Summary
Findings of randomized controlled trials and robust quasi-experimental and epidemiological studies suggest likely benefits of influenza vaccination for persons with asthma, with reductions in influenza infection, respiratory illness, asthma attacks, and emergency department visits and hospitalizations for influenza-related asthma complications.
Keywords: influenza, vaccination, immunization, asthma, laboratory confirmed influenza
Abstract
There is uncertainty about the effectiveness of influenza vaccination in persons with asthma and its impact on asthma outcomes, which may contribute to the suboptimal vaccination rates in persons with asthma. This systematic review and meta-analysis involved searching 12 international databases for randomized controlled trials (RCTs) and high-quality quasi-experimental and epidemiological studies (1970–2016). The risk of bias was low for 3 included RCTs. The quality of 3 included observational studies was moderate. The quality of evidence was very low for all study outcomes. Pooled vaccine effectiveness in 1825 persons with asthma from 2 test-negative design case-control studies was 45% (95% confidence interval [CI], 31%–56%) for laboratory-confirmed influenza. Pooled efficacy of live vaccines in reducing influenza was 81% (95% CI, 33%– 94%). Live vaccine reduced febrile illness by 72% (95% CI, 20%–90%). Influenza vaccine prevented 59%–78% of asthma attacks leading to emergency visits and/or hospitalizations. For persons with asthma, influenza vaccination may be effective in both reducing influenza infection and asthma attacks.
Influenza is an acute respiratory illness caused by infection with the influenza virus, which can be severe and, particularly in high-risk groups, may result in considerable disease and, in some cases, death [1]. Worldwide, influenza causes an estimated 5 million cases of severe illness and half a million deaths each year, costing the United States an estimated $87 billion per annum [2, 3]. In persons with asthma, chronic airway inflammation and type 2 immune responses are thought to impair antiviral immunity in the respiratory tract [4], resulting in susceptibility to severe influenza illness and associated bacterial infection. Mechanisms of increased susceptibility to influenza in asthma include weaker innate immune and T-helper 1 cell responses and a deficient interferon α response of plasmacytoid dendritic cells to influenza [5]. Furthermore, influenza infections can lead to severe asthma attacks, often requiring hospitalization [6].
Annual immunization with influenza vaccine is currently recommended by the World Health Organization and national immunization technical advisory groups in the United States and a number of European and other high-income countries [3, 7]; however, the uptake in persons with at-risk conditions—including asthma—is well below the target of 75% (eg, 40% in the United States in 2015–2016) [8–10]. The reasons for this lack of coverage are complex and multifactorial, but they include a lack of confidence among patients and healthcare providers in the effectiveness and safety of vaccines [11].
Important in this respect is the hypothesis that the defective mucosal and systemic immunity in asthma may reduce protection provided by influenza vaccines [12, 13]. There may be some grounds to this concern in the context of asthma, because a recent Cochrane systematic review [14] investigating the effectiveness of influenza vaccination in persons with asthma was inconclusive regarding the efficacy of influenza vaccines. It is also of concern that the safety of live influenza vaccines in infants with wheezing disorders or asthma has not yet been conclusively established [14]. Given that placebo randomized controlled trials (RCTs) of influenza vaccination are no longer undertaken in persons with asthma (the last placebo RCT was carried out in 2001 with none planned in future) [15], there is the need to also consider evidence from other study designs in addition to RCTs [16]. We therefore carried out a systematic review and meta-analysis of RCTs and robust quasi-experimental and epidemiological studies to evaluate the efficacy, effectiveness, and safety of influenza vaccination in persons with asthma.
METHODS
Selection Criteria and Search Strategy
Our methods have been described in detail in our published protocol [17] (PROSPERO [Prospective register of systematic reviews] registration, CRD42016037219). We searched the published literature (January 1970 to January 2016) for studies investigating the effectiveness of influenza vaccination in persons with asthma. Our start date was chosen because the evidence on this subject began to accrue after publication of the paper by Bell et al [18] in 1978 [19, 20]. See Supplementary Appendix I for search strategies.
Risk of Bias Assessment in Individual Studies
Two reviewers (E. V. and K. E. F.) independently assessed the risk of bias, and disagreements were resolved through discussion or by the involvement of a third reviewer (C. R. S.). The risk of bias of experimental studies was based on the suggested algorithm in the Cochrane Collaboration’s tool [21]. Overall low risk of bias was assigned to a study with low risk of bias for all 6 domains, overall unclear risk was assigned to a study with unclear risk of bias for ≥1 domain, and overall high risk of bias was assigned to a study with high risk of bias for ≥1 domain.
