Improvements in the immunologic tests performed on cerebrospinal fluid have increased the sensitivity and reduced the turnaround time for diagnosis of central nervous system histoplasmosis over prior antigen and antibody detection methods and culture.
Keywords: histoplasmosis, meningitis, antibody, antigen, diagnosis
Abstract
Background
Central nervous system (CNS) histoplasmosis is a life-threatening condition and represents a diagnostic and therapeutic challenge. Isolation of Histoplasma capsulatum from cerebrospinal fluid (CSF) or brain tissue is diagnostic; however, culture is insensitive and slow growth may result in significant treatment delay. We performed a retrospective multicenter study to evaluate the sensitivity and specificity of a new anti-Histoplasma antibody enzyme immunoassay (EIA) for the detection of IgG and IgM antibody in the CSF for diagnosis of CNS histoplasmosis, the primary objective of the study. The secondary objective was to determine the effect of improvements in the Histoplasma galactomannan antigen detection EIA on the diagnosis of Histoplasma meningitis.
Methods
Residual CSF specimens from patients with Histoplasma meningitis and controls were tested for Histoplasma antigen and anti-Histoplasma immunoglobulin G (IgG) and immunoglobulin M (IgM) antibody using assays developed at MiraVista Diagnostics.
Results
A total of 50 cases and 157 controls were evaluated. Fifty percent of patients with CNS histoplasmosis were immunocompromised, 14% had other medical conditions, and 36% were healthy. Histoplasma antigen was detected in CSF in 78% of cases and the specificity was 97%. Anti-Histoplasma IgG or IgM antibody was detected in 82% of cases and the specificity was 93%. The sensitivity of detection of antibody by currently available serologic testing including immunodiffusion and complement fixation was 51% and the specificity was 96%. Testing for both CSF antigen and antibody by EIA was the most sensitive approach, detecting 98% of cases.
Conclusions
Testing CSF for anti-Histoplasma IgG and IgM antibody complements antigen detection and improves the sensitivity for diagnosis of Histoplasma meningitis.
Central nervous system (CNS) involvement is present in 5%–10% of patients with disseminated histoplasmosis [1, 2]. Clinical presentations include meningitis, hydrocephalus, brain or spinal cord mass lesions, stroke, and encephalitis. CNS infection may occur with concomitant pulmonary or disseminated disease or may be present in isolation. Neurologic involvement may be detected at the initial presentation or may represent relapse after treatment of disseminated or pulmonary histoplasmosis. The time course may be rapid or protracted over several years [3]. Given these heterogeneous presentations, the disease is often unrecognized and diagnosis and treatment delayed, resulting in neurologic complications or death.
Even when CNS involvement is suspected, laboratory confirmation can be challenging. In the 2 largest reviews, cerebrospinal fluid (CSF) cultures were positive in only 28% of patients [1, 2], and growth was delayed for several weeks after presentation. Diagnosis is clinically important, as these patients require longer courses and higher doses of liposomal amphotericin B than patients with disseminated disease not involving the CNS [4].
The limitations of fungal cultures have resulted in attempts to identify more sensitive and rapid diagnostics for CNS histoplasmosis. Diagnostic techniques that have improved performance characteristics include detection of antigen in CSF using radioimmunoassay (RIA) and of antibody by complement fixation (CF) [5]. Testing of CSF using these techniques improved the sensitivity to 67%—significantly better than culture, but still imperfect [1]. Detection of anti-Histoplasma antibodies by RIA had a sensitivity of 89% [6], but this assay was never validated or offered for clinical testing.
The current Histoplasma antigen detection enzyme immunoassay (EIA) [7] is more sensitive than the original RIA [1] and antibody detection by EIA is more sensitive than immunodiffusion (ID) or CF [8]. With the availability of these newer diagnostics, we evaluated the accuracy of the antigen and antibody EIAs for the diagnosis of Histoplasma meningitis.
