We used a dynamic human immunodeficiency virus (HIV) transmission model to determine the cost-effectiveness of HIV care interventions in British Columbia, Canada, in 2011–2013. HIV testing and treatment initiation interventions were cost-effective, while the treatment retention intervention was not.
Keywords: cost-effectiveness, British Columbia, HIV, antiretroviral therapy, HIV testing
Abstract
Background
Recognition of the secondary preventive benefits of antiretroviral therapy (ART) has mobilized global efforts to “seek, test, treat, and retain” people living with human immunodeficiency virus [HIV]/AIDS (PLHIV) in HIV care. We aimed to determine the cost-effectiveness of a set of HIV testing and treatment engagement interventions initiated in British Columbia, Canada, in 2011–2013.
Methods
Using a previously validated dynamic HIV transmission model, linked individual-level health administrative data for PLHIV, and aggregate-level HIV testing data, we estimated the cost-effectiveness of primary care testing (hospital, emergency department [ED], outpatient), ART initiation, and ART retention initiatives vs a counterfactual scenario that approximated the status quo. HIV incidence, mortality, costs (in 2015$CDN), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios were estimated. Analyses were executed over 5- to 25-year time horizons from a government-payer perspective.
Results
ED testing was the best value at $30216 per QALY gained and had the greatest impact on incidence and mortality among PLHIV, while ART initiation provided the greatest QALY gains. The ART retention initiative was not cost-effective. Delivered in combination at the observed scale and sustained throughout the study period, we estimated a 12.8% reduction in cumulative HIV incidence and a 4.7% reduction in deaths among PLHIV at $55258 per QALY gained. Results were most sensitive to uncertainty in the number of undiagnosed PLHIV.
Conclusions
HIV testing and ART initiation interventions were cost-effective, while the ART retention intervention was not. Developing strategies to reengage PLHIV lost to care is a priority moving forward.
Following recent scientific findings on the secondary preventive benefits of combination antiretroviral treatment (ART) in observational [1–4] and experimental studies [5, 6], as well as confirmed individual health benefits from early ART initiation [7], international efforts are now focused on identifying and implementing evidence-based interventions to maximize population-level diagnosis, ART access, and, ultimately, viral suppression to address the global human immunodeficiency virus (HIV)/AIDS pandemic [8]. Substantial, coordinated efforts will be required to identify, implement, and evaluate effective interventions to treat and prevent HIV in order to achieve the goals of the ambitious United Nations 90-90-90 target, which calls for 90% of those living with HIV to be diagnosed, 90% of those diagnosed to access ART, and 90% of those on ART to reach viral suppression by 2020 [8]. While HIV testing to identify and reduce the size of the undiagnosed HIV-positive population remains critical [9], engaging and retaining people living with human immunodeficiency virus (PLHIV) on ART poses perhaps the greatest challenge to achieving the UN 90-90-90 target [10]. A range of interventions comprising a combination implementation strategy [11], accounting for the needs of specific demographic groups, and carefully considering the most practical means and opportune settings to intervene will be necessary in diverse settings globally.
On this basis, the British Columbia Ministry of Health launched the Seek and Treat for Optimal Prevention of HIV/AIDS (STOP HIV/AIDS) pilot program in 2 regional health authorities (Vancouver Coastal and Northern) in British Columbia (BC) in 2010. The pilot provided increased funding for HIV testing and treatment [12] and targeted funds for public health intervention and entailed a commitment to ongoing monitoring and evaluation, aided by comprehensive linked administrative databases [13, 14], to ascertain the value of implemented interventions and guide future investment decisions.
To establish an effective HIV response with long-term sustainability, there is an urgent need for efficiency in allocating scarce resources for the variety of public health interventions available in HIV/AIDS care [11]. Interventions to improve the HIV care cascade at its various stages may vary substantially in their incremental value. Further, the impact of individual interventions may be enhanced or diminished when delivered in combination with others (ie, their effects may not simply be additive), and this impact can change over time [15]. Prioritizing interventions for implementation, scale-up, or disinvestment on the basis of the incremental health benefits they provide per dollar invested provides clear and objective criteria to inform resource allocation decisions.
Our objective was to determine the cost-effectiveness of a set of HIV testing and treatment engagement interventions initiated in BC, as part of the STOP HIV/AIDS pilot program, which ran from 2011 to 2013. We drew on individual-level data and published information on the scale and effect of a selection of interventions executed in BC as part of the STOP HIV/AIDS pilot and satisfied our objective using a validated dynamic HIV transmission model built using population-level linked health administrative data.
METHODS
Model Description
We adapted and extended an existing deterministic transmission model previously used to estimate the health benefits and costs of expanded HIV screening and treatment in the United States [16] and BC [17]. The adult population of BC aged 15–64 years were partitioned into compartments on the basis of HIV risk behavior (men who have sex with men [MSM], people who inject drugs [PWID], MSM-PWID, and heterosexuals), screening status, and HIV infection status. Among those HIV infected, individuals were further classified as infected, diagnosed, on ART, and off-therapy and partitioned according to CD4 cell count (CD4≥500 µL, 350–499 µL, 200–349 µL, <200 µL). Disease progression was differentiated among those on ART [18] and not on ART [19] and estimated as a function of CD4 count, stratified into the 4 categories noted above. Health state transitions occurred at monthly intervals (Table 1).
Table 1.
Input Parameters for the Dynamic Compartmental Human Immunodeficiency Virus Transmission Model
| Description | Value | Source |
|---|---|---|
| Number of individuals in susceptible compartment i at time t (initial [1996]a value) | 2.64M | [14, 20, 21, 22] |
| Number of individuals in HIV-infected (undiagnosed) compartment i at time t (initial value) | 3694 | [14, 22, 23] |
| Number of individuals in HIV-diagnosed compartment i at time t (initial value) | 3844 | [14, 22, 23] |
| Number of individuals in HIV treatment compartment i at time t (initial value) | 0 | [14, 22, 23] |
| Number of individuals in HIV off-treatment compartment i at time t (initial value) | 0 | [14, 22, 23] |
| Monthly entry rate of individuals into compartment i | Time-varying | [20] |
| Average duration uninfected individuals in compartment i remain “identified” after screening | 12 months | Assumption |
| Monthly HIV screening rate for individuals in compartment i | Figure 1 | [1] |
| High risk (PWID, MSM/PWID) (multiplier) | 2.6 | [24] |
| Total sufficient contact rate | Time-varying | [3–6, 16, 21, 25, 26, 27–56] |
| Mortality and maturation rate for individuals in compartment i | ||
| Monthly mortality rate: heterosexual (susceptible) | 0.00027 | [57] |
| Monthly mortality rate: MSM (susceptible) | 0.00034 | [57] |
| Monthly mortality rate: PWID (susceptible) | 0.00238 | [27] |
| Monthly mortality rate: MSM/PWID (susceptible) | 0.00246 | [27] |
| Monthly mortality rate: heterosexual (infected/diagnosed) | ||
| CD4: ≥500 | 0.00079 | [58] |
| CD4: 350–499 | 0.00079 | [58] |
| CD4: 200–349 | 0.00136 | [59] |
| CD4: <200 | 0.00853 | [18] |
| Monthly mortality rate: MSM (infected/diagnosed) | ||