The Quality Assessment Tool for Quantitative Studies Dictionary developed by the Effective Public Health Practice Project was used to evaluate observational studies and nonrandomized controlled studies (non-RCTs) [22]. The overall quality was rated as strong in the absence of weak ratings in each of the 6 components, moderate overall rating in the presence of 1 weak rating, and weak overall rating in the presence of ≥2 weak ratings.
Data Analysis
Separate meta-analyses were performed for clinically and methodologically comparable experimental and observational studies to estimate the incidence or frequency of influenza infection (laboratory confirmed) and febrile illness. Random-effects models were used to summarize the findings depending on the degree of clinical heterogeneity of the studies. For dichotomous outcomes, the treatment effect was estimated using risk ratios (RRs) with 95% confidence intervals (CIs) or odds ratios (ORs) with 95% CIs. Vaccine efficacy/effectiveness (VE) is usually reported as a percentage, for example, (1 – OR) × 100. Safety data from cross-over trials could not be pooled together owing to lack of adequate data regarding the 2 cross-over periods. Statistical heterogeneity was assessed using the standard χ2 test and I2 statistic, which describes the proportion of dispersion across studies due to true heterogeneity rather than to a sampling error (0%–100% heterogeneity). We contacted authors of included studies that had missing data. All statistical analyses were undertaken using RStudio software, version 0.99.893 (RStudio, Inc) [23].
The CIs (for Supplementary Figures S1–S5 in Supplementary Appendix II) were produced using the generic inverse variance method for meta-analysis. We provided pooled estimates for each VE outcome, combining all study designs (regardless of their clinical or methodological heterogeneity). Owing to studies’ asymmetric 95% CIs, pooled treatment effects and their 95% CIs were provided using the log-relative estimates and standard errors as input.
RESULTS
Selection of Studies and Study Characteristics
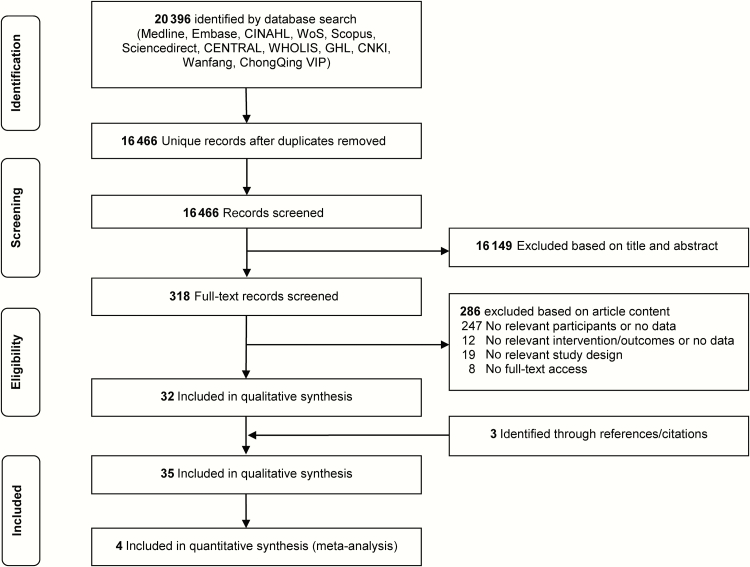
Our initial research identified 20396 unduplicated records. After screening titles and abstracts, 318 potentially eligible studies were selected for full review. Thirty-two studies eligible for inclusion were identified through database searches and another 3 through reference screening. We therefore included 35 studies enrolling 142519 patients with asthma in the qualitative synthesis and 4 studies in the meta-analyses (Figure 1). A brief summary of vaccine types per each end point is provided in Table 1. Full citations for these 35 articles [A1–A35] are provided in Supplementary Appendix II, along with detailed study characteristics and methodological critiques (Supplementary Tables S1–S8).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram.
Table 1.
