A dengue vaccine, CYD-TDV, is highly efficacious for all dengue serotypes among children >5 years of age who have acquired baseline immunity from previous exposure, and vaccine efficacy increases with age in this subgroup.
Keywords: dengue, vaccine efficacy, preexposure effect, age effect
Abstract
Background
A recombinant, live-attenuated, tetravalent dengue vaccine (CYD-TDV) was licensed for children aged ≥9 years in a few countries, but the dependence of vaccine efficacy on baseline immunity status and age groups has not been fully characterized.
Methods
Combining the 2 phase 3 trials CYD14 and CYD15, we estimated the vaccine efficacy for each of the 4 serotypes of dengue virus (DENV), as well as all serotypes combined, simultaneously stratified by baseline immunity status and age group, while accounting for uncertainty in the baseline immunity status of subjects.
Results
Baseline seropositive subjects showed high efficacy for all serotypes: 70.2% (95% confidence interval [CI], 57.4%–80.1%) for dengue serotype 1 (DENV-1), 67.9% (95% CI, 49.9%–82.0%) for DENV-2, 77.5% (95% CI, 64.3%–90.2%) for DENV-3, 89.9% (95% CI, 79.8%–99.9%) for DENV-4, and 75.4% (95% CI, 68.3%–81.6%) overall. In contrast, baseline seronegative subjects showed moderate efficacy against DENV-4 (51.2% [95% CI, 20.0%–72.8%]) but no significant efficacy against other serotypes. Among seropositive children, the overall efficacy tended to increase with age: 35.9% (95% CI, –7.6% to 69.3%) for children ≤5 years old, 65.6% (95% CI, 40.3%–84.2%) for those 6–8 years old, 73.4% (95% CI, 62.6%–82.1%) for those 9–11 years old, and 80.6% (95% CI, 72.9%–87.3%) for those 12 years or older.
Conclusions
The CYD-TDV vaccine was highly efficacious for all dengue serotypes among children aged >5 years who have acquired baseline immunity from previous exposure. Increasing vaccine efficacy with age was not fully explained by increasing prevalence of baseline immunity with age.
The epidemics of dengue, a mosquito-borne disease in most tropical and subtropical regions of the world, have been growing in recent decades, and the need for an effective vaccine has become increasingly urgent [1, 2]. A recombinant, live-attenuated, tetravalent dengue vaccine (CYD-TDV) had shown promising efficacies in 2 phase 3 clinical trials, CYD14 in Asia-Pacific countries among children 2–14 years old and CYD15 in Latin American countries among children 9–16 years old, and was subsequently licensed in a few countries [3–5]. In both trials, 3 doses of the vaccine were administered at months 0, 6, and 12, and the efficacy follow-up period was from month 13 to month 25, followed by a 4-year period of safety monitoring. To assess immunogenicity, about 20% of CYD14 and 10% of CYD15 participants were randomly selected to have blood drawn at months 0, 7, and 13, where the time points are also referred to as baseline, post–dose 2 (PD2), and post–dose 3 (PD3). On this subset, serotype-specific antibody levels were measured by a plaque reduction neutralization test, with a 50% reduction in the plaque count (PRNT50) as the neutralizing end point. During follow-up, dengue infection was tested on subjects with acute febrile illness (temperature ≥38°C on ≥2 consecutive days). PD3 immunogenicity was also assessed on virologically confirmed dengue cases. Both studies suggested the vaccine was efficacious against dengue virus (DENV) serotypes 3 and 4, with vaccine efficacy (VE) estimates ranging from 65% to 81% and moderately efficacious for the other 2 serotypes (VE, 35%–55%). Pooling the 2 trials together, a more recent analysis found that VE among baseline seropositive children was higher than that among baseline seronegative children, regardless of age group [6]. Meanwhile, controlling for baseline immunity status, older children tended to have higher VE. However, none of these differences showed statistical significance, likely because that analysis was performed only on the immunogenicity subset of participants whose baseline immunity status were measured. In addition, it is unclear how much baseline immunity affects VE for specific dengue serotypes. Despite the remaining questions about VE and its association with baseline immunity and age, children <9 years of age were excluded from the licensure of this vaccine, mostly due to the safety concerns raised by a relatively high hospitalization rate among vaccinated children aged ≤5 years in year 3 of CYD 14 [5–8]. Therefore, a more complete picture of the association of VE with baseline immunity status and age is needed, which is possible if the baseline immunity status unmeasured for the majority of the study populations can be inferred properly from observed information.