| CD4: ≥500 | 0.00045 | [28] |
| CD4: 350–499 | 0.00045 | [58] |
| CD4: 200–349 | 0.00175 | [59] |
| CD4: <200 | 0.00853 | [18] |
| Monthly mortality rate: PWID, including MSM/PWID (infected/diagnosed) | ||
| CD4: ≥500 | 0.00250 | [58] |
| CD4: 350–499 | 0.00250 | [58] |
| CD4: 200–349 | 0.00357 | [59] |
| CD4: <200 | 0.00856 | [18] |
| Monthly mortality rate: on ART (×1000–1) | Time-varying | [14, 22, 23] |
| Non-PWID | ||
| CD4: ≥500 | 0.474 (1996); 0.399 (2010) | |
| CD4: 350–499 | 0.608 (1996); 0.541 (2010) | |
| CD4: 200–349 | 1.167 (1996); 1.043 (2010) | |
| CD4: <200 | 8.259 (1996); 7.883 (2010) | |
| PWID | ||
| CD4: ≥500 | 0.973 (1996); 0.833 (2010) | |
| CD4: 350–499 | 1.181 (1996); 1.078 (2010) | |
| CD4: 200–349 | 2.557 (1996); 2.354 (2010) | |
| CD4: <200 | 8.288 (1996); 8.021 (2010) | |
| Monthly maturation rate | 0.00125 | [20] |
| Symptom-based monthly case finding rate for infected individuals in compartment i | ||
| Low risk (CD4: 200–349) | 0.00874 | [60] |
| High risk (CD4: <200) | 0.01842 | [60] |
| HIV disease progression rate for individuals not on ART | [19, 60–62] | |
| CD4: ≥500 to CD4: 350–499 | 0.02209 | |
| CD4: 350–499 to CD4: 200–349 | 0.02209 | |
| CD4: 200–349 to CD4 <200 | 0.02209 | |
| CD4: <200 to death | 0.00250 | |
| HIV disease progression rate for individuals on ART in compartment i | Supplementary Materials, Figure A6 | [14, 22, 23] |
| Rate of ART discontinuation | Figure 2, panels E and F | [14, 22, 23] |
| Probability of direct ART initiations immediately after diagnosis | Supplementary Materials, Figure A4 | [14, 22, 23] |
| Rate of individuals from diagnosed compartment i initiating antiretroviral treatment | Figure 2, panels A and B | [14, 22, 23] |
| Rate of ART reinitiation among those who had discontinued treatment | Figure 2, Panels C and D | [14, 22, 23] |
| Indicator variable for eligibility for HIV treatment initiation | Time-varying | [63, 64, 65–71] |
| 1996–1997 | CD4 <500 | |
| 1998–2001 | CD4 <350 | |
| 2002–2003 | CD4 <200 | |
| 2004–2007 | CD4 <350 | |
| 2008–2010 | CD4 <500 | |
| Post-2011 | All CD4 | |
| HIV transmission parameter | ||
| Needle-sharing parameters | ||
| Number of injections (monthly) at baseline | 19.5 | [21, 26] |
| Reduced injections due to opioid agonist treatment | 0.75 | [27, 72] |
| Probability of shared injection at baseline | 0.21 | [21, 28] |
| Probability of transmission per shared injection: CD4 >500 | 0.002 | [27, 29, 30] |
| Probability of transmission per shared injection: CD4: 350–499 | 0.002 | |
| Probability of transmission per shared injection: CD4: 200–349 | 0.003 | |
| Probability of transmission per shared injection: CD4 <200 | 0.003 | |
| Reduced probability of transmission on ART | 0.9 | [4] |
| Homosexual sex parameters | ||
| No sexual partners (annual) | 2.7 | [16, 21, 31] |
| Condom use probability | 0.5 | [25, 32–36] |
| Probability of transmission: CD4: >500 | 0.04 | [16, 37–40] |
| Probability of transmission: CD4: 350–499 | 0.04 | |
| Probability of transmission: CD4: 200–349 | 0.05 | |
| Probability of transmission: CD4: <200 | 0.1 | |
| Reduced probability of transmission on ART | 0.96 | [6] |
| Decreased number of sexual partners due to diagnosis | 0.5 | [41–44 |
| Condom effectiveness | 0.9 | [45] |
| Heterosexual sex parameters | ||
| No sexual partners: MSM | 0.08 | [32] |
| No sexual partners: MSM/PWID | 0.08 | [46, 47] |
| No sexual partners: PWID | 1.6 | [47, 48] |
| No sexual partners: heterosexual | 0.9 | [32, 49–51] |
| Condom use probability: MSM | 0.3 | [34, 36, 46] |
| Condom use probability: MSM/PWID | 0.3 | |
| Condom use probability: PWID | 0.3 | [35, 46] |
| Condom use probability: heterosexual | 0.3 | [51] |
| Probability of transmission: CD4: >500 | 0.025 | [3, 37, 52–56] |
| Probability of transmission: CD4: 350–499 | 0.025 | |
| Probability of transmission: CD4: 200–349 | 0.035 | |
| Probability of transmission: CD4: <200 | 0.065 | |
| Decreased number of sexual partners due to diagnosis | 0.5 | [44] |
| Reduced probability of transmission on ART | 0.96 | [5] |
| Condom effectiveness | 0.9 | [45] |
| Costs (2015$CDN) | ||
| ART costsc: PWID | Time dependentb | [73] |
| CD4: >500 | 435 (1996); 1276 (2010) | |
| CD4: 350–499 | 464 (1996); 1287 (2010) | |
| CD4: 200–349 | 483 (1996); 1287 (2010) | |
| CD4: <200 | 478 (1996); 1294 (2010) | |
| ART costsc: non-PWID | Time dependentb | [73] |
| CD4: >500 | 494 (1996); 1295 (2010) | |
| CD4: 350–499 | 523 (1996); 1306 (2010) | |
| CD4: 200–349 | 542 (1996); 1305 (2010) | |
| CD4: <200 | 537 (1996); 1313 (2010) | |
| Non-ART medical costsd: PWID | Time dependentb | [74] |
| CD4: >500 | 788 (1996); 745 (2010) | |
| CD4: 350–499 | 825 (1996); 784 (2010) | |
| CD4: 200–349 | 883 (1996); 1022 (2010) | |
| CD4: <200 | 1896 (1996); 1992 (2010) | |
| Non-ART medical costsd: non-PWID | Time dependentb | [74] |
| CD4: >500 | 317 (1996); 309 (2010) | |
| CD4: 350–499 | 362 (1996); 330 (2010) | |
| CD4: 200–349 | 471 (1996); 471 (2010) | |
| CD4: <200 | 1098 (1996); 763 (2010) | |
| HIV enzyme-linked immunosorbent assay antibody test | 13 | [75] |
| Confirmatory Western blot test | 21 | [75] |
| Medical care costsd: HIV-negative, MSM | 205 | [76] |
| Medical care costsd: HIV-negative, PWID | 518 | [73, 76] |
| Medical care costsd: HIV-negative, heterosexual | 217 | [76] |
| Intervention costse | Appendix | |
| Hospital-based care testing | 32081 (IMP); 12750 (SUS) | |
| Emergency department testing | 42798 (IMP); 9892 (SUS) | |
| Outpatient clinic testing | 49215 (IMP); 14785 (SUS) | |
| ART initiation | 66922 (IMP); 32271 (SUS) | |
| ART retention | 230734 (IMP); 111263 (SUS) | |
| Annual discount rate | 0.03 | |
| Quality-adjusted life years | ||
| Susceptible | 1.00 | [77] |
| Infected: CD4: ≥500 | 0.91 | [78–81] |
| Infected: CD4: 350–499 | 0.79 | [78–81] |
| Infected: CD4: 200–349 | 0.79 | [78–81] |
| Infected: CD4: <200 | 0.72 | [78–81] |
| Diagnosed: CD4: ≥500 | 0.87 | [78–81] |
| Diagnosed: CD4: 350–499 | 0.72 | [78–81] |
| Diagnosed: CD4: 200–349 | 0.72 | [78–81] |
| Diagnosed: CD4: <200 | 0.72 | [78–81] |
| On ART: CD4>500 | 0.87 | [78–81] |
| On ART: CD4: 350–499 | 0.83 | [78–81] |
| On ART: CD4: 200–349 | 0.83 | [78–81] |
| On ART: CD4: <200 | 0.82 | [78–81] |
| PWID multiplier | 0.90 | [27, 82] |
Abbreviations: ART, highly active antiretroviral treatment; CDN, Canadian dollars; HIV, human immunodeficiency virus; IMP, implementation phase; MSM, men who have sex with men; PWID, people who inject drugs; SUS, sustainment phase.
“Initial” values refer to 1996 values, the start of the model calibration period (1996–2010).
Figures presented for time-dependent parameters are 1996 and 2010 values.
Includes ART medication costs and associated pharmacy dispensation costs.
Includes non-ART medication costs and associated pharmacy dispensation costs, costs of physician billings for outpatient care, and hospitalization cost.
Intervention costs were differentiated between the implementation phase and the sustainment phase, with the latter excluding fixed costs of initiating the interventions.
HIV transmission occurred through homo- and heterosexual sex and needle sharing according to the assumption of proportional mixing [83, 84]. Table 1 provides the estimated baseline (1996) sexual and injection risk behaviors used to calibrate the model, with changes in these behaviors corresponding with proxies of injection and sexual risk behavior during the period [26, 27], as we have detailed previously [85].
The model was parameterized using comprehensive linked individual health administrative and registry data for the population of diagnosed PLHIV. Further details regarding the construction and composition of the HIV-positive cohort and available databases are provided elsewhere [13, 14]. The model was validated against population-level estimates of overall HIV prevalence, HIV incidence (overall and by risk group), the number of diagnosed PLHIV (overall and by ART receipt), the number of deaths among PLHIV by calendar year, and the size of the HIV-negative population during the period 1996–2010.
