We describe 13 periprosthetic joint infections (PJIs) caused by Propionibacterium avidum. The majority were hip-associated and occurred after hip arthroplasty surgery using an anterior surgical approach. Upon phylogenetic analysis, isolated strains clustered with P. avidum PJI strains from Sweden.
Keywords: Propionibacterium avidum, Cutibacterium avidum, periprosthetic joint infection, hip, whole-genome sequencing
Abstract
Background
Propionibacteria are important members of the human skin microbiota, but are also opportunistic pathogens associated with periprosthetic joint infection (PJI). While the role of Propionibacterium acnes in PJI has been widely described, insight into the capacity of Propionibacterium avidum to cause PJI is limited.
Methods
An unusual cluster of 4 hip PJIs caused by P. avidum in one orthopedic center in 2015 prompted us to retrospectively identify and analyze clinical data related to previous P. avidum PJI cases (1997–2015). We also characterized the hemolytic and biofilm-producing capacity of our 4 clinical P. avidum strains isolated in 2015, and investigated their phylogenetic relationships by whole-genome sequencing.
Results
We retrospectively identified 13 P. avidum PJIs, with the majority being hip-related infections (n = 11). Preoperative synovial fluid cultures were P. avidum positive in 63.6% of cases. Six of 12 patients (50%) with available case histories were treated with an exchange of the prosthesis. In all but 1 of the 6 patients treated with debridement-retention of the prosthesis, treatment failed, thus requiring a 2-stage revision. The isolated P. avidum strains showed a more pronounced hemolytic activity, but a similar biofilm-forming ability when compared to P. acnes. Whole-genome sequencing identified 2 phylogenetic clusters highly related to P. avidum PJI strains isolated in Sweden.
Conclusions
We describe the largest series of P. avidum PJI predominantly located in the hip. Phylogenetic similarity of our P. avidum strains to PJI strains isolated elsewhere suggests that these invasive lineages may be common.
Periprosthetic joint infections (PJIs) following prosthesis implantation result in high morbidity [1]. The incidence is rising due to the increasing life span of our population, resulting in high numbers of degenerative disorders requiring joint replacements. The most commonly isolated microorganisms in PJI are staphylococci, followed by streptococci, enterococci, gram-negative bacteria, and anaerobes [2].
Propionibacteria are gram-positive anaerobic bacteria and integral components of the normal human skin microbiota, but also cause opportunistic infections including PJI [3]. Of the 3 members of the cutaneous group of human propionibacteria, Propionibacterium acnes is by far the most frequent cause of PJI. Individual case reports of soft tissue and medical device–related infections due to Propionibacterium avidum (recently proposed as Cutibacterium avidum) and, even less commonly, Propionibacterium granulosum, have been described [4–11].
In 2015, we identified and treated a cluster of 4 patients with P. avidum PJIs occurring within a single orthopedic center. Due to this unusual observation, we decided to conduct a wider, and much needed, epidemiological and clinical assessment of patients with P. avidum PJI for potential risk factors and treatment outcomes. We also investigated P. avidum PJI strains for key virulence properties, and performed whole-genome analysis to examine their phylogenetic relationship to one another, as well as a small number of previously sequenced strains isolated from PJIs.
MATERIALS AND METHODS
Patients and Study Design
The Department of Orthopedics of the University Hospital Balgrist is a specialized tertiary care hospital with 120 beds. In 2015, approximately 5000 surgical procedures were performed, of which 326 procedures were primary hip arthroplasties. In 2015, we prospectively identified 4 patients with a hip P. avidum PJI. Patients’ clinical and epidemiological history was retrieved from the prospectively managed database on all infections from the infectious diseases consulting service, and from the hospital clinical information system. We also conducted a retrospective analysis of the microbiological laboratory database of the Institute of Medical Microbiology, University of Zurich, to identify further P. avidum infections at the University Hospital Balgrist (1997–2014). The clinical presentation of the patients with P. avidum isolated in tissue, synovial fluid, or sonicated fluid was reviewed. Infection was differentiated from contamination when P. avidum grew in at least 2 biopsy or sonication fluid samples [12]. We calculated the in-hospital incidence of P. avidum PJI as the number of P. avidum PJIs divided by number of total surgeries per year performed at the clinic Balgrist, taking into account the increasing number of primary implantation of arthroplasties or revision surgeries in recent years. Basic characteristics, clinical presentation at the time of diagnosis, diagnostic steps according to Musculoskeletal Infection Society (MSIS) criteria [13], surgical and antibiotic treatment, and outcome of PJI were analyzed.
Potential risk factors such as an association with a particular surgeon, changing the surgical incision approach, antibiotic prophylaxis, or patient body mass index (BMI) were studied. The surgical incision approach used in hip arthroplasty surgeries was changed from lateral to anterior in 2006. As a result, infection rate before and after introduction of this change was investigated to identify whether the surgical approach used was a potential risk factor. Perioperative intravenous antibiotic prophylaxis with cefuroxime 1.5 g was routinely administered 30–60 minutes prior to skin incision, followed by 2 additional doses. Skin was disinfected 3 times with a povidone-iodine solution (Betaseptic) throughout the entire study period.
The cantonal ethic authority of Zurich, Switzerland, approved the study protocol (Kantonale Ethikkommission numbers 2016-00145 and 2015-0357).
Bacteriology
Microbiological techniques and standard biochemical methods for the detection and identification of Propionibacterium species were performed as previously described [14]. In short, incubation time was 7 days for synovial fluid and sonication fluid and 10 days for tissue biopsies. Diagnosis of P. avidum included a positive reaction for catalase, CAMP factor, and esculin, and a negative test for indole to distinguish from other Propionibacterium species [15]. From 2012, strains were identified using matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Bruker MALDI Biotyper in combination with research-use-only versions of the MALDI Biotyper software package (version 3.0) and the reference database version 3.3.1.0 (4613 entries) or later database versions. When ordered by the infectious diseases specialist, antibiotic susceptibility testing was performed using Etest strips (bioMérieux) on Brucella agar plates (McFarland 0.5) cultivated for 48 hours.