METHODS
Details about designs and trial procedures of CYD14 and CYD15 have been described previously [3, 4]. We performed a modified intent-to-treat analysis by excluding individuals who either had missing dates for their first doses or had laboratory-confirmed dengue symptom onset at enrollment. Our analyses were based on 10271 subjects from CYD14 and 20854 from CYD15. The active efficacy period of each participant from his or her study entry time (also the first injection time) to 13 months after the third injection was included in all subsequent analyses.
Outcomes and Predictors
The outcomes in this analysis are the times from study entry to laboratory-confirmed onsets of dengue symptoms of all degrees of severity. For each individual, the outcome is represented by the number of laboratory-confirmed onsets and the timing of these onsets. Five predictors for the risk of dengue disease are considered in this analysis: country, age, vaccine status, and baseline and PD3 immunity status. Baseline and PD3 immunity status was dichotomized by whether any of the 4 serotype-specific PRNT50 was ≥10 (1 = seropositive, 0 = seronegative). PD3 immunity status was considered because it was measured on more participants than baseline immunity status and may be informative about missing values of baseline immunity status (Supplementary Table 2). For cases infected before dose 3, PD3 immunity status was not solely affected by vaccination and was therefore considered as missing. Previously reported association between VE and age was based on the division of the study populations into <9 years and ≥9 years [6]. To explore finer age groups while still maintaining sufficient number of subjects within each group, we divided the study population into 4 age groups: ≤5 years, 6−8 years, 9−11 years, and ≥12 years. The CYD15 trial recruited only the 2 older groups.
Statistical Analysis
Survival Analysis
To construct the survival curves for the times to symptomatic infection stratified by serotype, baseline immunity status and vaccine assignment, we aligned the country-specific timelines by setting the entry time of the first study participant to zero in each country, and then we pooled the countries in each study to generate the Fleming-Harrington type of survival curves [9]. These survival curves are interpreted as the average over the dengue seasons in all of the countries in the trials. Under the assumption that baseline immunity status was missing at random—that is, whether a value is missing does not depend on the value itself given the observed data [10]—the missing values were imputed using the logistic regression of baseline immunity on country and age group (Supplementary Note 1). Sex, vaccine status, and disease outcome were not significantly associated with baseline immunity status and were thus excluded.
Vaccine Efficacy Estimation Based on Cox Proportional Hazards Models
The hazard of symptomatic infection was stratified by country and serotype and was adjusted for age group, vaccine status, baseline immunity, and their interactions in a Cox proportional hazards model. The model, in particular the interaction term, may vary according to which variable VE is to be stratified on—for example, interaction of vaccine status with age group or baseline immunity or both. Two logistic regression models were added to form a hierarchy of models, one regressing the probability of being baseline seropositive on country and age group, and the other regressing the probability of being PD3 seropositive on baseline immunity and vaccine status. In the model for baseline immunity, the effect of age group was assumed to differ between countries in CYD14 and those in CYD15, as populations in the 2 regions likely had different dengue exposure history. These models were fitted jointly using an expectation-maximization (EM) algorithm to account for cases with missing serotype information and for missing values of baseline and PD3 immunity status (Supplementary Note 2) [11]. Confidence intervals (CIs) for estimated efficacies and hazards were obtained by bootstrapping. A simulation study was conducted to validate our estimation method (Supplementary Note 3). To show that the EM algorithm predicts missing values of baseline immunity satisfactorily, we compared the receiver operating characteristic (ROC) curves between the EM algorithm and a few logistic regression models in terms of (1) goodness of fit of the model to the data (ie, using all complete data to fit the model) and (2) cross-validation, repeatedly sampling partial data to fit the model and using the remaining data for validation (Supplementary Note 4). All analyses were performed with the statistical computing package R (version 3.3.0) [12].