Interventions Assessed
We focused on the following 5 distinct interventions that were part of the STOP HIV/AIDS initiative: HIV testing in hospital, emergency departments (EDs), and outpatient clinic settings; ART initiation initiatives; and ART retention initiatives (Table 2) [86–88]. Each intervention was delivered at the Vancouver Coastal Health Authority (VCHA), home to 25% of BC’s population and 50% of BC’s population of PLHIV [13, 14]. We used observed aggregate-level testing rates and individual-level ART initiation and reinitiation rates in VCHA during the study period to estimate the effect of these interventions.
Table 2.
Description of Selected British Columbia Ministry of Health Seek and Treat for Optimal Prevention of Human Immunodeficiency Virus/AIDS Interventions Delivered in 2011–2013
| Intervention | Description | Delivery |
|---|---|---|
| Hospital-based testing | Integrate the routine offering of HIV testing into clinical practice in hospitals | Simplified pretest guideline while maintaining verbal informed consent Included a new patient follow-up process allowing for post-test counseling, education, public health follow-up, and linkage to care to be delegated to Vancouver Coastal Health Authority, communicable disease control nurses |
| ED testing | Integrate the routine offering of HIV testing into clinical practice in EDs | Introduced similarly as hospital-based testing |
| Outpatient clinic testing | Increase the routine offering of HIV testing to adult patients who had not been tested in the last year or presented specific risk, clinical symptoms, or the diagnosis of another sexually transmitted disease | Simplified pretest guideline while maintaining verbal informed consent. Consisted of training, continuing medical education credits, approved by the College of Family Physicians of Canada, and ongoing in-practice support |
| ART initiation | Expanded support to help gain access to ART | Low-barrier patient-centric support to liaise with a continuum of health and social services was provided by a multidisciplinary team that included healthcare professionals, social workers, and trained peer navigators |
| ART retention | Expanded case management to maintain ART adherence and help ART reinitiation among patients who have discontinued ART | When required, one-on-one adherence monitoring and support was provided Following a referral for case management, outreach workers attempted to locate patients who had discontinued ART |
The STOP initiative period was 2011–2013.
Abbreviations: ART, highly active antiretroviral treatment; ED, emergency department; HIV, human immunodeficiency virus.
In order to construct the counterfactual “status quo” level of testing, ART initiation, and retention, we estimated and extended trends in annual rates of testing and ART initiation from 2006 to 2010 (excluding prior years when initiation was constrained by clinical guidelines [63, 64]), as well as the probabilities of ART dropout, derived using multistate Markov models [89, 90] (Figures 1 and 2). We derived the costs for the implementation and sustainment phases of each respective intervention using financial data provided by VCHA [91, 92]. We assumed no public health intervention costs for the status quo scenario. Further detail on the calculations used to estimate the scale of delivery across HIV risk groups, effectiveness, and costs are provided in the Supplementary Materials.
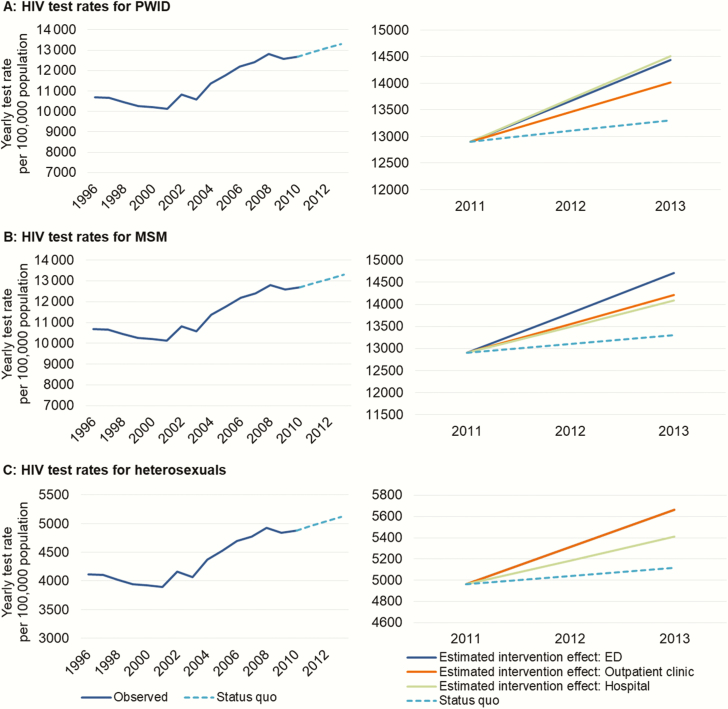
Figure 1.
Historical trends and estimated British Columbia Ministry of Health Seek and Treat for Optimal Prevention of HIV/AIDS intervention effects for human immunodeficiency virus (HIV) test initiatives. Historical aggregate-level HIV testing rates (left-hand sides of panels A–C) were extrapolated to generate counterfactual HIV testing rates, approximating the status quo (ie, no additional public health investments to scale-up testing) for the intervention period (dashed lines). Information on actual testing rates in Vancouver Coastal Health Authority, adjusted using external data, were used to estimate testing rates for each setting and by HIV risk group during the intervention period. Abbreviations: ED, emergency department; HIV, human immunodeficiency virus; MSM, men who have sex with men; PWID, people who inject drugs.
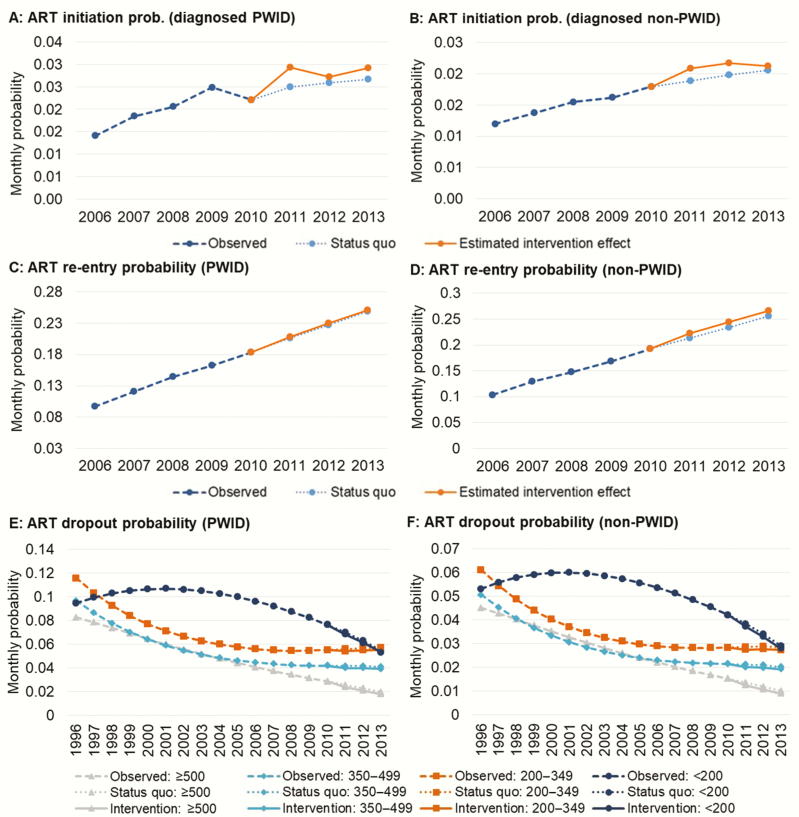
Figure 2.
Historical trends and estimated British Columbia Ministry of Health Seek and Treat for Optimal Prevention of human immunodeficiency virus/AIDS intervention effects for highly active antiretroviral treatment (ART) initiation and retention initiatives. The observed monthly probability of ART initiation among diagnosed people who inject drugs (PWID; panel A) and non-PWID (panel B) for British Columbia from 1996–2010 was used to generate counterfactual ART initiation probabilities approximating the status quo (ie, no additional public health investments to support ART initiation) for the intervention period (dashed line). ART initiation probabilities in Vancouver Coastal Health Authority, observed in linked individual-level data, were used to determine the intervention effect. The same procedure was used to determine probabilities of ART reentry (panels C and D). To adequately capture changes in the probability of ART dropout within the dynamic simulation model, we extrapolated counterfactual CD4-based transition probabilities using multistate Markov models for PWID and non-PWID (panels E and F) and estimated observed dropout probabilities during the intervention period using similar procedures as described above. Abbreviations: ART, highly active antiretroviral treatment; PWID, people who inject drugs.
