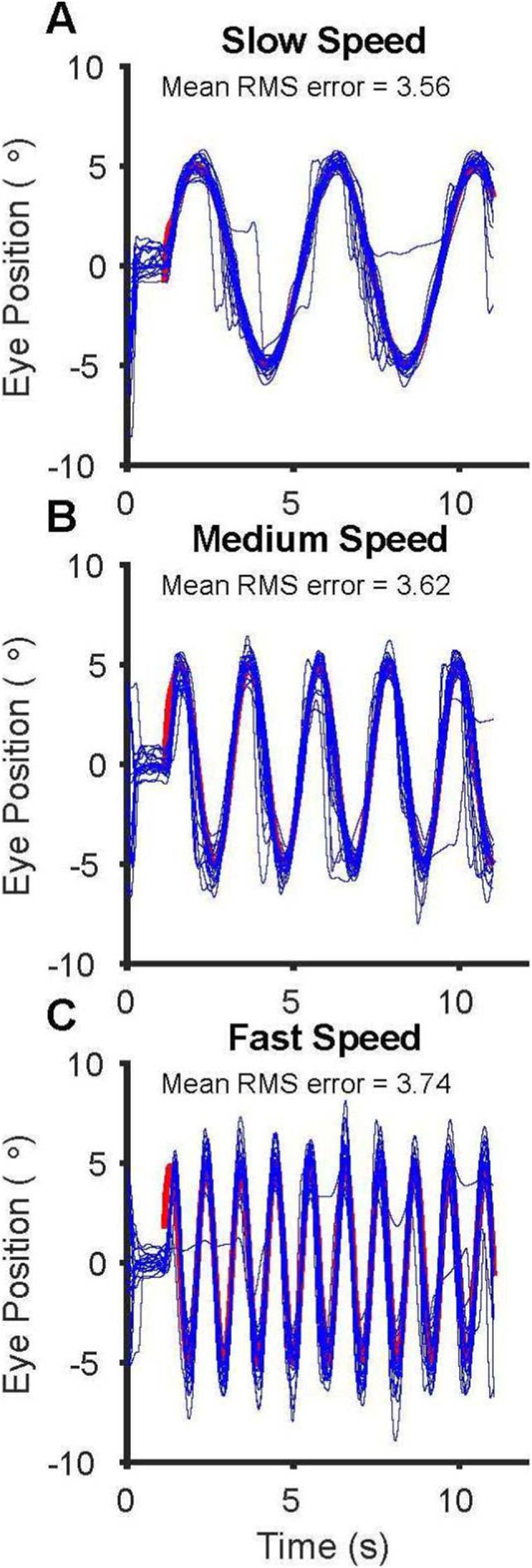
Example subject ocular-motor performance for the slow (A), medium (B), and fast stimulus conditions (C). The red trace underneath is the horizontal position of the stimulus and the blue traces are all 20° horizontal eye movement records. The mean RMS error for all 20 trials and for each stimulus condition is listed in the figure. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
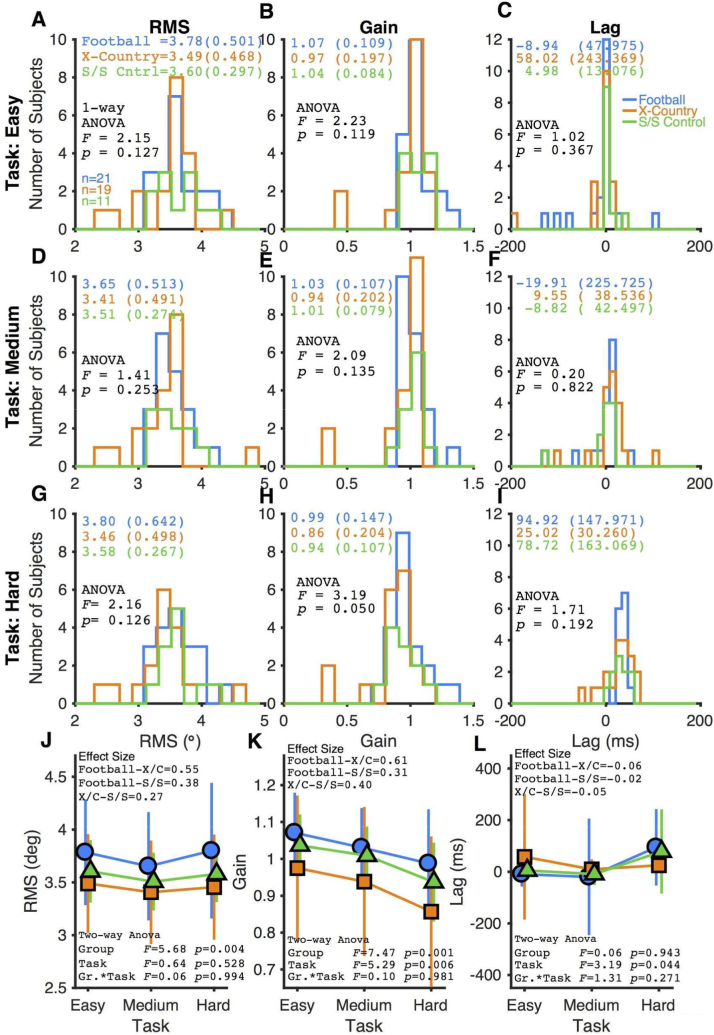
Fig. 2 summarizes the oculomotor findings for three groups of subjects. For no stimulus condition (slow, medium, or fast) or smooth pursuit performance metric (RMS, gain, and lag) was there a qualitative (shift in a histogram) or quantitative difference between the three subject groups (one-way ANOVA, Fig. 2). This study is a two-way design (subject group by task difficulty) and Fig. 2J–L summarizes the result for each smooth pursuit metric. A two-way ANOVA indicates a significant effect of subject group for smooth pursuit RMS, as well as significant effects for both subject group and task difficulty for smooth pursuit gain. Given the well documented shortcomings of interpreting results through the p-value of null-hypothesis significance testing (Hentschke and Stüttgen, 2011; Nakagawa and Cuthill, 2007), the effect size between the subject group was calculated for each smooth pursuit metric (Fig. 2J–L). Combining the data across task difficulty, the effect sizes of subject group differences ranges between 0.27 and 0.61 (Fig. 2J–L), which in general are in the small range (Hentschke and Stüttgen, 2011; Nakagawa and Cuthill, 2007). Thus, between the histograms of the raw data, the one-way ANOVAs, and the effect size, there appears to be no meaningful difference between our three subject groups in oculomotor performance during our smooth pursuit task. The degrees of freedom for subject group and task difficulty is 2.
Fig. 2.
