Abstract
To discriminate the feasible differences and find potential similarities and relationships of Citri Reticulatae Pericarpium (CRP), this work was accomplished by a comprehensive and reliable method using gas chromatography–mass spectrometer (GC–MS) to analyze the volatile oils and high‐performance liquid chromatography (HPLC) simultaneously to determine the contents of five bioactive flavonoids, namely hesperidin, nobiletin, 3,5,6,7,8,3′,4′‐heptamethoxyflavone, tangeretin, and 5‐hydroxy‐6,7,8,3′,4′‐pentamethoxyflavone in 25 batches of CRP samples of 10 cultivars collected from different regions in China. The GC–MS analyses indicated that 98 compounds were successfully identified from the volatile oils obtained and the major constituents of volatile oil are d‐limonene, γ‐terpinene, α‐pinene, linalool, and myrcene. Even 2‐(methylamino) benzoate was found in all cultivar samples harvested at maturation stage. Under the optimal condition, the quantitative analyses of five bioactive flavonoids were successfully performed by HPLC and hierarchical cluster analysis (HCA). Results showed significant differences among cultivars in the contents of five bioactive flavonoids mentioned earlier. The HCA and GC–MS results provided a convenient approach which might be applied for rapid similarity evaluation and also holds the potential for analysis of compounds present in other plants. Therefore, this work obtained offers scientific basis to control quality and develop medicinal value of the medicinal materials in CRP.
Keywords: Citri Reticulatae Pericarpium, cluster analysis, flavonoids, GC–MS, HPLC, volatile oil
1. INTRODUCTION
Citri Reticulatae Pericarpium (CRP, Chenpi in Chinese), the dried ripe fruits peel of Citrus reticulata Blanco and its cultivars collected between September and December, has been broadly applied to a famous traditional Chinese medicine (TCM) and widely added to food as a condiment in China because of its different pharmacologic effects, low toxicity, and costs (Committee of National Pharmacopoeia, 2015; Duan, Guo, Liu, Liu, & Li, 2014; Shen, 2002). As a vital Chinese herbal medicine, CRP possesses various therapeutic activities, including strengthening spleen, promoting qi, eliminating dampness and phlegm, and so forth (Committee of National Pharmacopoeia, 2015; Xue & Xu, 2005). Dried tangerine or orange peels are widely distributed in different regions, such as Guangdong Province, Fujian Province, Sichuan Province, Zhejiang Province, Jiangxi Province, Hunan Province, and so forth. Guang Chenpi (Citrus reticulata “Chachi”), Chuan Chenpi (C. reticulata “Dahongpao”), Zhe Chenpi (C. reticulata “Unshiu”), and Jian Chenpi (C. reticulata “Tangerina”) are recorded by Chinese pharmacopeia. Among of the main Chenpi cultivars, the dried ripe pericarp of C. reticulata “Chachi,” mainly produced in Xinhui district of Guangdong Province in China, named Guang Chenpi (GCP) in Chinese, is viewed as famous drug of the region on account of its superior quality (Tan et al., 2015).
Phytochemical studies showed that abundant components are present in CRP, such as flavonoids, alkaloids, phenolic acids, and essential oils (EOs), among which flavonoids were considered to be the primary bioactive components (Zheng et al., 2013). Moreover, the major components of CRP are dietary flavonoids, which are generally categorized into two groups: flavanone glycosides (primarily hesperidin) and polymethoxylated flavones (PMFs, primarily nobiletin and tangeretin) (Ho & Kuo, 2014; Zeng, Dua, Chen, Li, & Liu, 2017). Currently, hesperidin is used as a chemical reference for quality control of CRP in the Chinese pharmacopeia because of its extremely high concentration (over 3%) (Committee of National Pharmacopoeia, 2015). Besides, EOs are the other principal pharmacological components of CRP (Qin et al., 2013). EOs extracted from plant materials, such as flowers, roots, bark, seeds, fruit peels, and wood, are subtle and aromatic. By report, several methods have been applied for extracting EOs, namely steam distillation (SD) and supercritical fluid extraction‐CO2 (SFE‐CO2), and SD as the common method is recorded on Chinese pharmacopeia, while several studies have quantified flavonoids in Citrus herbs using thin‐layer chromatography (TLC), HPLC–UV, HPLC–DAD, HPLC–ECD, HPLC–MS, and CE–ECD, primarily focusing on the determination of flavonoids in fruits, juices, or CRP of different Citrus species (Camarda, Di Stefano, Del Bosco, & Schillaci, 2007; Careri, Elviri, Mangia, & Musci, 2000; Liu et al., 2013; Peng, Liu, & Ye, 2006; Sahraoui et al., 2011; Wang & Luo, 1989; Zheng et al., 2009). In recent studies, lots of attention is being paid to flavonoids because of its anticancer, antioxidant, anticonvulsant, and anti‐inflammatory properties (Chang et al., 2015; Devi et al., 2015; Dimpfel, 2006; Fu et al., 2017), whereas few on volatile oils which have abundantly valid biological activities on CRP (Duan et al., 2016; Yi, Dong, Liu, Yi, & Zhang, 2015). So far, there were a group of analyses of flavonoids including constituents and contents, and the results indicated obvious differences in CP and GCP (Lin, Li, Ho, & Lo, 2012; Liu et al., 2013; Xing, Zhao, Zhang, & Li, 2017; Zhang et al., 2012; Zheng et al., 2009, 2013).
Modern pharmacology study has demonstrated that volatile oils play critical roles in certain allergy and antitussive, expectorant, and anti‐inflammatory effects (Wang et al., 2014). In addition, most reports on volatile oils components of Chenpi are focused on the comparison in citrus, such as Citri Reticulatae Pericarpium (CRP) and Citri Reticulatae Pericarpium Viride (CRPV), but few is centered around different cultivars (Chen & Cui, 1998; Gao, 2011; Hu et al., 2014; Mao, Ou, & Wang, 2015; Wang & Li, 2015; Yi et al., 2015). Gas chromatography coupled with mass spectroscopy (GC–MS) and high‐performance liquid chromatography (HPLC) with dual wavelength detection have become a reasonable and powerful approach for the qualitative and quantitative analyses in tangerine peels. However, to the best of our knowledge, no such study was yet reported about HPLC method coupled with GC–MS approach to evaluate and discriminate the quality of CRP effectively and comprehensively among different cultivars in order to ensure its superior clinical use.
Thus, the objectives of this study were to develop a comprehensive, accurate, and reliable HPLC method for the simultaneous quantitative determination of five bioactive flavonoids (Figure 1), including hesperidin (C1), nobiletin (C2), 3,5,6,7,8,3′,4′‐heptamethoxyflavone (C3), tangeretin (C4), and 5‐hydroxy‐6,7,8,3′,4′‐pentamethoxyflavone (C5), as well as qualitative profiling of other secondary metabolites in 25 batches of tangerine peel samples of 10 cultivars collected from different major citrus‐producing areas in China, such as Guangdong Province, Guangxi Province, Sichuan Province, Fujian Province, Zhejiang Province, Jiangxi Province, Hubei Province, and Hunan Province. The reliability and adaptability of the method were verified by the determination of linear range, recovery, and reproducibility with CRP samples. And the results were evaluated and classified by hierarchical cluster analysis (HCA) based on the contents of five bioactive flavonoids. The results provide detailed information for identifying botanical origin, chemotaxonomic investigation of Citrus species, and potential perspective for quality control of complex matrices.
Figure 1.
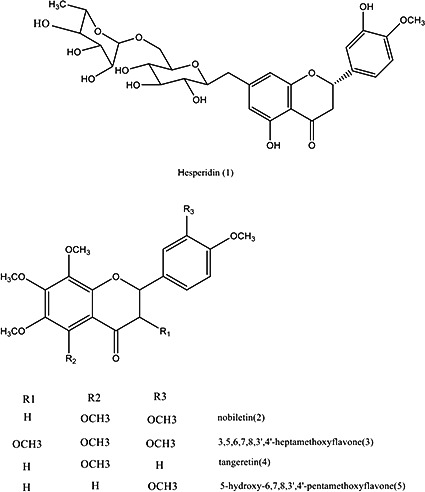
Chemical structure of bioactive flavonoids C1–C5
2. MATERIALS AND METHODS
2.1. Plant materials and chemicals
Twenty‐five samples including 10 different cultivars (collected between October 2013 and December 2015) were collected from different major citrus‐producing areas in China. The detailed information of the samples is presented in Table 1. Approximately 20 kg of fresh fruits was collected from each sampling area. Then, the citrus peels were removed and dried under the sun for about 5–7 days, which were used for testing the samples. And the voucher specimens, authenticated by Prof. Guodong Zheng, have been deposited at the Laboratory of Institute of Pharmaceutical Sciences, Guangzhou Medical University, Guangzhou University City, Guangdong Province, China.
Table 1.