Analyses
The primary model-projected outcomes included HIV incidence, mortality, and quality-adjusted life-years (QALYs) gained, as well as the costs of medical care, including costs attributable to ART (drug and pharmacy dispensation costs), non-ART medical care (HIV and non-HIV diagnostic tests, hospital-based care, family practice care, non-ART prescriptions), and costs attributable to HIV testing and public health intervention. The costs of ART and non-ART medical care among PLHIV were updated from previously published studies [93, 94]. We applied QALY weights derived from the peer-reviewed literature for HIV-negative individuals and PLHIV in and out of treatment, adjusting for injection drug use [76–81].
We estimated incremental cost-effectiveness ratios (ICERs) for each component intervention compared to the counterfactual status quo. In order to capture the long-term individual benefits of ART and second-order transmission effects, we considered 5-, 10-, and 25-year time horizons following the intervention period (2011–2013), for a (maximum) study period of 2011–2038. We considered a third-party payer perspective, presented all costs in 2015$CDN, and discounted costs and QALYs at an annual rate of 3%. Cost-effectiveness analysis was conducted according to well-established methods and conformed to guidelines on cost-effectiveness analysis and dynamic transmission modeling [95–97].
Otherwise, we executed sensitivity analyses on the size of the undiagnosed population [98], the uncertainty in the (unobserved) distribution of testing across HIV risk groups for each setting (hospital, outpatient clinic, ED), the effectiveness of each intervention, and the costs of the ART initiation and retention interventions. Further details are provided in the Supplementary Materials.
RESULTS
Model Validation
Estimated prevalence in the model was within 5.7% of the independently estimated Public Health Agency of Canada (PHAC) prevalence during the model calibration period (1996–2010), while estimated HIV incidence was within 2.7% of the PHAC estimate [98]. Overall model-estimated numbers of diagnosed cases were within 1.9% of observed figures at the midpoint of each calendar year, and the estimated number of deaths among diagnosed PLHIV was within 9% of observed figures. Finally, the modeled figures for the total susceptible population (aged 15–64 years) were within 1% of observed figures (further details provided in the Supplementary Materials).
HIV Testing Interventions
Overall, we estimated that the specified interventions increased testing rates from 222 to 1814 per 100000 BC residents aged 15–65 years during the intervention period. We further estimated the hospital-based testing initiative would result in 131 fewer incident HIV cases and 42 fewer deaths among PLHIV if the initiative were maintained at its current level over a 25-year time horizon (2011–2038; Figure 3). This intervention would result in higher ART and testing costs and would carry a public health intervention cost of $3.98 million but would offset non-ART medical costs by $4.83 million in present value over the 25-year study period. Also, it would result in an increment of 295.88 QALYs, for an ICER of $34544 per QALY gained (Table 3).
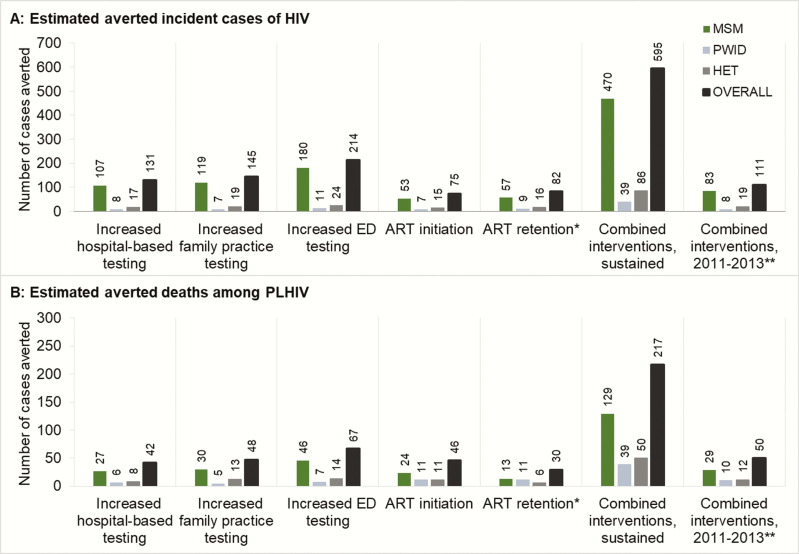
Figure 3.
Epidemiological effects of the British Columbia Ministry of Health Seek and Treat for Optimal Prevention of HIV/AIDS initiative interventions evaluated (25-year time horizon). Estimated effects of each human immunodeficiency virus (HIV) care intervention on averted incident cases (panel A) and deaths among people living with human immunodeficiency virus (panel B), by HIV risk group, over the complete study time horizon. *Combined initiatives to prevent treatment dropout and enhance reengagement. **Reverting back to the counterfactual status quo-levels of HIV testing and treatment engagement for the remainder of the study time horizon. Abbreviations: ART, highly active antiretroviral treatment; ED, emergency department; HET, heterosexual; HIV, human immunodeficiency virus; MSM, men who have sex with men; PLHIV, people living with human immunodeficiency virus; PWID, people who inject drugs.
Table 3.
Benefits, Costs, and Incremental Cost-Effectiveness of British Columbia Ministry of Health Seek and Treat for Optimal Prevention of Human Immunodeficiency Virus/AIDS Interventions
| Time Horizons Following the Intervention Period | ART Costs | Non-ART Costs | Human Immunodeficiency Virus Testing Costs | Interventiona Costs | Total Costs | QALYs | Incremental Cost- Effectiveness Ratiob |
|---|---|---|---|---|---|---|---|
| 5-year time horizon | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (B) | (Millions) | |
| Status quo | $871.47 | $601.30 | $16.75 | -- | $67.15 | 24.96 | -- |
| Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ QALYs | ||
| Hospital-based testing | 1.88 | –0.32 | 0.77 | 1.89 | 4.31 | 7.04 | $612051 |
| Outpatient clinic testing | 2.08 | –0.32 | 1.40 | 2.63 | 5.87 | 7.26 | $808749 |
| ED testing | 2.89 | –0.47 | 1.42 | 2.11 | 6.09 | 10.40 | $585985 |
| ART initiation | 6.82 | –1.69 | 0.00 | 4.28 | 9.67 | 94.91 | $101877 |
| ART retentionc | 5.90 | –0.47 | 0.00 | 14.75 | 20.34 | 43.16 | $471385 |
| Combined interventions, sustained | 19.55 | –3.27 | 3.58 | 25.67 | 46.26 | 164.12 | $281892 |
| Combined interventions, 2011–2013 onlyd | 10.67 | –2.19 | 0.83 | 15.18 | 25.02 | 122.30 | $204578 |
| 10-year time horizon | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (B) | (Millions) | |
| Status quo | $1429.18 | $896.87 | $26.08 | -- | $104.45 | 38.81 | -- |
| Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ QALYs | ||
| Hospital-based testing | 4.31 | –1.16 | 1.29 | 2.53 | 7.36 | 45.06 | $163396 |
| Outpatient clinic testing | 4.92 | –1.20 | 2.34 | 3.37 | 9.83 | 48.82 | $201410 |
| ED testing | 6.74 | –1.75 | 2.38 | 2.61 | 10.58 | 69.01 | $153352 |
| ART initiation | 9.52 | –2.60 | 0.00 | 5.90 | 13.50 | 204.29 | $66102 |
| ART retentionc | 9.52 | –0.92 | 0.00 | 20.35 | 29.38 | 92.42 | $317911 |
| Combined interventions, sustained | 34.50 | –7.47 | 5.99 | 34.77 | 70.23 | 457.78 | $153414 |
| Combined interventions, 2011–2013 onlyd | 12.67 | –3.48 | 0.83 | 15.18 | 26.45 | 253.30 | $104427 |
| 25-year time horizon | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (M) | $CDN (B) | (Millions) | |
| Status quo | $2778.53 | $1510.33 | $48.22 | -- | $193.01 | 71.69 | -- |
| Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ cost (M) | Δ QALYs | ||
| Hospital-based testing | 6.26 | –4.83 | 2.53 | 3.98 | 10.22 | 295.88 | $34544 |
| Outpatient clinic testing | 7.75 | –5.28 | 4.59 | 5.04 | 14.54 | 333.29 | $43623 |
| ED testing | 9.79 | –7.76 | 4.67 | 3.73 | 14.03 | 464.44 | $30216 |
| ART initiation | 11.79 | –4.10 | 0.00 | 9.55 | 19.60 | 586.52 | $33423 |
| ART retentionc | 16.56 | –2.82 | 0.00 | 32.93 | 48.51 | 304.02 | $159551 |
| Combined interventions, sustained | 49.74 | –23.28 | 11.74 | 55.23 | 105.34 | 1906.30 | $55258 |
| Combined interventions, 2011–2013 onlyd | 9.76 | –6.20 | 0.83 | 15.18 | 23.17 | 637.42 | $36356 |
Abbreviations: ART, combination antiretroviral therapy; B, billions; CDN, Canadian dollars; ED, emergency department; M, millions; QALY, quality-adjusted life-year.