RESULTS
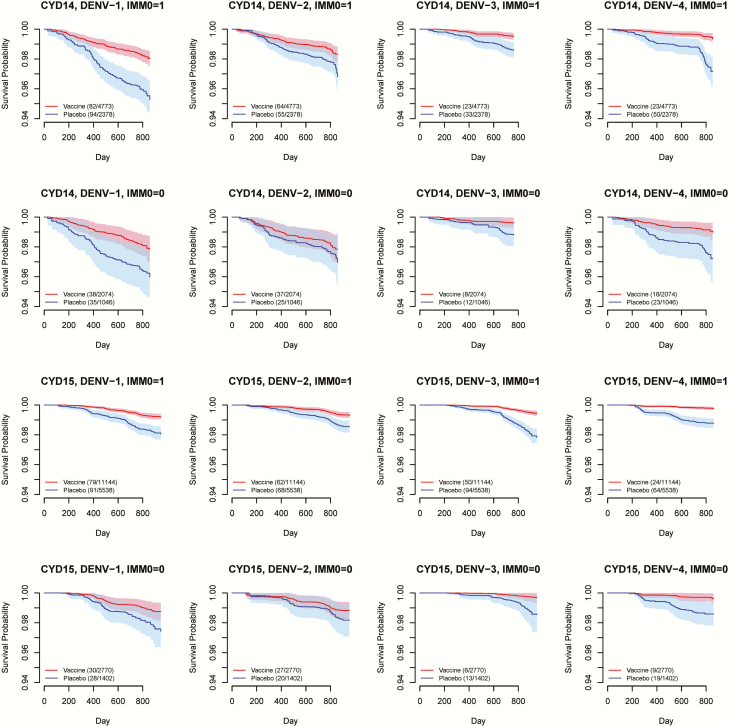
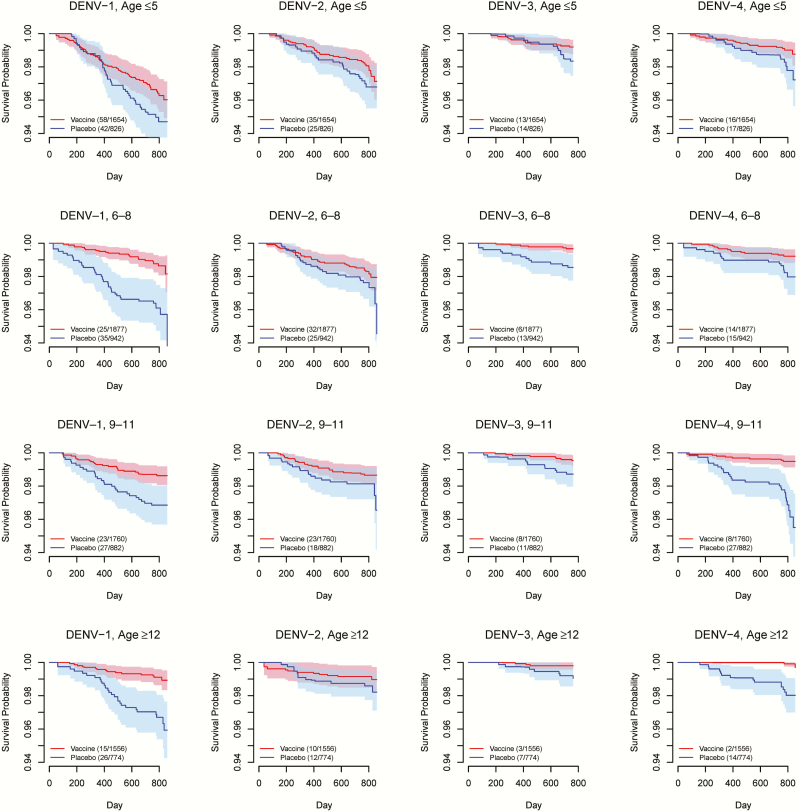
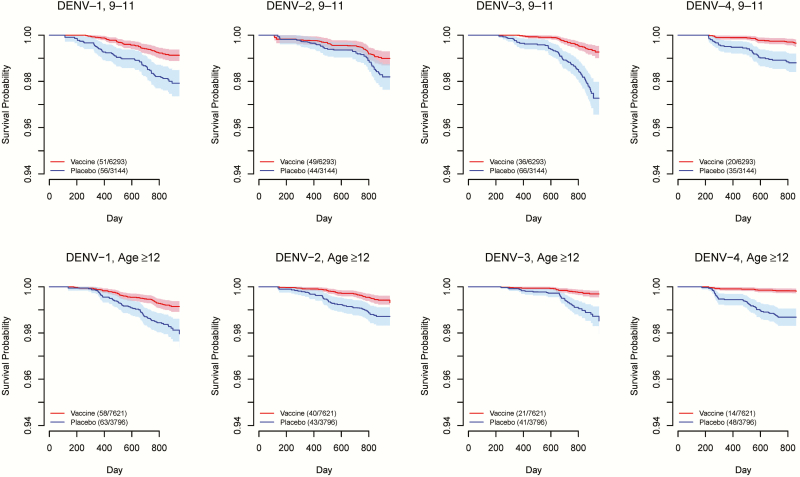
We summarized the age structure by study, vaccine status, and disease outcome according to the age grouping in this analysis (Supplementary Table 1). Figure 1 shows Fleming-Harrington survival curves among vaccinated subjects vs those on placebo for each combination of study, serotype, and baseline immunity status. In general, the CI bands for the 2 randomization groups are well separated for baseline seropositive subpopulations, except for DENV-2 in CYD14. In contrast, the CI bands mostly overlap for baseline seronegative subpopulations. These patterns imply that the vaccine protection is probably stronger if a vaccinated person were seropositive at baseline. Survival curves stratified by age groups are shown for CYD14 in Figure 2 and for CYD15 in Figure 3, suggesting a general trend of higher VE in older age groups. The increase in age does not seem to be associated with more separated survival curves for DENV-2 in CYD14, but the higher survival probabilities with increasing age for both arms and the relatively stable gap between them also imply high VE for older children. For CYD15, in which only children aged ≥9 years were enrolled, the survival curves and their CI bands are well separated for all serotypes except for DENV-2 in the age group of 9−11 years (Figure 3).
Figure 1.
Fleming-Harrington survival curves for time to virologically confirmed dengue symptoms in the vaccine arm (red) and the placebo arm (blue), stratified by dengue virus (DENV) serotype, baseline immunity status (IMM0), and study. Solid lines represent mean survival curves over 50 imputed data sets of unobserved baseline immunity status. Shaded areas are 95% confidence interval bands. Key shows number of events/number of study subjects in each arm.
Figure 2.
Fleming-Harrington survival curves for time to virologically confirmed dengue symptoms in the vaccine arm (red) and the placebo arm (blue) of CYD14, stratified by dengue virus (DENV) serotype and age group. Solid lines represent mean survival curves over 50 imputed data sets of unobserved baseline immunity status. Shaded areas are 95% confidence interval bands. Key shows number of events/number of study subjects in each arm.
Figure 3.
Fleming-Harrington survival curves for time to virologically confirmed dengue symptoms in the vaccine arm (red) and the placebo arm (blue) of CYD15, stratified by dengue virus (DENV) serotype and age group. Solid lines represent mean survival curves over 50 imputed data sets of unobserved baseline immunity status. Shaded areas are 95% confidence interval bands. Key shows number of events/number of study subjects in each arm.