Bacterial Strains
A total of 5 strains isolated from 4 patients in 2015 (clinical isolates [CIs] 828, 853, 878, 882, 855) were analyzed. We also examined 4 randomly selected clinical P. acnes strains (CI803, CI805, CI806, CI820) isolated from patients with hip PJI from our bacterial biobank for comparison of hemolytic activity. To compare P. avidum with P. acnes for biofilm formation, we used P. acnes strain ATCC 11827, which is known to adhere to foreign materials and produce biofilms in vivo [16].
Hemolysis and Biofilm Formation
Zones of hemolysis were measured (in mm) on Brucella agar plates. Biofilm formation was measured in vitro using a microtiter plate assay [17]. The results were compared to those obtained with the P. acnes strain ATCC 11827. The ratio of biofilm mass over total biomass of P. acnes and P. avidum, respectively, were calculated.
Whole-Genome Sequencing and Phylogenetic Analyses
Genomic DNA was extracted from P. avidum cultures using a QIAamp DNA Micro Kit (Qiagen) according to the manufacturer’s instructions. Sequencing libraries were prepared using a NexteraXT library kit (Illumina), pooled, and then sequenced on an Illumina MiSeq. Paired-end sequencing reads of 300 bp were assembled using Mimicking Intelligent Read Assembly version 4.0.2 [18], and the assemblies were manually refined using Consed and Gap5 [19]. Core genomic regions shared by all analyzed P. avidum strains were compared as described [20] using Nucmer [21]. The core single-nucleotide polymorphism (SNP) sites were concatenated and imported into MEGA5 [22] for phylogenetic tree analysis.
Comparison of the exopolysaccharide (EPS) encoding locus in P. avidum strains was performed using Nucmer; the 35-kb EPS region previously described in the strain T13 [5] was aligned against all P. avidum genomes. Homologous regions identified in each strain were manually assembled with Gap5 [19] using the T13 region as reference, and the resulting scaffolds were then aligned with MAFFT [23] for phylogenetic analysis in MEGA7.
Statistical Analysis
Wilcoxon rank-sum tests were used to compare continuous variables and Fisher exact test to compare categorical variables.
RESULTS
Cohort of Propionibacterium avidum Periprosthetic Joint Infection Cases
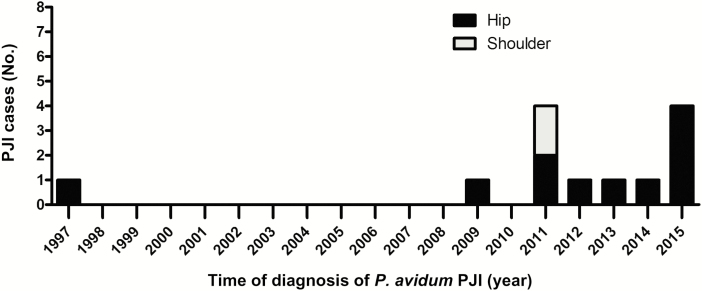
In 2015, we identified a cluster of 4 P. avidum PJIs that occurred after primary hip arthroplasty surgery, which was conducted via an anterior approach. Retrospective analysis of our microbiological database identified an additional 9 patients with a PJI due to P. avidum, and 2 patients with osteomyelitis and a soft tissue infection due to P. avidum (all between January 1997 and December 2014). Of the 13 PJIs (4 prospectively, 9 retrospectively), 11 were hip related (84.6%) and 2 shoulder related (Figure 1).
Figure 1.
Incidence and localization of Propionibacterium avidum prosthetic joint infection (PJI) among all patients treated at the University Hospital Balgrist at the time of diagnosis (1997 and 2015).
Arthroplasty surgery was performed by 13 surgical teams in 6 operating theaters excluding an association between postsurgical infection and a specific surgeon or team. Only 8 patients had surgery on the same joint preceding infection at the University Hospital Balgrist, labeled as in-house acquired infection. Among them, hip arthroplasties were more affected than shoulder (7 hip infections of a total of 6860 surgeries [0.10%] vs 1 shoulder infection of a total of 1963 surgeries [0.04%]) between 1997 and 2015. For hip arthroplasty, the standard surgical incision approach changed from lateral to anterior in 2006 within our clinic. We observed a lower ratio of postoperative infections compared to the number of total surgeries of 0.04% (1/2262) during the time period before changing the incision approach (1997–2005), as compared to 0.13% (6/4598) after the new approach was adopted (2006–2015). The risk for a postsurgical infection was 2.95 times higher in the latter time period (relative risk, 2.95 [95% confidence interval, .36–24.5]; P = .44). Except for the additional routine use of tranexamic acid since 2005, no hospital hygiene procedures (type of irrigation, skin cleansing, ventilation system) had been changed during the observed period. All patients routinely received perioperative antibiotic prophylaxis with cefuroxime.
One of the patients had a P. avidum PJI in 1997. Because the medical history records of this patient no longer exist, this patient was excluded from further analysis. Thus, we describe in detail a cohort of 12 patients treated for a P. avidum PJI at the same orthopedic center.