METHODS
Study Specimens
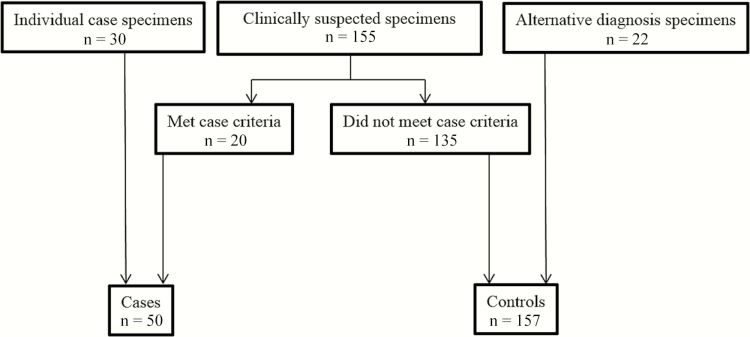
The study sample consisted of residual CSF from patients who had specimens submitted to MiraVista Diagnostics for Histoplasma antigen testing between 2000 and 2015. The study population comprised these groups (Figure 1):
Figure 1.
The study population and classification of cases and controls. Individual case specimens: patients with central nervous system (CNS) histoplasmosis accrued through clinical testing at MiraVista Diagnostics from outside institutions besides Indiana University Medical Center (IUMC), University of Kentucky Medical Center (UKMC), and Vanderbilt University Medical Center (VUMC). Clinical suspected specimens: unique patients with specimen submitted for testing at MiraVista Diagnostics from IUMC, UKMC, and VUMC based on clinical concern for CNS histoplasmosis. This group includes cases that met the criteria for diagnosis of meningitis (cases) and patients that did not meet those criteria (controls). Alternative diagnosis specimens: patients (controls) with no clinical suspicion for CNS histoplasmosis, in whom no specimens were submitted for testing at MiraVista Diagnostics but from whom cerebrospinal fluid specimens were obtained from the microbiology laboratories at IUMC.
Clinically suspected case and control specimens (n = 155): This group included all patients with CSF samples submitted to MiraVista Diagnostics for Histoplasma antigen testing between 2014 and 2015 from Indiana University Medical Center (IUMC), University of Kentucky Medical Center (UKMC), and Vanderbilt University Medical Center (VUMC), areas that are highly endemic for histoplasmosis. CSF obtained for Histoplasma antigen testing performed as part of clinical care specimens were stored frozen at MiraVista Diagnostics and prospectively tested for Histoplasma antibodies as part of the study protocol. Investigators blinded to results of the study-related testing performed chart review. Based on case definitions below, 20 patients met criteria for CNS histoplasmosis, and 135 were included as controls.
Individual case specimens (n = 30): This group was composed of residual CSF samples from 30 patients with previously diagnosed CNS histoplasmosis for whom treating physicians consulted with the medical director at MiraVista Diagnostics and provided clinical information.
Fungal meningitis not suspected (n = 22): This group included residual CSF specimens from patients at IUMC in whom fungal meningitis was not suspected and Histoplasma antigen testing was not ordered. These patients, in addition to the 135 patients in the clinically suspect group who did not meet case criteria, comprised the control group.
Case Definitions
Patients were categorized as CNS histoplasmosis cases if they had CNS inflammation (defined as CSF white blood cell count ≥5 cells/μL) or brain imaging abnormalities and supporting laboratory studies:
Confirmed: isolation of Histoplasma capsulatum from CSF;
Probable: detection of Histoplasma antigen by EIA or anti-Histoplasma antibodies in the CSF by ID or CF;
Possible: pulmonary or disseminated histoplasmosis without laboratory confirmation of CNS involvement (negative or absent culture, microscopy, detection of antigen or detection of antibody by ID or CF in the CSF). Similar classification has been previously described for histoplasmosis [1] and other endemic mycosis [9, 10].
Controls included patients with either:
Pulmonary or disseminated histoplasmosis [7] without CNS involvement (no clinical findings for meningitis, no pleocytosis or CNS imaging abnormalities, no diagnosis of or treatment for CNS histoplasmosis);
Negative testing for histoplasmosis (either with or without CSF pleocytosis).
Laboratory Methods
The EIA for detection of anti-Histoplasma antibodies has been described [8]. CSF was diluted 25-fold in StartingBlock buffer (Thermo Scientific, Rockford, Illinois). Results were expressed semi-quantitatively as EIA units by comparison to a standard curve. Results >10 units were considered positive and results <7.9 units were classified as negative. Results between 8.0 and 9.9 units were categorized as indeterminate but for analysis were classified as negative.