Sample information and the results of the volatile oil extraction (n = 25)
| Sample | Cultivars | Place of collection | Time of collection | Essential oil yield (g/kg) | Extract characters |
|---|---|---|---|---|---|
| S1 | Citrus reticulata “Chachi” | Sanjiang Town, Xinhui District, Jiangmen City, Guangdong Province | 2015/11/05 | 67.43 ± 0.08 | Colorless |
| S2 | C. reticulata “Chachi” | Sanjiang Town, Xinhui District, Jiangmen City, Guangdong Province | 2015/12/23 | 62.94 ± 0.04 | Colorless |
| S3 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2015/10/11 | 89.91 ± 0.35 | Colorless |
| S4 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2010 | 5.84 ± 0.13 | Pale yellow |
| S5 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 1.80 ± 0.05 | Aqua |
| S6 | C. reticulata “Chachi” | Dongjia Town, Xinhui District, Jiangmen City, Guangdong Province | 2010 | 4.50 ± 0.04 | Dark yellow |
| S7 | C. reticulata “Chachi” | Dongjia Town, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 2.70 ± 0.02 | Pale yellow |
| S8 | C. reticulata “Chachi” | Tianlu Village, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 17.98 ± 0.05 | Aqua |
| S9 | C. reticulata “Chachi” | Shijian Town, Xinhui District, Jiangmen City, Guangdong Province | 1995 | 7.19 ± 0.01 | Yellow‐green |
| S10 | C. reticulata “Chachi” | Meijiang Town, Xinhui District, Jiangmen City, Guangdong Province | 1995 | 4.50 ± 0.02 | Yellow‐green |
| S11 | C. reticulata “Chachi” | Huaiji County, Zhaoqing City, Guangdong Province | 2015/11/26 | 2.70 ± 0.01 | Pale yellow |
| S12 | C. reticulata “Chachi” | Longmen County, Huizhou City, Guangdong Province | 2015/11/10 | 20.21 ± 0.09 | Colorless |
| S13 | C. reticulata “Unshiu” | Lingui County, Guilin City, Guangxi Zhuang Autonomous Region | 2013/10/03 | 2.25 ± 0.02 | Aqua |
| S14 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2014/11/30 | 2.25 ± 0.11 | Yellow‐green |
| S15 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2015/10/21 | 2.02 ± 0.05 | Green |
| S16 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2015/11/30 | 1.35 ± 0.01 | Yellow‐green |
| S17 | C. reticulata “Speciosa” | Dianjun District, Yichang City, Hubei Province | 2015/10/11 | 0.90 ± 0.03 | Yellow‐green |
| S18 | C. reticulata “Speciosa” | Dianjun District, Yichang City, Hubei Province | 2015/12/11 | 4.04 ± 0.02 | Yellow‐green |
| S19 | C. reticulata “Speciosa” | Pujiang County, Chengdu City, Sichuan Province | 2015/10/15 | 3.59 ± 0.05 | Aqua |
| S20 | C. reticulata “Kinokuni” | Nanfeng County, Fuzhou City, Jiangxi Province | 2015/10/10 | 9.00 ± 0.13 | Aqua |
| S21 | C. reticulata “Dahongpao” | Pujiang County, Chengdu City, Sichuan Province | 2015/10/17 | 6.74 ± 0.05 | Yellow‐green |
| S22 | C. reticulata “Subcompressa” | Yongquan Town, Linhai County, Taizhou City, Zhejiang Province | 2014/10/15 | 0.90 ± 0.06 | Yellow‐green |
| S23 | C. reticulata C. reticulata “Tangerina” | Dongzhang Town, Fuqing County, Fujian Province | 2015/12/24 | 4.50 ± 0.04 | Yellow‐green |
| S24 | Citrus reticulata “Ponkan” | Yongchun County, Quanzhou City, Fujian Province | 2015/12/07 | 0.315 ± 0.09 | Yellow‐green |
| S25 | C. reticulata “Shiyueju” | Huaiji County, Zhaoqing City, Guangdong Province | 2015/11/26 | 38.25 ± 0.20 | Pale green |
The solvents, HPLC grade n‐hexane and acetonitrile, were purchased from Honeywell (America). Deionized water (18 mol/L−1·Ω) was prepared by distilled water through a Milli‐Q system (Millipore, Milford, MA, USA). The reference standards of flavonoids C1–C5 (hesperidin, nobiletin, 3,5,6,7,8,3′,4′‐heptamethoxyflavone, tangeretin, and 5‐hydroxy‐6,7,8,3′,4′‐pentamethoxyflavone) purchased from Must (Chengdu, China) were isolated and purified from “Chachi” CRP by conventional column chromatography, and their structures were identified by EI‐MS, 1HNMR, and 13C NMR in comparison with the data from the literature. Their purities were determined to be 98% based on HPLC analysis using a peak area normalization method.
2.2. Sample preparation for HPLC analysis
The tested samples were cut into smaller pieces and further ground into powder (100 mesh). Each sample powder (0.5 g) was weighed accurately and extracted with 50 ml methanol using ultrasonicator at room temperature for 30 min at 320 W. After that, the sample was filtered and the 10 ml volume of solution was set at 25 ml volumetric flask. The obtained solution was filtered through a 0.22‐μm filter membrane, and 20 μl of the filtrate was subjected to HPLC analysis.
2.3. Sample preparation for GC–MS analysis
The peels were manually removed, sun‐dried, and stored under dry conditions. Before extraction, the peels were powdered using a mill (Xuyang Equipment Manufacture Company, China) and passed through a 24 mesh sieve. Approximately 200 g of every sample powder we collected were swollen by soaking in 2,000 ml of distilled water for 12 hr prior to hydrodistillation for 5 hr using a standard extractor. Then, the EOs were prepared according to the standard method described in Chinese pharmacopeia (2015 version). The volatile oil was collected in a sterilized glass vial. Water was removed from the volatile oil using anhydrous sodium sulfate. The extraction yield was calculated (in milliliter of oil), was stored at 4°C in the dark, and then dissolved in n‐hexane prior to GC–MS analysis. EO yield (g/kg) was calculated with the following formula: EO yield (g/kg) = [mass of EOs obtained (g)/mass of dry matter (kg)].
2.4. Instruments and experimental conditions
GC–MS analysis was performed using an Agilent Technologies 7890A GC coupled with a fused silica capillary column (HP‐5MS, 0.25 mm × 30 m, film thickness 0.25 μm) and equipped with a 5975C MS detector (Agilent Technologies, Palo Alto, CA, USA). The column temperature was set at 60°C initially (maintained for 2 min), which was then increased to 80°C at a rate of 1°C/min for 10 min, subsequently raised to 25°C at a rate of 5°C/min, and finally to 300°C at a rate of 20°C/min for 1 min. The inlet temperature was 270°C. The carrier gas was helium with a constant flow rate of 1.0 ml/min in a split ratio of 10:1 and the injection volume was 5 μl. To the experimental conditions of the mass spectrometer, electron impact (EI+) mass spectra were operated at 70 eV. Injector and detector temperatures were set at 270°C, respectively. Scan at 0.2 scan/s from m/z 30 to 550 amu and solvent was delayed by 4 min. All the data analyses were carried out on a personal computer. The library searches and spectral matching of the resolved pure components were conducted on the NIST (National Institute of Standards and Technology) 08s.L MS database.
2.5. HPLC analysis
The flavonoids were detected at 283 nm and 330 nm by the UV detector using an Agilent 1260 Series HPLC system (Shimadzu Corporation, Japan). The compounds were separated on a Dikma Diamonsil C18 column (4.6 mm × 250 mm i.d., 5 μm) and at a column temperature of 25°C. The solvent system consisted of acetonitrile (B) and water (A) with the following gradient elution program: 0 min 85% A + 15% B; 15 min 60% A + 40% B; 35 min 50% A + 50% B; 40 min 25% A + 75% B; 50 min 15% A + 85% B. The mobile phase conditions were modified from a previously reported method (Zheng et al., 2009). The flow rate was 1.0 ml/min, and the injection volume of the sample was 20 μl. The effluent was monitored by UV detection at 283 nm for compound C1 and 330 nm for compounds C2–C5.
2.6. Establishment of calibration curves
To build the calibration curves, the mixed standard stock solution was prepared by dissolving the reference compounds (C1–C5) in methanol with the final concentrations of each compound at 483.6, 203.2, 160.8, 163.6, and 160.8 μg/ml, respectively. Working standard solutions containing each of the five compounds were prepared by diluting the stock solutions with methanol to a series of proper concentrations. Resulting solutions were filtered through a 0.22‐μm nylon syringe filters, and aliquots of 20 μl were injected in the chromatographic system for analyses. These solutions were stored at 4°C for further HPLC analysis. The standard solutions were analyzed in triplicates, and peak areas were used as analytical signal. The calibration curves were constructed by plotting the peak areas versus the concentrations of standards.
2.7. Method validation
The method was validated for linearity, sensitivity, repeatability, and accuracy. The limits of detection (LOD) and limits of quantification (LOQ) under the present conditions were determined at an S/N (signal to noise) of about 3 and 10, respectively. Intraday variations were chosen to determine the precision of the developed assay. For intraday variability test, three different amounts (high, middle, and low levels) of reference compounds were analyzed for six replicates within 1 day. Variations were expressed by the relative standard deviation (RSD) of the data. Recovery was used to evaluate the accuracy of the method. Six different amounts of the standard solutions were added to sample S1, and the recovery was measured in triplicate. For comparison, unspiked S1 sample was concurrently prepared and analyzed. The recovery was calculated as follows: Recovery (%) = (Detection − Original Amount)/Addition × 100%. For the stability test, the sample solution was analyzed using the established method at 0, 2, 4, 6, 8, 10, 12, 24, and 48 hr, respectively, the peak areas of five analytes were recorded, and the RSD of peak areas at different times were calculated.