Clinical Characteristics of Patients With Propionibacterium avidum Periprosthetic Joint Infection
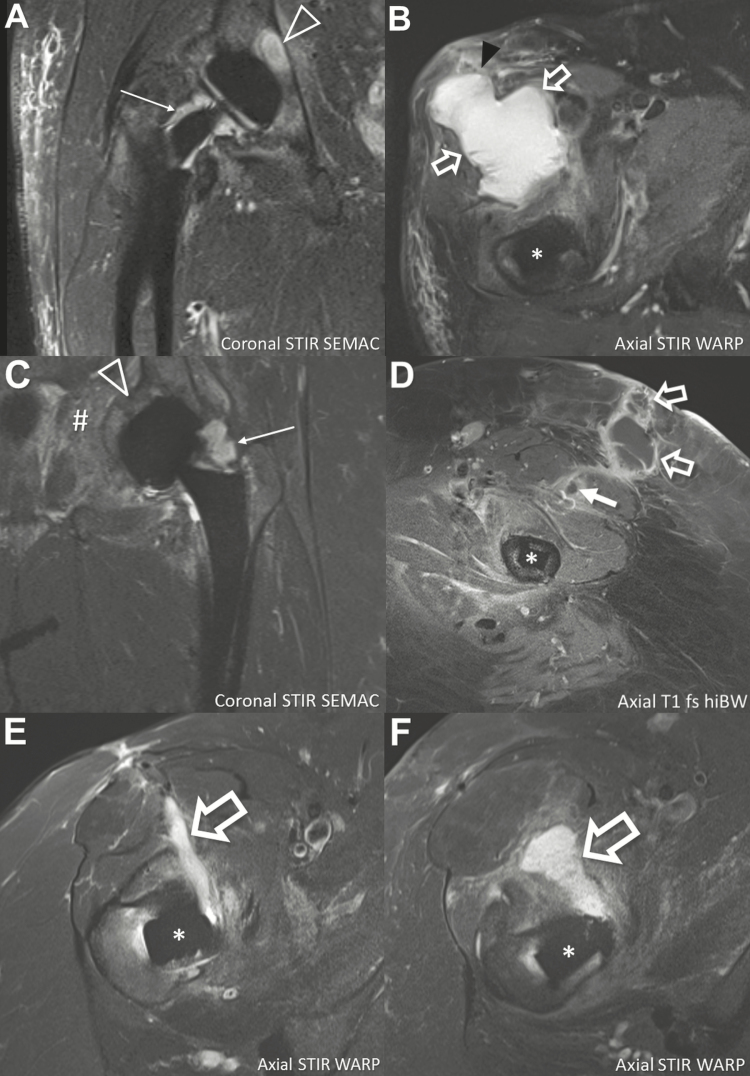
The median age of our 12 patients (7 females) was 61 years at the time of diagnosis. Nine of 12 patients (75%) with a P. avidum PJI were obese with a BMI >30 kg/m2 (Table 1 and Supplementary Table 1). All patients presented with pain, 6 with wound secretion or sinus tract formation, and 4 with local signs of inflammation, such as skin erythema and swelling. Fever was reported in 4 patients. In 4 patients with a preoperative magnetic resonance imaging, periprosthetic soft tissue abscess and joint effusion were observed, with communication between the abscess and the joint effusion (Figure 2; Supplementary Table 1); a further 2 cases (numbers 2 and 4) showed osteomyelitis of the acetabulum. The majority of infections (75%) were delayed (ie, presenting ≥1 month after joint surgery).
Table 1.
Clinical Characteristics of 12 Patients With Prosthetic Joint Infection Caused by Propionibacterium avidum, Either as a Monomicrobial (n = 8) or Part of a Polymicrobial (n = 4) Infection, at the Time of Initial Surgical Treatment of Infection at the University Hospital Balgrist
| Characteristic | No. (%) |
|---|---|
| Age, y, median (range) | 61 (45–81) |
| Female sex | 7 (58.3) |
| BMI, kg/m2, median (range) | 34.0 (27.9–40.6) |
| Obesity grade 1 (>30–<35) | 3 (25) |
| Obesity grade 2 (>35–<40) | 5 (41.7) |
| Obesity grade 3 (>40) | 1 (8.3) |
| Underlying joint disorder for arthroplasty | |
| Degenerative | 9 (75) |
| Trauma | 2 (16.7) |
| “Head necrosis” | 1 (8.3) |
| Place of last arthroplasty surgery before infection | |
| University Hospital of Balgrist | 7 (58.3) |
| Other hospital | 5 (41.7) |
| Localization of infection | 12 |
| Hip PJI | 10 (83.3) |
| Shoulder PJI | 2 (16.7) |
| Signs and symptoms | |
| Pain | 12 (100) |
| Wound secretion or sinus tract | 6 (50) |
| Swelling, skin erythema | 4 (33.3) |
| Fever | 4 (33.3) |
| Time to diagnosis of infection after last surgical revision of arthroplastya | |
| Median (range), wk | 13.1 (2.3–63.2) |
| Time to initial septic surgery after last surgical revision of arthroplasty | |
| Median (range), wk | 30.2 (2.9–100.3) |
| Surgical treatment | |
| DAIR including partial exchange of prosthesis | 5 (41.6) |
| 1-stage exchange | 2 (16.7) |
| 2-stage exchange | 4 (33.3) |
| Antibiotic treatment only | 1 (8.3) |
| Per oral antibiotic treatment after initial IV treatment | |
| Clindamycin | 4 (33.3) |
| Levofloxacin/rifampin | 4 (33.3) |
| Ciprofloxacin/rifampin | 2 (16.7) |
| Clindamycin/rifampin | 2 (16.7) |
Abbreviations: BMI, body mass index; DAIR, debridement-antibiotics-irrigation-retention; IV, intravenous; PJI, prosthetic joint infection.
aTime to first microbiological diagnosis of P. avidum (either preoperative synovial puncture or intraoperative tissue samples) with confirmation of infection due to Musculoskeletal Infection Society criteria [13].
Figure 2.
Spectrum of magnetic resonance imaging findings of 4 patients with Propionibacterium avidum prosthetic joint infections. A and B, An 81-year-old man (patient 4; Table 2) 2 years after total hip arthroplasty of the right hip. Coronal image demonstrates joint effusion (thin arrow) adjacent to the neck of the prosthesis and osteomyelitis (triangle) of the acetabulum. Axial image in the same patient shows large soft tissue abscess (outlined arrows) anterior to the hip joint breaking through the superficial muscle fascia (black arrowhead). C and D, A 53-year-old woman (patient 2) 6 months after total hip arthroplasty (THA) of the left hip. Coronal image demonstrates joint effusion (thin arrow) and extensive bone marrow edema in the acetabulum consistent with osteomyelitis (triangle). Furthermore, extension of the infection into the soft tissue of the pelvis is seen (#). Axial image after intravenous gadolinium administration at the level of the middle third of the femoral shaft shows anterior intramuscular abscess (solid arrow) and epifascial abscess (outlined arrows) extending to the dermis, with typical peripheral contrast enhancement. E, A 59-year-old woman (patient 1) 2 years after THA of the right hip with soft tissue abscess (outlined arrow) along the anterior surgical approach, extending to the femoral neck. F, A 64-year-old woman (patient 3) 19 days after THA of the right hip with soft tissue abscess (outlined arrow) along the anterior surgical approach, extending to the femoral neck. *Femoral component of THA. Abbreviations: fs, fat-saturated; hiBW, high readout bandwidth; SEMAC, slice-encoding for metal artifact correction; STIR, short tau inversion recovery; T1, T1-weighted; WARP, optimized inversion pulse.
