Summary
Long-term, heavy marijuana use was associated with increased cardiovascular events in human immunodeficiency virus (HIV)–infected men aged 40–60 independent of tobacco smoking, viral load, and other risk factors, while there was no significant association with HIV disease markers, progression to AIDS, or mortality.
Keywords: HIV, AIDS, marijuana, cardiovascular disease
Abstract
Background
Marijuana use is prevalent among persons infected with human immunodeficiency virus (HIV), but its long-term effects on HIV disease progression and comorbidities are unknown.
Methods
In this prospective study of 558 HIV-infected men enrolled in the Multicenter AIDS Cohort Study between 1990 and 2010, there were 182 HIV seroconverters and 376 with viral suppression on combination antiretroviral therapy (ART). Associations between heavy marijuana use and HIV disease markers or white blood cell (WBC) count were examined using mixed-effects and linear regression models. Effects of marijuana use on cardiovascular (CV) events and other endpoints were estimated using Kaplan-Meier and logistic regression analyses.
Results
The median baseline age of participants was 41, 66% were white, 79% had education >12 years, and 20% reported heavy marijuana use at ≥50% of biannual visits during follow-up. Long-term heavy marijuana use showed no significant associations with viral load, CD4 counts, AIDS, cancer, or mortality in both cohorts but was independently associated with increased CV events between ages 40–60 after adjusting for age, tobacco smoking, viral load, and traditional risk factors (odds ratio [OR], 2.5; 95% confidence interval [CI] 1.3, 5.1). Marijuana and tobacco use were each independently associated with higher WBC counts in adjusted models (P < .01); the highest quartile of WBC counts (≥6500 cells/µL) was associated with increased CV events (OR 4.3; 95% CI, 1.5, 12.9).
Conclusions
Heavy marijuana use is a risk factor for CV disease in HIV-infected men ages 40–60, independent of tobacco smoking and traditional risk factors.
Marijuana use for recreational and medicinal purposes is prevalent among individuals infected with human immunodeficiency virus type 1 (HIV). Recent estimates in cohorts of HIV-infected (HIV+) men who have sex with men (MSM) range from 24% to 62% [1–3]. Despite this high prevalence, few studies have investigated health effects of long-term heavy marijuana use among HIV+ individuals [4]. Observational studies reported contradictory findings regarding associations between marijuana use and HIV viral load [5–9] or CD4 cell counts [1, 3, 5, 6, 10]. Animal model studies also report inconsistent effects of tetrahydrocannabinol administration on viral replication and disease progression [11, 12]. Thus, the effects of marijuana use on HIV disease and associated comorbidities remain unclear.
Cardiovascular (CV) disease is a comorbidity of particular concern in HIV+ populations [13], given that the estimated incidence is up to 2-fold higher than that of age-matched uninfected controls [14, 15]. The high prevalence of tobacco smoking and other traditional CV risk factors, combination antiretroviral therapy (ART)–associated dyslipidemia, and healthcare disparities explain only a portion of this elevated risk [16–21]. Thus, identifying nontraditional risk factors that explain elevated CV risk in HIV-infected populations is a priority for improving health outcomes [13–15]. Marijuana smoking has been associated with myocardial infarction and other adverse CV events in a few studies of HIV-uninfected individuals [22–25], yet the evidence regarding its effects on CV disease remains unclear [4]. To our knowledge, its effects on CV disease in HIV+ individuals have been evaluated in only 1 study [20].
Our aims in this longitudinal study were to examine the effect of long-term heavy marijuana use on HIV disease markers in men with acute and chronic HIV infection and to evaluate differences in CV events and other health outcomes during long-term follow-up.
METHODS
Study Cohort
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of HIV-infected and HIV-uninfected MSM [26, 27]. Participants undergo standardized interviews detailing behavioral characteristics, medical treatments or conditions, physical examinations, and collection of biological specimens at biannual study visits. All participants provided written informed consent, and each site’s institutional review board approved the study protocols.
In this study we examined 2 independent cohorts from the MACS public data release P23 using visits from 1990 through 2010 (Figure 1A): HIV-negative (HIV−) participants who seroconverted after MACS enrollment (HIV seroconverters) and HIV+ participants with baseline viral suppression on combination ART (chronic HIV). Inclusion criteria for HIV seroconverters were first HIV antibody positive visit after 1990; baseline was anchored to midpoint between last documented HIV− (1989 or later) and first HIV+ visit. Inclusion criteria for those with chronic HIV were a baseline visit in 1996 or later with at least 1 year of prior combination ART use and baseline viral load <400 HIV RNA copies/mL and CD4 cell count ≥300 cells/µL. Hepatitis C virus (HCV) antibody–positive patients and those reporting 1 year or more of other heavy drug use (daily nitrite inhalants [poppers] or uppers use, daily or weekly cocaine or heroin use) were excluded from the study.