Histogram comparing ocular motor performance for the 3 subject groups. The first row is for the easy stimulus, the second row is for the medium stimulus, and the third row is for the hard stimulus. The ANOVAs in the first three rows is a one-way ANOVA examining the effect of subject group. The mean and standard deviation of each distribution are listed in the panel. The fourth row shows a summary for each subject group for three task difficulties. The two-way ANOVAS in the fourth row test for the effects of subject group and task difficulty, and an interaction of the two variables. There are two degrees of freedom for both subject group and task difficulty. Effect size is calculated using Cohen's d metric. The first column analyzes smooth pursuit root mean squared metric (RMS), the middle column analyzes pursuit gain, and the third column analyzes pursuit lag. In each panel, cyan represents values from the football subjects, orange represents cross country subjects, and green represents SES matched nonathletes. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Functional data
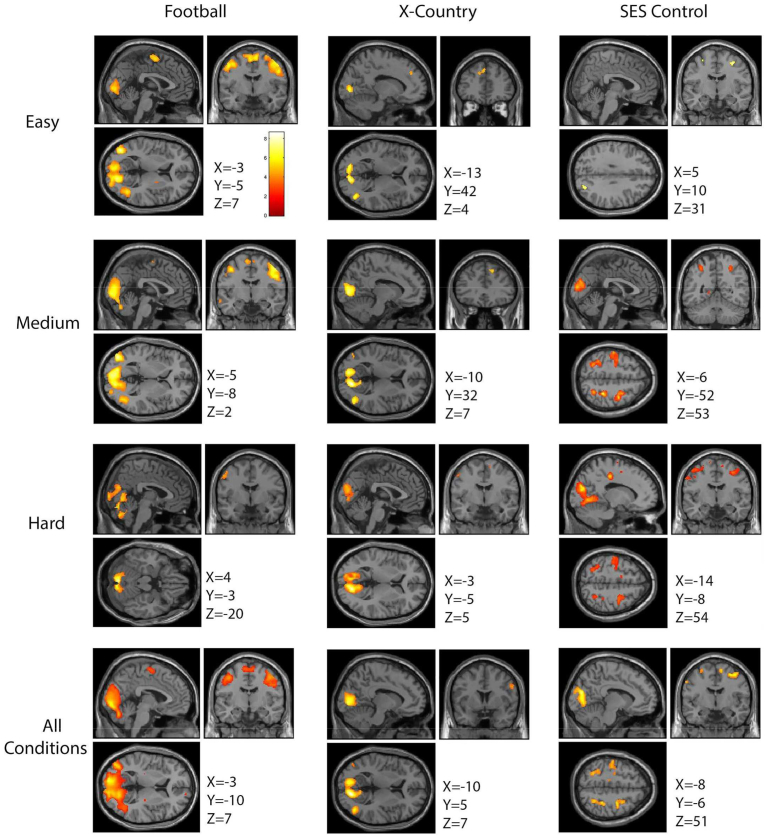
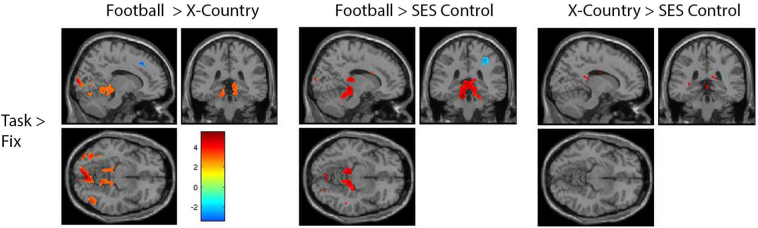
Fig. 3 illustrates the oculomotor function activation for each subject group and stimulus condition. For the three stimulus conditions (easy, medium, and hard), there is stronger activation (increase in p value) and recruitment of more brain regions (more voxels) for each group (p < 0.005, k = 10), reflecting task difficulty (Fig. 3). Qualitatively, football players consistently showed greater activation during the easy and medium conditions than either the cross country runners or SES non-athlete controls. When examining the hard stimulus condition alone, the football players show greater activation in the cerebellar regions, whereas the cross-country runners and SES control non-athletes show greater activation in frontal regions such as the FEFs (Fig. 3). The last row of Fig. 3 combines the stimulus conditions together to highlight the differences between the three subjects groups by increasing the signal to noise ratio of functional activation.
Fig. 3.
A within group comparison of all conditions separately and collapsed. All contrasts are condition greater than fixation. The first column shows the activation of football players during each of the three conditions of the task as well as the activation when all conditions are collapsed and compared to fixation. The second column shows the activation of cross country runners for all three conditions separately as well as collapsed. The third column shows the activation of SES matched non-athletes for all three conditions separately as well as collapsed.
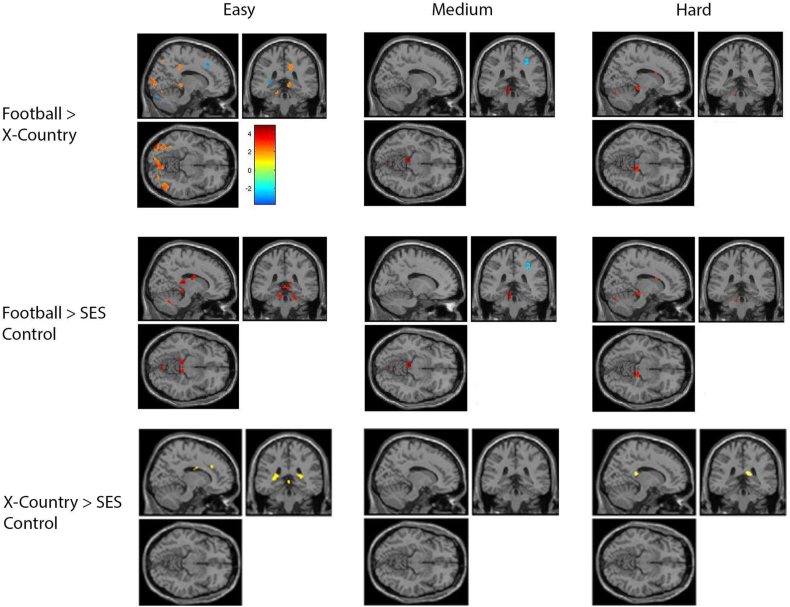
In Fig. 4, for each stimulus condition, the differences between groups are shown. The top row contrasts football players minus cross-country runners, illustrating some activation difference in the cerebellum. The middle row contrasts football players minus SES non-athletes, also illustrating activation differences in the cerebellum. The bottom row contrasts cross-country runners minus SES non-athletes, illustrating no consistent difference.
Fig. 4.
Comparison between groups during each condition of the task (x = 14, y = −36, z = −10). The top row shows the activation of football players during each condition subtracted by the activation of the cross country runners during the same condition. The middle row shows the activation of football players during each condition subtracted by the activation of the SES matched non-athletes during the same condition. The bottom row shows the activation of cross country runners during each condition subtracted by the activation of the SES matched non-athletes during the same condition.
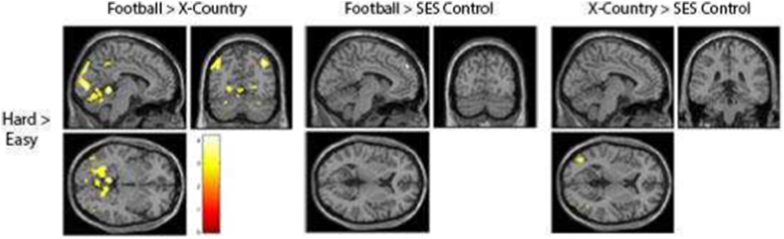
Fig. 5 summarizes the activation differences of the hard stimulus minus the easy stimulus conditions, contrasted between subject groups. In the left panel, football players have more activation in the FEF, occipital lobe, and cerebellum than cross-country runners. In the middle and right panels, we see no systematic differences when football players are contrasted with SES non-athletes, or when cross-country runners are contrasted with SES non-athletes, respectively.