Diagnosis was confirmed preoperatively in 5 patients with ≥3 minor criteria according to MSIS PJI definition criteria [13]. Among the 11 cases in which a preoperative puncture of synovial fluid had been performed, P. avidum was cultivated from 7 (63.6%) of the patients. Five of these patients (71.4%) showed elevated leukocytes >3000 (range, 8000–308000) cells/µL in the synovial fluid cell, and 6 (85.7%) showed ≥80% neutrophil granulocytes (Table 2). PJI was found to be monomicrobial in 8 patients and polymicrobial in 4 patients (Table 2).
Table 2.
Diagnostic Characteristics of 12 Patients With Prosthetic Joint Infection Caused by Propionibacterium avidum at Time of Septic Surgery at the University Hospital Balgrist
| No. | Age, y, Sex | PJI Site | Preoperative | Intraoperative | P. avidum Strain | Polymicrobial Infection (Other Pathogen) | |||
|---|---|---|---|---|---|---|---|---|---|
| Blood | Synovial Fluid | Tissue Biopsies | Sonication Fluid | ||||||
| CRP (mg/l) , ESR (mm/h) | P. avidum Growth | Leukocyte Count (% Neutrophils) | P. avidum Positive/ Total Taken | Positive P. avidum, CFU/mL | |||||
| Prospective (cluster in 2015) | |||||||||
| 1 | 59, F | Hip | 4.9, 31 | Negative | ND (80) | 4/6 | ≥100 | CI878 | No |
| 2 | 53, F | Hip | 200, 103 | Positive | 308000 (80) | 2/5 | ≥100 | CI882 | No |
| 3 | 64, F | Hip | 74, 82 | Positive | 48800 (80) | 5/5 | 60 | CI853, CI855 | No |
| 4 | 81, M | Hip | 62.7, 82 | Positive | 248000 (80) | 4/6 | In broth | CI828 | Yes (S. aureus) |
| Retrospective (1997–2014) | |||||||||
| 5 | 57, F | Hip | 7.6, 52 | Positive | 8800 (80) | 2/8 | 20 | NA | No |
| 6 | 72, F | Hip | 176, ND | Positive | ND | 3/4 | NA | NA | Yes (F. magna) |
| 7 | 45, F | Hip | 17, 21 | Positive | 38300 (80) | 2/3 | Negative | NA | No |
| 8 | 56, M | Hip | 15.2, 54 | Negative | 2500 (ND) | 2/4 | Negative | NA | Yes (F. magna) |
| 9 | 65, F | Hip | 14, 37 | Negative | ND | 1/5 | ≥100 | NA | No |
| 10 | 69, M | Shoulder | 119, ND | Positive | ND | 3/3 | NA | NA | No |
| 11 | 56, M | Shoulder | 38/ND | Negative | ND | 1/4 | ≥100 | NA | No |
| 12 | 63, F | Hip | 95/ND | ND | ND | 0/7a | 20 and 27 | NA | Yes (S. epidermidis) |
Abbreviations: CFU, colony-forming units; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; F, female; F. magna, Finegoldia magna; M, male; NA, not available; ND, not done (possible reasons include dry aspirate or hemolytic sample); P. avidum, Propionibacterium avidum; PJI, prosthetic joint infection; S. aureus, Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis.
aDiagnosis of P. avidum PJI was based on clinical presentation of sinus tract as well as previous growth of P. avidum in another hospital.
All but 1 of the 12 P. avidum strains were susceptible to clindamycin, levofloxacin, and rifampin (Table 3) [24]. In 1 patient, we found that the initially isolated strain was resistant to clindamycin and 6 months later, after levofloxacin and rifampin treatment, also resistant to ciprofloxacin and levofloxacin. Following surgical debridement or exchange of the prosthesis, all patients were treated intravenously for approximately 2 weeks with a β-lactam (or vancomycin in case of allergies), followed by an oral therapy. Oral treatment and its duration was chosen according to minimum inhibitory concentration values and the surgical approach adopted, respectively. Thus, antibiotics were given for a total of 3 months for debridement-antibiotics-irrigation-retention (DAIR) as well as 1-stage exchange and, for 6 weeks, for 2-stage exchange.
Table 3.
Median Minimum Inhibitory Concentration and Resistance Pattern of 12 Propionibacterium avidum Strains From Prosthetic Joint Infections Using European Committee on Antimicrobial Susceptibility Testing Breakpoints for Gram-Positive Anaerobes
| Antibiotic | Samples, No. | MIC50, mg/L (range) | MIC Breakpoint, mg/L | ||
|---|---|---|---|---|---|
| EUCAST Breakpoint | Resistant, No. (%) | ||||
| Sensitive | Resistant | ||||
| Penicillina | 5 | 0.064 (0.03–0.125) | ≤0.25 | >0.5 | 0 (0) |
| Clindamycina | 12 | 0.032 (<0.016 to >256) | ≤4 | >4 | 1 (8.3) |
| Ciprofloxacinb | 12 | 0.25 (0.19 to >32) | ≤0.5 | >1 | 1 (8.3) |
| Levofloxacinb | 10 | 0.125 (0.094 to >32) | ≤1 | >2 | 1 (10) |
| Rifampin | 12 | 0.004 (0.003–0.008) | … | … | |
| Cefuroximeb | 4 | 0.38 (0.38–0.5) | ≤4 | >8 | 0 (0) |
Abbreviations: EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC50, median minimum inhibitory concentration.
aEUCAST clinical breakpoints (Table version 6.0, valid from 1 January 2016) are shown for gram-positive anaerobes.
bIf no clinical breakpoints for gram-positive anaerobes exist, breakpoints from pharmacokinetic/pharmacodynamic data (non-species related) were taken [24].