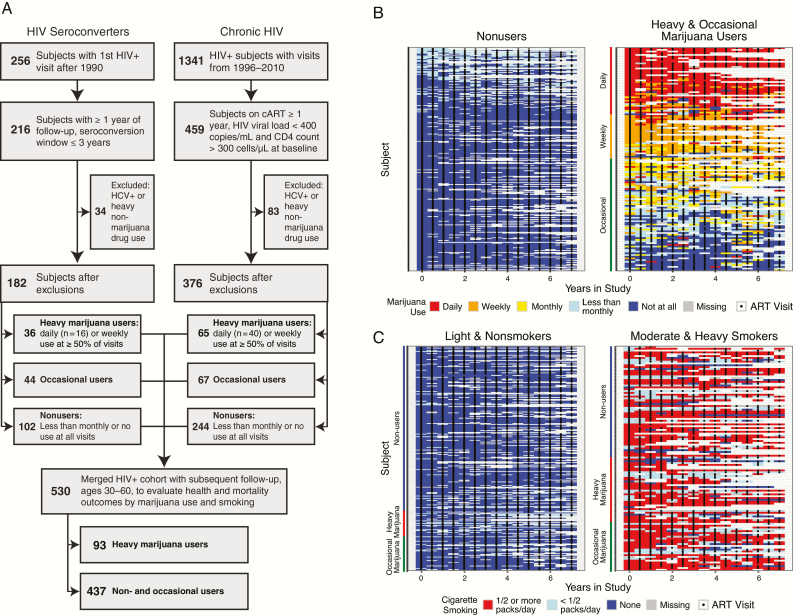
Figure 1.
Cohort selection and longitudinal patterns of marijuana and smoking exposures. A, Cohort selection flowchart. B, Lasagna plots illustrating self-reported patterns of marijuana use (top panel) and cigarette smoking (bottom panel) among participants in the chronic HIV cohort. Each row depicts visit-level exposure data for an individual in the study interval used for group classification. Abbreviations: cART, combination antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Exposure Variables
Marijuana was the primary exposure of interest. Participants were classified based on self-reported frequency of marijuana use as heavy users (defined as daily or weekly use at ≥50% of biannual visits), nonusers (less than monthly or no use at all visits), and occasional users (remaining participants). Participants who reported smoking an average of 0.25 cigarette packs/day or more were classified as moderate/heavy tobacco smokers.
Outcome and Covariate Definitions
HIV disease markers included plasma viral load, CD4+ and CD8+ T lymphocyte cell counts, and CD4:CD8 ratio [20]. White blood cell (WBC) counts were examined given prior association with cigarette smoking and marijuana use [28, 29]. Ascertainment of health outcomes in the MACS, including AIDS, cancer, and CV diagnoses, has been described elsewhere [19, 27, 30, 31]. Study endpoints for each participant were defined as follows: first incident event after age 30 for AIDS diagnoses, cancer, and mortality; first incident event after age 40 for CV events; loss to follow-up, last visit in 2010, or age 60. These age ranges were selected based on the known association of age ≥40 with CV risk and limited follow-up or events before age 30 and after age 60. AIDS diagnoses included participants with CD4 counts <200 cells/µL during follow-up. Cancer diagnoses were classified using International Classification of Diseases for Oncology, third edition (ICD-O-3), codes [31]. A CV composite endpoint was comprised of the following ICD-ninth edition, codes [16]: myocardial infarction (410), ischemic heart disease (411, 414, 429.2), angina pectoris (413), cerebrovascular disease (433, 434, 435), atherosclerosis (440), and heart failure (428). Traditional CV risk factors (diabetes mellitus, hypertension, and hypercholesterolemia) were defined based on reported use of medications for these conditions for 1 year or more during 10 years prior to endpoint or based on mean laboratory test values at the 2 most recent visits prior to endpoint within 5 years: diabetes, hemoglobin A1C ≥6.5%; hypertension, systolic blood pressure >140 or diastolic blood pressure >90; and hypercholesterolemia, total cholesterol >240.
Statistical Methods
Mixed-effects models were fit for each time-varying HIV disease marker as a continuous dependent variable. Marijuana use, smoking, age at baseline, race, and education were time-invariant categorical covariates. Duration in study was a time-varying covariate. Additional models for time-varying WBC and neutrophil count fit for the merged HIV+ cohort with follow-up between ages 40 and 60 included viral load and total cholesterol as time-varying categorical covariates. All models included a random intercept and slope.
Stratified Kaplan-Meier curves were used to assess differences in mortality and health outcomes in the merged HIV+ cohort by marijuana use or smoking, with age as the time axis and allowing staggered entry. Individuals with events prior to entry age were excluded from analyses; log-rank test was used to evaluate statistical significance of differences between groups.
Case-control data at endpoint was used to assess associations between marijuana use, CV events, and WBC count in the merged HIV+ cohort with follow-up between ages 40 and 60. Logistic and linear regression models were fit with CV event and time-updated log2-transformed WBC count, respectively, as dependent variables. Predictors included time-updated marijuana use, tobacco smoking, age, race, HIV viral load, and traditional risk factors; linear regression models also included total cholesterol. All analyses were performed using R, version 3.2.
RESULTS
Clinical Characteristics
A total of 558 individuals met eligibility requirements for the HIV seroconverter (n = 182) and chronic HIV (n = 376) cohorts, respectively; heavy marijuana users comprised approximately 20% of both cohorts (Figure 1A). Participants were predominantly white, with >12 years of education, median age 40.8 years at baseline, and median duration between last HIV− and first HIV+ visit 1.0 years for HIV seroconverters (Table 1). Heavy marijuana use was associated with cigarette smoking in both cohorts (P = .035 and P = <.001). There were no significant associations between heavy marijuana use and other demographic and baseline characteristics, including age, race, education, alcohol use, Center for Epidemiological Studies Depression Scale (CES-D) depression scores, viral load, and CD4 count. Self-reported ART use, adherence, and regimens were similar between marijuana users and nonusers (Table 1 and Supplementary Table 1). The patterns of both marijuana and tobacco use remained generally consistent over time within individual participants (Figure 1B).
Table 1.