Δ incremental costs/QALYs.
Costs of public health intervention.
Incremental cost-effectiveness ratio (ICER) of the intervention vs the counterfactual status quo: ICER = (Costintervention – Costsstatus quo)/(QALYintervetnion – QALYstatus quo).
ART retention includes the initiatives targeting preventing ART dropouts and enhancing reengagement among treatment-discontinued people living with human immunodeficiency virus.
Reverting back to the counterfactual status quo-levels of human immunodeficiency virus testing and treatment engagement for the remainder of the study time horizon.
In contrast, we estimated the outpatient clinic testing initiative will result in a reduction of 145 incident cases and 48 deaths among PLHIV for an ICER of $43623 per QALY gained if sustained through 2038. Finally, given the enhanced ability to reach populations at higher risk of HIV seroconversion (PWID and MSM), we estimated the ED testing initiative would have the largest impact on provincial HIV incidence, resulting in 214 cases averted and 67 deaths among PLHIV for an ICER of $30216 per QALY gained if sustained at the current scale throughout the study period.
ART Engagement Initiatives
We estimated a 10.6% increase in ART initiation for PWID and 7.9% increase for non-PWID from 2011 to 2013 vs the status quo counterfactual. The effects of the ART retention initiative were limited, including a 0.9%/4.2% increase in the probability of reengaging PWID/non-PWID on ART and a 4.7%/5.6% reduction in the probability of dropout among PWID/non-PWID (overall, across CD4 strata).
If sustained at the current scale throughout a 25-year time horizon, we estimated the ART initiation intervention would avert 75 incident cases and 46 deaths, resulting in an increment cost of $19.60 million in present value, with an additional $11.79 million attributable to ART costs and $9.55 million in public health intervention costs. However, this would be offset by $4.10 million in savings in non-ART medical costs. For an aggregate gain of 586.52 QALYs, we estimated an incremental cost-effectiveness of $33423 per QALY gained. Otherwise, we anticipated the ART retention initiative will avert 82 incident cases and 30 deaths at a cost of $159551 per QALY gained.
Combined Effect of Selected STOP HIV/AIDS Interventions
We considered the effects of the combination of HIV testing and ART engagement initiatives if sustained throughout the study period and if discontinued (reverting back to pre-STOP levels) after the 2011–2013 intervention period. If sustained, we anticipate 595 incident HIV cases averted and 217 deaths averted among PLHIV, at a cost of $55258 per QALY gained. If the intervention was to be discontinued after the 3-year pilot period, the intervention would only avert 111 incident cases and 50 deaths among PLHIV, at a cost of $36356 per QALY gained.
Incremental cost-effectiveness ratios for the majority of the interventions at shorter time horizons (5, 10 years) were outside the cost-effective range. However, the ART initiation initiative remained around $100000 per QALY even at a 5-year time horizon, demonstrating the more immediate value of engaging PLHIV in ART and the longer-term benefits that result from earlier detection of HIV and averted HIV incidence resulting from HIV testing interventions.
Sensitivity Analyses
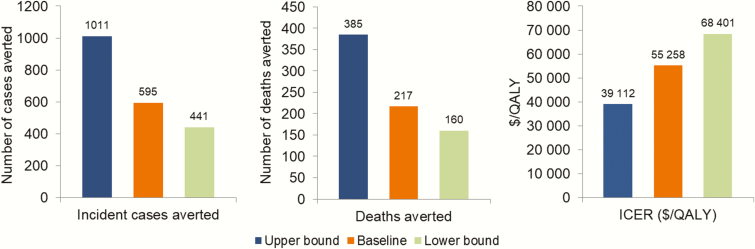
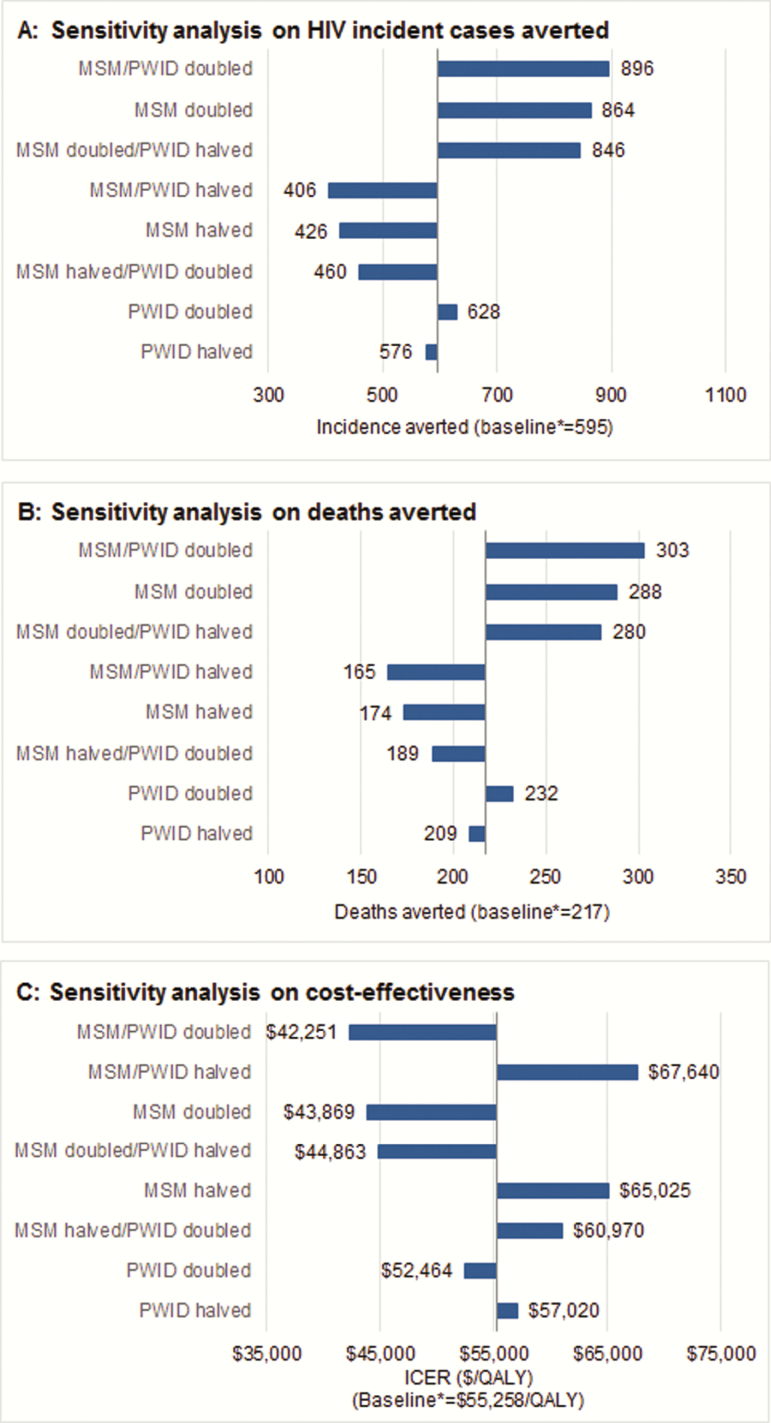
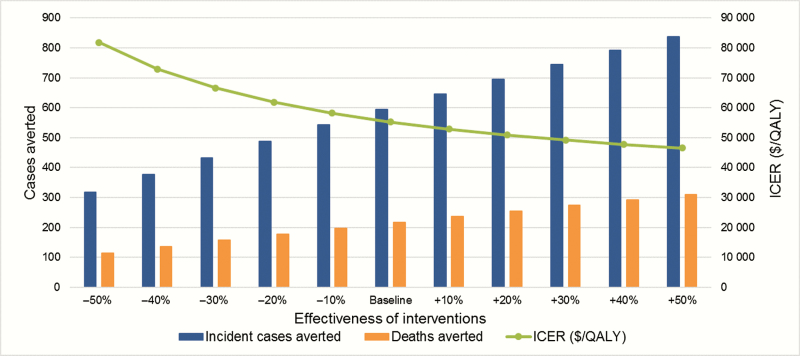
The results of our sensitivity analyses are presented in Figures 4–6. Focusing on the sustained implementation of the combined interventions, use of the upper and lower bounds of PHAC-estimated HIV prevalence for the province of BC in 2010 resulted in substantial differences in the estimated number of incident HIV cases averted (595 at baseline; 441 and 1011 at the lower and upper bounds) and deaths averted (217; 160–385), with the baseline ICER of $55258, ranging from $68401 to $39112 (Figure 4). Attributing a greater proportion of HIV tests toward MSM and PWID improved (decreased) ICERs for the HIV testing initiatives, while attributing a greater proportion of tests toward heterosexuals increased ICERs (Figure 5); however, ICERs remained within a cost-effective range in all instances. Removing implementation costs for each intervention resulted in small changes in ICERs, most notably decreasing the ICER for the ART retention initiative to $145403 per QALY. In 2-way sensitivity analysis, the ART retention initiative never fell below $50000, even after simultaneously doubling effectiveness and decreasing costs by 90%. Finally, improving the effectiveness of each intervention jointly increased the number of incident cases and deaths averted and decreased the ICER, while decreasing effectiveness by 50% increased the ICER to $81837 per QALY gained (Figure 6). Detailed results are presented in the Supplementary Materials.