Summary of Publications Reporting the Effectiveness and Safety of Influenza Vaccines
| Outcomea | Publications per Vaccine Type, No.a | |||
|---|---|---|---|---|
| Inactivated | Live | Both | Not Specified | |
| Influenzab (7 publications) | 1 (Sugaya 1994 [A26]) | 2 (Miyazaki 1993 [A12] and Tanaka 1993 [A20]) | 1 (Fleming 2006 [A6]) | 3 (McLean 2015 [A31], Ohmit 2014 [A32], and Otero 2009 [A33]) |
| Asthma exacerbationb (7 publications) | 4 (Bueving 2004 [A3], Abadoglu 2004 [A21], Sugaya 1994 [A26], and Jaiwong 2015 [A28]) | 0 | 0 | 3 (Gharagozlou 2006 [A7], Kramarz 2001 [A29], and Watanabe 2005 [A35]) |
| Hospitalization (6 publications) | 4 (Bell 1978 [A2], Abadoglu 2004 [A21], Sugaya 1994 [A26], and Jaiwong 2015 [A28]) | 0 | 0 | 2 (Gharagozlou 2006 [A7] and Christy 2004 [A27]) |
| Consultations (2 publications) | 0 | 0 | 1 (Fleming 2006 [A6]) | 1 (Christy 2004 [A27]) |
| Emergency visits (3 publications) | 1 (Jaiwong 2015 [A28]) | 0 | 0 | 2 (Gharagozlou 2006 [A7] and Christy 2004 [A27]) |
| Respiratory illness (8 publications) | 5 (Bueving 2004 [A3], Abadoglu 2004 [A21], Sugaya 1994 [A26], Jaiwong 2015 [A28], and Smits 2002 [A34]) | 2 (Miyazaki 1993 [A12] and Tanaka 1993 [A20]) | 0 | 1 (Jaiwong 2015 [A28]) |
| Asthma medication (2 publications) | 1 (Jaiwong 2015 [A28]) | 0 | 0 | 1 (Gharagozlou 2006 [A7]) |
| Pulmonary function (1 publication) | 1 (Abadoglu 2004 [A21]) | 0 | 0 | 0 |
| School/work absence (1 publication) | 0 | 0 | 1 (Fleming 2006 [A6]) | 0 |
| Safety (24 publications) | 17 (Bell 1978 [A2], Bueving 2004 [A4], Castro 2001 [A5], Govaert 1993 [A8], Hahn 1980 [A9], Kmiecik 2007 [A10], Miller 2003 [A11], Nicholson 1998 [A13], Ortwein 1987 [A14], Pedroza 2009 [A15], Reid 1998 [A17], Sener 1999 [A18], Stenius 1986 [A19], Campbell 1984 [A22], Chiu 2003 [A23], Kava 1987 [A24], and Kim 2003 [A25]) | 4 (Atmar 1990 [A1], Miyazaki 1993 [A12], Redding 2002 [A16], and Tanaka 1993 [A20]) | 1 (Fleming 2006 [A6]) | 2 (Gharagoszlou 2006 [A7] and Kramarz 2000 [A30]) |