The Histoplasma antigen EIA was performed as previously reported for serum [7]. Pretreatment of the CSF with ethylenediaminetetraacetic acid (EDTA) was implemented in 2009 [11]. One hundred microliters of 4% EDTA was added to 300 µL of CSF, and the mixture was vortexed and placed in a heat block at 104°C for 6 minutes. The heat-treated CSF was centrifuged and the supernatant was tested for antigen. The purpose of this step was to dissociate antigen–antibody immune complexes and denature the antibody, freeing the antigen for detection by EIA.
An optical density above the cutoff for positivity was considered positive. Results above the 0.4 ng/mL standard were reported as ng/mL. Those between the cutoff and the 0.4 ng/mL standard were reported as positive, below the limit of quantification (LOQ). Results above the 19 ng/mL standard were reported as positive, above the LOQ. Levels below the LOQ were assigned a concentration of 0.3 ng/mL and those above the LOQ were assigned a value of 19.0 ng/mL for statistical analysis and illustration.
Histoplasma antibody testing by CF or ID was performed as part of clinical care through commercial laboratories employed by the facility at which the patient was evaluated.
Statistical Analysis
SigmaPlot software (Systat Software, San Jose, California) was used for transformation of optical density values into EIA units. MedCalc for Windows version 12.3.0 (Ostend, Belgium) was performed to calculate predictive values for 155 clinically suspected specimens, representing all patients at IUMC, UKMC, and VUMC for whom CSF was submitted to MiraVista Diagnostics for Histoplasma antigen testing. A χ2 analysis was used to compare subgroups using MedCalc software. P values <.05 were considered significant.
Ethical Considerations
The study was approved by the institutional review boards (IRBs) at the following institutions: IUMC, UKMC, VUMC, and the University of California, San Francisco Medical Center. IRB committees at the other institutions reviewed the protocol and concluded that IRB approval was not required. Inclusion of a case from University of Tokyo Hospital was approved by the Hospital Ethics Committee.
RESULTS
A comparison of baseline characteristics is presented in Table 1. There were 9 confirmed cases, 36 probable cases, and 5 possible cases. The median age and sex and presence of immunocompromising conditions or medications were similar between groups, but cases were less likely to have comorbid diseases than controls.
Table 1.
Baseline Characteristics of Cases and Controls
| Variable | Cases (n = 50) |
Controls (n = 157) |
P Value |
|---|---|---|---|
| Age, y, median (range) | 43.0 (1 mo–77 y) | 49.0 (1 mo–85 y) | .14 |
| Sex, male | 30 (60) | 91 (58) | .93 |
| Immunocompromisea | 25 (50) | 67 (42.7) | .46 |
| Nonimmunocompromising medical conditionsb | 7 (14) | 69 (43.9) | .0003 |
| No recognized underlying condition | 18 (36) | 21 (13.4) | .0008 |
Data are presented as No. (%) unless otherwise indicated.
aImmunodeficiency state or immunosuppressive therapy. Immunocompromising conditions included AIDS in 13, solid organ transplantation in 6, immunosuppressive therapy for inflammatory disorders in 5, and an uncharacterized immunodeficiency in 1 patient.
bNonimmunocompromising conditions included cerebral palsy, chronic pulmonary disease, hepatitis C, malignancy, pregnancy, and head trauma in 1 patient each.
Fungal culture of CSF was positive for H. capsulatum in 19% of cases (Table 2). Cultures were positive more often in the individual cases (28.6%) than the clinically suspected cases (5.3%), but the difference was not significant (P = .1066).
Table 2.
Histoplasma Diagnostic Testing in the Cerebrospinal Fluid
| Test | Sensitivitya | Specificityb | PPV, % | NPV, % |
|---|---|---|---|---|
| Culture | 9/47 (19.1) | 119/119 (100)c | 100 | 91.8 |
| Antigen | 39/50 (78) | 140/145 (96.6) | 71.8 | 97.5 |
| EIA IgG antibody | 37/45 (82.2) | 145/153 (94.8) | 55.9 | 97.9 |
| EIA IgM antibody | 14/45 (31.1) | 149/153 (97.4) | 57.1 | 92.7 |
| EIA IgG or IgM antibodyd | 37/45 (82.2) | 142/153 (92.8) | 52.1 | 97.9 |
| Antigen or antibody EIAe | 48/49 (98.0) | 139/153 (90.8) | 54.7 | 100 |
| ID antibody | 19/43 (44.2) | 13/13 (100) | 100 | 94.1 |
| CF antibody | 5/10 (50) | 13/14 (92.9) | 43.9 | 94.4 |
| ID or CF antibodyd | 22/43 (51.2) | 22/23 (95.6) | 56.4 | 94.6 |
| Antigen or antibody ID or CFe | 39/43 (90.7) | 18/23 (78.3) | 31.7 | 98.7 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CF, complement fixation; EIA, enzyme immunoassay; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M; NPV, negative predictive value; PPV, positive predictive value.