We first estimated nonstratified serotype-specific and overall VE for CYD14 and CYD15 separately and jointly (Supplementary Table 3). DENV-4 is associated with the highest VE estimates for both studies, followed by DENV-3. With the studies combined, the estimated VE reaches 76.9% (95% CI, 70%–83%) for DENV-4 and 71.6% (95% CI, 63.7%–78.3%) for DENV-3. The vaccine shows moderate efficacies for both DENV-1 and DENV-2, in the range of 35%−55%. The overall VE, regardless of serotype, was estimated to be 59.9% (95% CI, 55.1%–64.0%).
The largest differences in VE are observed when stratified by baseline immunity for the 2 studies combined, as shown in Table 1. Among seronegative subjects, the vaccine provides significant protection only for DENV-4, with an estimated VE of 51.2% (95% CI, 20.0%–72.8%). The VE against DENV-3 has a similar magnitude but lacks statistical significance. The efficacies against DENV-1 and DENV-2 are essentially zero in this subpopulation. In contrast, the vaccine shows high and significant efficacies against all serotypes among the seropositive subpopulation. The estimated VE reaches 89.9% (95% CI, 79.8%–99.9%) for DENV-4, followed by 77.5% (95% CI, 64.3%–90.2%), 70.2% (95% CI, 57.4%–80.1%), and 67.9% (95% CI, 49.9%–82.0%) for DENV-3, DENV-1, and DENV-2, respectively. The benefit of baseline immunity on the VE is statistically significant for DENV-1, DENV-2, and DENV-4 with well-separated CIs. The overall VE among baseline seropositive subjects is 75.4% (95% CI, 68.3%–81.6%).
Table 1.
Estimates of Vaccine Efficacy Stratified by Baseline Immunity Status for CYD14 and CYD15 Jointly
| Serotype | Baseline Seronegative |
Baseline Seropositive | ||
|---|---|---|---|---|
| Est. VE, % | (95% CI) | Est. VE, % | (95% CI) | |
| 1 | 15.4 | (–23.3 to 45.0) | 70.2 | (57.4–80.1) |
| 2 | –12.5 | (–42.2 to 26.8) | 67.9 | (49.9–82.0) |
| 3 | 45.3 | (–20.2 to 99.7) | 77.5 | (64.3–90.2) |
| 4 | 51.2 | (20.0–72.8) | 89.9 | (79.8–99.9) |
| All | 21.9 | (–5.5 to 42.6) | 75.4 | (68.3–81.6) |
Country- and serotype-specific baseline hazards were adjusted for baseline immunity status and age group.
Abbreviations: CI, confidence interval; VE, vaccine efficacy.
Table 2 shows that age appears to be another important determinant for the levels of VE. For all serotypes as well as overall, there is a clear trend of increasing VE estimates with increasing age. For example, the VE against DENV-4 increases from 56.8% (95% CI, 26.5%–78.3%) among children ≤5 years old, to 64.6% (95% CI, 40.9%–81.1%) among children 6−8 years of age, to 78.3% (95% CI, 67.5%–86.7%) among children 9−11 years of age, and to 86.5% (95% CI, 78.2%–93%) among children ≥12 years old. Comparing the age group ≤5 years to the 2 age groups ≥9 years, the overall VE differs significantly: 38.9% (95% CI, 17.3%–53.8%) vs 61.3% (95% CI, 54.2%–68.1%) for 9–11 years old and 67.7% (95% CI, 60.5%–73.6%) for ≥12 years old. The age effect on the overall VE seems to be driven primarily by the age effect on the VE specific to DENV-4. The VE estimates are relatively robust to changes in the model (Supplementary Table 4).
Table 2.