aSee Supplementary Appendix II for details.
bPrimary outcome.
Risk of Bias Assessment in Individual Studies
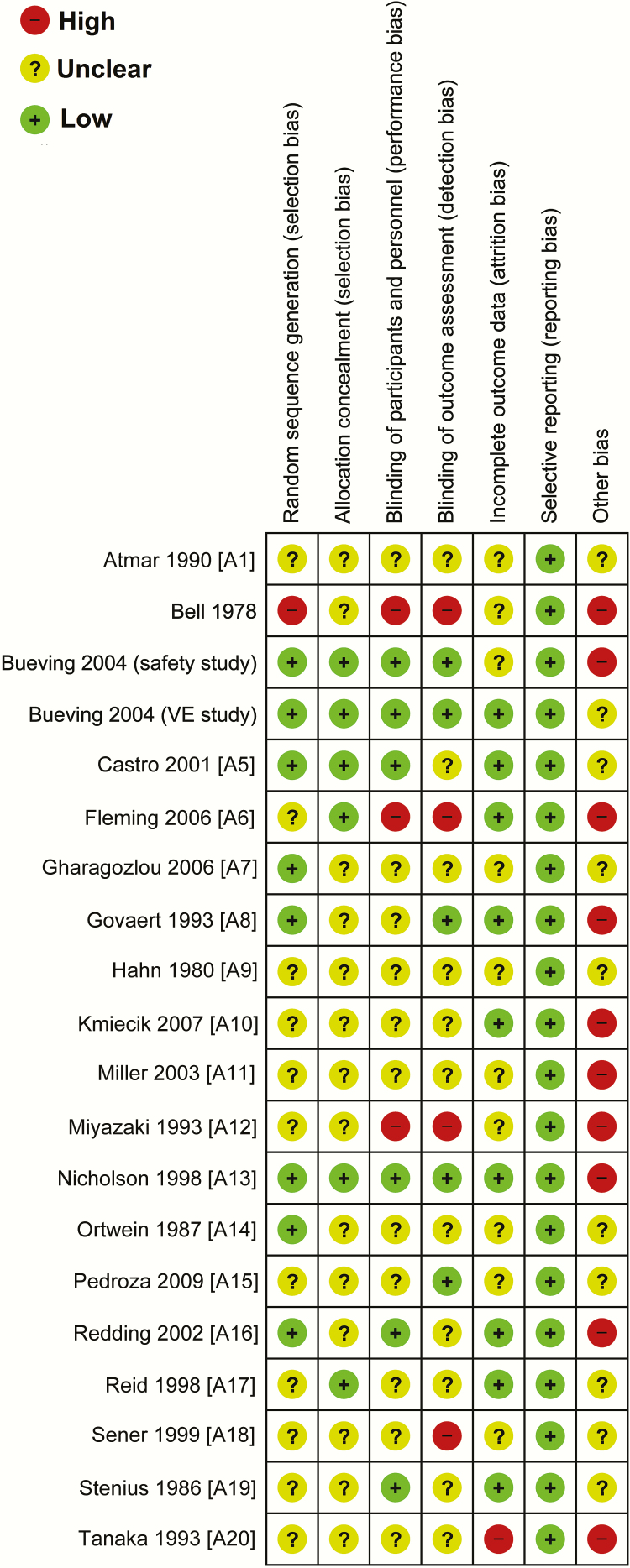
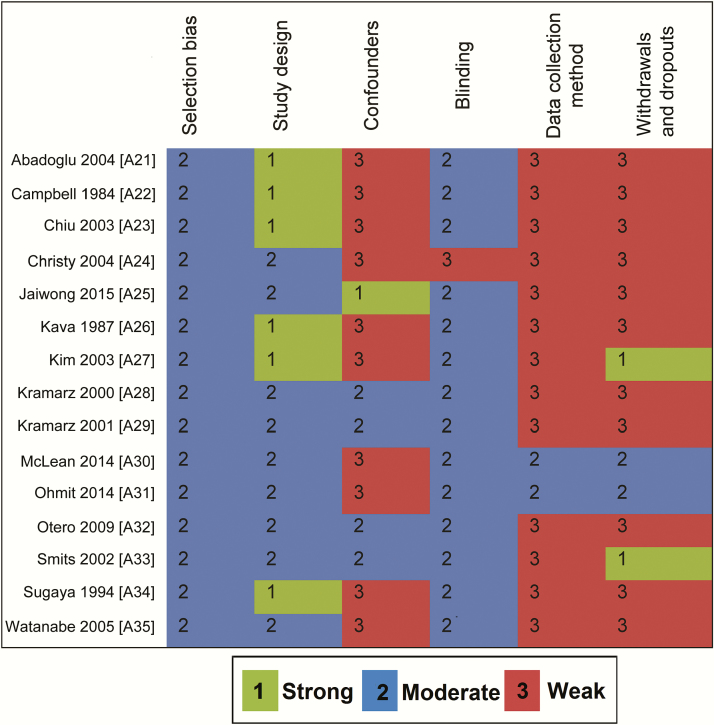
The overall risk of bias was high in 5 RCTs, unclear in 12, and low in 3 (Figure 2). The overall quality of 12 studies (6 non-RCTs and 6 cohort studies) was rated as “weak.” In 2 case-control studies and 1 cohort study, the overall quality was rated as “moderate” (Figure 3) (Supplementary Tables S2–S5).
Figure 2.
Risk of bias summary. Review authors’ judgments about each risk of bias item are shown for each randomized controlled trial. This rating was based on the Cochrane guideline. VE, Vaccine efficacy/effectiveness.
Figure 3.
Quality assessment of the nonrandomized controlled trials and observational studies using the Effective Public Health Practice Project quality assessment tool [22].
Overall Quality of Evidence
The body of evidence regarding influenza VE and safety regarding primary and secondary outcomes was rated, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as being of very low quality owing to inconsistency, indirectness, and imprecision across studies. In addition, the strength of evidence for the protective effects of vaccination against pulmonary function and school or work absenteeism was rated as very low because the evidence was based on single studies. Thus, the consistency, directness, and precision of the pooled overall estimation could not be assessed. Similarly, the evidence of safety of influenza vaccination against influenza infection and respiratory tract illness was assigned as very low because it was provided by single studies (Supplementary Table S6).