Fig. 5.
Comparison within groups between hard and easy conditions (x = −6, y = −66, z = 10). This contrast compared the activation during the hard condition minus the easy conditions in order to demonstrate increased activation accompanying the increase in cognitive demand. The first column shows the activation of football players subtracted by the cross country players for this contrast. The second column shows the activation of the football players subtracted by the SES matched non-athletes for this contrast. The third column shows the activation of cross country runners subtracted by the SES matched non-athletes for this contrast.
Fig. 6 summarizes activation differences between our subject groups with the stimulus conditions combined. The left panel contrasts football players minus cross-country runners, illustrating greater activation in occipital cortex and the cerebellum. The middle panel contrasts football players minus SES non-athletes, illustrating greater activation in the cerebellum. The right panel illustrates no systematic differences in activation between cross-country runners and SES non-athletes.
Fig. 6.
Between group comparison during the task (x = 14, y = −36, z = −10). All three conditions were collapsed representing the task as a whole. The task activation was then subtracted by the fixation and this resulting activation was then compared between groups. The first column shows the task-based activation of the football players subtracted by the cross country runners. The second column shows the task based activation of the football players subtracted by the SES matched non-athlete controls. The third column shows the task-based activation of the cross country runners subtracted by the SES matched non-athlete controls.
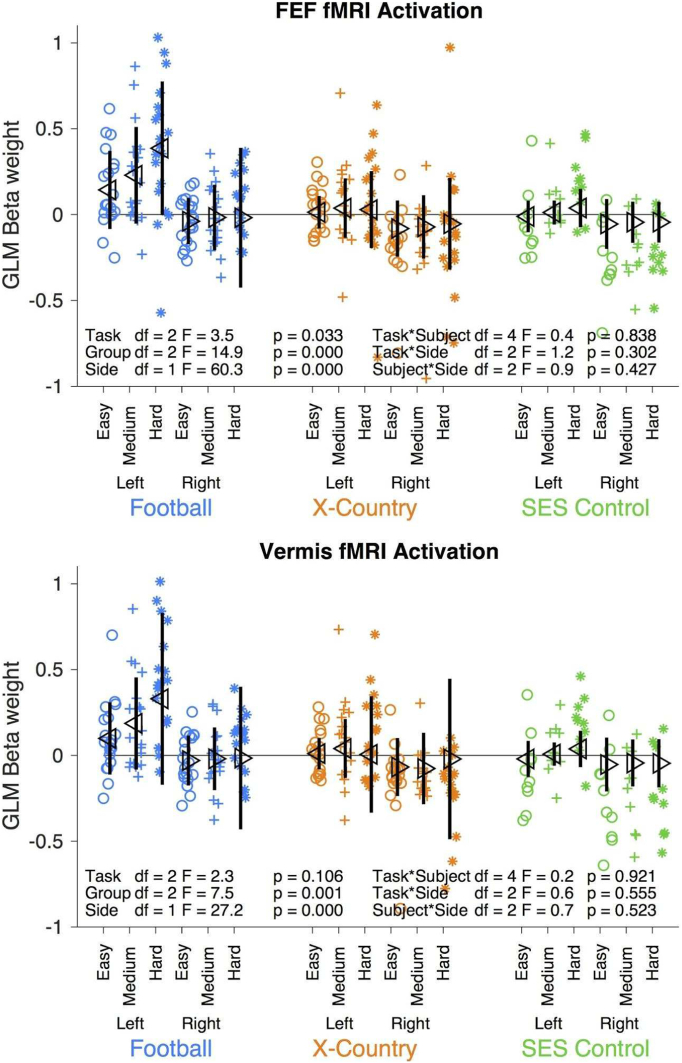
Fig. 7A quantifies the differences in FEF activation for each hemisphere between our three groups for each stimulus condition by examining the GLM beta weights from Fig. 6. Football players have a significantly greater activation pattern in comparison to the runners and non-athletes. There is a significant effect of subject group and side on activation. There is greater left hemisphere FEF activation for all three subjects groups in comparison to the right hemisphere FEF. In addition, there is a possible qualitative effect of stimulus condition (p = 0.034). There are no significant interactions.
Fig. 7.
GLM beta weight from Fig. 6 for each group and condition in the bilateral frontal eye fields (FEF) and vermis. The football players are plotted in ceyan, the cross-country runners are plotted in orange, and the SES matched non-athletes are plotted in green. The easy task condition is plotted with circles, the medium condition is plotted with plus signs, and the hard condition is plotted with stars. The left and right sides of the central nervous system are plotted separately. Each symbol is an individual subject. The mean and standard deviation are plotted in black. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Finally, Fig. 7B quantifies the differences in cerebellar vermis activation for each side between our three groups for each stimulus condition. Football players have a significantly greater activation pattern in comparison to the runners and non-athletes. There is greater left vermis activation in comparison to the right vermis. In addition, there is a possible qualitative but not quantitative effect of stimulus condition on vermis activation. There are no significant interactions.
4. Discussion
The aim of the current study was to determine whether oculomotor differences are observed in athletes who play a concussion-prone sport in comparison to other groups. The smooth pursuit task was chosen because it has been demonstrated to show reduced performance in concussion patients. Functional MRI was also used in order to explore for any differences in functional brain activation while subjects performed the visual motor task. Both athletes in a non-concussion-prone sport (cross country runners) and SES-matched non-athletes were used as two separate control groups.
No meaningful differences were found among the groups for any of the three standard analyses used to assess smooth pursuit: root mean squared error, gain, and lag. Only one research group has published studies examining eye movements in relation to subconcussion. Kawata et al. (2016b) studied the acute effects of repetitive head impacts (heading a soccer ball) on near point convergence among twenty soccer players. They found a decrease in performance both immediately after and at 24-h post-exposure. Interpretations of this study are limited by the lack a control “placebo” activity group (e.g., a sport-based but non-heading activity) and the lack of blinding of researchers and subjects, especially for what is a subjective visual motor test. However, in a second study, Kawata et al. (2016a) used mouthguard-based accelerometers to divide twenty-nine football players into high versus low head impact (22 high; 7 low) during training camp. The acute effect on near point convergence was measured at baseline (pre-season) and before and after non-contact practice and contact practices, and again at postseason. For the high impact players but not for the low impact players, they found a significant performance impairment, though their performance had returned to baseline by the postseason follow-up. Unfortunately, the authors provide no histogram demonstrating how and why the two groups were chosen, nor do they provide reassurance regarding the degree of blinding by the experimenter collecting the eye movement data. In general, eye movement measures that can be objectively quantified are preferable to those that rely on subjective report, in part because they minimize experimenter and subject bias.
It is important to note that our study focused on the accumulated effects of subconcussion compared to athletes and non-athletes without this exposure. The football players we recruited had not experienced a concussion in the previous season, and data was collected just prior to the upcoming season in order to allow the maximum amount of whatever healing would take place to occur. In other words, the players were tested at the moment in time during which they should have shown the least amount of acute effects possible, e.g., a ‘lifetime exposure’ approach rather than an immediate post-season or even post-acute approach (as per Kawata).