2.8. Hierarchical cluster analysis
HCA is a statistical approach to distinguish homogeneous groups of cases based on tested characteristics. HCA is carried out to study the distances between pairs of samples, in order to highlight groupings between them through the Euclidean distance algorithm using single linkage clustering. When distance between samples is relatively small, it implies that samples are similar. Therefore, the contents of the five analytes were defined as five characteristics in the analysis to differentiate and classify the 25 CRP accessions examined during experiment. HCA of samples were performed by SPSS software (SPSS 16.0 for Windows, SPSS Inc., USA).
3. RESULTS
3.1. Analytical method validation of HPLC
To establish informative and reliable HPLC condition of tangerine peels, detailed information regarding calibration curves, linear ranges, LOD, and LOQ is displayed in Table 2. For all the examined compounds, all five calibration curves exhibited good linearity (R 2 > .9990), the intraday precisions, repeatability, stability, recovery calculated as relative standard deviation (RSD) were all <3%, and the accuracy ranged from 98.81% to 100.08% which are displayed in Tables 3, 4, 5, respectively. The results revealed the developed method was applied to the determination of the five flavonoids in samples collected from different regions in China. Representative HPLC chromatograms of standard mixture and sample under the optimized conditions are shown in Figure 2.
Table 2.
Calibration curve data for reference compounds 1–5 (n = 3)
| Compounds | Regression equation (y = ax + b)a | R 2 | Linear range (μg/ml) | LOD (μg/ml) | LOQ (μg/ml) |
|---|---|---|---|---|---|
| 1 | y = 34,031x + 75,594 | .9993 | 48.36–338.52 | 0.02 | 0.05 |
| 2 | y = 76,076x + 55,702 | .9993 | 4.06–101.60 | 0.02 | 0.06 |
| 3 | y = 56,695x + 1699 | .9996 | 0.08–21.00 | 0.02 | 0.08 |
| 4 | y = 83,002x + 19,904 | .9996 | 3.27–81.80 | 0.01 | 0.04 |
| 5 | y = 59,354x − 6578 | .9990 | 0.08–5.00 | 0.02 | 0.08 |
y and x denote the peak area and corresponding injection concentration (μg/ml), respectively. a and b denote the slope and intercept of the regression line, respectively.
Table 3.
Intraday precision of the developed method (n = 6)
| Compounds | Concentration (μg/ml) | Intraday | ||
|---|---|---|---|---|
| Detecteda (μg/ml) | Accuracy (%) | RSD (%) | ||
| 1 | 290.16 | 291.61 ± 0.50 | 100.50 | 0.17 |
| 338.52 | 337.32 ± 0.44 | 99.65 | 0.13 | |
| 483.60 | 510.07 ± 1.04 | 105.47 | 0.20 | |
| 2 | 121.92 | 127.31 ± 0.02 | 104.42 | 0.02 |
| 142.24 | 147.25 ± 0.19 | 103.52 | 0.13 | |
| 203.20 | 221.49 ± 1.14 | 109.00 | 0.50 | |
| 3 | 96.48 | 103.73 ± 0.03 | 107.51 | 0.03 |
| 112.56 | 119.89 ± 0.10 | 106.51 | 0.08 | |
| 160.80 | 173.19 ± 0.57 | 107.71 | 0.33 | |
| 4 | 98.16 | 100.82 ± 0.02 | 102.71 | 0.02 |
| 114.52 | 116.60 ± 0.10 | 101.82 | 0.09 | |
| 163.60 | 176.12 ± 0.59 | 107.65 | 0.34 | |
| 5 | 96.48 | 101.64 ± 0.21 | 105.35 | 0.21 |
| 112.56 | 117.81 ± 0.06 | 104.66 | 0.05 | |
| 160.80 | 176.48 ± 0.19 | 109.75 | 0.11 | |
Data are represented as the mean ± SD.
Table 4.
Analysis repeatability and stability of the developed method
| Compounds | Repeatability (n = 6) | Stability (n = 8) | ||||
|---|---|---|---|---|---|---|
| RT (min)a | Content (mg/g)a | RSD (%) | RT (min)a | Content (mg/g)a | RSD (%) | |
| 1 | 15.17 ± 0.29 | 3.03 ± 0.06 | 2.07 | 15.10 ± 0.34 | 3.02 ± 0.03 | 1.03 |
| 2 | 36.49 ± 0.47 | 0.46 ± 0.01 | 1.97 | 36.37 ± 0.55 | 0.47 ± 0.01 | 0.95 |
| 3 | 40.17 ± 0.46 | 0.04 ± 0.01 | 2.727 | 39.97 ± 0.52 | 0.05 ± 0.01 | 2.53 |
| 4 | 42.09 ± 0.28 | 0.30 ± 0.01 | 2.25 | 42.00 ± 0.32 | 0.31 ± 0.01 | 0.81 |
| 5 | 45.00 ± 0.16 | 0.03 ± 0.01 | 2.74 | 44.95 ± 0.18 | 0.04 ± 0.01 | 2.53 |
Data are represented as the mean ± SD.
Table 5.
Recovery data of the developed method (n = 6)
| Compounds | Concentration of analyte | Recovery (%)a | RSD (%) | ||
|---|---|---|---|---|---|
| Original (μg/ml)a | Spiked (μg/ml) | Found (μg/ml)a | |||
| 1 | 70.89 ± 2.06 | 87.05 | 158.01 ± 2.90 | 100.08 ± 1.54 | 1.54 |
| 2 | 9.02 ± 0.26 | 36.58 | 45.24 ± 0.77 | 99.03 ± 2.12 | 2.14 |
| 3 | 1.69 ± 0.06 | 28.94 | 30.63 ± 0.34 | 99.98 ± 1.21 | 1.21 |
| 4 | 12.08 ± 0.32 | 29.45 | 41.18 ± 0.62 | 98.81 ± 2.61 | 2.64 |
| 5 | 1.35 ± 0.037 | 28.94 | 30.20 ± 0.52 | 99.67 ± 1.78 | 1.79 |
Data are represented as the mean ± SD.
Figure 2.
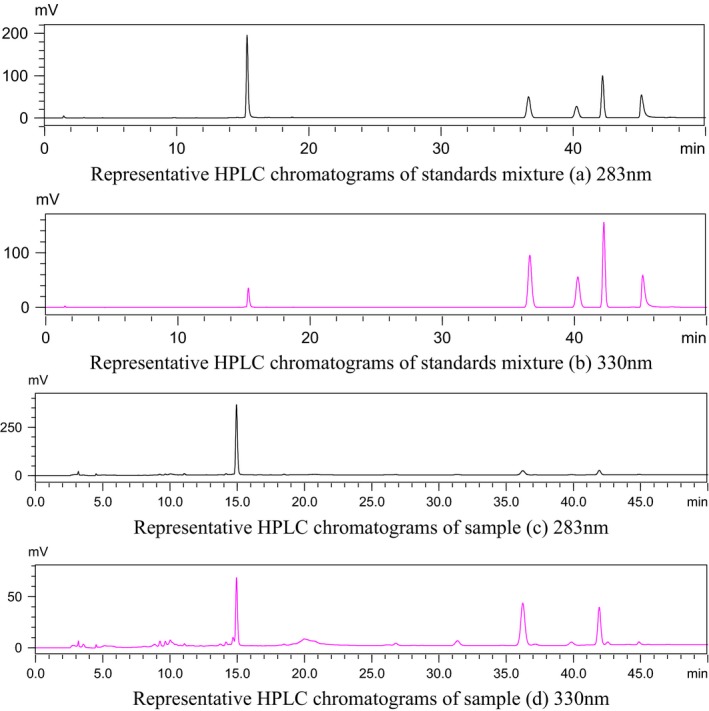
HPLC chromatograms of standard mixture (a) and sample (b). The numbers indicate bioactive flavonoids C1–C5
3.2. Quantitative analysis of samples in HPLC
The developed analytical method was subsequently applied to the simultaneous determination of above five flavonoids in 25 batches of CRP collected from different major citrus‐producing regions in China. The contents of oil and five analytical compounds presented as the average value ± SD are summarized in Table 6. The data indicated that the content of each flavonoid varied significantly among the different cultivars and regions. Among the cultivars tested, the content of hesperidin (compound 1) is the richest in 25 samples varied from 16.6 ± 0.01 to 79.6 ± 0.04 mg/g, but the content of hesperidin was found to be much less in the peel of Citrus reticulata “Chachi” than in other cultivars. And nobiletin and tangeretin are the major PMF components in CRP. These results are in line with the results of Zheng et al. (2009) and Liu et al. (2013).
Table 6.