Vaccine Efficacy/Effectiveness Against Influenza Infection
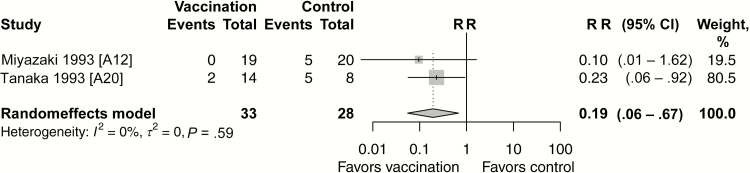
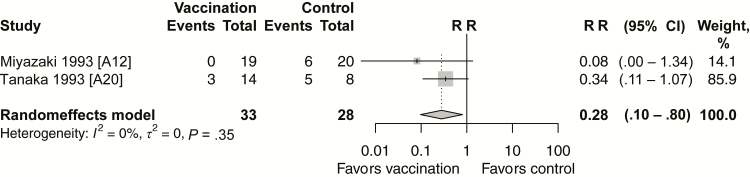
Nosocomial outbreaks of A (H1N1) and B subtypes were observed during 2 consecutive years (1988–1989 and 1989–1990) among 84 children with asthma [A12, A20]. Protection provided by live attenuated influenza vaccine (LAIV) in these children against laboratory-confirmed influenza was found in 2 small RCTs (pooled VE, 81%; 95% CI, 33%–94%; Figure 4). A large multicenter RCT evaluated the efficacy of the live vaccine compared with the inactivated vaccine against community-acquired culture-confirmed influenza illness in children (aged 6–17 years) [A6]. LAIV efficacy was significantly higher than that of the inactivated influenza vaccine. LAIV efficacy against influenza subtypes antigenically similar to those included in the vaccine was 35% (95% CI, 4%–56%).
Figure 4.
Vaccine efficacy/effectiveness against influenza infection for live attenuated influenza vaccine versus no vaccine (randomized controlled trials). Abbreviations: CI, confidence interval; RR, risk ratio.
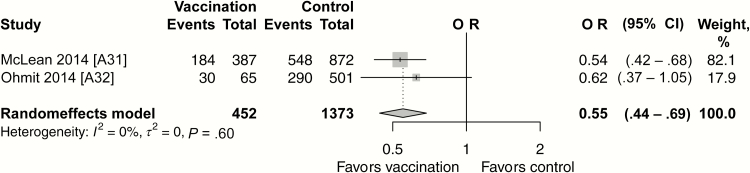
A meta-analysis was undertaken of 2 test-negative design (TND) studies performed in the United States during the seasons 2011–2012 and 2012–2013 [A31, A32]. In 2011–2012, the influenza vaccine in the United States was well matched and influenza A H3N2 predominated, with A H1N1 and both influenza B (Victoria and Yamagata) also circulating [24]. In 2012–2013, H3N2 again predominated, with a late season predominance of influenza B [25]. The influenza VE for persons with asthma ranged from 38% (95% CI, 0%–63.0%) in 2011–2012 to 46% (32%–58%) in 2012–2013. Once these results were pooled, we found an overall VE of 45% (95% CI, 31%–56%; Figure 5) in preventing laboratory-confirmed influenza (confirmed with real-time polymerase chain reaction) in 1825 individuals with asthma (aged ≥6 months) [A31, A32].
Figure 5.
Vaccine efficacy/effectiveness against laboratory-confirmed (confirmed with real-time polymerase chain reaction) influenza infection for seasonal influenza vaccine versus no vaccine (test-negative design studies). Abbreviations: CI, confidence interval; OR, odds ratio.
One prospective cohort study assessed the effectiveness of the influenza vaccine in preventing influenza in 338 children (2005–2006 season). There were no laboratory-confirmed influenza infection cases in the vaccinated group, but 8 (4.4%) of the unvaccinated children had an infection [A33]. In an non-RCT, the efficacy of inactivated vaccine was 42% (95% CI, 21%–57%) against influenza infection (diagnosed by means of virus isolation or hemagglutination inhibition antibody titer increase) in 137 children (aged 2–14 years) [A26].
Vaccine Efficacy/Effectiveness Against Asthma Attacks and Other Clinical Outcomes
Protective effects of vaccination against asthma exacerbation were also observed in 4 studies [A7, A28, A29, A35]. One RCT [A7] found that influenza vaccine protected against the incidence, frequency and duration of asthma attacks in 201 children (aged 1–15 years). The incidence of acute asthma attacks was lower in the vaccinated group than in the unvaccinated group (occurring in 39 of 79 vs 82 of 122 children; RR 0.73; 95% CI, .57–.95).
VE against asthma attacks was also studied in 3 observational cohort studies [A28, A29, A35]. In the first study, inactivated influenza vaccine provided greater protection against asthma attacks (defined as wheezing episodes) (mean [standard deviation (SD)], 1.6 [1.6]) than in the unimmunized group (6.2 [3.9]) (P < .001) [A28]. The second study found a reduction in attacks after controlling for asthma severity and other confounders. Protective incidence rate ratios were observed for the 1994–1995 (0.59; 95% CI, .43–.81) and 1995–1996 (0.65; .52–.80) seasons but not for the 1993–1994 season (0.78; .55–1.10) [A29]. In the third study, during the 2002–2003 season (but not the 2001–2002 season) the rate of asthma attacks was significantly (P = .04) lower in the vaccine group (mean [SD], 0.14 [0.4]) than in the control group (0.35 [0.61]) [A35].