A possible limitation of our study is the maximum pursuit speed, which may not have been sufficiently high, resulting in a low ceiling effect. This likely occurred due to the small size of the computer screen within the scanner. Future studies should perform a full psychophysical pilot to insure adequate increased error rates for faster stimuli. On the other hand, all groups showed a consistent increase in the intensity and volume of activation with increased difficulty, implying that the task did become more demanding as the speed increased (Fig. 3), just maybe not sufficiently so to reveal meaningful between group differences.
Another limitation in our study is the assumption of repeated head impact exposure. We did not have the ability or resources to do a full size instrumented accelerometer study, therefore can only assume the starting junior and senior football players experienced repeated head impacts. Future work would be enhanced by recording accelerometer data in both groups and correlating changes in activation with differences in exposure.
For the fMRI results, we found an increase in the amount of tissue area activated during the oculomotor task among football players as compared to athlete and non-athlete controls. Areas of greater activation included the oculomotor region of the cerebellum (vermis) and areas in the FEF. No differences were found between the two control groups. All groups displayed the expected bilateral activation in the occipital lobe, both in the cuneus and lateral regions, as well the FEF and SEF in the frontal lobes; however, only the football players showed significant activation in the cerebellum, specifically the vermis. Vermal activation was expected in all groups and both control groups revealed activation when the significance threshold was lowered, suggesting that the vermis was recruited in all participants but to a higher degree by the football players.
Only one prior study has examined functional activation maps in relationship to a behaviorally relevant task among subjects who have accumulated subconcussive hits. Talavage et al. (2014) instrumented twenty-one high school football players' helmets with accelerometers for one season of play. The study included pre-season and in-season neurocognitive testing with ImPACT and fMRI during one season of high school football. Of the eleven subjects, three were diagnosed with concussion by the team physician. Of the remaining eight non-concussed subjects, four had a normal in-season ImPACT and four had an abnormal in-season ImPACT. The functional fMRI task was the 1 or 2-Back working memory test. Of the eight non-concussed subjects, those who demonstrated abnormalities on the ImPACT showed a decrease in fMRI activation in the dorsolateral pre-frontal cortex and the cerebellum compared to those who did not have an impaired ImPACT score. Issues arise with regard to the use the ImPACT test as a means of categorizing subjects, however, due to a published false positive rate of 40% (Nelson et al., 2016). The size of the sample also contributes to concerns about the validity and reliability of the results. As per the previously discussed work of Kawata and colleagues, this study focuses on the acute rather than long-term effects of subconcussive hits to the brain. Finally, it differs from our study in that the task used, while certainly relevant in terms of known post-concussive effects, is not sensorimotor in nature.
Johnson and colleagues, in a series of two papers, examined the oculomotor deficits and functional brain activation (fMRI) of 9 concussed athletes both acutely (within 7 days of the injury) and subacutely (~30 days post-injury) (Johnson et al., 2015a, Johnson et al., 2015b). At the acute time course, they report longer saccadic latencies during anti-saccades, worse positional errors during pro-saccades, anti-saccades, and memory guided saccades, and fewer saccades during the self-paced saccade task. Examining the effect size, they found a strong effect (>2.0) for directional errors in the anti-saccade task, directional errors in the memory guided task, and the number of saccades in the self-paced saccade task. Interestingly, they did not find any deficits in two smooth pursuit tasks (sinusoidal and circular pursuit), which is in contrast to other studies of smooth pursuit and concussions (Cifu et al., 2015; Heitger et al., 2009, Heitger et al., 2006; Hoffer et al., 2017). In their fMRI results, Johnson and colleague's concussed group demonstrated a larger activation and recruitment of new sites in the brain, in comparison to the normal volunteers.
In Johnson and colleagues follow-up manuscript, they examined the eye movements and brain activation of the same concussed athletes at 30 days post injury (Johnson et al., 2015a, Johnson et al., 2015b). The concussed athletes (7 participated in the follow-up study) were clinically asymptomatic and showed oculomotor improvements compared to their acutely concussed measured eye movement. Nevertheless, oculomotor differences remained between the subacute group and normal volunteers. The concussed group demonstrated increased and larger areas of brain activation in fMRI, similar to when the athletes were acutely concussed.
In regards to the eye movement analysis, both papers are limited by the small number of subjects. The lack of raw data (no histograms or scatter graphs) in the papers also leaves open the possibility the results are driven by a single subject. Finally, the normal volunteer group does not appears to be matched for sport with student athletes. Notwithstanding these limitations, their results are in agreement with ours in terms of an increase in and larger area of brain activation, including in the cerebellum.
To the best of our knowledge, then, our study is the first to examine groups of athletes in the off-season using a sensorimotor task and fMRI. Off-season data collection was used in order to minimize the acute effects of recent hit exposure and better isolate the effects of long-term exposure. Our sample of twenty athletes per athlete group compares well to fMRI studies in general, which tend to have limited N's primarily due to the costs involved. The lower number of SES matched subjects (11) resulted from the difficulty of recruiting students who's SES matched that of the football players. As expected, the football team had a higher percentage of students from lower SES strata than the student population at large. The difficulty we experienced even in recruiting sufficient numbers of matched non-athlete controls highlights the relevance of including SES matching in future studies.
The finding of greater cerebellar activity among football players has at least two possible interpretations. First, the football players may be working harder to compensate for some subtle, long-term subconcussive deficits (e.g., a “compensation” theory). Animal studies demonstrate that, after a concussion, a metabolic slowdown is produced that parallels the symptom trajectory. As one of the most metabolically active areas of the brain, the cerebellum would be expected to be particularly affected by such a slow down. The cerebellar areas of football players experiencing subconcussive symptoms might therefore show added activation as their brains attempt to compensate for these losses. This interpretation would not explain the greater activation found for the FEF, however, because those areas have only average metabolic demand. A finding that supports the compensation theory is the fact that differences in activation between the football players and the control groups increased with task difficulty. This implies that not only were football players required to recruit different brain regions such as the cerebellum to perform the task, but also as the task increased in difficulty, they were required to make more than expected recruitment in already active areas in response to this increase of demand.
An equally valid interpretation of our fMRI findings, however, is that our sample was comprised of top athletes in a sport requiring high visual motor skill. As such, more of their cerebellum and FEF may be devoted to oculomotor task performance regardless of their history of hits to the brain. This has been demonstrated for other high-skill activities, such as musicians and the proportion of the brain dedicated to finger control in musicians (Landau and D'esposito, 2006; Münte et al., 2002), and taxi drivers and the proportion of the brain dedicated to mental maps (Woollett et al., 2009). One possible method for surmounting this difficulty would be to repeat this study with a within-sport design, using accelerometer data to differentiate those who experienced greater and lesser hits to the brain during the season. This approach would, however, relate again to acute rather than life-time cumulative effects of subconcussion hits. To examine life-time effects, one might examine groups of retired football players with greater and lesser previous seasons of play, although again one would need to insure that the reason for participating in fewer seasons was not due to lesser athletic ability.