Six of 12 patients were primarily treated with a complete 1- or 2-stage exchange of the prosthesis, 5 with a DAIR procedure, and 1 with antibiotics alone. In all but 1 of the 6 patients treated with either an initial DAIR or antibiotic alone, treatment failed necessitating a 2-stage revision of the prosthesis with a good clinical outcome.
Phenotypic Analysis of Propionibacterium avidum Strains From a Cluster of Periprosthetic Joint Infection
Hemolysis and Biofilm Production
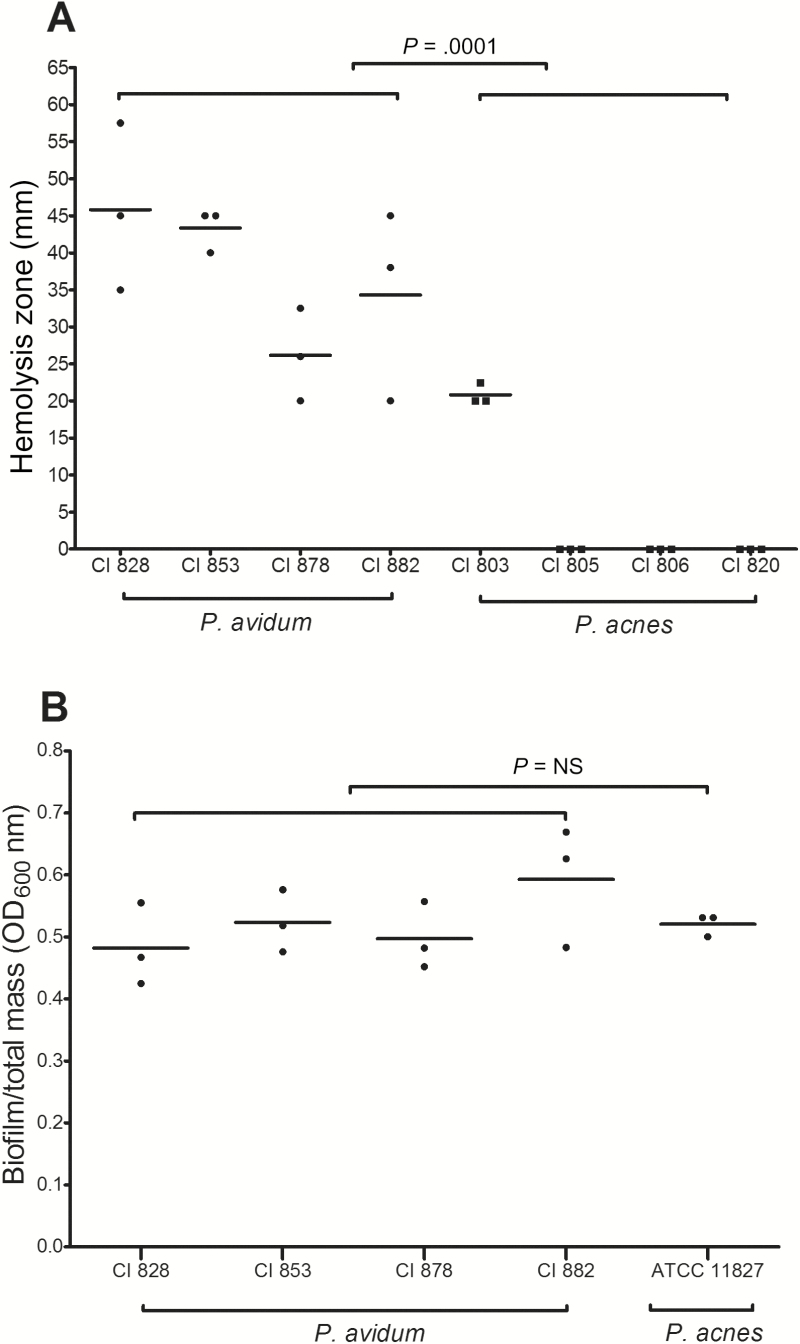
The P. avidum strains isolated from a cluster of PJIs in our clinic in 2015 showed a strong hemolytic reaction, which was significantly greater than that of the P. acnes strains isolated from other hip PJIs (Figure 3A; P = .01). The ratio of biofilm to total mass of the P. avidum strains was, however, not significantly different from the P. acnes strain ATCC 11827 (Figure 3B).
Figure 3.
Phenotypic characterization of Propionibacterium avidum strains isolated in 2015 from hip prosthetic joint infections (PJIs) (clinical isolates 828, 853, 878, 882). A, Hemolysis activity of 4 P. avidum strains compared to 4 Propionibacterium acnes strains on Brucella agar zone diameter (mm) measured (unpaired t test, Mann-Whitney test) using serial dilution from a starting inoculum of 2–5 × 108 colony-forming units/mL. B, Biofilm formation of 4 P. avidum PJI strains compared to the P. acnes biofilm strain ATCC 11827 using a static biofilm assay (unpaired t test, Mann-Whitney test). Data shown are from 3 experiments done in technical triplicates. Abbreviations: CI, clinical isolate; OD, optical density.
Whole-Genome Sequencing and Phylogenetic Analysis
In 1 of the 4 patients presenting with a PJI in 2015, we detected 2 phenotypically different P. avidum strains (CI853 and CI855). To characterize the genetic diversity of our 5 PJI P. avidum strains, and therefore determine their relatedness, we performed whole-genome sequencing followed by phylogenetic analysis; publicly available P. avidum genomes from 2 PJIs (T13, T14, T15) and 5 various non-PJI sources (MJR7694, 44067, ATCC25577, TM16, UCD/PD2) were also included for comparison. Illumina sequencing generated draft genome sequences that consisted of 69–106 contigs depending on the strain considered (Table 4). All 5 strains had almost identical G + C content (63.4%–63.5%) and genome sizes ranging from 2.48–2.54 Mbp (Table 4). A total of 172 genomic regions totaling 2.1 Mbp were found to be shared among the 13 P. avidum strains.
Table 4.