Six studies assessed VE in preventing hospitalizations from asthma attacks or respiratory infections [A2, A7, A21, A26-A28]. A RCT assessed the duration of hospitalization for influenzalike illness (ILI) accompanied by asthma, ILI alone, and asthma alone in 93 children (aged 6–16 years). The duration of hospitalization for ILI alone (P < .01) and ILI accompanied by asthma (P < .05) was significantly shorter in the bivalent inactivated vaccine group than in the unvaccinated group [A2]. In a cohort study, the mean [SD] number of hospitalizations was 0.2 [0.6] among inactivated vaccine recipients and 1.3 [1.5] among controls (P < .001) [A28].
Two studies [A6, A27] assessed the protective effects of vaccination against asthma or respiratory illness consultations. A retrospective cohort study reported higher visits to a pediatric clinic among vaccine recipients (2.14) than in the unvaccinated ones (0.71; OR, 2.9; 95% CI, 2.0–4.1) [A27].
VE against respiratory illness was found in 4 studies [A26, A28, A33, A34]. Pooled estimates regarding live attenuated VE against febrile illness were estimated from 2 RCTs [A12, A20]. A pooled VE of 72% (95% CI, 20%–90%; Figure 6) was observed against febrile illness during 2 nosocomial outbreaks with A (H1N1) and B subtypes [A12, A20]. In another trial, the clinical efficacy of inactivated subunit vaccine against febrile influenza illness was 49% (95% CI, 24%–66%) in 137 children (aged 2–14 years) (P < .01). A higher VE (74%) was observed in children ≤7 years old (P < .01) [A26].
Figure 6.
Vaccine efficacy/effectiveness against febrile illness for live attenuated influenza vaccine versus no vaccine (randomized controlled trials). Abbreviations: CI, confidence interval; RR, risk ratio.
Three cohort studies reported protective effects of vaccination against respiratory illness. In the first study, the number of respiratory tract illnesses was significantly lower (mean [SD], 2.2 [2.1]) in the inactivated vaccine recipients than in the unvaccinated group (6.9 [3.9]) (P < .001) [A28]. The second study found that 0.6% of vaccine recipients had a respiratory syncytial virus infection, compared with 2.5% of controls. In addition, protective effects of the vaccine were also observed against other respiratory infections (RR: 0.61; 95% CI, .29–.95) and bronchiolitis (0.47; .26–.84) [A33]. In the last study, the effectiveness of the inactivated subunit vaccine was 56% (95% CI, 18%– 76%) against acute respiratory disease (defined as ILI, bronchitis, bronchiolitis, asthma exacerbation, or otitis media) during the 1996–1997 season. In particular, a higher VE of 77% (95% CI, 35%–92%) was found in younger children (<6 years old) [A34].
The VE in preventing asthma-related emergency department (ED) visits was evaluated in 3 studies [A7, A27, A28]. A cohort study observed fewer ED visits for asthma exacerbations among inactivated vaccine recipients (mean number of visits [SD], 0.4 [0.9]) than in the unvaccinated group (2.2 [2.6]) (P < .001) [A28]. In contrast, another cohort study of vaccinated children had more ED visits for asthma or pneumonia (OR 2.0; 95% CI, 1.2–3.1) [A27].
The protective effects against increased use of asthma medication were also reported in 2 studies [A7, A28]. In an RCT, the frequency of bronchodilator use was lower in the vaccinated group (35 of 79) than in the unvaccinated group (77 of 122; VE, 50%; 95% CI, 34%–64%) [A7]. A cohort study reported significantly more bronchodilator administrations in the unvaccinated group than in the inactivated vaccine group (mean [SD], 6.2 [3.9] vs 1.6 [1.6]), and significantly more prednisolone administrations (1.1 [1.2] vs 0.1 [0.3]; respectively) (both P < .001) [A28]. No improvements in pulmonary function or reductions in work/school absenteeism were found with influenza vaccine [A6, A21].