Figure 4.
Results of sensitivity analyses on human immunodeficiency virus (HIV) prevalence. The size of population of people living with HIV in 2010 was set to the lower or upper bounds of provincial HIV prevalence estimates in 2010 from the public health agency of Canada. All results were for the combined intervention, sustained over the complete study time horizon. Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-years.
Figure 5.
Sensitivity analysis on proportion of risk groups in human immunodeficiency virus (HIV) testing in hospital, outpatient clinic, and emergency department (ED) settings. The baseline distribution of HIV testing in each setting was as follows: hospital-based: 92.7% heterosexual, 3.3% men who have sex with men (MSM), 4.0% people who inject drugs (PWID); outpatient clinic: 96.6% heterosexual, 2.1% MSM, 1.3% PWID; ED: 94.7% heterosexual, 3.2% MSM, 2.0% PWID. The proportions of heterosexuals tested in each scenario were adjusted accordingly. All results were for the combined intervention, sustained over the complete study time horizon. Abbreviations: HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; MSM, men who have sex with men; PWID, people who inject drugs; QALY, quality-adjusted life-years.
Figure 6.
Sensitivity analysis on the effectiveness of the combined British Columbia Ministry of Health Seek and Treat for Optimal Prevention of human immunodeficiency virus/AIDS pilot interventions. All results were for the combined intervention, sustained over the complete study time horizon. Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-years.
DISCUSSION
We estimated the cost-effectiveness of a selected set of HIV testing and treatment engagement initiatives implemented as part of BC’s STOP HIV/AIDS pilot from 2011 to 2013. Using World Health Organization criteria for assessing cost-effectiveness [99] with a 25-year time horizon, the ICERs we found to represent 59%–312% of BC’s Gross Domestic Product (GDP) per capita ($51135), suggesting, by international standards [100], that all but the ART retention initiative can be classified as “very cost-effective” (<1× GDP per capita: hospital-based, outpatient clinic, and ED testing; ART initiation and combined STOP pilot) or “cost effective” (<3× GDP per capita: combined STOP pilot). Delivered in combination at the current scale and sustained throughout our study period, we estimated the interventions could reduce cumulative HIV incidence by 13% and deaths among PLHIV by 5% at an incremental cost of $105 million in present value, or $3.8 million per year over the 25-year study time horizon.
Individually, ED testing provided the best value for the money and the greatest impact on preventing HIV incidence and mortality among PLHIV, while the ART initiation strategy provided the greatest gain in QALYs. Despite large increases in testing, we did not observe “saturation” in testing due to the limited scale of these interventions and the projected growth in the BC population [20]. Further, while we could only attribute tests across risk groups with indirect estimates, results were robust in sensitivity analyses, varying the proportion of tests across risk groups. These points underline the value of HIV testing, which is likely to be even higher in settings with larger populations of undiagnosed PLHIV.
The ART retention initiative was the least successful, notably leading to a very modest 0.9% increase in ART reinitiation rates among treatment-discontinued PWID and was only considered (marginally) cost-effective by excluding startup costs or drastically increasing effectiveness. While we could only estimate the effectiveness of this intervention coarsely—a subset of Vancouver Coastal Health clients not identified in our databases was targeted—the intervention’s impact was nonetheless not detectable at the Health Authority level. This may be partially attributable to an increasingly selective client base that the HIV care system continues to struggle to retain in ART, despite a high level of public health programming and fully covered medication costs. This context is important in considering the generalizability of our results to other settings. Nonetheless, a recent systematic review of interventions to improve ART adherence demonstrated that while some interventions were effective, their estimated effects were modest and waned over time [101]. Refined interventions and, possibly, new medication technologies [102, 103] will be critical to the success of HIV treatment and prevention strategies in BC and elsewhere moving forward.
We note that we have not provided an exhaustive account and evaluation of all interventions executed as part of the STOP HIV/AIDS pilot project but rather a selection of those with sufficient reported information to provide reasonable estimates of scale of delivery and effectiveness. In particular, several smaller-scale targeted HIV testing campaigns were executed, including a highly effective small-scale bathhouse testing campaign and a peer-driven testing campaign that had a 0.2% HIV-positivity rate and that reconnected 324 individuals (6.8%) into care [104, 105].
Given the demonstrated value of the assessed interventions, the feasibility of scale-up and sustainment of these and other interventions across the province must be considered carefully. Our findings pertain to interventions executed by VCHA, whose underlying epidemic has a distinct profile from that of other health authorities. The STOP HIV/AIDS initiative is now an ongoing provincial program, and each of the 4 other health authorities have implemented similar programs according to the gaps in their regional care cascades [106]. It is not necessarily the case that these interventions, at their observed scale and effectiveness, would have a comparable impact in other regions within or outside BC, which may also have different underlying levels of existing services.
This analysis featured several limitations worthy of further discussion. First, the interventions assessed were delivered on a nonrandomized basis, and information on the distribution of tests among the key HIV risk groups had to be estimated. We emphasize that our counterfactual status quo scenario—a characterization of what testing and treatment engagement rates would have been in the absence of the STOP pilot—were estimates based on historical trends in these rates over time. Despite these limitations, a flexible modeling framework that is amenable to sensitivity analysis and can capture long-term outcomes provides perhaps the best opportunity to assess such interventions within a causal framework [107].
Second, while the majority of our model was built with linked individual-level data for the population of PLHIV in BC, the size of the undiagnosed population and its distribution across risk groups was drawn from a national modeling effort from the Public Health Agency of Canada [98], which did not include much of the population-level data available to the STOP HIV/AIDS study team. Ascertaining accurate estimates of the size of the undiagnosed population of PLHIV and the distribution of this population across HIV risk groups is of paramount importance in establishing the value of HIV testing campaigns, in particular, in absolute terms and relative to other interventions.
Otherwise, we have previously outlined limitations due to infectivity being modeled indirectly through CD4-based stages of disease progression and no explicit account of higher infectivity in the 6 months following seroconversion. Our model was nonetheless able to produce risk group–specific incidence estimates and reproduce key aspects of the HIV epidemic at the population level in BC with a high degree of precision [17].
We have demonstrated the cost-effectiveness and estimated the long-term epidemiological impact of 5 key components of a coordinated combination HIV prevention strategy executed in BC. Our results demonstrate the substantial value these programs have added despite limited-scale implementation and underline the need to expand and sustain public health intervention efforts to curb the HIV epidemic in BC.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We acknowledge Michelle Olding for assistance with manuscript preparation, as well as all British Columbia Ministry of Health and Vancouver Coastal Health Decision support staff who were involved in data access and procurement, including Miranda Compton Vancouver Coastal Health Authority (VCHA), Theodora Consolacion (BC-CDC), Monika Lindegger, (BC-CDC), Al Cassidy (BC Ministry of Health), and Joleen Wright and Karen Luers (VCHA). We also acknowledge Ciro Panessa, Nancy South, and Mark Gilbert for their contributions to the STOP/HIV AIDS study group. Bohdan Nosyk is a Michael Smith Foundation for Health Research Scholar.
The STOP HIV/AIDS Study Group is comprised of the following: Rolando Barrios, VCHA; Patty Daly, VCHA; Reka Gustafson, VCHA; Perry RW Kendall, British Columbia (BC) Ministry of Health; Gina McGowan, British Columbia Ministry of Health; Irene Day, BC Centre for Excellence in HIV/AIDS; Kate Heath, BC Centre for Excellence in HIV/AIDS; Robert S Hogg, BC Centre for Excellence in HIV/AIDS; Julio SG Montaner, BC Centre for Excellence in HIV/AIDS; and Bohdan Nosyk, BC Centre for Excellence in HIV/AIDS.
Disclaimer. The funders had no direct role in the conduct of the analysis or the decision to submit the manuscript for publication.