Genome Assembly Characteristics of Propionibacterium avidum Prosthetic Joint Infection Isolates Compared in This Study
| Strain | Accessiona | Assembly Coverage | Size, Mb | Contigs | Genes | Proteins | GC% |
|---|---|---|---|---|---|---|---|
| ATCC 25577 | AGBA01 | 29× | 2.55 | 7 | 2290 | 2200 | 63.3 |
| 44067 | CP005287.1 | 375× | 2.53 | 1 | 2297 | 2184 | 63.5 |
| CI853 | NBIS00000000 | 114× | 2.53 | 105 | 2394 | 2224 | 63.5 |
| CI855 | NBIR00000000 | 219× | 2.54 | 106 | 2406 | 2227 | 63.5 |
| CI828 | NBIQ00000000 | 249× | 2.48 | 76 | 2313 | 2152 | 63.4 |
| CI882 | NBIP00000000 | 327× | 2.50 | 69 | 2360 | 2182 | 63.4 |
| CI878 | NBIO00000000 | 190× | 2.54 | 89 | 2422 | 2251 | 63.4 |
| MJR7694 | LRVD01 | 76× | 2.47 | 16 | 2235 | 2118 | 63.4 |
| TM16 | AOUA01 | 34× | 2.54 | 420 | 2441 | 2133 | 63.4 |
| UCD-PD2 | LYSN01 | 76× | 2.67 | 51 | 2442 | 2304 | 63.4 |
| T13 | LLJH01 | 50× | 2.46 | 15 | 2223 | 2109 | 63.4 |
| T15 | LLJJ01 | 50× | 2.46 | 14 | 2223 | 2110 | 63.4 |
| T14 | LLJI01 | 50× | 2.52 | 9 | 2290 | 2184 | 63.4 |
Abbreviation: GC, G + C content.
aNational Center for Biotechnology Information whole-genome sequencing accession number. For 44067, the NCBI nucleotide accession number is shown.
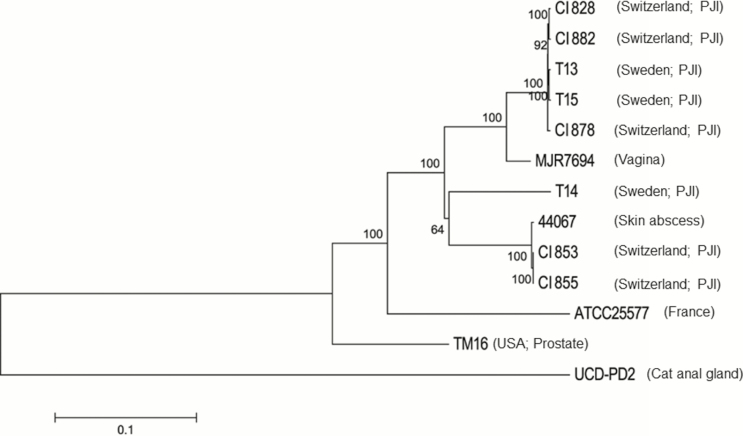
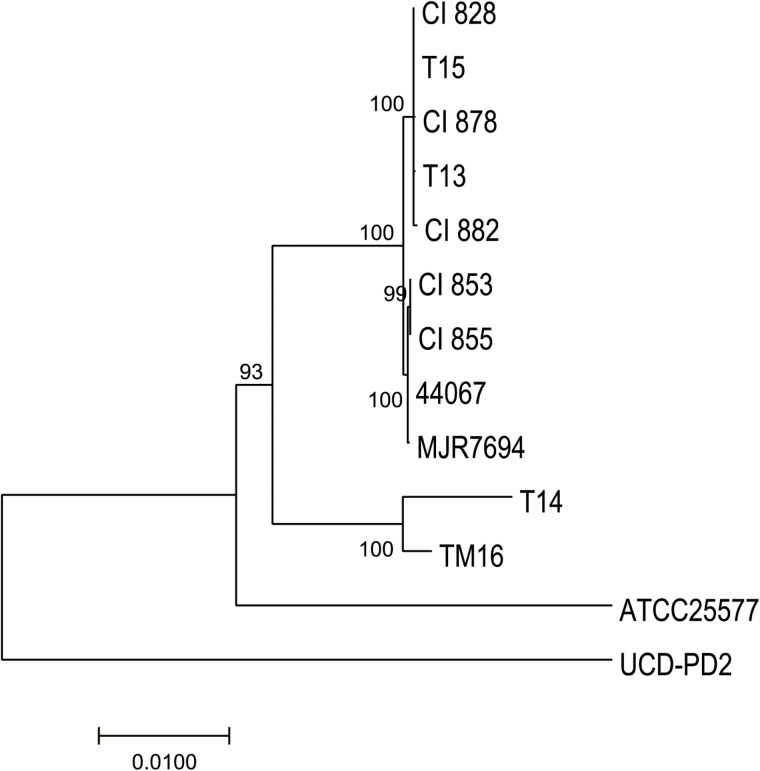
Comparison of the core genomic regions showed that CI853 and CI855, which were isolated from the same patient, differed only by a single SNP, and CI828, CI882 and CI878, which were isolated from 3 different patients, similarly displayed a very high degree of relatedness to each other with 99.6%–99.8% identity at SNP sites (Figure 4). CI828, CI878 and CI882 also clustered together with the recently described P. avidum T13 and T15 strains recovered from patients with a hip PJI in Sweden. CI853 and CI855 were found to be similar to a further PJI strain also isolated in Sweden (T14) (89.9% SNP identity). Compared with an average identity of 55.1% among the 5 non-PJI strains at these sites, this high degree of similarity suggests that the isolates within each group may be clonal. All the sequenced strains harbored a gene cluster encoding EPS synthesis as previously described (Figure 5) [5], as well as genes involved in survival, fitness, and defense.
Figure 4.
A neighbor-joining phylogenetic tree constructed on the single-nucleotide polymorphisms (SNPs) in the core genome regions of the Propionibacterium avidum strains, including 5 P. avidum strains isolated from 4 patients with prosthetic joint infection (PJI) described in this study (clinical isolates 828, 853, 855, 878, 882), and previously sequenced strains (T13, T14, T15, ATCC25577, UCD-PD2, MJR7694, 44067, and TM16). Horizontal bar represents p-distances based on substitutions at the SNP sites. Bootstrap values are based on 500 replicates.