Contents (mg/g, n = 3)a of five bioactive flavonoids in CRP samples collected from different regions in China
| Sample | Cultivars | Place of collection | Time of collection | Hesperidin | Nobiletin | 3,5,6,7,8,3′,4′‐Heptamethoxyflavone | Tangeretin | 5‐Hydroxy‐6,7,8,3′,4′‐pentamethoxyflavone |
|---|---|---|---|---|---|---|---|---|
| S1 | Citrus reticulata “Chachi” | Sanjiang Town, Xinhui District, Jiangmen City, Guangdong Province | 2015/11/05 | 24.34 ± 0.03 | 2.92 ± 0.00 | 0.27 ± 0.00 | 1.81 ± 0.00 | 0.14 ± 0.00 |
| S2 | C. reticulata “Chachi” | Sanjiang Town, Xinhui District, Jiangmen City, Guangdong Province | 2015/12/23 | 16.60 ± 0.01 | 2.22 ± 0.00 | 0.16 ± 0.00 | 1.41 ± 0.00 | 0.10 ± 0.00 |
| S3 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2015/10/11 | 29.61 ± 0.06 | 4.09 ± 0.01 | 0.38 ± 0.00 | 2.73 ± 0.00 | 0.20 ± 0.00 |
| S4 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2010 | 25.33 ± 0.01 | 3.33 ± 0.00 | 0.21 ± 0.00 | 2.70 ± 0.00 | 0.23 ± 0.00 |
| S5 | C. reticulata “Chachi” | Gujing Village, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 21.82 ± 0.02 | 3.05 ± 0.01 | 0.28 ± 0.00 | 2.51 ± 0.00 | 0.22 ± 0.00 |
| S6 | C. reticulata “Chachi” | Dongjia Town, Xinhui District, Jiangmen City, Guangdong Province | 2010 | 29.54 ± 0.02 | 3.34 ± 0.00 | 0.26 ± 0.00 | 2.84 ± 0.00 | 0.24 ± 0.00 |
| S7 | C. reticulata “Chachi” | Dongjia Town, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 27.93 ± 0.01 | 3.88 ± 0.00 | 0.26 ± 0.00 | 3.04 ± 0.00 | 0.27 ± 0.00 |
| S8 | C. reticulata “Chachi” | Tianlu Village, Xinhui District, Jiangmen City, Guangdong Province | 2005 | 29.33 ± 0.01 | 3.76 ± 0.00 | 0.30 ± 0.00 | 2.98 ± 0.00 | 0.27 ± 0.00 |
| S9 | C. reticulata “Chachi” | Shijian Town, Xinhui District, Jiangmen City, Guangdong Province | 1995 | 32.01 ± 0.01 | 3.59 ± 0.00 | 0.23 ± 0.00 | 3.02 ± 0.00 | 0.30 ± 0.00 |
| S10 | C. reticulata “Chachi” | Meijiang Town, Xinhui District, Jiangmen City, Guangdong Province | 1995 | 36.94 ± 0.00 | 3.76 ± 0.00 | 3.96 ± 0.00 | 3.03 ± 0.00 | 0.27 ± 0.00 |
| S11 | C. reticulata c | Huaiji County, Zhaoqing City, Guangdong Province | 2015/11/26 | 47.99 ± 0.02 | 5.09 ± 0.00 | 0.04 ± 0.00 | 2.13 ± 0.00 | 0.38 ± 0.00 |
| S12 | C. reticulata “Chachi” | Longmen County, Huizhou City, Guangdong Province | 2015/11/10 | 38.32 ± 0.00 | 3.70 ± 0.00 | 0.27 ± 0.00 | 3.11 ± 0.00 | 0.32 ± 0.00 |
| S13 | C. reticulata “Unshiu” | Lingui County, Guilin City, Guangxi Zhuang Autonomous Region | 2013/10/03 | 57.07 ± 0.01 | 0.40 ± 0.00 | 0.69 ± 0.00 | 0.19 ± 0.00 | 0.02 ± 0.00 |
| S14 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2014/11/30 | 65.36 ± 0.01 | 0.32 ± 0.00 | 0.34 ± 0.00 | 0.17 ± 0.00 | 0.02 ± 0.00 |
| S15 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2015/10/21 | 59.62 ± 0.01 | 0.37 ± 0.00 | 0.47 ± 0.00 | 0.18 ± 0.00 | 0.02 ± 0.00 |
| S16 | C. reticulata “Erythrosa” | Shimen County, Changde City, Hunan Province | 2015/11/30 | 57.61 ± 0.05 | 0.27 ± 0.00 | 0.38 ± 0.00 | 0.11 ± 0.00 | 0.01 ± 0.00 |
| S17 | C. reticulata “Speciosa” | Dianjun District, Yichang City, Hubei Province | 2015/10/11 | 68.07 ± 0.01 | 0.60 ± 0.00 | 0.35 ± 0.00 | 0.30 ± 0.00 | 0.03 ± 0.00 |
| S18 | C. reticulata “Speciosa” | Dianjun District, Yichang City, Hubei Province | 2015/12/11 | 73.29 ± 0.05 | 0.46 ± 0.00 | 0.29 ± 0.00 | 0.24 ± 0.00 | 0.02 ± 0.00 |
| S19 | C. reticulata “Speciosa” | Pujiang County, Chengdu City, Sichuan Province | 2015/10/15 | 72.76 ± 0.03 | 0.26 ± 0.00 | 0.32 ± 0.00 | 0.14 ± 0.00 | 0.02 ± 0.00 |
| S20 | C. reticulata “Kinokuni” | Nanfeng County, Fuzhou City, Jiangxi Province | 2015/10/10 | 44.39 ± 0.02 | 6.91 ± 0.00 | 0.18 ± 0.00 | 4.82 ± 0.00 | 0.69 ± 0.00 |
| S21 | C. reticulata “Dahongpao” | Pujiang County, Chengdu City, Sichuan Province | 2015/10/17 | 60.52 ± 0.00 | 5.75 ± 0.00 | 0.10 ± 0.00 | 2.54 ± 0.00 | 0.43 ± 0.00 |
| S22 | C. reticulata “Subcompressa” | Yongquan Town, Linhai County, Taizhou City, Zhejiang Province | 2014/10/15 | 79.65 ± 0.04 | 0.22 ± 0.00 | 0.37 ± 0.00 | 0.11 ± 0.00 | 0.02 ± 0.00 |
| S23 | C. reticulata “Tangerina” | Dongzhang Town, Fuqing County, Fujian Province | 2015/12/24 | 49.59 ± 0.01 | 4.66 ± 0.01 | 0.11 ± 0.00 | 2.51 ± 0.00 | 0.34 ± 0.00 |
| S24 | C. reticulata “Ponkan” | Yongchun County, Quanzhou City, Fujian Province | 2015/12/07 | 44.91 ± 0.01 | 4.77 ± 0.00 | 0.04 ± 0.00 | 3.53 ± 0.00 | 0.84 ± 0.00 |
| S25 | C. reticulata “Shiyueju” | Huaiji County, Zhaoqing City, Guangdong Province | 2015/11/26 | 60.58 ± 0.07 | 3.83 ± 0.00 | 0.04 ± 0.00 | 3.60 ± 0.00 | 0.15 ± 0.00 |
3.3. Quality assessment of CRP by HCA
To evaluate the change in similarity of five flavonoids displayed by the 25 CRP samples of different cultivars, an HCA based on the major five flavonoids was performed. The HCA is a multivariate procedure that allows the classification of variables into groups based on Euclidean distances between cases. The dendrogram of the 25 CRP samples is shown in Figure 3. The HCA results demonstrated a better hierarchical classification with all the samples well grouped according to their species. Obviously, as given in Figure 3, CRP samples were apparently classified as one of two groups so that this analysis provided further profound understanding of the distribution of the multiple chemical compounds in CRP. Two major clusters, viz., clusters 1 and 2, can be found in Figure 3. Cluster 1 was composed of 15 samples, while cluster 2 was composed of 10 samples. Although the concentrates of the samples S1–S12 (C. reticulata “Chachi”) from Guangdong Province are distinguishing, they could be classified into one cluster. In other words, the chemical ingredients of C. reticulata “Chachi” are similar. It can be seen that cluster 2 includes C. reticulata “Speciosa,” C. reticulata “Dahongpao,” C. reticulata “Subcompressa,” C. reticulata “Erythrosa,” C. reticulata “Unshiu,” and C. reticulata “Shiyueju,” which indicates that the six species possess the similarities and relationships to some extent. In addition, it can be useful for us to choose the appropriate dried tangerine peels with desired content of components as well as to choose alternate cultivars with more similarity in the absence of a desired variety at the time of requirement.
Figure 3.
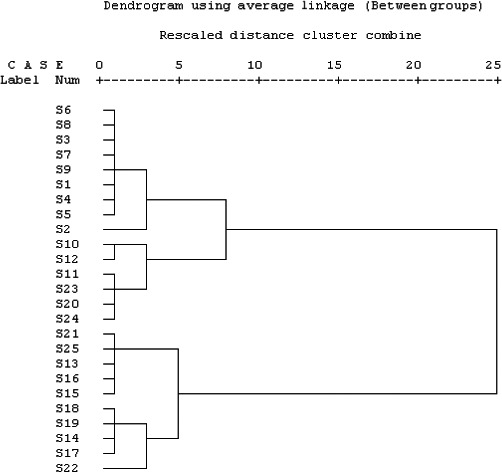
Dendrogram of hierarchical cluster analysis for 25 Citri Reticulatae Pericarpium samples in HPLC collected from different major citrus‐producing areas in China. Rescaled distance cluster combine was selected as a measurement. Citri Reticulatae Pericarpium samples were divided into two main clusters
3.4. Global yields of volatile oil in CRP
In the experiment, EOs were extracted from a total of 15 batches of 10 varieties of dried tangerine peels by using extraction method of EOs in Chinese pharmacopeia (2015 edition). The contents of oil are presented as the average value ± SD (W/W). The EOs extraction yield results acquired among the 25 CRP samples based on a dry weight are present in Table 1. Obviously, as can be seen that a total of EOs obtained were transparent and oily liquid with a rich smell, whose extraction yield approximately ranges from 0.90 to 67.43 (g/kg). And there existed significant differences in EOs contents and their color, which may be affected by the cultivars and environmental factors, such as storage conditions, time of collection, and preservation. According to Table 1, it is noticeable that C. reticulata “Chachi” which is mainly produced in Xinhui, Guangdong are rich in EOs except for C. reticulata “Shiyueju,” even the highest EOs yield reach up to 67.43 g/kg, whereas the lowest is only 0.90 g/kg recorded for the C. reticulata “Speciosa” picked from Dianjun District, Yichang City, Hubei Province and C. reticulata “Subcompressa” collected from Yongquan Town, Linhai County, Taizhou City, Zhejiang Province, respectively. In addition, the Table 1 showed an obvious difference in the EOs extraction ratio of C. reticulata “Chachi” produced in diverse regions, and found that the extracting rate of C. reticulata “Chachi” producing in Xinhui district, Guangdong Province is higher than other areas in Guangdong Province. Moreover, it was discovered that the less EOs contents, the deeper the color in this test, and a tentative inference on this result is that the color of EOs may have certain correlation with oil content.