Baseline and Updated Cohort Characteristics
| Characteristic | HIV Seroconverters | Chronic HIV | ||||||
|---|---|---|---|---|---|---|---|---|
| Nonusers | Heavy Marijuana | Occasional Marijuana | P Valuea | Nonusers | Heavy Marijuana | Occasional Marijuana | P Valuea | |
| (n = 102) | (n = 36) | (n = 44) | (n = 244) | (n = 65) | (n = 67) | |||
| Length of follow-up, mean (standard deviation), y | 7.7 (2.6) | 7.5 (2.3) | 7.9 (2.1) | .715 | 5.5 (2.1) | 5.1 (2.1) | 5.82 (1.9) | .157 |
| Seroconversion window, median (IQR), yb | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | .500 | — | — | — | — |
| Age, median (IQR), y | 40.5 (33–47) | 33.3 (28–42) | 37.8 (32–41) | .015 | 42 (37–46) | 41 (35–49) | 41.5 (36–47) | .771 |
| Racial/ethnic group | .161 | .411 | ||||||
| White | 83 (81.4) | 23 (63.9) | 36 (81.8) | 146 (59.8) | 33 (50.8) | 42 (62.7) | ||
| Black | 12 (11.8) | 9 (25.0) | 7 (15.9) | 55 (22.5) | 21 (32.3) | 17 (25.4) | ||
| Other | 7 (6.9) | 4 (11.1) | 1 (2.3) | 43 (17.6) | 11 (16.9) | 8 (11.9) | ||
| Education >12 years | 86 (84.3) | 32 (88.9) | 38 (86.4) | .789 | 187 (76.6) | 49 (75.4) | 50 (74.6) | .934 |
| Heavy or binge alcohol usec | 26 (25.5) | 13 (36.1) | 12 (27.3) | .471 | 54 (22.1) | 17 (26.2) | 22 (32.8) | .19 |
| Moderate/heavy cigarette smokingd | 27 (26.5) | 18 (50.0) | 15 (34.1) | .035 | 60 (24.6) | 35 (53.8) | 27 (40.3) | <.001 |
| CES-D score, median (IQR) | 9 (4–16) | 9 (3–16) | 13 (2–20) | .981 | 8 (3–16) | 7.5 (5–16) | 13 (7–20) | .023 |
| CES-D score ≥16 | 26 (26.3) | 11 (30.6) | 14 (34.1) | .628 | 63 (26.2) | 18 (28.1) | 26 (40.6) | .078 |
| CD4 count (cells/µL), median (IQR) | 629 (455–855) | 667 (507–834) | 680 (544–828) | .552 | 559 (445–741) | 548 (432–620) | 553 (435–790) | .312 |
| HIV viral load ≥400 copies/mL | 78 (89.7) | 30 (93.8) | 30 (88.2) | .728 | 0 (0.0) | 0 (0.0) | 0 (0.0) | — |
| Any ART use in study periode | 82 (80.4) | 29 (80.6) | 33 (75) | .742 | 244 (100) | 65 (100) | 67 (100) | — |
| ART adherence at ≥95% of visitse | 75 (91.5) | 27 (93.1) | 25 (75.8) | .04 | 167 (68.4) | 43 (66.15) | 41 (61.2) | .533 |
| AIDS diagnosesf | 32 (31.4) | 8 (22.2) | 17 (38.6) | .344 | 16 (6.6) | 6 (9.2) | 4 (6.0) | .690 |
| Mortalitiesf | 20 (19.6) | 6 (16.6) | 10 (22.7) | .794 | 9 (3.7) | 3 (4.6) | 3 (4.5) | .794 |
| One or more cancer diagnosesf | 14 (13.7) | 3 (8.3) | 7 (15.9) | .634 | 13 (5.3) | 4 (6.0) | 3 (4.5) | .891 |
| One or more cardiovascular eventsf | 8 (11.1) | 3 (14.3) | 1 (2.9) | .311 | 13 (6.7) | 14 (29.2) | 5 (10.9) | <.001 |
Data are n (%) at baseline visit (index date of the analysis) unless otherwise specified. Study period for seroconverter and chronic HIV cohorts: −4 years to +7 years relative to baseline and baseline to +7 years, respectively.
Abbreviations: ART, antiretroviral therapy; CES-D, Center for Epidemiological Studies Depression Scale; HIV, human immunodeficiency virus; IQR, interquartile range.
aχ2 or Fisher exact test, analysis of variance, or Kruskal-Wallis rank sum test for categorical, normally distributed continuous, and nonnormally distributed continuous variables, respectively.
bDuration between last HIV− and first HIV+ visits.
cHeaviest reported usage at ≥2 visits in study period: heavy, >14 drinks/week; binge, 5 or more drinks/occasion at least monthly.
dAverage ≥0.25 packs/day during study period.
eSelf-reported current ART medication use.
fIncident events during post-baseline follow-up, restricted to ages 40–60 years for cardiovascular events.
An all-by-all comparison of baseline and demographic characteristics stratified by marijuana use (Supplementary Figure 1) indicated more cigarette smoking among heavy marijuana or alcohol users, yet no other associations were observed. CES-D depression scores were lower with increasing age, yet these trends did not vary by marijuana use.
Marijuana Use and HIV-Related Outcomes
Marijuana use showed no significant association with mean trajectories of HIV disease markers, including no dose–response relationship when stratifying by daily vs weekly use (Figure 2A). In mixed-effects models adjusted for age, smoking, race, and education, daily marijuana use was associated with lower CD4:CD8 ratio at baseline in the chronic HIV cohort (P = .009; Supplementary Table 2), but this effect was not reproduced in HIV seroconverters (P = .457). There was no significant association between daily or weekly use and other HIV disease markers.