Estimates of Vaccine Efficacy Stratified by Age Group for CYD14 and CYD15 Jointly
| Serotype | Age Group, y | |||||||
|---|---|---|---|---|---|---|---|---|
| ≤5 | 6–8 | 9–11 | ≥12 | |||||
| Est. VE, % | (95% CI) | Est. VE, % | (95% CI) | Est. VE, % | (95% CI) | Est. VE, % | (95% CI) | |
| 1 | 29.8 | (1.1–52.9) | 62.6 | (40.8–79.1) | 60.0 | (47.4–69.9) | 58.0 | (43.9–68.2) |
| 2 | 22.1 | (–17.5 to 49.9) | 28.6 | (–8.7 to 54.7) | 43.8 | (26.5–59.1) | 54.5 | (38.0–67.3) |
| 3 | 54.7 | (17.7–79.1) | 76.1 | (52.3–93.3) | 67.3 | (54.3–77.2) | 79.5 | (70.4–88.0) |
| 4 | 56.8 | (26.5–78.3) | 64.6 | (40.9–81.1) | 78.3 | (67.5–86.7) | 86.5 | (78.2–93.0) |
| All | 38.9 | (17.3–53.8) | 57.3 | (41.4–68.9) | 61.3 | (54.2–68.1) | 67.7 | (60.5–73.6) |
Country- and serotype-specific baseline hazards were adjusted for baseline immunity status and age group.
Abbreviations: CI, confidence interval; VE, vaccine efficacy.
We examined whether the dependence of VE on baseline immunity persists after controlling for age in Table 3. Serotypes were ignored due to the limited number of people with observed baseline immunity status. Among seropositive subjects, older children tend to have higher estimated VE than the younger ones. For example, VE was estimated to be 35.9% (95% CI, –7.6% to 69.3%) for children ≤5 years, vs 65.6% (95% CI, 40.3%–84.2%), 73.4% (95% CI, 62.6%–82.1%), and 80.6% (95% CI, 72.9%–87.3%) for children in age groups 6–8 years, 9–11 years, and ≥12 years old, respectively. The VE estimate for children 5 years or younger is not statistically significant and has wide CIs, partly because very few children in this group were seropositive at baseline. Among seronegative children, there are no statistically meaningful differences between the age groups. Controlling for age group, a significant difference in VE between seropositive and seronegative subjects is only seen in children ≥9 years old. In particular, the differences seem more pronounced among older children. The ratios of VEs between seropositive and seronegative subjects are 1.23, 1.69, 2.12, and 3.42 for the 4 age groups from younger to older, respectively.
Table 3.
Estimates of Vaccine Efficacy Stratified by Both Baseline Immunity Status and Age Group for CYD14 and CYD15 Jointly
| Age Group, y |
Baseline Immunity Status | |||
|---|---|---|---|---|
| Seropositive | Seronegative | |||
| Est. VE, % | (95% CI) | Est. VE, % | (95% CI) | |
| ≤5 | 35.9 | (–7.6 to 69.3) | 29.2 | (–13.5 to 63.7) |
| 6–8 | 65.6 | (40.3–84.2) | 38.9 | (–6.3 to 74.7) |
| 9–11 | 73.4 | (62.6–82.1) | 34.6 | (3.6–59.4) |
| ≥12 | 80.6 | (72.9–87.3) | 23.6 | (–15.3 to 54.3) |
Serotype information is ignored and the VE estimated is for any symptomatic dengue infection. Country-specific baseline hazards were adjusted for baseline immunity status and age group.
Abbreviations: CI, confidence interval; VE, vaccine efficacy.
Our method shows substantially larger areas under the ROC curve and thus has more satisfactory predictive power than traditional regression-based approaches in terms of both goodness of fit of the model to the data and cross-validation (Supplementary Figure 1). The assumptions about the dependency of VE on baseline immunity status, age group, or both do not affect the performance of predicting missing baseline immunity status, and the use of PD3 immunity status does benefit the prediction (Supplementary Figure 2).