3.5. Typical total ion chromatogram of volatile oil in CRP
GC–MS analysis has been the most popular and powerful assistant technique to identify volatile constituents of EOs. In this study, methanol extracts of CRP samples collected from different citrus‐producing areas in China were analyzed by GC–MS analysis under optimal conditions. A total of typical total ion chromatograms (TICs) from CRP samples obtained are shown in Figure 4, which exhibits the differences in TICs of the volatile oils of CRP samples among different cultivars collected from different major citrus‐producing areas in China. Apparently, as can be seen, each TIC is completely complex and diverse analytic system. Additionally, from Figure 1, there are significant differences on the peak height of the same retention time in CRP samples. It seems that different peak height is on behalf of the concentration of chemical constituent, and with all the spectra available, the chromatograms of several chief peaks can be easily detected. Then, the higher peak cluster represents the stronger content of components. Eventually, a library search was carried out for all the peaks using the NIST 08 s.L MS database Figure 5.
Figure 4.
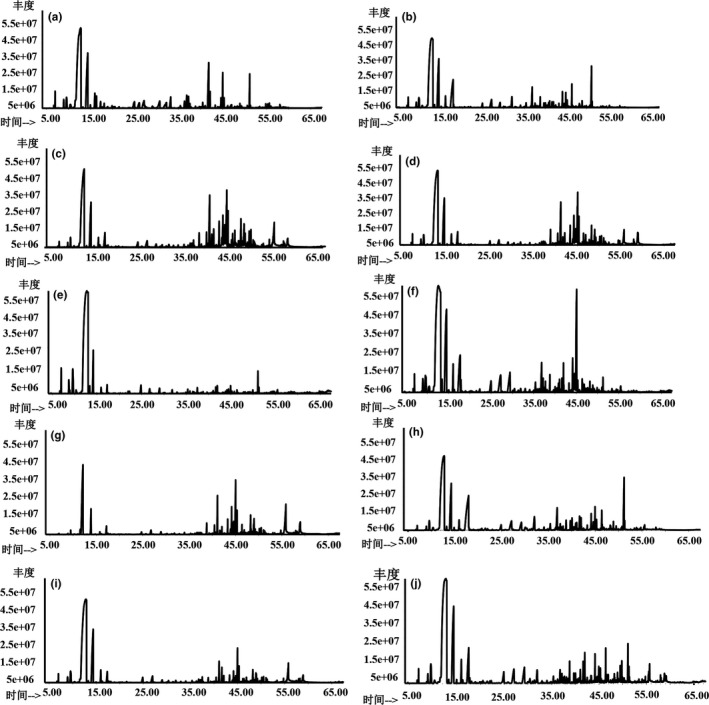
Total ion chromatography of volatile oil from CRP. Temperature programmed gas chromatography (TPGC) and electron impact (EI) ion source were used for the analysis of complex samples that we described in the Materials and Methods. Constituents were identified by comparison of authentic compounds with reference spectra in the computer library (NIST 08s.L MS database) and confirmed by comparison of those authentic compounds with data in literature. CRP of (a) Citrus reticulata “Chachi,” (b) C. reticulata “Dahongpao,” (c) C. reticulata “Subcompressa,” (d) C. reticulata “Erythrosa,” (e) C. reticulata “Shiyueju,” (f) C. reticulata “Kinokuni,” (g) C. reticulata “Speciosa,” (h) C. reticulata “Tangerina,” (i) C. reticulata “Unshiu,” and (j) C. reticulata “Ponkan”
Figure 5.
Graphic for table of contents
3.6. Comparative analysis of volatile components in CRP
Using GC–MS analysis, the quantity of the constituents tested was reach up to 98 with pretty good match which were identified via similarity comparison with compounds of database NIST 08 s.L MS database in this work. The 98 chemical compositions which have more than 90% similarity were identified for the EO from CRP peels extracted by SD, demonstrated in Table 7. For example, d‐limonene, γ‐terpinene, and 2‐(methylamino) benzoate were confirmed at 12.7, 14.4, and 41.7 min, respectively, by comparison of credible components with reference spectra in the computer library NIST 08 s.L MS database and identified by comparison of those authentic compounds with data in literature. Moreover, from Table 7, we have observed that the main constituents of the volatile oil of tangerine peels were almost monoterpenes and sesquiterpenes. Monoterpenes were d‐limonene, γ‐terpinene, myrcene, camphene, terpinolene, α‐pinene, and α‐phellandrene. Sesquiterpenes were elemene, α‐farnesene, α‐cubebene, valencene, α‐gurjunene, α‐caryophyllene, (+)‐aromadendrene, and aristolene. In addition, the fatty acid principally include (Z,Z)‐9,12‐tadecadienoic acid, (Z,Z,Z)‐9,12,15‐tadecatrienoic acid, octadecanoic acid, and hexadecanoic acid. Immediately following, gas chromatography area normalization method for the determination of the relative content of each component was used. The relative percentage concentrations of the principal chromatographic peak were measured with peak area normalization, which was summarized in Table 8. The types and contents of volatile oil components in part are similar, but they have certain differences in CRP from various regions. The data in Table 8 visibly indicated that the content of each volatile constituent is varied apparently among the diverse cultivars and regions, particularly among different disparate cultivars. Although d‐limonene (24.54%–73.09%) is the most ample volatile component, γ‐terpinene (3.69%–17.71%) the second in all samples obtained in CP, their percentage was diversified, based on the origins of the plant which are important index of volatile oil, in accordance with the literature (Duan et al., 2016; Zhou, Huang, Mo, & Liao, 2009). Therefore, the content differences of volatile constituents may result in various pharmacological effects of CP. Moreover, the content of d‐limonene was found to be much higher in the peel of Citrus reticulata “Shiyueju” than in other cultivars examined. On the contrary, the content of γ‐terpinene was discovered to be lower in the CRP cultivars. In short, the results of the components of the peel oil of CRP gained from this study are nearly in accordance with other prior researches. Furthermore, what is noteworthy is that in the whole examined, the percentage of 2‐dimethylamino methyl benzoate, the possessed chemical component is varied completely. The cultivars of GCP cultivated in Guangdong Province contain plentiful of specific component of 2‐dimethylamino‐methyl benzoate. Similarly, S9 and S12 have a considerable high concentration severally. Nevertheless, what is not consistent with previous conclusion (Zhou et al., 2009), was recorded that it existed only in the GCP. In summary, the data displayed crucial variations in the content of these EO constituents in the samples tested from various regions in China. Therefore, a significant difference in chemical components of the EOs might also be attributed to various factors, for instance, growth environment, genetic source, and geographical and environmental conditions, particularly genetic source.
Table 7.