Safety
There was no increased risk of serious local or systemic adverse reactions or vaccine-related asthma exacerbations or symptoms (eg, wheeze) or respiratory illnesses [A1, A4-20, A22-25, A30]. One trial comparing live with inactivated vaccine found a significant increase in wheezing symptoms in the inactivated vaccine group [A6]. In 2 [A2, A15] of 16 studies, deterioration in pulmonary function was found after vaccination, although these changes were not accompanied by asthma symptoms or increased use of medication or healthcare services. We found 4 non-RCTs [A22–A25] and 1 observational study [A30] (not included in the review by Cates and Row [14]). These found that influenza vaccine led to no increase in postvaccine asthma attack or symptoms when compared with placebo (for non-RCTs) or no vaccine (observational studies) (Supplementary Table S7).
DISCUSSION
Our findings indicate that influenza vaccination prevents influenza and other clinically important health outcomes in persons with asthma. Pooled estimates from observational TND studies suggest that influenza vaccination is beneficial against laboratory-confirmed influenza (VEs ranging from 38% to 46%, with a pooled estimate of 45%) [A31, A32]. Influenza vaccination reduced asthma exacerbations, healthcare use, respiratory illnesses, and medications for asthma [A2, A7, A12, A20, A26, A28, A29, A33, A35]. However, much of this evidence comes from observational studies, and therefore bias and residual confounding are alternative possible explanations. For each outcome, the quality of the body of evidence (across all included studies using GRADE) was also very low.
There are several reasons why evidence from robust quasi-experimental and observational studies must be considered. A Cochrane review of RCTs on this subject, which found inconclusive evidence to support influenza vaccination in those with asthma [14], was well conducted but still of limited value to decision makers, clinicians, or patients. This is because there have been no relevant placebo RCTs over the last 15 years and none are in progress or planned, because it has been considered unethical to withhold vaccination, particularly from those most at risk of severe influenza illness. Furthermore, observational TND studies are used to help inform national advisory bodies on their influenza vaccination programs. For instance, the US Advisory Committee on Immunization Practices did not recommend the use of LAIV for the 2016–2017 season owing to evidence of no effectiveness (3%) for LAIV in US-based TND studies [7]. Among children with a history of asthma or wheezing, however, LAIV was found to have superior efficacy compared with trivalent inactivated influenza vaccine [26]. Therefore, further research using observational study data is required to establish the effectiveness of LAIV among children with asthma [27].
Strengths and Limitations
Most studies differed by recruitment methods, vaccine ascertainment methods, type of vaccines, and outcome definitions (in some cases outcomes were not described). In particular, the definition and evaluation of asthma exacerbations are important points of variability across studies. An additional file (Supplementary Table S8) shows further characteristics of included studies. Most studies (experimental and observational) also recruited children or adults <65 years old. Thus, only a few studies have assessed influenza vaccination in older persons with asthma.
In 3 RCTs, the low sensitivity of viral culture tests to confirm influenza infection may have affected the accuracy of the results [A12, A20, A26]. Furthermore in 3 studies, residual immunity from previous vaccination or influenza exposure from previous seasons may have affected VE estimates [A31, A32, A34].
With the small number of studies included in each meta-analysis, publication bias could not be adequately assessed. Planned subgroup and sensitivity analysis (eg, VE against influenza B and A subtypes) could not be carried out owing to lack of data from the included studies [17]. In addition, more in-depth analyses are required, including the number, nature, and antigenic distance specified by virus mutations across sequential circulating variants and vaccine components and the role of prior vaccination [28]. This will require larger TND studies with pooling of data across regions and countries. We did not found new substantive evidence for LAIV safety, beyond those studies reviewed by Cates and Row [14].
In conclusion, public health initiatives are required to improve the current low vaccine uptake in persons with asthma [10]. Evidence from clinical trials and observational studies suggests that the influenza vaccine is safe and that it probably benefits persons with asthma against influenza infection, respiratory illness, asthma attacks and other influenza-related asthma complications. including asthma-related ED visits and hospitalizations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. E. V. wrote this review with help from A. S., C. R. S., and C. B. K. E. F. screened studies, extracted data, and appraised the quality of the studies. B. v. W., J. M., and L. R. commented critically on a draft of the review. J. S., N. G. P., and S. L. J. helped write the review. L. T. helped create the early drafts.
Disclaimer. The funding body had no role in the design of the study, review process, analysis, interpretation, or reporting of data.
Financial support. The work was funded by the Chief Scientist Office of the Scottish Government under the grant (AUKCAR/14/03). This work is carried out with the support of the Asthma UK Centre for Applied Research (AUK-AC-2012-01).