DISCUSSION
Our analyses confirmed the dependence of VE on baseline serostatus as found previously [6, 7]. However, our results differ notably from previous findings based solely on the observed immunogenicity subset [6]. Regarding the differences in VE between baseline seropositive and seronegative subgroups, we estimated a larger gap among children 9 years or older (73.4% vs 34.6% for 9–11 years old, and 80.6% vs 23.6% for ≥12 years old), compared to previously reported 81.9% vs 52.5% for ≥9 years old. On the other hand, we estimated a smaller gap among children <9 years old (35.9% vs 29.2% for ≤5 years old, and 65.6% vs 38.9% for 6–8 years old), compared to previously reported 70.1% vs 14.4% for <9 years old. In particular, our VE estimates, 23.6%–34.6% for seronegative children ≥9 years old and 35.9%–65.6% for seropositive children <9 years old, are lower than their previously reported counterparts, 52.5% and 70.1%, though the differences are not statistically significant. A possible reason for these differences is the differentiation of VE across finer age groups, particularly between ≤5 years old and 6–8 years old. Age is a confounder for baseline immunity in term of their effects on VE. As a result, if the variation of VE by age is not adequately accounted for in the analysis, the variation of VE by baseline immunity status could be estimated with bias, as confirmed in a simulation study (see Supplementary Note 3 and Supplementary Table 5). By regressing partially observed baseline immunity status on fully observed age group and country, our method is able to provide much more efficient VE estimates compared to using the immunogenicity subset alone.
Our analysis is the first one to stratify VE by baseline immunity and age simultaneously so that the exact association of one factor with VE can be assessed when controlling for the other. Such simultaneous stratification is crucial for accurate estimation, since the increase in VE by baseline immunity partly contributed to the apparent age effect on VE, as older children in dengue-hyperendemic areas are more likely to have been infected and acquired certain levels of immunity. In addition to the dependence of VE on baseline immunity, we found further increasing trend of VE with increasing age among baseline seropositive children. It was recently reported that baseline serotypic antibody titers in the 2 studies increase with age, raising an open research question about whether higher VE was driven by higher baseline titers rather than just seropositivity [13].
It is interesting that, among children aged ≤5 years, the VE does not depend much on the baseline serostatus. Children in this age group are known to have a greater risk to vascular permeability and dengue shock upon secondary dengue infections, a phenomenon known as antibody-dependent enhancement (ADE), than their older peers [14–16]. The possibility of vaccine-induced ADE among seronegative children could partially explain the higher dengue-related hospitalization in the vaccinated arm of this age group in some of the safety follow-up years of the CYD-TDV trials, particularly in year 3 of CYD14 [5–8, 17, 18]. However, CYD-DTV did not seem to sensitize younger children much more than older children for general dengue disease during the efficacy period. Among baseline seronegative vaccinees in the immunogenicity subset, the attack rate of dengue disease, regardless of severity, was 5.66% in those 2–5 years old, not significantly higher than 4.82%, 4.48%, and 3.76% in 6–8, 9–11, and ≥12 years old, respectively. Furthermore, the VE estimates for seronegatives are similar between 2–5 years old and other age groups (Table 3). On the other hand, our analyses included only the active efficacy phase of CYD14 and CYD15, and therefore have no implication for the long-term efficacies of CYD-DTV. Whether disease enhancement by the vaccine mainly occurred in dengue-naive young children and how vaccine efficacies against mild and severe disease evolve in the long run need further investigation in ongoing and postlicensure studies, using careful extension of our analytic frameworks to link various data sources.
Our analyses would have been further improved if more relevant data were available for the imputation of unmeasured baseline immunity. The most informative and completely observed variables used for the imputation are country and age group. Sex was not associated with baseline immunity in the immunogenicity subset and was thus excluded. PD3 antibody level was informative about baseline immunity (Supplementary Figure 2), but itself was subject to a large amount of missing values. Because blood samples were collected from all participants in both trials at month 13, more PD3 antibody levels may potentially be measured to refine the analyses when feasible. In future vaccine trials, we recommend more exposure-risk–related variables be collected, such as population and mosquito density, socioeconomic status, and historical dengue surveillance data. However, measuring baseline immunity for as many subjects as possible, or preferably all, should be the first priority. More refined baseline immunity profiles have been used in recent analyses, for example, naive, monotypic immunity, and multitypic immunity [17, 19]. In view of the complex cross-reactivity in immunity among dengue serotypes, potential misclassification between the latter 2 categories needs to be handled carefully.