Volatile compounds of essential oils of CRP studied
| Peak number | Retention time | Compounds | Molecular formula | Relative molecular mass |
|---|---|---|---|---|
| 1 | 6.942 | α‐Pinene | C10H16 | 136 |
| 2 | 7.612 | Camphene | C10H16 | 136 |
| 3 | 8.657 | α‐Phellandrene | C10H16 | 136 |
| 4 | 8.687 | Sabinene | C10H16 | 136 |
| 5 | 8.912 | β‐Pinene | C10H16 | 136 |
| 6 | 9.53 | Myrcene | C10H16 | 136 |
| 7 | 10.319 | Octana | C8H16O | 128 |
| 8 | 11.168 | α‐Terpinene | C10H16 | 136 |
| 9 | 11.536 | 1‐Methyl‐4‐(1‐methylethyl)benzene | C10H14 | 134 |
| 10 | 12.717 | d‐Limonene | C10H16 | 136 |
| 11 | 13.144 | Benzylcarboxaldehyde | C8H8O | 120 |
| 12 | 13.304 | 13,7‐dimethyl‐3,6‐Octatriene | C10H16 | 136 |
| 13 | 13.316 | Ocimene | C10H16 | 136 |
| 14 | 14.355 | γ‐Terpinene | C10H16 | 136 |
| 15 | 14.462 | N‐Methylaniline | C7H9N | 107 |
| 16 | 15.043 | 1‐Octanol | C8H16O | 130 |
| 17 | 15.969 | Terpinolene | C10H16 | 136 |
| 18 | 16.064 | 1‐Methyl‐4‐(1‐methylethylidene)cyclohexene | C10H16 | 136 |
| 19 | 16.337 | 1‐Methyl‐4‐(1‐methylethenyl)‐benzene | C10H12 | 132 |
| 20 | 16.492 | Benzoic acid methyl ester | C8H8O2 | 136 |
| 21 | 17.536 | Linalool | C10H18O | 132 |
| 22 | 17.75 | 1‐Nonanal | C9H18O | 142 |
| 23 | 18.124 | 1,3,8‐p‐Menthatriene | C10H14 | 134 |
| 24 | 18.622 | Bicyclo[2.2.1]heptan‐2‐ol,1,3,3‐trimethyl‐ | C10H18O | 154 |
| 25 | 18.711 | 3,5,5‐Trimethyl‐2‐cyclohexen‐1‐one; | C9H14O | 138 |
| 26 | 19.821 | Limonene oxide | C10H16O | 152 |
| 27 | 19.21 | 2‐Cyclohexen‐1‐ol, 1‐methyl4‐(1‐methylethyl)‐trans‐ | C10H18O | 154 |
| 28 | 20.949 | Campho | C10H16O | 152 |
| 29 | 21.406 | 1‐Methyl‐4‐(1‐methylethenyl) cyclohexanol | C10H18O | 154 |
| 30 | 21.982 | Citronella | C10H18O | 154 |
| 31 | 23.667 | Borneol | C10H18O | 154 |
| 32 | 24.932 | 4‐Terpineol | C10H18O | 154 |
| 33 | 27.163 | α‐Terpineol | C10H18O | 154 |
| 34 | 29.276 | Decanal | C10H20O | 156 |
| 35 | 34.03 | cis‐Carveol | C10H16O | 152 |
| 36 | 31.383 | Neml | C10H18O | 154 |
| 37 | 31.941 | Citronellol | C10H20O | 156 |
| 38 | 32.956 | Carvone | C10H14O | 150 |
| 39 | 33.751 | 3‐Methyl‐6‐(1‐methylethyl)‐2‐cyclohexen‐1‐one | C10H16O | 152 |
| 40 | 34.019 | Geraniol | C10H18O | 154 |
| 41 | 35.2 | Citral | C10H16O | 152 |
| 42 | 35.354 | 4‐(1‐Methylethenyl)‐1‐cyclohexene‐1‐carboxaldehyde | C10H14O | 150 |
| 43 | 35.461 | 1‐Cyclohexene‐1‐carboxaldehyde,4‐(1‐methylethyl)‐ | C10H16O | 152 |
| 44 | 36.054 | Nonanoic acid | C9H18O2 | 158 |
| 45 | 36.268 | 2‐Methyl‐5‐(1‐methylethyl)phenol | C10H14O | 150 |
| 46 | 36.761 | Thymol | C10H14O | 150 |
| 47 | 37.004 | Perillyl alcohol | C10H16O | 152 |
| 48 | 37.129 | 3‐Methyl‐4‐isopropylphenol | C10H14O | 150 |
| 49 | 37.532 | 2‐Methoxy‐4‐vinylphenol | C9H10O2 | 150 |
| 50 | 37.71 | Undecanal | C11H22O | 170 |
| 51 | 38.161 | 2,4‐Decadienal | C10H16O | 152 |
| 52 | 38.363 | (R)‐(+)‐Citronellic acid | C10H18O2 | 170 |
| 53 | 38.874 | Methyl 2‐aminobenzoate | C8H9NO2 | 151 |
| 54 | 39.265 | 2,4,6‐Trichlorophenol | C6H3Cl3O | 197 |
| 55 | 39.307 | α‐Cubebene | C15H24 | 204 |
| 56 | 39.503 | Eugenol | C10H12O2 | 164 |
| 57 | 39.693 | 2,6‐Octadiene,2,6‐dimethyl‐ | C10H18 | 138 |
| 58 | 40.043 | cis‐3,7‐Dimethyl‐2,6‐octadien‐1‐ol–acetate | C12H20O2 | 196 |
| 59 | 40.215 | Benzene,4‐ethenyl‐1,2‐dimethoxy‐ | C10H12O2 | 164 |
| 61 | 40.458 | Copaene | C15H24 | 204 |
| 62 | 40.583 | Decanoic acid | C10H20O2 | 172 |
| 63 | 40.868 | trans‐3,7‐Dimethyl‐2,6‐octadien‐1‐yl ethanoate | C12H20O2 | 196 |
| 64 | 41.657 | 2‐(Methylamino)benzoate | C9H11NO2 | 165 |
| 65 | 41.96 | Cyclodecane | C10H20 | 140 |
| 66 | 41.978 | Dodecanal | C12H24O | 184 |
| 67 | 42.999 | Isovaleric acid octyl ester | C13H26O2 | 214 |
| 68 | 43.301 | α‐Caryophyllene | C15H24 | 204 |
| 69 | 43.45 | (+)‐Aromadendrene | C15H24 | 204 |
| 70 | 43.883 | Undecanoic acid | C11H22O2 | 186 |
| 71 | 44.138 | Bicyclosesquiphellandrene | C15H24 | 204 |
| 72 | 44.951 | α‐Farnesene | C15H24 | 204 |
| 73 | 45.527 | Methyl laurate | C13H26O2 | 214 |
| 74 | 45.913 | α‐Calacorene | C15H20 | 200 |
| 75 | 46.382 | Elemene | C15H24 | 204 |
| 76 | 45.682 | α‐Gurjunene | C15H24 | 204 |
| 77 | 45.8 | Valencene | C15H24 | 204 |
| 78 | 45.919 | Aristolene | C15H24 | 204 |
| 79 | 46.512 | Nemlidol | C15H26O | 222 |
| 81 | 46.892 | Dodecanoic acid | C12H24O2 | 200 |
| 82 | 47.142 | (−)‐Globulol | C15H26O | 222 |
| 83 | 47.883 | Tetradecanal | C14H28O | 212 |
| 84 | 48.097 | Longifolene | C15H24 | 204 |
| 85 | 48.887 | α‐Eudesmol | C15H26O | 222 |
| 86 | 49.379 | Spathulenol | C15H24O | 202 |
| 87 | 51.617 | 1,4‐Dimethyl‐7‐isopropylazulene | C15H18 | 198 |
| 88 | 51.724 | Phenanthrene/Anthracene | C14H10 | 178 |
| 89 | 52.27 | Nootkatone | C15H22O | 218 |
| 90 | 54.774 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 |
| 91 | 54.952 | Tetradecanoic acid | C14H28O2 | 228 |
| 92 | 55.635 | Hexadecanoic acid | C16H32O2 | 256 |
| 93 | 56.116 | Octadecanoic acid | C18H36O2 | 284 |
| 94 | 57.991 | 9,12‐Octadecadienoic acid(Z,Z)‐,methyl ester | C19H34O2 | 294 |
| 95 | 58.092 | (Z,Z,Z)‐9,12,15‐Octadecatrienoic acid methyl ester | C19H32O2 | 292 |
| 96 | 58.870 | (Z,Z,Z)‐9,12,15‐Octadecatrienoic acid | C18H30O2 | 278 |
| 97 | 58.976 | (Z,Z)‐9,12‐Octadecadienoic acid | C18H32O2 | 280 |
| 98 | 59.202 | Ethyl linoleate | C20H36O2 | 308 |
Table 8.