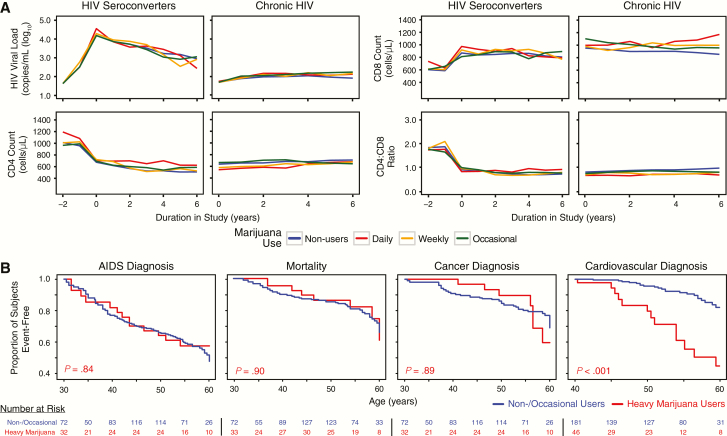
Figure 2.
Association between marijuana use, HIV disease marker trajectories, and health outcomes. A, Mean values of HIV disease markers by marijuana use. B, Kaplan-Meier curves of AIDS diagnoses, mortality, cancer diagnoses, and cardiovascular events for the merged HIV+ cohort stratified by marijuana use. P values denote pair-wise log-rank test vs control group. Abbreviations: HIV, human immunodeficiency virus.
Next, we evaluated associations between marijuana use and HIV-related outcomes during long-term follow-up. There were no significant associations between heavy marijuana use and progression to AIDS, cancer diagnoses, or mortality in either cohort (Table 1). AIDS-related causes of death comprised 68.1% of all mortalities; the AIDS-defining malignancies (ADMs) Kaposi sarcoma (32.8%) and non-Hodgkin lymphoma (20.7%) comprised the majority of cancer diagnoses. By contrast, a higher proportion of heavy marijuana users had CV events between ages 40 and 60 years compared with non- and occasional users in both cohorts (P = .311 and P < .001; Table 1). Consistent with these findings, Kaplan-Meier curves for the merged HIV+ cohort between ages 40 and 60 showed elevated rates of incident CV events among heavy marijuana users (P < .001; Figure 2B), while there was no significant difference in rates of AIDS diagnoses, cancer, or mortality (Figure 2B).
Marijuana Use, Increased WBC Count, and Cardiovascular Events
Previous studies demonstrated significant associations between tobacco smoking, elevated WBC count, and coronary heart disease [28, 29, 32], but these associations have not been explored in the context of marijuana use during HIV infection. We therefore evaluated mean trajectories of WBC counts stratified by marijuana and tobacco use over the first 6 years of follow-up in both cohorts. Mean WBC counts were elevated in cigarette smokers vs nonsmokers; the highest mean WBC counts were observed in marijuana/tobacco co-users in both cohorts (Supplementary Figure 2). In mixed-effects models adjusted for age, race, education, viral load, and total cholesterol, cigarette smoking was associated with higher WBC count (estimate = .153; P < .0001), and heavy marijuana use was associated with increasing WBC count in the merged HIV+ cohort between ages 40 and 60 (marijuana × age interaction: estimate = .020; P < .001; Figure 3A and Supplementary Table 3). Comparable estimates were observed for neutrophil counts (Figure 3A and Supplementary Table 3), but not monocyte or lymphocyte counts, in models adjusted for the same covariates. Thus, neutrophils are the main leukocyte population explaining higher WBC counts. Using case-control data at endpoint, heavy marijuana use (estimate = 1.11; P = .01) and cigarette smoking (estimate = 1.15; P < .0001) were each independently associated with increased WBC count in linear regression models (Table 2). The interaction between marijuana use and smoking was not significant in mixed-effects and linear regression models, providing further evidence for independent effects of these factors.
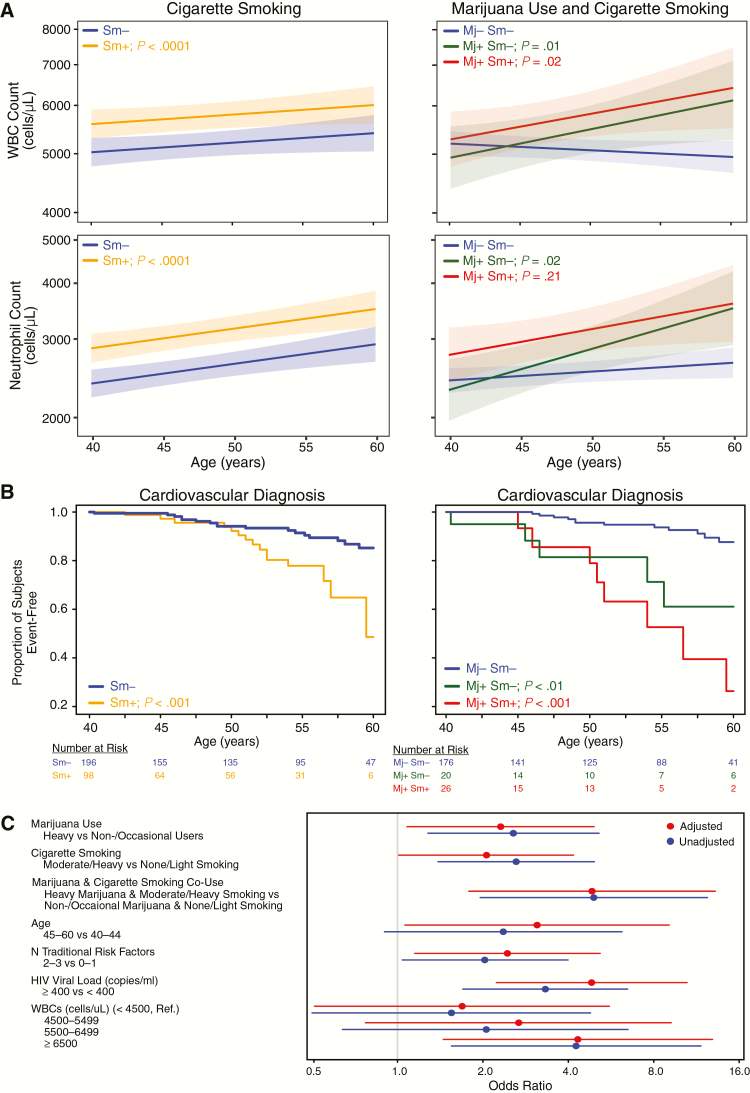
Figure 3.