Figure 5.
A neighbor-joining phylogenetic tree constructed based on nucleotide differences within the Propionibacterium avidum–specific exopolysaccharide-encoding genomic island of 13 sequenced P. avidum strains. Horizontal bar represents p-distances based on substitutions at 16217 positions aligned across all strains. Bootstrap values are based on 500 replicates.
DISCUSSION
This is the first clinical study describing a large cohort of patients with PJI due to the skin commensal P. avidum. We observed that P. avidum was predominantly diagnosed in association with hip arthroplasty (85%). This is in contrast to published data on P. acnes, which mainly causes shoulder PJI infections (prosthesis and postarthroscopy related) but rarely hip infections [14]. We interpreted these results as a consequence of preferential skin colonization by P. avidum of sweat glands in the moist groin and perianal regions [25] and contrast to relatively low abundance on the face [26]. As we found P. avidum PJI predominantly in the hip of obese patients, we hypothesize that this association results from P. avidum overgrowth in the moist skin folds typically found in the groin region of obese individuals. This may facilitate their entry into the surgical wound, even after preoperative skin antisepsis, leading to downstream infection.
Synovial fluid analysis showed a high number of leukocytes and a positive culture for P. avidum in 67% of cases. Thus, in contrast to P. acnes, preoperative differentiation of septic from aseptic loosening of the prosthesis was much easier [27]. Debridement in conjunction with antibiotics was not sufficient to treat the infections. All 5 infections with a delayed presentation, and treated with debridement or antibiotics alone, required a subsequent 2-stage exchange of the prosthesis. This indicates that the surgical treatment approach in P. avidum PJI should be the same as that described for other bacterial species [1]—that is, when duration of symptoms is >3 weeks, a sinus tract is present, or the implant is already loose, 1- or 2-stage revision of the arthroplasty is required for a successful treatment outcome.
We observed obesity as a potential risk factor for P. avidum PJI after hip arthroplasty. A high BMI was noted in all but 1 patient, which is in line with a recent publication describing hip PJIs due to P. avidum [5]. Overall, obesity with a BMI >35 kg/m2 or >100 kg is a risk for orthopedic infections in general [28]. Most of our infections were associated with hip arthroplasties. We did not observe a significant increase in infection when the incision approach was changed, in line with the study of Ilchmann et al [29]; however, since the combined numbers of both studies are small, it is yet unclear whether the incision approach for hip arthroplasty represents a risk factor for PJI. In general, better characterization between P. acnes and P. avidum strains may be an important factor; however, at our institution, except for the introduction of MALDI in 2012 diagnostic methods, remained the same.
While the pathogenicity of P. avidum infections is poorly understood, we found a more pronounced hemolytic activity by P. avidum strains as compared to P. acnes, in line with reports in the literature [30]. Underlying its potential importance, hemolysis was recently described as a “clinical marker” to better distinguish orthopedic infections with P. acnes vs a contaminated culture, although this association is controversial [31,32]. A potentially key virulence trait of P. avidum in relation to PJIs is its ability to form biofilms on medical implants. The extracellular polymeric matrix of such biofilms is different from that produced by P. acnes biofilms [33]. In addition, P. avidum produces a capsule that is unique and has not been described for P. acnes nor P. granulosum [33]. This capsule may protect against phagocytosis. In the genomes of all 5 P. avidum isolates described in this study, we identified homologs of an EPS-encoding island previously found to be present in P. avidum, but not other cutaneous propionibacteria, which may be potentially important in adherence. This island is flanked by transfer RNA genes, suggesting acquisition by horizontal gene transfer. One PJI isolate, T14, contains an EPS island that clusters with that from the prostate-derived isolate TM16 [34] upon phylogenetic analysis. Thus, expression of this locus may be a general virulence determinant not just related to PJI.
Phylogenetic analysis showed that our sequenced strains formed 2 distinct clusters along with strains isolated from patients with hip PJIs in Sweden [5], and non-PJI strains isolated from the vaginal microbiota [25] and a skin abscess [36]. Currently a detailed understanding of the population genetic structure of P. avidum is lacking. We speculate that major phylogenetic divisions and clonal lineages of P. avidum with varying disease potential or ecological specialization occur, similar to that observed with P. acnes phylogroups I, II, and III recently proposed as distinct bacterial subspecies [37]. This is tentatively supported by the observation of at least 2 distinct serotypes of P. avidum with a cell wall composition that mirrors P. acnes types I and II [38, 39].
In conclusion, this is the first description of a large series of PJI caused by P. avidum. We show that the skin commensal P. avidum predominantly caused delayed PJIs, which were only resolved by 2-stage revisions. We did not identify a specific risk factor for the increasing number of P. avidum PJIs in recent years. Further studies evaluating skin colonization with P. avidum might help to select patients at higher risk for invasive P. avidum infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank Melissa Kaspar of the University of Zurich; Bettina Schulthess, Thomas Klein, and the technicians of the Institute of Medical Microbiology of the University of Zurich for expert help and assistance; Sabrina Catanzaro of the University Hospital Balgrist for assistance regarding data acquisition; and Marianne Kästli, Zentrallabor Zurich, for providing data of synovial fluid cell counts.