In summary, with the caveat that our smooth pursuit task might not have achieved sufficient difficulty to reveal group differences, our results do not provide support for the idea that cumulative subconcussive hits accrued during participation in a concussion-prone sport affect the behaviorally relevant, sensory motor task of smooth pursuit compared to cross country runner and non-athletes. And while differences were found with regard to fMRI imaging, the results are difficult to interpret. The combination of fMRI and a behaviorally relevant task is a powerful one. Future studies are needed for smooth pursuit and other behaviorally relevant cognitive and sensorimotor tasks, and controls should be SES matched when possible. Within the literature, it is important to differentiate studies examining acute versus life-time cumulative effects of subconcussion.
Acknowledgements
Indiana Spinal Cord and Brain Injury Research Fund, NIH R21DC013974. This research was partially supported by NSF IIS-1636893, BCS-1734853 and NIH NIMH ULTTR001108 to FP. The Indiana University Imaging Research Facility partially supported data acquisition and preprocessing.
References
- Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., Robinson M.E. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. 2015;5:91–101. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Abreau F., Templer D.I., Schuyler B.A., Terry Hutchison H. Neuropsychological assessment of soccer players. Neuropsychology. 1990;4:175–181. [Google Scholar]
- Adams J., Adler C.M., Jarvis K., DelBello M.P., Strakowski S.M. Evidence of anterior temporal atrophy in college-level soccer players. Clin. J. Sport Med. 2007;17:304–306. doi: 10.1097/JSM.0b013e31803202c8. [DOI] [PubMed] [Google Scholar]
- Bahrami N., Sharma D., Rosenthal S., Davenport E.M., Urban J.E., Wagner B., Jung Y., Vaughan C.G., Gioia G.A., Stitzel J.D., Whitlow C.T., Maldjian J.A. Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology. 2016;281:919–926. doi: 10.1148/radiol.2016160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- Balaban C., Hoffer M.E., Szczupak M., Snapp H., Crawford J., Murphy S., Marshall K., Pelusso C., Knowles S., Kiderman A. Oculomotor, vestibular, and reaction time tests in mild traumatic brain injury. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G.R. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008;68:309–326. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zhu T., Zhong J., Janigro D., Rozen E., Roberts A., Javien H., Merchant-Borna K., Abar B., Blackman E.G. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger H.G., Vanderploeg R.D., McAllister T. Subconcussive blows to the head: a formative review of short-term clinical outcomes. J. Head Trauma Rehabil. 2016;31:159–166. doi: 10.1097/HTR.0000000000000138. [DOI] [PubMed] [Google Scholar]
- Brahm K.D., Wilgenburg H.M., Kirby J., Ingalla S., Chang C.-Y., Goodrich G.L. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom. Vis. Sci. 2009;86:817–825. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- Broglio S.P., Guskiewicz K.M., Sell T.C., Lephart S.M. No acute changes in postural control after soccer heading. Br. J. Sports Med. 2004;38:561–567. doi: 10.1136/bjsm.2003.004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio S.P., Schnebel B., Sosnoff J.J., Shin S., Fend X., He X., Zimmerman J. Biomechanical properties of concussions in high school football. Med. Sci. Sports Exerc. 2010;42:2064–2071. doi: 10.1249/MSS.0b013e3181dd9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capó-Aponte J.E., Tarbett A.K., Urosevich T.G., Temme L.A., Sanghera N.K., Kalich M.E. Effectiveness of computerized oculomotor vision screening in a military population: pilot study. J. Rehabil. Res. Dev. 2012;49:1377–1398. doi: 10.1682/jrrd.2011.07.0128. [DOI] [PubMed] [Google Scholar]
- Capó-Aponte J.E., Urosevich T.G., Temme L.A., Tarbett A.K., Sanghera N.K. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil. Med. 2012;177:804–813. doi: 10.7205/milmed-d-12-00061. [DOI] [PubMed] [Google Scholar]
- Carroll E.J. PUNCH-DRUNK. Am J Med Sci. 1936;191:706–712. [Google Scholar]
- Chappell M.H., Uluğ A.M., Zhang L., Heitger M.H., Jordan B.D., Zimmerman R.D., Watts R. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J. Magn. Reson. Imaging. 2006;24:537–542. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- Chappell M.H., Brown J.A., Dalrymple-Alford J.C., Uluğ A.M., Watts R. Multivariate analysis of diffusion tensor imaging data improves the detection of microstructural damage in young professional boxers. Magn. Reson. Imaging. 2008;26:1398–1405. doi: 10.1016/j.mri.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Cifu D.X., Wares J.R., Hoke K.W., Wetzel P.A., Gitchel G., Carne W. Differential eye movements in mild traumatic brain injury versus normal controls. J. Head Trauma Rehabil. 2015;30:21–28. doi: 10.1097/HTR.0000000000000036. [DOI] [PubMed] [Google Scholar]
- Collins M.W., Kontos A.P., Reynolds E., Murawski C.D., Fu F.H. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg. Sports Traumatol. Arthrosc. 2014;22:235–246. doi: 10.1007/s00167-013-2791-6. [DOI] [PubMed] [Google Scholar]
- Crisco J.J., Fiore R., Beckwith J.G., Chu J.J., Brolinson P.G., Duma S., McAllister T.W., Duhaime A.-C., Greenwald R.M. Frequency and location of head impact exposures in individual collegiate football players. J. Athl. Train. 2010;45:549–559. doi: 10.4085/1062-6050-45.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.M., Whitlow C.T., Urban J.E., Espeland M.A., Jung Y., Rosenbaum D.A., Gioia G.A., Powers A.K., Stitzel J.D., Maldjian J.A. Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma. 2014;31:1617–1624. doi: 10.1089/neu.2013.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E.M., Apkarian K., Whitlow C.T., Urban J.E., Jensen J.H., Szuch E., Espeland M.A., Jung Y., Rosenbaum D.A., Gioia G.A., Powers A.K., Stitzel J.D., Maldjian J.A. Abnormalities in diffusional kurtosis metrics related to head impact exposure in a season of high school varsity football. J. Neurotrauma. 2016;33:2133–2146. doi: 10.1089/neu.2015.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan A., Halterman C., Langan J., Drew A.S., Osternig L.R., Chou L.-S., van Donkelaar P. Cancelling planned actions following mild traumatic brain injury. Neuropsychologia. 2007;45:406–411. doi: 10.1016/j.neuropsychologia.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dompier T.P., Kerr Z.Y., Marshall S.W., Hainline B., Snook E.M., Hayden R., Simon J.E. Incidence of concussion during practice and games in youth, high school, and collegiate American football players. JAMA Pediatr. 2015;169:659–665. doi: 10.1001/jamapediatrics.2015.0210. [DOI] [PubMed] [Google Scholar]
- Downs D.S., Abwender D. Neuropsychological impairment in soccer athletes. J. Sports Med. Phys. Fitness. 2002;42:103–107. [PubMed] [Google Scholar]