Heavy marijuana use and white blood cell (WBC) count are independently associated with increased odds of cardiovascular (CV) events. A, Estimated mean trajectories for WBC (top panels) and neutrophil counts (bottom panels) by cigarette smoking (left panel) or by marijuana use and cigarette smoking (right panel), for the merged HIV+ cohort with follow-up between ages 40 and 60 years from mixed effects models adjusted for viral load, race, age at entry, education, and total cholesterol (Supplementary Table 3). B, Kaplan-Meier curves of first CV event stratified by smoking only (left panel) or by marijuana use and cigarette smoking (right panel) for the merged HIV+ cohort with follow-up between ages 40 and 60. Mj+ denotes heavy marijuana users, Mj−, occasional or nonusers, Sm+, moderate or heavy smokers (≥0.25 packs/day, average), Sm−, light or nonsmokers, P values, pair-wise log-rank test vs control group. C, Forest plot showing estimated odds of adverse CV events for the indicated covariates from logistic regression model 2 (Table 3) with WBC values by quartiles. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; Mj, marijuana; Sm, smoking; WBC, white blood cells.
Table 2.
Factors Associated With Increased White Blood Cell Count
| Unadjusted Models | Adjusted Model | |||
|---|---|---|---|---|
| Predictor | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value |
| Heavy marijuana usea | 1.09 (1.01, 1.18) | .023 | 1.10 (1.02, 1.19) | .012 |
| Moderate/heavy cigarette smokingb | 1.12 (1.05, 1.19) | <.0001 | 1.15 (1.08, 1.22) | <.0001 |
| Age 45–60 vs 40–44 | 1.05 (0.98, 1.13) | .152 | 1.05 (0.98, 1.13) | .172 |
| Race | ||||
| White | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| Black | 0.86 (0.80, 0.92) | <.0001 | 0.87 (0.81, 0.94) | <.001 |
| Other | 0.96 (0.88, 1.05) | .331 | 0.97 (0.89, 1.05) | .425 |
| Diabetes risk factorc | 1.03 (0.91, 1.16) | .677 | — | — |
| Hypertension risk factord | 1.04 (0.98, 1.11) | .179 | — | — |
| Total cholesterol (mg/dL) | ||||
| <180 | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| 180–229 | 1.13 (1.06, 1.21) | <.001 | 1.11 (1.04, 1.18) | .002 |
| ≥230 | 1.11 (1.01, 1.21) | .023 | 1.10 (1.01, 1.20) | .022 |
| HIV viral load ≥400 copies/mL | 0.88 (0.82, 0.96) | .002 | 0.90 (0.83, 0.97) | .006 |
Models were fit using linear regression with time-updated log2-transformed white blood cell count (cells/µL) as the dependent variable, from the merged HIV+ cohort at first incident cardiovascular event after age 40, loss to follow-up, last visit in 2010, or age 60. All laboratory test values were the mean of the 2 most recent visits prior to endpoint.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
aDaily or weekly marijuana use at ≥50% of visits in period 10 years prior to endpoint.
bAverage ≥0.25 packs/day in period 10 years prior to endpoint.
cHemoglobin A1C ≥6.5% or use of diabetes medications for at least 1 year in the 10 years prior to endpoint.
dSystolic blood pressure >140 or diastolic blood pressure >90, or use of antihypertensive medications for at least 1 year in the 10 years prior to endpoint.
Given the high prevalence of cigarette smoking among marijuana users, we evaluated associations between marijuana use, cigarette smoking, and CV event rates in the merged HIV+ cohort between ages 40 and 60. CV events occurred in 19.7% of heavy marijuana users compared with 8.7% of occasional and nonusers (P = .012), while demographics and HIV disease characteristics were similar at endpoint (Supplementary Table 4). Kaplan-Meier curves showed increased CV and non-AIDS–defining malignancy rates among cigarette smokers (P < .001 and P < .05; Figure 3B, left panel, and Supplementary Figure 3), while there was no significant effect on progression to AIDS, mortality, or ADM diagnoses (Supplementary Figure 3). CV event rates were also increased among marijuana users who did not smoke cigarettes compared with nonsmokers and were highest among marijuana/tobacco co-users (P < .01 and P < .001; Figure 3B, right panel). Among cigarette smokers, average cigarette packs/day was similar between marijuana users and nonusers (mean 0.88 vs 0.96 packs/day, respectively; P > .3, t test), indicating Kaplan-Meier estimates were not influenced by more cigarette smoking among marijuana/tobacco co-users. In logistic regression models adjusted for age, smoking, viral load, and 2–3 vs 0–1 traditional CV risk factors, time-updated heavy marijuana use was associated with 2.5-fold increased odds of CV events (95% confidence interval [CI], 1.2–5.3; P = .016). Effects of marijuana/tobacco co-use on increased odds of CV events were additive in this model (odds ratio, 4.8 [95% CI, 1.8–12.7]; P < .01; Table 3). The association between heavy marijuana use and CV risk was not modified by detectable HIV viral load (estimate for interaction = 0.740; P = .358). Other risk factors were not significantly associated with increased odds of CV events, except for diabetes and elevated platelet count (Supplementary Table 5), which were not significant in adjusted models. Similar to results in logistic regression models, time-varying heavy marijuana use was associated with increased incident CV events in Cox proportional hazard models adjusted for time-varying cigarette smoking, viral load, and traditional risk factors (hazard ratio, 2.16; 95% CI, 1.04–4.51; P = .039)
Table 3.