Financial support. This study was funded by the BC Ministry of Health–funded “Seek and Treat for Optimal Prevention of HIV & AIDS” pilot project, a grant from the National Institutes of Health/National Institute on Drug Abuse (R01-DA-041747), and by Genome Canada (142HIV).
Potential conflicts of interest. J. S. G. M. has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, the MAC AIDS FUND, and ViiV Healthcare. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
STOP HIV/AIDS Study Group:
Rolando Barrios, Patty Daly, Reka Gustafson, Perry R W Kendall, Gina McGowan, Irene Day, Kate Heath, Robert S Hogg, Julio S G Montaner, and Bohdan Nosyk
References
- 1. Montaner JS, Lima VD, Barrios R et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 2010; 376:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Das M, Chu PL, Santos GM et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quinn TC, Wawer MJ, Sewankambo N et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 4. Wood E, Kerr T, Marshall BDL et al. Longitudinal community plasma HIV-1-RNA concentrations and incidence of HIV-1 among injecting drug users: a prospective cohort study. BMJ 2009; 338:1191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodger A, Bruun T, Cambiano V et al. HIV transmission risk through condomless sex if HIV positive partner is on suppressive ART: PARTNER Study. CROI; Boston, 2014. [Google Scholar]
- 7. INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. NEJM 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joint United Nations Programme on HIV/AIDS (UNAIDS). 90–90–90 An ambitious treatment target to help end the AIDS epidemic 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [PubMed]
- 9. Karch DL, Hall HI, Tang T, Hu X, Mermin J. Comparative mortality among people diagnosed with HIV infections or AIDS in the U.S., 2001–2010. Public Health Rep 2015; 130:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59 Suppl 1:S21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang LW, Serwadda D, Quinn TC, Wawer MJ, Gray RH, Reynolds SJ. Combination implementation for HIV prevention: moving from clinical trial evidence to population-level effects. Lancet Infect Dis 2013; 13:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gustafson R, Montaner J, Sibbald B. Seek and treat to optimize HIV and AIDS prevention. CMAJ 2012; 184:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nosyk B, Colley G, Yip B et al. ; STOP HIV/AIDS Study Group. Application and validation of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One 2013; 8:e54416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heath K, Samji H, Nosyk B et al. ; STOP HIV/AIDS Study Group. Cohort profile: seek and treat for the optimal prevention of HIV/AIDS in British Columbia (STOP HIV/AIDS BC). Int J Epidemiol 2014; 43:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nosyk B, Krebs E, Eyawo O, Min JE, Barrios R, Montaner JS. Cost-effectiveness analysis along the continuum of HIV care: how can we optimize the effect of HIV treatment as prevention programs?Curr HIV/AIDS Rep 2014; 11:468–78. [DOI] [PubMed] [Google Scholar]
- 16. Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 2010; 153:778–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nosyk B, Min JE, Lima VD, Hogg RS, Montaner JS; STOP HIV/AIDS Study Group. Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study. Lancet HIV 2015; 2:e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner JS. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. J Acquir Immune Defic Syndr 2013; 63:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mellors JW, Muñoz A, Giorgi JV et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126:946–54. [DOI] [PubMed] [Google Scholar]
- 20. BC Statistics. Population Estimates. Available at: http://www.bcstats.gov.bc.ca/StatisticsBySubject/Demography/PopulationEstimates.aspx. Accessed January 2015.
- 21. Lima VD, Johnston K, Hogg RS et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis 2008; 198:59–67. [DOI] [PubMed] [Google Scholar]
- 22. Nosyk B, Colley G, Yip B et al. ; STOP HIV/AIDS Study Group. Application and validation of case-finding algorithms for identifying individuals with human immunodeficiency virus from administrative data in British Columbia, Canada. PLoS One 2013; 8:e54416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nosyk B, Montaner JSG, Colley G et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis 2014; S1473-3099:70254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. Persons tested for HIV—United States, 2006. MMWR 2008; 57:845–9. [PubMed] [Google Scholar]
- 25. BC Centre for Disease Control. HIV and Sexually Transmitted Infections 2010. Vancouver: BC Centre for Disease Control, 2010. [Google Scholar]
- 26. Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. J Subst Abuse Treat 2010; 39:22–31. [DOI] [PubMed] [Google Scholar]
- 27. Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health 2000; 90:1100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spittal PM, Craib KJ, Wood E et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ 2002; 166:894–9. [PMC free article] [PubMed] [Google Scholar]
- 29. Wall SD, Olcott EW, Gerberding JL. AIDS risk and risk reduction in the radiology department. AJR Am J Roentgenol 1991; 157:911–7; discussion 919–21. [DOI] [PubMed] [Google Scholar]
- 30. Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr 1992; 5:1116–8. [PubMed] [Google Scholar]
- 31. Ekstrand ML, Stall RD, Paul JP, Osmond DH, Coates TJ. Gay men report high rates of unprotected anal sex with partners of unknown or discordant HIV status. Aids 1999; 13:1525–33. [DOI] [PubMed] [Google Scholar]
- 32. Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–25. [DOI] [PubMed] [Google Scholar]
- 33. Harawa NT, Greenland S, Bingham TA et al. Associations of race/ethnicity with HIV prevalence and HIV-related behaviors among young men who have sex with men in 7 urban centers in the United States. J Acquir Immune Defic Syndr 2004; 35:526–36. [DOI] [PubMed] [Google Scholar]
- 34. Kral AH, Lorvick J, Ciccarone D et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health 2005; 82:i43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacKellar DA, Valleroy LA, Behel S et al. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS 2006; 20:1637–44. [DOI] [PubMed] [Google Scholar]
- 36. Rietmeijer CA, Wolitski RJ, Fishbein M, Corby NH, Cohn DL. Sex hustling, injection drug use, and non-gay identification by men who have sex with men. Associations with high-risk sexual behaviors and condom use. Sex Transm Dis 1998; 25:353–60. [DOI] [PubMed] [Google Scholar]
- 37. Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. Aids 1996; 10(Suppl A): 75–82. [DOI] [PubMed] [Google Scholar]
- 38. Caceres CF, van Griensven GJ. Male homosexual transmission of HIV-1. AIDS 1994; 8:1051–61. [DOI] [PubMed] [Google Scholar]
- 39. Jacquez JA, Koopman JS, Simon CP, Longini IM Jr. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr 1994; 7:1169–84. [PubMed] [Google Scholar]
- 40. Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol 1999; 150:306–11. [DOI] [PubMed] [Google Scholar]
- 41. The NIMH Multisite HIV Prevention Trial: Reducing HIV Sexual Risk Behavior. The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. Science 1998; 280:1889–94. [DOI] [PubMed] [Google Scholar]
- 42. Kamb ML, Fishbein M, Douglas JM Jr et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA 1998; 280:1161–7. [DOI] [PubMed] [Google Scholar]
- 43. Cleary PD, Van Devanter N, Rogers TF et al. Behavior changes after notification of HIV infection. Am J Public Health 1991; 81:1586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Higgins DL, Galavotti C, O’Reilly KR et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA 1991; 266:2419–29. [PubMed] [Google Scholar]
- 45. Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002; 1:CD003255. [DOI] [PubMed] [Google Scholar]
- 46. Bacon O, Lum P, Hahn J et al. Commercial sex work and risk of HIV infection among young drug-injecting men who have sex with men in San Francisco. Sex Transm Dis 2006; 33:228–34. [DOI] [PubMed] [Google Scholar]
- 47. Tyndall MW, Patrick D, Spittal P, Li K, O’Shaughnessy MV, Schechter MT. Risky sexual behaviours among injection drugs users with high HIV prevalence: implications for STD control. Sex Transm Infect 2002; 78Suppl 1:i170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fryar CD, Hirsch R, Porter KS, Kottiri B, Brody DJ, Louis T. Drug use and sexual behaviors reported by adults: United States, 1999–2002. Adv Data 2007; 384:1–14. [PubMed] [Google Scholar]
- 49. Brisson M, Boily MC, Mâsse BR, Adrien A, Léaune V. Highlights of the sexual activity of the heterosexual population in the province of Quebec. Sex Transm Infect 1999; 75:296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson AM, Mercer CH, Erens B et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet 2001; 358:1835–42. [DOI] [PubMed] [Google Scholar]
- 51. Davis JO, Smith TW.. General Social Surveys (GSS), 1972–2008: National Opinion Research Surveys.