Notably, our results suggest that the vaccine efficacies among those 6–8 years old are comparable to those 9–11 years old, regardless of baseline immunity status. Meanwhile, during the 5 years of follow-up of CYD14, those 6–8 years old in the vaccine group experienced an average annual hospitalization incidence of 0.43%, much lower than 0.71% in ≤5 years old and comparable to 0.32% in 9–11 years old [8]. In addition, the relative risk of hospitalization between vaccinated vs control was 0.54 in those 6–8 years old, again much lower than 1.26 in ≤5 years old and comparable to 0.54 in 9–11 years old [8]. These facts imply that children in this age group in dengue-endemic countries could be considered for vaccine coverage. To curb the increasing global burden of dengue, research on how to integrate this partially protective vaccine into a variety of intervention strategies, such as case surveillance and vector control, should be carried out, which will certainly benefit from a comprehensive understanding of the efficacies and safety of the vaccination and its interplay with dengue transmission at the population level [19, 20].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number R37-AI032042) and the National Institute of General Medical Sciences (MIDAS grant number U54-GM111274). The authors thank Sanofi Pasteur for providing the data.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilder-Smith A, Gubler DJ. Public health. Dengue vaccines at a crossroad. Science 2015; 350:626–7. [DOI] [PubMed] [Google Scholar]
- 3. Capeding MR, Tran NH, Hadinegoro SR et al. CYD14 Study Group Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358–65. [DOI] [PubMed] [Google Scholar]
- 4. Villar L, Dayan GH, Arredondo-García JL et al. CYD15 Study Group Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. [DOI] [PubMed] [Google Scholar]
- 5. Gailhardou S, Skipetrova A, Dayan GH et al. Safety overview of a recombinant live-attenuated tetravalent dengue vaccine: pooled analysis of data from 18 clinical trials. PLoS Negl Trop Dis 2016; 10:e0004821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hadinegoro SR, Arredondo-García JL, Capeding MR et al. CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 7. Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016; 14:45–54. [DOI] [PubMed] [Google Scholar]
- 8. SAGE Working Group on Dengue Vaccines and WHO Secretariat. Background paper on dengue vaccines. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 9. Fleming TH, Harrington DP. Nonparametric estimation of the survival distribution in censored data. Comm Stat 1984; 13: 2469–86. [Google Scholar]
- 10. Little RJA, Rubin DB.. Statistical analysis with missing data. 2nd ed Hoboken, New Jersey: John Wiley & Sons, 2002. [Google Scholar]
- 11. Yang Y, Halloran ME, Chen Y, Kenah E. A pathway EM-algorithm for estimating vaccine efficacy with a non-monotone validation set. Biometrics 2014; 70:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. Available at: http://www.R-project.org/. Accessed 1 May 2017. [Google Scholar]
- 13. Vigne C, Dupuy M, Richetin A et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum Vaccin Immunother 2017. doi:10.1080/21645515.2017.1333211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guzmán MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 2002; 6:118–24. [DOI] [PubMed] [Google Scholar]
- 15. Dinh The T, Le Thi Thu T, Nguyen Minh D et al. Clinical features of dengue in a large Vietnamese cohort: intrinsically lower platelet counts and greater risk for bleeding in adults than children. PLoS Negl Trop Dis 2012; 6: e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guzman MG, Harris E. Dengue. Lancet 2015; 385:453–65. [DOI] [PubMed] [Google Scholar]
- 17. Halstead SB, Russell PK. Protective and immunological behavior of chimeric yellow fever dengue vaccine. Vaccine 2016; 34:1643–7. [DOI] [PubMed] [Google Scholar]
- 18. Halstead SB. Critique of World Health Organization recommendation of a dengue vaccine. J Infect Dis 2016; 214:1793–5. [DOI] [PubMed] [Google Scholar]
- 19. Coudeville L, Baurin N, Vergu E. Estimation of parameters related to vaccine efficacy and dengue transmission from two large phase III studies. Vaccine 2016; 34:6417–25. [DOI] [PubMed] [Google Scholar]
- 20. Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015; 33:7100–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.