Association Between Marijuana Use, White Blood Cell Count, and Cardiovascular Events
| Cardiovascular Events | Unadjusted Models | Adjusted Model 1 | Adjusted Model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Controls (n = 370), n (%) | Cases (n = 44), n (%) | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Marijuana use | ||||||||
| None/occasional | 313 (84.6) | 30 (68.2) | 1.00 (Ref.) | — | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| Heavya | 57 (15.4) | 14 (31.8) | 2.56 (1.28, 5.13) | .008 | 2.51 (1.18, 5.31) | .016 | 2.31 (1.08, 4.93) | .031 |
| Cigarette smoking | ||||||||
| None/light | 261 (70.5) | 21 (47.7) | 1.00 (Ref.) | — | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| Moderate/heavyb | 109 (29.5) | 23 (52.3) | 2.62 (1.39, 4.94) | .007 | 2.55 (1.29, 5.04) | .007 | 2.06 (1.01, 4.17) | .045 |
| Age | ||||||||
| 40–44 | 86 (23.2) | 5 (11.4) | 1.00 (Ref.) | — | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| 45–60 | 284 (76.8) | 39 (88.6) | 2.36 (0.9, 6.18) | .080 | 3.35 (1.18, 9.57) | .024 | 3.10 (1.06, 9.05) | .039 |
| Traditional risk factorsc | ||||||||
| 0–1 | 295 (79.7) | 29 (65.9) | 1.00 (Ref.) | — | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| 2–3 | 75 (20.3) | 15 (34.1) | 2.03 (1.04, 3.99) | .039 | 2.59 (1.23, 5.43) | .012 | 2.44 (1.15, 5.18) | .020 |
| HIV viral load (copies/mL) | ||||||||
| <400 | 311 (84.1) | 27 (61.4) | 1.00 (Ref.) | — | 1.00 (Ref.) | — | 1.00 (Ref.) | — |
| ≥400 | 59 (16.0) | 17 (38.6) | 3.32 (1.70, 6.47) | <.001 | 3.73 (1.80, 7.72) | <.001 | 4.83 (2.23, 10.48) | <.001 |
| WBC count—quartiles (cells/µL) | ||||||||
| <4500 | 93 (25.1) | 5 (11.4) | 1.00 (Ref.) | — | — | — | 1.00 (Ref.) | — |
| 4500–5499 | 108 (29.2) | 9 (20.4) | 1.55 (0.5, 4.79) | .445 | — | — | 1.69 (0.51, 5.66) | .390 |
| 5500–6499 | 73 (19.7) | 8 (18.2) | 2.04 (0.64, 6.49) | .228 | — | — | 2.67 (0.77, 9.20) | .120 |
| ≥6500 | 96 (26.0) | 22 (50.0) | 4.26 (1.55, 11.73) | .005 | — | — | 4.32 (1.45, 12.89) | .009 |
Time-updated values from the merged HIV+ cohort at first incident cardiovascular event after age 40, loss to follow-up, last visit in 2010, or age 60. Models were fit using logistic regression.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; WBC, white blood cell.
aDaily or weekly marijuana use at ≥50% of visits in period 10 years prior to endpoint.
bAverage ≥0.25 packs/day in period 10 years prior to endpoint.
cNumber of cholesterol, diabetes, or hypertensive risk factors (see Materials and Methods).
In a separate multivariate logistic regression model, higher WBC count was associated with increased odds of CV events (Table 3 and Figure 3C). Time-updated WBC count in the highest quartile (≥6500 cells/µL) was associated with 4.3-fold increased odds of a CV event compared with the lowest quartile (<4500 cells/µL; 95% CI, 1.45–12.89; P = .009). When modeled as a continuous covariate, each 1000-cells/µL WBC increment was associated with 25% increased odds of a CV event independent of marijuana use, cigarette smoking, age, viral load, and traditional risk factors (95% CI, 1.05–1.49; P = .012); including WBC count as a covariate significantly improved model fit (P = .013, likelihood-ratio test).
DISCUSSION
In this longitudinal nested study of HIV+ men on ART, heavy marijuana use was associated with increased rates of CV events in men aged 40–60 independent of cigarette smoking and other known risk factors. Heavy marijuana use was also associated with elevated WBC count within the normal physiological range, an indicator of systemic inflammation that has been previously associated with increased CV disease in the general population [32, 33]. Furthermore, this elevation in WBC counts was explained by increased neutrophil counts. Elevated rates of CV events were observed among HIV+ heavy marijuana users beginning at relatively young ages (<50 years) and were further increased in marijuana/tobacco co-users compared with users of only 1 substance. Further studies are needed to determine whether the effect of long-term heavy marijuana use on CV events is related to proinflammatory effects of nontobacco smoke exposure, noninflammatory effects (eg, transient tachycardia and/or hypertension, elevated carboxyhemoglobin, hemodynamic effects, effects on platelets) [22–24], or a combination of these effects.