Financial support. This work was supported by the US National Institutes of Health (grant number R01GM099530 to H. L.). A. S. Z. was supported by the Swiss National Foundation (grant number 310030_146295). Y. A. was supported by the academic career program “Filling the Gap” of the Medical Faculty of the University of Zurich. A. M. was supported by a grant awarded to Professor Tony Bjourson from the European Union Regional Development Fund European Union Sustainable Competitiveness Programme for Northern Ireland, Northern Ireland Public Health Agency (Health and Social Care Research & Development), and Ulster University.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351:1645–54. [DOI] [PubMed] [Google Scholar]
- 2. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014; 27:302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 2014; 27:419–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loureiro-Amigo J, Pons S, Sierra M, Meije Y. Prosthetic valve with infective endocarditis caused by Propionibacterium avidum. A case report. Enferm Infecc Microbiol Clin 2017; 35:196–7. [DOI] [PubMed] [Google Scholar]
- 5. Wildeman P, Bruggemann H, Scholz CF, Leimbach A, Soderquist B. Propionibacterium avidum as an etiological agent of prosthetic hip joint infection. PloS One 2016; 11:e0158164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kritikos A, Pagin M, Borens O, Voide C, Orasch C. Identification of Propionibacterium avidum from a breast abscess: an overlooked etiology of clinically significant infections. New Microbes New Infect 2015; 4:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Million M, Roux F, Cohen Solal J et al. . Septic arthritis of the hip with Propionibacterium avidum bacteremia after intraarticular treatment for hip osteoarthritis. Joint Bone Spine 2008; 75:356–8. [DOI] [PubMed] [Google Scholar]
- 8. Panagea S, Corkill JE, Hershman MJ, Parry CM. Breast abscess caused by Propionibacterium avidum following breast reduction surgery: case report and review of the literature. J Infect 2005; 51:e253–5. [DOI] [PubMed] [Google Scholar]
- 9. Wang TK, Woo PC, Yuen KY. Perianal abscess caused by Propionibacterium avidum in a cirrhotic patient. New Microbiol 2002; 25:239–42. [PubMed] [Google Scholar]
- 10. Estoppey O, Rivier G, Blanc CH, Widmer F, Gallusser A, So AK. Propionibacterium avidum sacroiliitis and osteomyelitis. Revue du rhumatisme 1997; 64:54–6. [PubMed] [Google Scholar]
- 11. Dunne WM Jr., Kurschenbaum HA, Deshur WR et al. . Propionibacterium avidum as the etiologic agent of splenic abscess. Diagn Microbiol Infect Dis 1986; 5:87–92. [DOI] [PubMed] [Google Scholar]
- 12. Osmon DR, Berbari EF, Berendt AR et al. . Diagnosis and management of prosthetic joint infection: clinical practice. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 13. Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone & Joint J 2013; 95-b:1450–2. [DOI] [PubMed] [Google Scholar]
- 14. Bossard DA, Ledergerber B, Zingg PO et al. . Optimal length of cultivation time for isolation of Propionibacterium acnes in suspected bone and joint infections is more than 7 days. J Clin Microbiol 2016; 54:3043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cummins CS. Identification of Propionibacterium acnes and related organisms by precipitin tests with trichloroacetic acid extracts. J Clin Microbiol 1976; 2:104–10. [PMC free article] [PubMed] [Google Scholar]
- 16. Achermann Y, Tran B, Kang M, Harro JM, Shirtliff ME. Immunoproteomic identification of in vivo-produced Propionibacterium acnes proteins in a rabbit biofilm infection model. Clin Vaccine Immunol 2015; 22:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foulston L, Elsholz AK, DeFrancesco AS, Losick R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. mBio 2014; 5:e01667–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chevreux B, Pfisterer T, Drescher B et al. . Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res 2004; 14:1147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonfield JK, Whitwham A. Gap5 – editing the billion fragment sequence assembly. Bioinformatics 2010; 26:1699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomida S, Nguyen L, Chiu BH et al. . Pan-genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. mBio 2013; 4:e00003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurtz S, Phillippy A, Delcher AL et al. . Versatile and open software for comparing large genomes. Genome Biol 2004; 5:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28:2731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 2002; 30:3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European Committee on Antimicrobial Susceptibility Testing. Setting breakpoints for existing antimicrobial agents. EUCAST SOP 6.0. Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing, 2016. [Google Scholar]
- 25. McGinley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Appl Environ Microbiol 1978; 35:62– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnard E, Shi B, Kang D, Craft N, Li H. The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep 2016; 6:39491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levy O, Iyer S, Atoun E et al. . Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis?J Shoulder Elbow Surg 2013; 22:505–11. [DOI] [PubMed] [Google Scholar]
- 28. Lubbeke A, Zingg M, Vu D et al. . Body mass and weight thresholds for increased prosthetic joint infection rates after primary total joint arthroplasty. Acta Orthopaedic 2016; 87:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ilchmann T, Zimmerli W, Bolliger L, Graber P, Clauss M. Risk of infection in primary, elective total hip arthroplasty with direct anterior approach or lateral transgluteal approach: a prospective cohort study of 1104 hips. BMC Musculoskelet Disord 2016; 17:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol 1977; 6:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nodzo SR, Hohman DW, Crane JK, Duquin TR. Hemolysis as a clinical marker for Propionibacterium acnes orthopedic infection. Am J Orthop 2014; 43:E93–7. [PubMed] [Google Scholar]
- 32. Corvec S, Luchetta J, Aubin GG. Is hemolysis a clinical marker of Propionibacterium acnes orthopedic infection or a phylogenetic marker?Am J Orthoped 2015; 44:E61–2. [PubMed] [Google Scholar]
- 33. Mak TN, Schmid M, Brzuszkiewicz E et al. . Comparative genomics reveals distinct host-interacting traits of three major human-associated propionibacteria. BMC Genomics 2013; 14:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mak TN, Yu SH, De Marzo AM, Bruggemann H, Sfanos KS. Multilocus sequence typing (MLST) analysis of Propionibacterium acnes isolates from radical prostatectomy specimens. Prostate 2012; 73:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis AL, Deitzler GE, Ruiz MJ et al. . Genome sequences of 11 human vaginal Actinobacteria strains. Genome Announc 2016: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ordogh L, Hunyadkurti J, Voros A et al. . Complete genome sequence of Propionibacterium avidum strain 44067, isolated from a human skin abscess. Genome Announc 2013; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDowell A, Barnard E, Liu J, Li H, Patrick S. Proposal to reclassify Propionibacterium acnes type I as Propionibacterium acnes subsp. acnes subsp. nov. and Propionibacterium acnes type II as Propionibacterium acnes subsp. defendens subsp. nov. Int J Syst Evol Microbiol 2016; 66:5358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson JL, Cummins CS. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol 1972; 109:1047–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goodsell ME, Toth J, Johnson JL, Cummins CS. Two types of Propionibacterium avidum with different isomers of diaminopimelic acid. Curr Microbiol 1991; 22:225–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.