- Drew A.S., Langan J., Halterman C., Osternig L.R., Chou L.-S., van Donkelaar P. Attentional disengagement dysfunction following mTBI assessed with the gap saccade task. Neurosci. Lett. 2007;417:61–65. doi: 10.1016/j.neulet.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Ellemberg D., Leclerc S., Couture S., Daigle C. Prolonged neuropsychological impairments following a first concussion in female university soccer athletes. Clin. J. Sport Med. 2007;17:369–374. doi: 10.1097/JSM.0b013e31814c3e3e. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Turner R., Frackowiak R.S. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Fukushima J., Warabi T., Barnes G.R. Cognitive processes involved in smooth pursuit eye movements: behavioral evidence, neural substrate and clinical correlation. Front. Syst. Neurosci. 2013;7:4. doi: 10.3389/fnsys.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R.M., Gwin J.T., Chu J.J., Crisco J.J. Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery. 2008;62:789–798. doi: 10.1227/01.neu.0000318162.67472.ad. (discussion 798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Broglio S.P., Cantu R.C., Kirkendall D.T. No evidence of impaired neurocognitive performance in collegiate soccer players. Am. J. Sports Med. 2002;30:157–162. doi: 10.1177/03635465020300020201. [DOI] [PubMed] [Google Scholar]
- Gysland S.M., Mihalik J.P., Register-Mihalik J.K., Trulock S.C., Shields E.W., Guskiewicz K.M. The relationship between subconcussive impacts and concussion history on clinical measures of neurologic function in collegiate football players. Ann. Biomed. Eng. 2012;40:14–22. doi: 10.1007/s10439-011-0421-3. [DOI] [PubMed] [Google Scholar]
- Haran F.J., Tierney R., Wright W.G., Keshner E., Silter M. Acute changes in postural control after soccer heading. Int. J. Sports Med. 2013;34:350–354. doi: 10.1055/s-0032-1304647. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Anderson T.J., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., Ardagh M.W. Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain. 2004;127:575–590. doi: 10.1093/brain/awh066. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., Ardagh M.W., Anderson T.J. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj. 2006;20:807–824. doi: 10.1080/02699050600676354. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Jones R.D., Dalrymple-Alford J.C., Frampton C.M., Ardagh M.W., Anderson T.J. Mild head injury—a close relationship between motor function at 1 week post-injury and overall recovery at 3 and 6 months. J. Neurol. Sci. 2007;253:34–47. doi: 10.1016/j.jns.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Jones R.D., Frampton C.M., Ardagh M.W., Anderson T.J. Recovery in the first year after mild head injury: divergence of symptom status and self-perceived quality of life. J. Rehabil. Med. 2007;39:612–621. doi: 10.2340/16501977-0100. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Jones R.D., Macleod A.D., Snell D.L., Frampton C.M., Anderson T.J. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132:2850–2870. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- Helmer K.G., Pasternak O., Fredman E., Preciado R.I., Koerte I.K., Sasaki T., Mayinger M., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., Echlin P.S. Hockey concussion education project, part 1. Susceptibility-weighted imaging study in male and female ice hockey players over a single season. J. Neurosurg. 2014;120:864–872. doi: 10.3171/2013.12.JNS132093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke H., Stüttgen M.C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 2011;34:1887–1894. doi: 10.1111/j.1460-9568.2011.07902.x. [DOI] [PubMed] [Google Scholar]
- Hoffer M.E., Balaban C., Szczupak M., Buskirk J., Snapp H., Crawford J., Wise S., Murphy S., Marshall K., Pelusso C., Knowles S., Kiderman A. Laryngoscope Investigative Otolaryngology. 2017. The use of oculomotor, vestibular, and reaction time tests to assess mild traumatic brain injury (mTBI) over time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D.R., Osternig L.R., Chou L.-S. Single-task and dual-task tandem gait test performance after concussion. J. Sci. Med. Sport. 2017;20:622–626. doi: 10.1016/j.jsams.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Hutton S.B. Cognitive control of saccadic eye movements. Brain Cogn. 2008;68:327–340. doi: 10.1016/j.bandc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Janda D.H., Bir C.A., Cheney A.L. An evaluation of the cumulative concussive effect of soccer heading in the youth population. Inj. Control. Saf. Promot. 2002;9:25–31. doi: 10.1076/icsp.9.1.25.3324. [DOI] [PubMed] [Google Scholar]
- John Leigh R., Zee D.S. Oxford University Press; USA: 2015. The Neurology of Eye Movements. [Google Scholar]
- Johnson B., Neuberger T., Gay M., Hallett M., Slobounov S. Effects of subconcussive head trauma on the default mode network of the brain. J. Neurotrauma. 2014;31:1907–1913. doi: 10.1089/neu.2014.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Hallett M., Slobounov S. Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology. 2015;85:1163–1166. doi: 10.1212/WNL.0000000000001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Zhang K., Hallett M., Slobounov S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2015;9:564–573. doi: 10.1007/s11682-014-9316-x. [DOI] [PubMed] [Google Scholar]
- Kaminski T.W., Cousino E.S., Glutting J.J. Examining the relationship between purposeful heading in soccer and computerized neuropsychological test performance. Res. Q. Exerc. Sport. 2008;79:235–244. doi: 10.1080/02701367.2008.10599486. [DOI] [PubMed] [Google Scholar]
- Kawata K., Rubin L.H., Lee J.H., Sim T., Takahagi M., Szwanki V., Bellamy A., Darvish K., Assari S., Henderer J.D., Tierney R., Langford D. Association of football subconcussive head impacts with ocular near point of convergence. JAMA Ophthalmol. 2016;134:763–769. doi: 10.1001/jamaophthalmol.2016.1085. [DOI] [PubMed] [Google Scholar]
- Kawata K., Tierney R., Phillips J., Jeka J.J. Effect of repetitive sub-concussive head impacts on ocular near point of convergence. Int. J. Sports Med. 2016;37:405–410. doi: 10.1055/s-0035-1569290. [DOI] [PubMed] [Google Scholar]
- Kemp S., Duff A., Hampson N. The neurological, neuroimaging and neuropsychological effects of playing professional football: results of the UK five-year follow-up study. Brain Inj. 2016;30:1068–1074. doi: 10.3109/02699052.2016.1148776. [DOI] [PubMed] [Google Scholar]