- 52. Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J Acquir Immune Defic Syndr 2006; 41:632–41. [DOI] [PubMed] [Google Scholar]
- 53. Downs AM, De Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. European Study Group in Heterosexual Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol 1996; 11:388–95. [DOI] [PubMed] [Google Scholar]
- 54. Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis 2008; 198:687–93. [DOI] [PubMed] [Google Scholar]
- 55. Kaplan EH. Modeling HIV infectivity: must sex acts be counted?J Acquir Immune Defic Syndr 1990; 3:55–61. [PubMed] [Google Scholar]
- 56. Nicolosi A, Corrêa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Italian Study Group on HIV Heterosexual Transmission. Epidemiology 1994; 5:570–5. [DOI] [PubMed] [Google Scholar]
- 57. Statistics Canada. Available at: http://www.statcan.gc.ca/pub/84-537-x/4064441-eng.htm.
- 58. Lodwick RK, Sabin CA, Porter K et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet 2010; 376:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simmons RD, Ciancio BC, Kall MM, Rice BD, Delpech VC. Ten-year mortality trends among persons diagnosed with HIV infection in England and Wales in the era of antiretroviral therapy: AIDS remains a silent killer. HIV Med 2013; 14:596–604. [DOI] [PubMed] [Google Scholar]
- 60. Sanders GD, Bayoumi AM, Sundaram V et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med 2005; 352:570–85. [DOI] [PubMed] [Google Scholar]
- 61. Vlahov D, Graham N, Hoover D et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load CD4+ cell count. JAMA 1998; 279:35–40. [DOI] [PubMed] [Google Scholar]
- 62. Hughes MD, Johnson VA, Hirsch MS et al. Monitoring plasma HIV-1 RNA levels in addition to CD4+ lymphocyte count improves assessment of antiretroviral therapeutic response. ACTG 241 Protocol Virology Substudy Team. Ann Intern Med 1997; 126:929–38. [DOI] [PubMed] [Google Scholar]
- 63. Hammer SM, Saag MS, Schechter M et al. ; International AIDS Society-USA Panel. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA 2006; 296:827–43. [DOI] [PubMed] [Google Scholar]
- 64. Yeni PG, Hammer SM, Hirsch MS et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 2004; 292:251–65. [DOI] [PubMed] [Google Scholar]
- 65. Carpenter CC, Fischl MA, Hammer SM et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA 1996; 276:146–54. [PubMed] [Google Scholar]
- 66. Carpenter CC, Fischl MA, Hammer SM et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA 1997; 277:1962–9. [PubMed] [Google Scholar]
- 67. Carpenter CC, Cooper DA, Fischl MA et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA panel. JAMA 2000; 283:381–90. [DOI] [PubMed] [Google Scholar]
- 68. Hammer SM, Eron JJ Jr, Reiss P et al. ; International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008; 300:555–70. [DOI] [PubMed] [Google Scholar]
- 69. Thompson MA, Aberg JA, Cahn P et al. ; International AIDS Society-USA. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010; 304:321–33. [DOI] [PubMed] [Google Scholar]
- 70. Carpenter CC, Fischl MA, Hammer SM et al. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA panel. JAMA 1998; 280:78–86. [DOI] [PubMed] [Google Scholar]
- 71. Yeni PG, Hammer SM, Carpenter CC et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA panel. JAMA 2002; 288:222–35. [DOI] [PubMed] [Google Scholar]
- 72. Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver’s supervised injection facility. CMAJ 2008; 179:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nosyk B, Yip B, Lima VD, Hogg RS, Montaner JSG, STOP HIV/AIDS Study Group. Antiretroviral drug costs and prescription patterns in British Columbia, Canada: 1996–2011. Med Care 2014; 52:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nosyk B, Lima V, Colley G, Yip B, Hogg RS, Montaner JS. Costs of health resource utilization among HIV-positive individuals in British Columbia, Canada: results from a population-level study. Pharmacoeconomics 2015; 33:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Centres for Medicaid and Medicare Services. Medicaid Fee Schedule 2013. Available at: www.med-quest.us/PDFs/Provider%20Memos/Medicaid%20Fee% 20Schedule.pdf.
- 76. Canadian Institutes for Health Information. National Health Expenditure Trends:1974–2014, 2014.
- 77. Fryback DG, Dasbach EJ, Klein R et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making 1993; 13:89–102. [DOI] [PubMed] [Google Scholar]
- 78. Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:54–62. [DOI] [PubMed] [Google Scholar]
- 79. Honiden S, Sundaram V, Nease RF et al. The effect of diagnosis with HIV infection on health-related quality of life. Qual Life Res 2006; 15:69–82. [DOI] [PubMed] [Google Scholar]
- 80. Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making 2002; 22:27–38. [DOI] [PubMed] [Google Scholar]
- 81. Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making 2002; 22:475–81. [DOI] [PubMed] [Google Scholar]
- 82. Long EF, Brandeau ML, Galvin CM et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS 2006; 20:2207–15. [DOI] [PubMed] [Google Scholar]
- 83. Hethcote HW, Vanark JW. Epidemiologic models for heterogeneous populations—proportionate mixing, parameter-estimation, and immunization programs. Math Biosci 1987; 84:85–118. [Google Scholar]
- 84. Nold A. Heterogeneity in disease-transmission modeling. Math Biosci 1980; 52:227–40. [Google Scholar]
- 85. Nosyk B, Zang X, Min JE et al. Relative effects of antiretroviral therapy and harm reduction initiatives on HIV incidence in British Columbia, Canada, 1996–2013: a modelling study. Lancet HIV 2017;4:e303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vancouver Coastal Health. Hope to Health Routine HIV Testing Report 2015. Available at: http://www.vch.ca/media/Q4_2014_Routine_HIV_Testing_Report.pdf.
- 87. Vancouver Coastal Health. STOP HIV/AIDS Semi-Annual Monitoring Report 2014. Available at: http://www.vch.ca/media/Semi_Annual_HIV_Monitoring_Report_Through_December_31_2013_Final.pdf.
- 88. CATIE. Shifting the paradigm: the history of the Vancouver STOP HIV/AIDS Project 2013. Available at: http://www.catie.ca/sites/default/files/stop-exec-summary.pdf.
- 89. Jackson CH. Multi-state models for panel data: the msm package for R. J Stat Softw 2011; 38:1–28. [Google Scholar]
- 90. Peirce JM, Petry NM, Stitzer ML et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry 2006; 63:201–8. [DOI] [PubMed] [Google Scholar]
- 91. BC STOP HIV/AIDS Collaborative Implementation Committee. Summative Financial Reports by Health Authority and Quarter FISCAL YEAR 2013–2014, 2014June 30, 2014.
- 92. Personal Communication Compton M. Regional Manager, HIV/AIDS Services, VCH/PHC Hope to Health Initiative 2016.
- 93. Schackman BR, Leff JA, Barter DM et al. Cost-effectiveness of rapid hepatitis C virus (HCV) testing and simultaneous rapid HCV and HIV testing in substance abuse treatment programs. Addiction 2015; 110:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sloan CE, Champenois K, Choisy P et al. ; Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Investigators. Newer drugs and earlier treatment: impact on lifetime cost of care for HIV-infected adults. AIDS 2012; 26:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pitman R, Fisman D, Zaric GS et al. ; ISPOR-SMDM Modeling Good Research Practices Task Force. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–5. Value Health 2012; 15:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ramsey S, Willke R, Briggs A et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005; 8:521–33. [DOI] [PubMed] [Google Scholar]
- 97. Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models. A suggested framework and example of application. Pharmacoeconomics 2000; 17:461–77. [DOI] [PubMed] [Google Scholar]
- 98. Nosyk B, Montaner JS, Colley G et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis 2014; 14:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tan-Torres Edejer T, Baltussen R, Adam T et al. Making choices in health: WHO guide to cost-effectiveness analysis 2003.
- 100. World Health Organization. Choosing interventions that are cost-effective (WHOCHOICE): cost-effectiveness thresholds Available at: http://www.who.int/choice/costs/CER_thresholds/en/index.html.
- 101. Kanters S, Park JJ, Chan K et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV 2017; 4:e31–40. [DOI] [PubMed] [Google Scholar]
- 102. Spreen WR, Margolis DA, Pottage JC Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baert L, van ‘t Klooster G, Dries W et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm 2009; 72:502–8. [DOI] [PubMed] [Google Scholar]
- 104. Canadian AIDS Treatment Information Exchange. Peer HIV Testing Available at: http://www.catie.ca/en/pc/program/peer-testing-project?tab=evaluation. Accessed March 10, 2016.
- 105. Canadian AIDS Treatment Information Exchange. Bathhouse and “Know On The Go” Mobile HIV Testing Projects Available at: http://www.catie.ca/en/pc/elements/kotg. Accessed March 10, 2016.
- 106. Lourenço L, Colley G, Nosyk B, Shopin D, Montaner JS, Lima VD; STOP HIV/AIDS Study Group. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS One 2014; 9:e115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet 2011; 378:515–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.