In healthy individuals, relatively few physical health outcomes have been clearly linked to marijuana use [4, 34–36]. However, adverse CV and cerebrovascular effects of recent marijuana use have been reported in some studies of the general population [4, 22–25]. The association between long-term heavy marijuana use and increased CV event rates has not been previously reported in HIV+ individuals. A study examining associations between substance use and coronary plaque measures in the MACS reported no strong effect of marijuana [20]. However, the study design was different from ours in several respects including the narrower definition of CV disease and less stringent criteria for defining marijuana exposure. Furthermore, we excluded HCV+ individuals and other heavy illicit drug users from our study cohort. The association between marijuana use and CV events we detected was weaker than that of other known CV risk factors including age, cigarette smoking, and viral load >400 copies/mL [14, 15, 17–19, 37]. In contrast to some prior studies [14, 15, 18, 19], we found weak or no significant associations between CV events and hypertension or cholesterol risk factors, which may reflect the younger age and high smoking prevalence in our study cohort.
The association we found between heavy marijuana use and elevated WBC has been reported in healthy young men [28], but to our knowledge has not previously been reported in HIV+ individuals. In line with previous studies of HIV-uninfected populations [28, 29, 32, 33], cigarette smoking was associated with elevated WBC count in our cohort of mostly middle-aged HIV+ men. The mechanism by which marijuana exposure increases WBC count remains poorly defined but may be related to toxic or proinflammatory effects of smoke combustion products [36, 38].
The lack of association between marijuana use and HIV disease markers, progression to AIDS, mortality, or cancer reported here is consistent with prior observational [1, 3, 6, 8, 39] and case-control [5] studies. However, other studies reported associations between marijuana use and higher CD4 counts and/or lower viral load [9], higher CD4 counts [10], and slightly lower viral loads during the first year post-seroconversion [7]. While differences between these studies likely reflect differences in study populations, selection criteria, adjustment for confounders, and length of follow-up, the results reported here are consistent with those from other studies in MSM cohorts [1, 3, 6].
Strengths of this study include more selective inclusion and exclusion criteria compared with most previous studies. The chronic HIV cohort had controlled disease characteristics at baseline (CD4 count >300 cells/µL, viral load <400 copies/mL), and individuals with heavy use of other illicit drugs and HCV coinfection were excluded. These factors have substantial effects on HIV disease markers and other outcomes and therefore could confound efforts to detect effects of marijuana exposure. A further strength was the classification of long-term heavy marijuana use over years of follow-up and strict criteria for classifying heavy and nonusing participants over this time span.
Limitations of this study include those inherent to studies of longitudinal cohorts, including the possibility that results may be specific to the MSM population recruited for the MACS and nonrandom ascertainment and dropout biases. Available measures of marijuana exposure were self-reported frequency of use with no information regarding route of administration, source, quantity, and potency. Based on available knowledge, the predominant mode of exposure was likely via marijuana smoking. These concerns are mitigated in part by the evaluation of 2 independent cohorts selected from differing calendar periods, which nonetheless show a consistent lack of association between marijuana use and HIV disease markers. Furthermore, we assessed CV events using time-updated data for marijuana and tobacco exposure during 10 years prior to endpoint.
In summary, we found no significant association between marijuana use and HIV disease progression or mortality. However, long-term heavy marijuana use was associated with increased midlife CV events in HIV+ men, independent of cigarette smoking and traditional risk factors. Furthermore, the increase in CV events was additive in marijuana/tobacco co-users. These findings suggest that clinicians should consider heavy marijuana smoking as a modifiable risk factor when optimizing preventive care, particularly for individuals already at increased risk. In recent years, the prevalence of cigarette smoking has declined, while the prevalence of heavy marijuana use has remained high or increased in HIV-infected populations [3, 10, 40]. Given that traditional factors are not sufficient to explain elevated CV risk among HIV+ individuals [13, 15, 22], the identification of heavy marijuana smoking as a nontraditional risk factor helps to explain a portion of this elevated risk and warrants further investigation in other HIV-infected and uninfected populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The data for this manuscript were obtained by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (principal investigator [PI]), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, David Ostrow, Frank J. Palella, Jr, Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (co-PI), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan.
Financial support. This work was supported by National Institutes of Health (NIH) grants to D.G. (DP1 DA028994 and R01 DA30985). A.D. was supported in part by NIH T32-AI007386. S.S.M was supported in part by Harvard Catalyst Master’s Program in Clinical and Translational Investigation funded by the NIH Clinical and Translational Science Award Program (1UL1-TR001102); biostatistical consultation was provided by the Harvard Center for AIDS Research (P30 AI060354). The MACS is funded by the National Institute of Allergy and Infectious Diseases (U01-AI35039, U01-AI35040; U01-AI35041; U01-AI35042; and UM1-AI35043), with additional cofunding from the National Cancer Institute, National Institute on Drug Abuse, and National Institute of Mental Health at the NIH. MACS data collection is also supported by UL1-TR000424 (Johns Hopkins University NIH Clinical and Translational Science Award).