- Killam C., Cautin R.L., Santucci A.C. Assessing the enduring residual neuropsychological effects of head trauma in college athletes who participate in contact sports. Arch. Clin. Neuropsychol. 2005;20:599–611. doi: 10.1016/j.acn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., Shenton M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Lin A.P., Willems A., Muehlmann M., Hufschmidt J., Coleman M.J., Green I., Liao H., Tate D.F., Wilde E.A., Pasternak O., Bouix S., Rathi Y., Bigler E.D., Stern R.A., Shenton M.E. A review of neuroimaging findings in repetitive brain trauma. Brain Pathol. 2015;25:318–349. doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Hufschmidt J., Muehlmann M., Tripodis Y., Stamm J.M., Pasternak O., Giwerc M.Y., Coleman M.J., Baugh C.M., Fritts N.G., Heinen F., Lin A., Stern R.A., Shenton M.E. Cavum Septi pellucidi in symptomatic former professional football players. J. Neurotrauma. 2016;33:346–353. doi: 10.1089/neu.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte I.K., Nichols E., Tripodis Y., Schultz V., Lehner S., Igbinoba R., Chuang A.Z., Mayinger M., Klier E., Muehlmann M., Kaufmann D., Lepage C., Heinen F., Schulte-Koerne G., Zafonte R.D., Shenton M.E., Sereno A.B. Impaired cognitive performance in youth athletes exposed to repetitive head impacts. J. Neurotrauma. 2017 doi: 10.1089/neu.2016.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos A.P., Deitrick J.M., Collins M.W., Mucha A. Review of vestibular and oculomotor screening and concussion rehabilitation. J. Athl. Train. 2017;52:256–261. doi: 10.4085/1062-6050-51.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M.F., Little D.M., Donnell A.J., Reilly J.L., Simonian N., Sweeney J.A. Oculomotor function in chronic traumatic brain injury. Cogn. Behav. Neurol. 2007;20:170–178. doi: 10.1097/WNN.0b013e318142badb. [DOI] [PubMed] [Google Scholar]
- Landau S.M., D'esposito M. Sequence learning in pianists and nonpianists: an fMRI study of motor expertise. Cogn. Affect. Behav. Neurosci. 2006;6:246–259. doi: 10.3758/cabn.6.3.246. [DOI] [PubMed] [Google Scholar]
- Lencer R., Trillenberg P. Neurophysiology and neuroanatomy of smooth pursuit in humans. Brain Cogn. 2008;68:219–228. doi: 10.1016/j.bandc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Lipton M.L., Kim N., Zimmerman M.E., Kim M., Stewart W.F., Branch C.A., Lipton R.B. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston D.B., Wong L.R., Stone L.S. Oculometric assessment of sensorimotor impairment associated with TBI. Optom. Vis. Sci. 2017;94:51–59. doi: 10.1097/OPX.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher M.E., Hutchison M., Cusimano M., Comper P., Schweizer T.A. Concussions and heading in soccer: a review of the evidence of incidence, mechanisms, biomarkers and neurocognitive outcomes. Brain Inj. 2014;28:271–285. doi: 10.3109/02699052.2013.865269. [DOI] [PubMed] [Google Scholar]
- Mangus B.C., Wallmann H.W., Ledford M. Analysis of postural stability in collegiate soccer players before and after an acute bout of heading multiple soccer balls. Sports Biomech. 2004;3:209–220. doi: 10.1080/14763140408522841. [DOI] [PubMed] [Google Scholar]
- Martland H.S. PUNCH DRUNK. J. Am. Med. Assoc. 1928;91:1103. [Google Scholar]
- Maruta J., Jaw E., Modera P., Rajashekar U., Spielman L.A., Ghajar J. Frequency responses to visual tracking stimuli may be affected by concussion. Mil. Med. 2017;182:120–123. doi: 10.7205/MILMED-D-16-00093. [DOI] [PubMed] [Google Scholar]
- Master C.L., Scheiman M., Gallaway M., Goodman A., Robinson R.L., Master S.R., Grady M.F. Vision diagnoses are common after concussion in adolescents. Clin. Pediatr. 2016;55:260–267. doi: 10.1177/0009922815594367. [DOI] [PubMed] [Google Scholar]
- Matser J.T., Kessels A.G., Jordan B.D., Lezak M.D., Troost J. Chronic traumatic brain injury in professional soccer players. Neurology. 1998;51:791–796. doi: 10.1212/wnl.51.3.791. [DOI] [PubMed] [Google Scholar]
- Matser E.J., Kessels A.G., Lezak M.D., Jordan B.D., Troost J. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282:971–973. doi: 10.1001/jama.282.10.971. [DOI] [PubMed] [Google Scholar]
- Matser J.T., Kessels A.G., Lezak M.D., Troost J. A dose-response relation of headers and concussions with cognitive impairment in professional soccer players. J. Clin. Exp. Neuropsychol. 2001;23:770–774. doi: 10.1076/jcen.23.6.770.1029. [DOI] [PubMed] [Google Scholar]
- Mayinger M.C., Merchant-Borna K., Hufschmidt J., Muehlmann M., Weir I.R., Rauchmann B.-S., Shenton M.E., Koerte I.K., Bazarian J.J. White matter alterations in college football players: a longitudinal diffusion tensor imaging study. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9672-4. [DOI] [PubMed] [Google Scholar]
- McAllister T.W., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Tosteson T.D., Crisco J.J., Brolinson P.G., Duma S.M., Duhaime A.-C., Grove M.R., Turco J.H. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78:1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Tosteson T.D., Crisco J.J., Brolinson P.G., Duma S.M., Duhaime A.-C., Grove M.R., Turco J.H. Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology. 2012;78:1777–1784. doi: 10.1212/WNL.0b013e3182582fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., Jain S. Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology. 2014;82:63–69. doi: 10.1212/01.wnl.0000438220.16190.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O.L., Castro-Schilo L., Aguilar-Gaxiola S. Determinants of mental health and self-rated health: a model of socioeconomic status, neighborhood safety, and physical activity. Am. J. Public Health. 2014;104:1734–1741. doi: 10.2105/AJPH.2014.302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.R., Adamson G.J., Pink M.M., Sweet J.C. Comparison of preseason, midseason, and postseason neurocognitive scores in uninjured collegiate football players. Am. J. Sports Med. 2007;35:1284–1288. doi: 10.1177/0363546507300261. [DOI] [PubMed] [Google Scholar]
- Millspaugh J.A. Dementia Pugilistica. US Naval Med. Bull. 1937;35 [Google Scholar]
- Miyashita T.L., Diakogeorgiou E., Marrie K. Correlation of head impacts to change in balance error scoring system scores in division I men's lacrosse players. Sports Health. 2017;1941738116685306 doi: 10.1177/1941738116685306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha A., Collins M.W., Elbin R.J., Furman J.M., Troutman-Enseki C., DeWolf R.M., Marchetti G., Kontos A.P. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am. J. Sports Med. 2014;42:2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münte T.F., Altenmüller E., Jäncke L. The musician's brain as a model of neuroplasticity. Nat. Rev. Neurosci. 2002;3:473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Cuthill I.C. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Nelson L.D., LaRoche A.A., Pfaller A.Y., Lerner E.B., Hammeke T.A., Randolph C., Barr W.B., Guskiewicz K., McCrea M.A. Prospective, head-to-head study of three computerized neurocognitive assessment tools (CNTs): reliability and validity for the assessment of sport-related concussion. J. Int. Neuropsychol. Soc. 2016;22:24–37. doi: 10.1017/S1355617715001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor K.L., Rowson S., Duma S.M., Broglio S.P. Head-impact-measurement devices: a systematic review. J. Athl. Train. 2017;52:206–227. doi: 10.4085/1062-6050.52.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H.L. Traumatic encephalopathy (‘punch drunk’) of professional pugilists. J. Neurol. Psychopathol. 1934;15:20–28. doi: 10.1136/jnnp.s1-15.57.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce K.L., Sufrinko A., Lau B.C., Henry L., Collins M.W., Kontos A.P. Near point of convergence after a sport-related concussion: measurement reliability and relationship to neurocognitive impairment and symptoms. Am. J. Sports Med. 2015;43:3055–3061. doi: 10.1177/0363546515606430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L., Haxby J.V. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J. Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Milea D., Müri R.M. Eye movement control by the cerebral cortex. Curr. Opin. Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Putukian M., Echemendia R.J., Mackin S. The acute neuropsychological effects of heading in soccer: a pilot study. Clin. J. Sport Med. 2000;10:104–109. doi: 10.1097/00042752-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Rutherford A., Stephens R., Fernie G., Potter D. Do UK university football club players suffer neuropsychological impairment as a consequence of their football (soccer) play? J. Clin. Exp. Neuropsychol. 2009;31:664–681. doi: 10.1080/13803390802484755. [DOI] [PubMed] [Google Scholar]