Main components’ area normalization results (n = 25)
| Sample NO Peak NO | Relative percentage content % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 8 | 10 | 14 | 21 | 30 | 32 | 34 | 36 | 64 | |
| S1 | 2.49 | 1.96 | 3.01 | 0.08 | 64.64 | 16.86 | 0.18 | 0.04 | 0.61 | 0.26 | 0.01 | 1.82 |
| S2 | 2.35 | 1.98 | 2.97 | 0.23 | 63.80 | 17.57 | 0.20 | 0.10 | 0.29 | 0.19 | 0.00 | 0.83 |
| S3 | 2.61 | 2.00 | 3.03 | 0.08 | 67.29 | 15.33 | 0.18 | 0.04 | 0.43 | 0.20 | 0.02 | 9.78 |
| S4 | 1.21 | 1.15 | 2.02 | 0.10 | 55.15 | 14.40 | 0.80 | 0.02 | 1.06 | 0.57 | 0.03 | 5.57 |
| S5 | 0.52 | 0.28 | 1.38 | 0.02 | 51.61 | 8.63 | 1.78 | 0.01 | 0.93 | 0.32 | 0.05 | 0.40 |
| S6 | 0.58 | 0.82 | 0.66 | 0.10 | 32.58 | 4.76 | 1.17 | 0.00 | 0.4 | 0.57 | 0.00 | 0.23 |
| S7 | 0.44 | 0.56 | 1.48 | 0.06 | 41.61 | 10.85 | 1.70 | 0.02 | 1.2 | 0.45 | 0.04 | 0.35 |
| S8 | 1.08 | 1.20 | 2.06 | 0.04 | 55.48 | 16.92 | 0.62 | 0.02 | 1.21 | 0.33 | 0.04 | 5.30 |
| S9 | 0.48 | 0.68 | 0.00 | 0.05 | 49.65 | 13.97 | 0.48 | 0.02 | 0.77 | 0.36 | 0.00 | 3.24 |
| S10 | 0.59 | 0.59 | 0.00 | 0.03 | 48.16 | 11.12 | 0.50 | 0.00 | 1.29 | 0.30 | 0.00 | 1.20 |
| S11 | 0.66 | 0.73 | 1.06 | 0.00 | 38.85 | 9.19 | 2.93 | 0.07 | 2.56 | 0.71 | 0.29 | 0.46 |
| S12 | 0.98 | 1.09 | 2.20 | 0.18 | 60.15 | 19.41 | 0.61 | 0.08 | 0.72 | 0.38 | 0.05 | 3.57 |
| S13 | 0.31 | 0.43 | 1.50 | 0.50 | 55.40 | 9.73 | 1.04 | 0.02 | 0.72 | 0.38 | 0.11 | 0.86 |
| S14 | 0.38 | 0.38 | 1.14 | 0.03 | 43.17 | 8.15 | 1.48 | 0.03 | 0.57 | 0.42 | 0.14 | 5.47 |
| S15 | 0.25 | 0.37 | 1.21 | 0.02 | 45.62 | 8.39 | 2.15 | 0.03 | 0.93 | 0.64 | 0.15 | 0.07 |
| S16 | 0.35 | 0.33 | 1.21 | 0.11 | 47.06 | 7.09 | 1.02 | 0.01 | 0.56 | 0.29 | 0.06 | 0.54 |
| S17 | 0.06 | 0.08 | 0.00 | 0.05 | 24.54 | 3.69 | 1.29 | 0.03 | 0.58 | 0.66 | 0.13 | 0.70 |
| S18 | 0.71 | 0.56 | 1.18 | 0.05 | 60.15 | 7.75 | 2.14 | 0.02 | 0.62 | 0.50 | 0.08 | 0.21 |
| S19 | 0.39 | 0.50 | 1.71 | 0.04 | 54.53 | 10.59 | 1.61 | 0.05 | 0.65 | 0.64 | 0.12 | 4.77 |
| S20 | 0.40 | 0.77 | 0.65 | 0.03 | 46.62 | 8.85 | 4.70 | 0.04 | 1.02 | 2.48 | 0.12 | 0.26 |
| S21 | 0.41 | 0.45 | 1.43 | 0.16 | 64.64 | 9.61 | 8.37 | 0.08 | 0.61 | 0.86 | 0.14 | 0.33 |
| S22 | 0.17 | 0.23 | 0.73 | 0.05 | 33.54 | 6.43 | 1.61 | 0.02 | 0.72 | 0.43 | 0.14 | 0.63 |
| S23 | 0.16 | 0.24 | 1.02 | 0.07 | 41.63 | 7.59 | 12.04 | 0.12 | 0.86 | 1.59 | 0.23 | 0.79 |
| S24 | 0.34 | 0.43 | 1.54 | 0.04 | 45.65 | 9.71 | 3.67 | 0.12 | 1.17 | 2.03 | 0.22 | 0.41 |
| S25 | 1.02 | 0.24 | 3.16 | 0.08 | 73.09 | 3.82 | 0.62 | 0.17 | 0.97 | 0.70 | 0.02 | 0.60 |
4. DISCUSSION
GC–MS and HPLC approaches are of high sensitivity and accurate quantitative analysis method which are particularly suitable for rapidly analyzing phytochemical. EOs and flavonoids from CRP are the complex mixture of compounds with usage in health and medical sciences. In this work, all results suggested that the extraction method and GC–MS and HPLC analysis conditions applied to evaluate the quality comprehensively in 25 CRP samples collected from different districts in China were reliable. The variation in chemical compositions of the volatile oils and five bioactive flavonoids might be attributed to different geographic and environmental conditions (Chen & Cui, 1998). The chemical ingredients of volatile oil are not only an important source of spices, but also have a wealth of pharmacological activities, which are mainly composed of terpenoid, alcohols, and aldehydes from Citrus Reticulatae Pericarpium. Previous studies have shown that the extracts from CRP can be responsible for the antibacterial action, anticancer activity, insecticidal effect, and anti‐inflammatory and antioxidant ability in modern medicine. In addition, many studies show that terpenes have antifeedant activities, repellent actions, oviposition deterrent, contact toxicity activity, and fumigant activities in plant EOs (Xie, Yuan, Li, & Tang, 2001; Xu & Zhao, 1995; Xu, Zhao, Zhou, Ding, & Yu, 1994; Zhou et al., 2009). Plentiful of literatures concerning its efficacy in recent years have been published on a wide variety of articles. Moreover, chemical components may lead to different bioactive effects to some extent, especially the main bioactive compounds such as d‐limonene, α‐pinene, myrcene, and so on. In fact, the amounts of bioactive compounds play more important role in curing some diseases in TCMs used in China. A large and growing body of literature has investigated that the d‐limonene plays a significant role in antibacterial action, aroma enhancement function, and antitussive, expectorant, anti‐asthmatic, anti‐cancer, and other effects (Asamoto et al., 2002; Bezerra, Costa, & Nogueira, 2013; Huang, Sun, Long, & Sun, 2015; Shen, 2002). Significant antimicrobial activity of α‐terpineol has been proved (Cosentino et al., 1999). Previous studies have reported α‐pinene has antitussive, expectorant, antibacterial, and antifungal effects (Xia & Yu, 2000). There is a large volume of published studies describing the role of linalool has analgesic, anxiolytic, sedative hypnosis, anti‐inflammatory, antitumor, antibacterial, and other pharmacological activities (Jiang, Zhu, & Yu, 2015). Dried tangerine peel will possess great research value and broad market prospects of traditional Chinese medicine on account of a variety of pharmacological effects.
A wide variety of dried tangerine peels widely distributed throughout the country, whose medicine last for a long history. Profound traditional medicine, dried tangerine or orange peel, plays a significant role in curing respiratory system disease in China. Hence, it is necessary to further develop other medicinal value of orange peels owing to the insufficiency of viable Citrus reticulata Pericarpium. The China pharmacopeia (2015 version) has recorded the main cultivars of C. reticulata Blanco are C. reticulata “Chachi,” C. reticulata “Unshiu,” C. reticulata “Tangerina,” and C. reticulata “Dahongpao” in China. Our previous study has preliminarily investigated that C. reticulata “Ponkan” and C. reticulata “Shiyueju” generally are not applied to medicinal value as dried tangerine or orange peel possess a high amount of polymethoxylated flavones (PMFs), even the contents of those higher than the traditional famous region drug of GCP (Luo et al., 2017), similarly this experiment also found that the EO extraction yield and main chemical ingredients such as limonene of C. reticulata “Ponkan” and C. reticulata “Shiyueju” have come to the same conclusion.
5. CONCLUSION
In this study, a simple and sensitive GC–MS method provided a comprehensive analysis in aroma compositions and HPLC method was developed with five bioactive flavonoids contents in 25 batches of peel samples of 10 cultivars. The results obtained from this study provide a reference for developing other cultivars as a new type of medicinal resource to exert officinal value and health care value in the future.
ACKNOWLEDGMENTS
The work was greatly supported by the National Natural Science Foundation of China (no. 31401613), the Excellent Youth Development Program for higher education institution of Guangdong Province [(2014) 145].
Luo M, Luo H, Hu P, Yang Y, Wu B, Zheng G. Evaluation of chemical components in Citri Reticulatae Pericarpium of different cultivars collected from different regions by GC–MS and HPLC. Food Sci Nutr. 2018;6:400–416. https://doi.org/10.1002/fsn3.569
Contributor Information
Bo Wu, Email: gykyc@163.com.
Guodong Zheng, Email: gd200237@126.com.