Potential conflicts of interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chao C, Jacobson LP, Tashkin D et al. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend 2008; 94:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mimiaga MJ, Reisner SL, Grasso C et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health 2013; 103:1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okafor CN, Cook RL, Chen X et al. Trajectories of marijuana use among HIV-seropositive and HIV-seronegative MSM in the Multicenter AIDS Cohort Study (MACS), 1984–2013. AIDS Behav 2017; 21:1091–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): The National Academies Press, 2017. [PubMed] [Google Scholar]
- 5. Abrams DI, Hilton JF, Leiser RJ et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med 2003; 139:258–66. [DOI] [PubMed] [Google Scholar]
- 6. Di Franco MJ, Sheppard HW, Hunter DJ, Tosteson TD, Ascher MS. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men’s Health Study. Ann Epidemiol 1996; 6:283–9. [DOI] [PubMed] [Google Scholar]
- 7. Milloy MJ, Marshall B, Kerr T et al. High-intensity cannabis use associated with lower plasma human immunodeficiency virus-1 RNA viral load among recently infected people who use injection drugs. Drug Alcohol Rev 2015; 34:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okafor CN, Zhou Z, Burrell LE 2nd et al. Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am J Drug Alcohol Abuse 2016: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care 2016; 28:628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Souza G, Matson PA, Grady CD et al. Medicinal and recreational marijuana use among HIV-infected women in the Women’s Interagency HIV Study (WIHS) cohort, 1994–2010. J Acquir Immune Defic Syndr 2012; 61:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amedee AM, Nichols WA, LeCapitaine NJ et al. Chronic Δ⁹-tetrahydrocannabinol administration may not attenuate simian immunodeficiency virus disease progression in female rhesus macaques. AIDS Res Hum Retroviruses 2014; 30:1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molina PE, Amedee AM, LeCapitaine NJ et al. Modulation of gut-specific mechanisms by chronic δ(9)-tetrahydrocannabinol administration in male rhesus macaques infected with simian immunodeficiency virus: a systems biology analysis. AIDS Res Hum Retroviruses 2014; 30:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freiberg MS, So-Armah K. HIV and cardiovascular disease: we need a mechanism, and we need a plan. J Am Heart Assoc 2016; 4:e003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep 2016; 13:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin-Iguacel R, Llibre JM, Friis-Moller N. Risk of cardiovascular disease in an aging HIV population: where are we now? Curr HIV/AIDS Rep 2015; 12:375–87. [DOI] [PubMed] [Google Scholar]
- 16. Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003; 348:702–10. [DOI] [PubMed] [Google Scholar]
- 17. Currier JS, Taylor A, Boyd F et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr 2003; 33:506–12. [DOI] [PubMed] [Google Scholar]
- 18. Freiberg MS, Chang CC, Kuller LH et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaplan RC, Kingsley LA, Sharrett AR et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007; 45:1074–81. [DOI] [PubMed] [Google Scholar]
- 20. Kelly SG, Plankey M, Post WS et al. Associations between tobacco, alcohol, and drug use with coronary artery plaque among HIV-infected and uninfected men in the Multicenter AIDS Cohort Study. PLoS One 2016; 11:e0147822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monroe AK, Haberlen SA, Post WS et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. AIDS 2016; 30:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am J Cardiol 2014; 113:187–90. [DOI] [PubMed] [Google Scholar]
- 23. Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann Vasc Surg 2013; 27:996–1005. [DOI] [PubMed] [Google Scholar]
- 24. Jouanjus E, Lapeyre-Mestre M, Micallef J; French Association of the Regional Abuse and Dependence Monitoring Centres (CEIP-A) Working Group on Cannabis Complications Cannabis use: signal of increasing risk of serious cardiovascular disorders. J Am Heart Assoc 2014; 3:e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001; 103:2805–9. [DOI] [PubMed] [Google Scholar]
- 26. Detels R, Jacobson L, Margolick J et al. The multicenter AIDS cohort study, 1983 to…. Public Health 2012; 126:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 28. Friedman GD, Tekawa I, Grimm RH, Manolio T, Shannon SG, Sidney S. The leucocyte count: correlates and relationship to coronary risk factors: the CARDIA study. Int J Epidemiol 1990; 19:889–93. [DOI] [PubMed] [Google Scholar]
- 29. Sunyer J, Muñoz A, Peng Y et al. Longitudinal relation between smoking and white blood cells. Am J Epidemiol 1996; 144:734–41. [DOI] [PubMed] [Google Scholar]
- 30. Hessol NA, Kalinowski A, Benning L et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis 2007; 44:287–94. [DOI] [PubMed] [Google Scholar]
- 31. Seaberg EC, Wiley D, Martinez-Maza O et al. Cancer incidence in the Multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer 2010; 116:5507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 2004; 44:1945–56. [DOI] [PubMed] [Google Scholar]
- 33. Twig G, Afek A, Shamiss A et al. White blood cell count and the risk for coronary artery disease in young adults. PLoS One 2012; 7:e47183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall W, Weier M. Assessing the public health impacts of legalizing recreational cannabis use in the USA. Clin Pharmacol Ther 2015; 97:607–15. [DOI] [PubMed] [Google Scholar]
- 35. Meier MH, Caspi A, Cerdá M et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiatry 2016; 73:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med 2014; 371:879. [DOI] [PubMed] [Google Scholar]
- 37. Post WS, Budoff M, Kingsley L et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei B, Alwis KU, Li Z et al. Urinary concentrations of PAH and VOC metabolites in marijuana users. Environ Int 2016; 88:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcellin F, Lions C, Rosenthal E et al. ; HEPAVIH ANRS CO13 Study Group No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort). Drug Alcohol Rev 2017; 36:227–38. [DOI] [PubMed] [Google Scholar]
- 40. Akhtar-Khaleel WZ, Cook RL, Shoptaw S et al. Long-term cigarette smoking trajectories among HIV-seropositive and seronegative MSM in the Multicenter AIDS Cohort Study. AIDS Behav 2016; 20:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.