- Salinas C.M., Webbe F.M., Devore T.T. The epidemiology of soccer heading in competitive youth players. J. Clin. Sport Psychol. 2009;3:15–33. [Google Scholar]
- Samadani U., Ritlop R., Reyes M., Nehrbass E., Li M., Lamm E., Schneider J., Shimunov D., Sava M., Kolecki R., Burris P., Altomare L., Mehmood T., Smith T., Huang J.H., McStay C., Todd S.R., Qian M., Kondziolka D., Wall S., Huang P. Eye tracking detects disconjugate eye movements associated with structural traumatic brain injury and concussion. J. Neurotrauma. 2015;32:548–556. doi: 10.1089/neu.2014.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D.M., Hertel J., Evans T.A., Olmsted L.C., Putukian M. Effect of an acute bout of soccer heading on postural control and self-reported concussion symptoms. Int. J. Sports Med. 2004;25:326–331. doi: 10.1055/s-2004-819941. [DOI] [PubMed] [Google Scholar]
- Schütz A.C., Braun D.I., Gegenfurtner K.R. Eye movements and perception: a selective review. J. Vis. 2011;11 doi: 10.1167/11.5.9. [DOI] [PubMed] [Google Scholar]
- Shin W., Mahmoud S.Y., Sakaie K., Banks S.J., Lowe M.J., Phillips M., Modic M.T., Bernick C. Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am. J. Neuroradiol. 2014;35:285–290. doi: 10.3174/ajnr.A3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund G.P., Guskiewicz K.M., Marshall S.W., DeMarco A.L., Bonin S.J. Laboratory validation of two wearable sensor systems for measuring head impact severity in football players. Ann. Biomed. Eng. 2016;44:1257–1274. doi: 10.1007/s10439-015-1420-6. [DOI] [PubMed] [Google Scholar]
- Slobounov S.M., Walter A., Breiter H.C., Zhu D.C., Bai X., Bream T., Seidenberg P., Mao X., Johnson B., Talavage T.M. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: a multi-modal neuroimaging study. NeuroImage: Clin. 2017;14:708–718. doi: 10.1016/j.nicl.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick S.D., Schacter D.L. A sensory signature that distinguishes true from false memories. Nat. Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Sortland O., Tysvaer A.T. Brain damage in former association football players. An evaluation by cerebral computed tomography. Neuroradiology. 1989;31:44–48. doi: 10.1007/BF00342029. [DOI] [PubMed] [Google Scholar]
- Stephens R., Rutherford A., Potter D., Fernie G. Neuropsychological consequence of soccer play in adolescent U.K. school team soccer players. J. Neuropsychiatr. Clin. Neurosci. 2010;22:295–303. doi: 10.1176/jnp.2010.22.3.295. [DOI] [PubMed] [Google Scholar]
- Storey E.P., Master S.R., Lockyer J.E., Podolak O.E., Grady M.F., Master C.L. Near point of convergence after concussion in children. Optom. Vis. Sci. 2017;94:96–100. doi: 10.1097/OPX.0000000000000910. [DOI] [PubMed] [Google Scholar]
- Straume-Naesheim T.M. Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br. J. Sports Med. 2005;39:i70–i77. doi: 10.1136/bjsm.2005.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume-Naesheim T.M., Andersen T.E.,.K., Holme I.M., McIntosh A.S., Dvorak J., Bahr R. Do minor head impacts in soccer cause concussive injury? A prospective case-control study. Neurosurgery. 2009;64:719–725. doi: 10.1227/01.NEU.0000340681.12949.6D. (discussion 725) [DOI] [PubMed] [Google Scholar]
- Talavage T.M., Nauman E.A., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K.E., Feuer H., Leverenz L.J. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma. 2014;31:327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer A.A., Straumann D., Brugger P., Feddermann-Demont N. Persistent effects of playing football and associated (subconcussive) head trauma on brain structure and function: a systematic review of the literature. Br. J. Sports Med. 2016 doi: 10.1136/bjsports-2016-096593. [DOI] [PubMed] [Google Scholar]
- Tedeschi C.G. Cumulative effects of repeated head trauma of minimal intensity.* Observations on experimental animals. Exp. Biol. Med. 1944;57:264–266. [Google Scholar]
- Thiagarajan P., Ciuffreda K.J., Ludlam D.P. Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic Physiol. Opt. 2011;31:456–468. doi: 10.1111/j.1475-1313.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- Tsushima W.T., Geling O., Arnold M., Oshiro R. Are there subconcussive neuropsychological effects in youth sports? An exploratory study of high- and low-contact sports. Appl. Neuropsychol. Child. 2016;5:149–155. doi: 10.1080/21622965.2015.1052813. [DOI] [PubMed] [Google Scholar]
- Tysvaer A.T., Løchen E.A. Soccer injuries to the brain. A neuropsychologic study of former soccer players. Am. J. Sports Med. 1991;19:56–60. doi: 10.1177/036354659101900109. [DOI] [PubMed] [Google Scholar]
- Tysvaer A.T., Storli O.-V. Soccer injuries to the brain. Am. J. Sports Med. 1989;17:573–578. doi: 10.1177/036354658901700421. [DOI] [PubMed] [Google Scholar]
- Ursache A., Noble K.G., Blair C. Socioeconomic status, subjective social status, and perceived stress: associations with stress physiology and executive functioning. Behav. Med. 2015;41:145–154. doi: 10.1080/08964289.2015.1024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann Jones S.A., Breakey R.W., Evans P.J. Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. Br. J. Sports Med. 2014;48:159–161. doi: 10.1136/bjsports-2013-092758. [DOI] [PubMed] [Google Scholar]
- Witol A.D., Webbe F.M. Soccer heading frequency predicts neuropsychological deficits. Arch. Clin. Neuropsychol. 2003;18:397–417. [PubMed] [Google Scholar]
- Woollett K., Spiers H.J., Maguire E.A. Talent in the taxi: a model system for exploring expertise. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009;364:1407–1416. doi: 10.1098/rstb.2008.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.R., Red S.D., Lin A.H., Patel S.S., Sereno A.B. Evidence of cognitive dysfunction after soccer playing with ball heading using a novel tablet-based approach. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S.L., Lee Y.M., Odom M.J., Forbes J.A., Solomon G.S., Sills A.K. Sports-related concussion in helmeted vs. Unhelmeted athletes: who fares worse? Int. J. Sports Med. 2015;36:419–425. doi: 10.1055/s-0034-1395587. [DOI] [PubMed] [Google Scholar]