REFERENCES
- Asamoto, M. , Toriyama‐Baba, H. , Ohnishi, T. , Ota, T. , Naito, A. , Ando, A. , Tsuda, H. (2002). Journal of Cancer Research, 93, 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, P. Bezerra , Emmanoel, V. Costa , & Paulo Cesar, L. Nogueira . (2013). Essential oil constituents: Biodiversity and their applicability for cancer therapy Antitumor potential and other emerging medicinal properties of natural compounds (pp. 285–300). Netherlands, Springer. [Google Scholar]
- Camarda, L. , Di Stefano, V. , Del Bosco, S. F. , & Schillaci, D. (2007). Antiproliferative activity of Citrus juices and HPLC evaluation of their flavonoid composition. Fitoterapia, 78, 426–429. [DOI] [PubMed] [Google Scholar]
- Careri, M. , Elviri, L. , Mangia, A. , & Musci, M. (2000). Spectrophotometric and coulometric detection in the high performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. Journal of Chromatography A, 881, 449–460. [DOI] [PubMed] [Google Scholar]
- Chang, C. Y. , Lin, T. Y. , Lu, C. W. , Huang, S. K. , Wang, Y. C. , Chou, S. S. , Wang, S. J. (2015). Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology, 50, 157–169. [DOI] [PubMed] [Google Scholar]
- Chen, Y. G. , & Cui, F. S. (1998). The study on constituents of volatile oils in four CRP. Journal of JiangXi College of Traditional Chinese Medicine, 10, 32–33. [Google Scholar]
- Committee of National Pharmacopoeia . (2015). Pharmacopoeia of RP China. 1 Beijing: Chemical Industry Press; (p.1329). [Google Scholar]
- Cosentino, S. , Tuberoso, C. , Pisano, B. , Satta, M. , Mascia, V. , Arzedi, E. , Palmas, F. (1999). In‐vitro antimicrobial activity and chemical composition of Sardinian thymus essential oils. Letters in Applied Microbiology, 29, 130–135. [DOI] [PubMed] [Google Scholar]
- Devi, K. P. , Rajavel, T. , Nabavi, S. F. , Setzer, W. N. , Ahmadi, A. , Mansouri, K. , Nabavi, S. M. (2015). Hesperidin: A promising anticancer agent from nature. Industrial Crops and Products, 76, 582–589. [Google Scholar]
- Dimpfel, W. (2006). Different anticonvulsive effects of hesperidin and its aglycone hesperetin on electrical activity in the rat hippocampus in‐vitro. Journal of Pharmacy and Pharmacology, 58, 375–379. [DOI] [PubMed] [Google Scholar]
- Duan, L. , Guo, L. , Dou, L. L. , Zhou, C. L. , Xu, F. G. , Zheng, G. D. , Liu, E. H. (2016). Discrimination of Citrus reticulata Blanco and Citrus reticulata “Chachi” by gas chromatograph‐mass spectrometry based metabolomics approach. Food Chemistry, 212, 123–127. [DOI] [PubMed] [Google Scholar]
- Duan, L. , Guo, L. , Liu, K. , Liu, E. H. , & Li, P. (2014). Characterization and classification of seven citrus herbs by liquid chromatography‐quadrupole time‐of‐flight mass spectrometry and genetic algorithm optimized support vector machines. Journal of Chromatography A, 1339, 118–127. [DOI] [PubMed] [Google Scholar]
- Fu, M. Q. , Xu, Y. J. , Chen, Y. L. , Wu, J. J. , Yu, Y. S. , Zou, B. , Xiao, G. S. (2017). Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata “Chachi”) during storage. Food Chemistry, 230, 649–656. [DOI] [PubMed] [Google Scholar]
- Gao, B. (2011). Studies on components identification and biological activities of flavonoids and essential oils in PCR “Chachi” peels. Huazhong Agricultural University. [Google Scholar]
- Ho, S. C. , & Kuo, C. T. (2014). Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti‐neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food and Chemical Toxicology, 71, 176–182. [DOI] [PubMed] [Google Scholar]
- Hu, J. T. , Zhao, Z. M. , Tang, T. X. , Yang, Y. T. , Luo, H. J. , & Jiang, L. (2014). Content variation of volatile components in Xinhui Citrus Reticulate “Chachi” of different storage time. Chinese Journal of Experimental Traditional Medical Formulae, 20, 62–65. [Google Scholar]
- Huang, Q. J. , Sun, Z. G. , Long, Y. , & Sun, Q. (2015). Recent progress in research on anticancer mechanism of D‐limonene. Food Science, 36, 240–244. [Google Scholar]
- Jiang, D. M. , Zhu, Y. , & Yu, X. M. (2015). Advances in research of pharmacological effects and formulation studies of linalool. China Journal of Chinese Materia Medica, 40, 3530–3533. [PubMed] [Google Scholar]
- Lin, Y. S. , Li, S. , Ho, C. T. , & Lo, C. Y. (2012). Simultaneous analysis of six polymethoxyflavones and six 5‐hydroxy‐polymethoxyflavones by high performance liquid chromatography combined with linear ion trap mass spectrometry. Journal of Agriculture and Food Chemistry, 60, 12082–12087. [DOI] [PubMed] [Google Scholar]
- Liu, E. H. , Zhao, P. , Duan, L. , Zheng, G. D. , Guo, L. , Yang, H. , & Li, P. (2013). Simultaneous determination of six bioactive flavonoids in Citri Reticulatae Pericarpium by rapid resolution liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry. Food Chemistry, 141, 3977–3983. [DOI] [PubMed] [Google Scholar]
- Luo, H. J. , Yang, Y. T. , Li, X. W. , Luo, M. X. , Hu, P. J. , Chen, H. , Zheng, G. D. (2017). Determination of polymethoxylated flavones in Citri Reticulatae Pericarpium from eleven cultivar origins. Chinese Traditional Patent Medicine, 39, 565–569. [Google Scholar]
- Mao, L. , Ou, X. Q. , & Wang, J. (2015). Comparative study on GC–MS fingerprints of volatile oil in Citrus reticulata “Chachi” stored less than 3 years and more than 3 years. Lishizhen Medicine and Materia Medica Research, 26, 895–897. [Google Scholar]
- Peng, Y. , Liu, F. , & Ye, J. (2006). Quantitative and qualitative analysis of flavonoid markers in Fructus aurantii of different geographical origin by capillary electrophoresis with electrochemical detection. Journal of Chromatography B, 830, 224–230. [DOI] [PubMed] [Google Scholar]
- Qin, K. M. , Zheng, L. J. , Cai, H. , Cao, G. , Lao, Y. , Lu, T. , Cai, B. (2013). Characterization of chemical composition of Citri Reticulatae Pericarpium volatile oil by comprehensive two‐dimensional gas chromatography with high‐resolution time‐of‐flight mass spectrometry. Evidence‐Based Complementary and Alternative Medicine, 2013, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraoui, N. , Vian, M. A. , Vian, M. A. , Maataoui EI, M. , Boutekedjiret, C. , Chemat, F. (2011). Valorization of citrus by‐products using microwave steam distillation (MSD). Innovative Food Science & Emerging Technologies, 12, 163–170. [Google Scholar]
- Shen, Y. (2002). Pharmacology of Traditional Chinese Medicine (pp. 108–111). Shanghai: Shanghai Publishing House of Science and Technology. [Google Scholar]
- Tan, H. , Hu, D. , Song, J. , Xu, Y. , Cai, S. , Chen, Q. , Xu, H. (2015). Distinguishing radix Angelica sinensis from different regions by HS‐SFME/GC–MS. Food Chemistry, 186, 200–206. [DOI] [PubMed] [Google Scholar]
- Wang, J. , & Li, P. (2015). Comparison among volatile oil compositions from tangerine peels and their kindreds. Chin Tradit Pat Med, 37, 364. [Google Scholar]
- Wang, Y. S. , & Luo, W. Y. (1989). Studies on quantitative determination of total flavonoid in qingpi, zhiqiao and zhishi by TLC‐densitometric methods. China Journal of Chinese Materia Medica, 14, 230–2. [PubMed] [Google Scholar]
- Wang, J. Y. , Yang, J. , Zhang, T. , Xiang, L. Y. , Wang, H. M. , & Hou, J. W. (2014). Study on quality control method of volatile oil from Citri Reticulatae Pericarpium. Chinese Archives of Traditional Chinese Medicine, 32, 139–141. [Google Scholar]
- Xia, Z. D. , & Yu, J. L. (2000). The influence of alpha pinene to the biosynthesis of Candida albicans . Journal of Modern Medicinal Chemistry, 10, 44–46. [Google Scholar]
- Xie, Y. S. , Yuan, M. X. , Li, B. T. , & Tang, L. M. (2001). The research on insecticidal activity and chemical compounds of Artemisia selengensis . Jiangxi Plant Protection, 24, 105. [Google Scholar]
- Xing, T. T. , Zhao, X. J. , Zhang, Y. D. , & Li, Y. F. (2017). Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of Citrus using UPLC‐Q‐TOF‐MS. Journal of Agriculture and Food Chemistry, 65, 2615–2627. [DOI] [PubMed] [Google Scholar]
- Xu, H. H. , & Zhao, S. H. (1995). The study on pests in the cream kills the eggs action in five kinds of essential oil. Journal of the Chinese Cereals and Oils Association, 10, 2. [Google Scholar]
- Xu, H. H. , Zhao, S. H. , Zhou, J. , Ding, J. K. , & Yu, X. J. (1994). The effective constituents of insecticidal is acted on Artemisia scoparia oil. Acta Entomologica Sinica, 37, 411. [Google Scholar]
- Xue, F. , & Xu, Z. M . (2005). The volume of TCM. Drug encyclopedia in China (pp. 290–291). Beijing: People's Medical Publishing House. [Google Scholar]
- Yi, L. , Dong, N. , Liu, S. , Yi, Z. , & Zhang, Y. (2015). Chemical features of Citri Reticulatae Pericarpium and Citri Reticulatae Pericarpium Viride revealed by GC–MS metabolomics analysis. Food Chemistry, 186, 192–199. [DOI] [PubMed] [Google Scholar]
- Zeng, S. L. , Dua, L. , Chen, B. Z. , Li, P. , & Liu, E. H. (2017). Chemicalome and metabolome profiling of polymethoxylated flavonoids in Citri Reticulatae Pericarpium based on an integrated strategy combining background subtraction and modified mass defect filter in a microsoft excel platform. Journal of Chromatography A, 1508, 106–120 https://doi.org/10.1016/j.chroma.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Zhang, J. Y. , Zhang, Q. , Zhang, H. X. , Ma, Q. , Liu, J. Q. , & Qiao, Y. J. (2012). Characterization of polymethoxylated flavonoids (PMFs) in the peels of ‘Shatangju’ mandarin (Citrus reticulata Blanco) by online high‐performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. Journal of Agriculture and Food Chemistry, 60, 9023–9034. [DOI] [PubMed] [Google Scholar]
- Zheng, G. D. , Yang, D. P. , Wang, D. M. , Zhou, F. , Yang, X. , & Jiang, L. (2009). Simultaneous determination of five bioactive flavonoids in Citri Reticulatae Pericarpium from China by high‐performance liquid chromatography with dual wavelength detection. Journal of Agriculture and Food Chemistry, 57, 6552–6557. [DOI] [PubMed] [Google Scholar]
- Zheng, G. D. , Zhou, P. , Yang, H. , Li, Y. , Li, P. , & Liu, E. H. (2013). Rapid resolution liquid chromatography electrospray ionisation tandem mass spectrometry method for identification of chemical constituents in Citri Reticulatae Pericarpium. Food Chemistry, 136, 604–611. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Huang, Q. H. , Mo, Y. Y. , & Liao, S. M. (2009). Analysis on the volatile oil of Xinhui Citri Reticulatae Pericarpium in different years by GC/MS. Journal of Chinese Medicinal Materials, 32, 24–26. [PubMed] [Google Scholar]