Potential conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nair H, Brooks WA, Katz M et al. . Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 2. Molinari NA, Ortega-Sanchez IR, Messonnier ML et al. . The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–96. [DOI] [PubMed] [Google Scholar]
- 3. Public Health England. Influenza: the green book. Chapter 19. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/385226/Green_Book_Chapter_19_v8_2.pdf. Accessed 10 March 2015. [Google Scholar]
- 4. Ritchie AI, Jackson DJ, Edwards MR, Johnston SL. Airway epithelial orchestration of innate immune function in response to virus infection: a focus on asthma. Ann Am Thorac Soc 2016; 13(suppl 1):S55–63. [DOI] [PubMed] [Google Scholar]
- 5. Gill MA, Bajwa G, George TA et al. . Counterregulation between the FεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010; 184:5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papadopoulos NG, Christodoulou I, Rohde G et al. . Viruses and bacteria in acute asthma exacerbations—a GA² LEN-DARE systematic review. Allergy 2011; 66:458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grohskopf LA, Sokolow LZ, Broder KR et al. . Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep 2016; 65:1–54. [DOI] [PubMed] [Google Scholar]
- 8. National early season flu vaccination coverage, United States, November 2015 Available at: http://www.cdc.gov/flu/fluvaxview/nifs-estimates-nov2015.htm. Accessed 8 June 2016.
- 9. Prevention and control of influenza pandemics and annual epidemics Available at: http://apps.who.int/gb/archive/pdf_files/WHA56/ea56r19.pdf. Accessed 4 August 2016.
- 10.Commission of the European Communities. Proposal for a council recommendation on seasonal influenza vaccination 2009. Available at: http://ec.europa.eu/health/ph_threats/com/Influenza/docs/seasonflu_rec2009_en.pdf. Accessed 4 August 2016.
- 11. Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–8. [DOI] [PubMed] [Google Scholar]
- 12. Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol 2014; 134:247–57; quiz 58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ritchie AI, Farne HA, Singanayagam A, Jackson DJ, Mallia P, Johnston SL. Pathogenesis of viral infection in exacerbations of airway disease. Ann Am Thorac Soc 2015; 12(suppl 2):S115–32. [DOI] [PubMed] [Google Scholar]
- 14. Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev 2013; 2:CD000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bueving HJ, Bernsen RM, de Jongste JC et al. . Influenza vaccination in children with asthma: randomized double-blind placebo-controlled trial. Am J Respir Crit Care Med 2004; 169:488–93. [DOI] [PubMed] [Google Scholar]
- 16. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 17. Vasileiou E, Sheikh A, Butler C et al. . Effectiveness of influenza vaccination for preventing influenza-related complications in people with asthma: a systematic review protocol. BMJ Open 2016; 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell TD, Chai H, Berlow B, Daniels G. Immunization with killed influenza virus in children with chronic asthma. Chest 1978; 73:140–5. [DOI] [PubMed] [Google Scholar]
- 19. Rothbarth PH, Kempen BM, Sprenger MJ. Sense and nonsense of influenza vaccination in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 151:1682–6. [DOI] [PubMed] [Google Scholar]
- 20. Cates CJ, Jefferson TO, Bara AI, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev 2000; CD000364. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated March 2011. The Cochrane Collaboration. Available at: http://handbook.cochrane.org/. Accessed 8 May 2015. [Google Scholar]
- 22. Effective Public Health Practice Project Quality assessment tool for quantitative studies. Available at: http://www.ephpp.ca/tools.html. Accessed 8 May 2015.
- 23. RStudio Team. RStudio: integrated development for R. Boston, MA: RStudio 2015. Available at: http://www.rstudio.com/. Accessed 1 March 2015.
- 24.Centers for Disease Control and Prevention. The 2011–2012 Influenza season Available at: http://www.cdc.gov/flu/pastseasons/1112season.htm. Accessed 3 August 2016.
- 25.Centers for Disease Control and Prevention. The 2012–2013 influenza season Available at: http://www.cdc.gov/flu/pastseasons/1213season.htm. Accessed 3 August 2016.
- 26. Ambrose CS, Dubovsky F, Yi T, Belshe RB, Ashkenazi S. The safety and efficacy of live attenuated influenza vaccine in young children with asthma or prior wheezing. Eur J Clin Microbiol Infect Dis 2012; 31:2549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson CR, Lone NI, Kavanagh K et al. . Evaluating the effectiveness, impact and safety of live attenuated and seasonal inactivated influenza vaccination: protocol for the Seasonal Influenza Vaccination Effectiveness II (SIVE II) study. BMJ Open 2017; 7:e014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skowronski DM, Janjua NZ, De Serres G. Understanding suboptimal influenza vaccine effectiveness within the agent, host, and environment paradigm. Clin Infect Dis 2013; 57:476–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.