aPositive test results/patients with central nervous system (CNS) histoplasmosis.
bNegative test result/patients without CNS histoplasmosis.
cDenominators vary because specimen volume was inadequate to perform the anti-Histoplasma antibody EIA and culture or antibody detection by ID and CF testing was not performed as part of clinical care in some patients.
dComparison of antibody detection by EIA and ID or CF: sensitivity, P = .0029; specificity, P = 1.000.
eComparison of antigen or antibody detection by EIA and antigen or antibody by ID or CF: sensitivity, P = .1813; specificity, P = .0801.
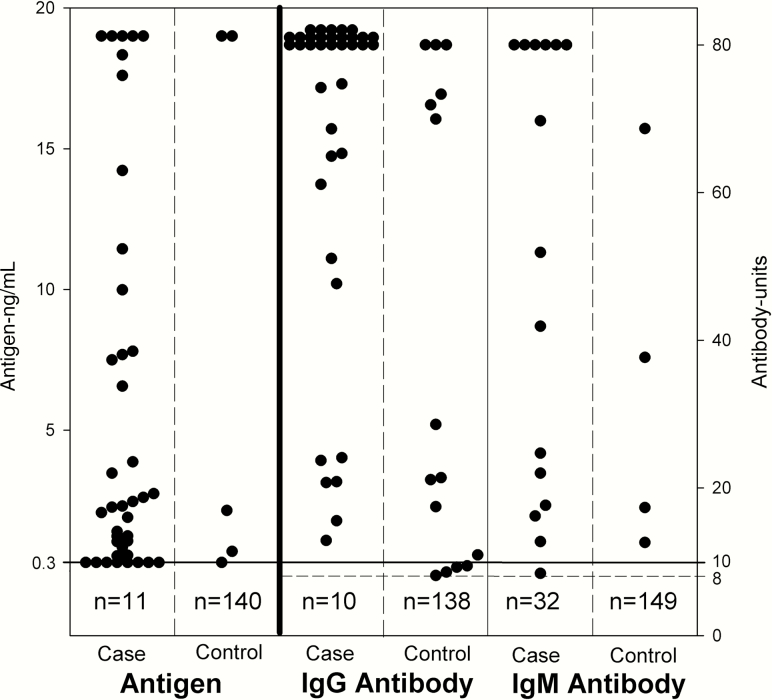
Antigen was detected in the CSF in 78% of cases, including 78% of confirmed cases, 89% of probable cases, and 0% of possible cases. To avoid incorporation bias, we also calculated the sensitivity of CSF antigen testing after excluding 23 cases in which antigen testing was the sole means of laboratory diagnosis, resulting in a sensitivity of 78% in confirmed or probable cases established through culture or antibody testing by ID or CF. Antigen was detected in 33 of 39 (85%) of the CSF specimens that were treated with EDTA compared with 5 of 11 (45%) that were tested before the EDTA treatment step was implemented (P = .0187). Quantitative antigen concentrations are shown in Figure 2. Histoplasma antigen was detected in urine or serum in 36 of 47 (77%) cases.
Figure 2.
Histoplasma antigen and anti-Histoplasma antibody results in cerebrospinal fluid in the cases and controls. For antigen testing, results <0.3 ng/mL (indicated by the solid line shown at 0.3 ng/mL) were considered negative, whereas results of ≥0.3 ng/mL were positive, below the limit of quantification at 0.4 ng/mL. Antigen results >19 ng/mL were above the limit of quantification. Results indicated by the number at the bottom of each of the antigen columns represent negative results because the space below the line designating the cutoff for positivity would not permit depiction of individual results for the controls. For antibody testing, results <8 units (indicated by broken horizontal line) were negative, results between 8 and 9.9 units were indeterminate, and results of 10 units (indicated by solid horizontal line shown at 10 units) or higher were positive. Results indicated by the number at the bottom of each of the antibody columns represent negative results because the space below the line designating the cutoff for positivity would not permit depiction of individual results for the controls, and the 32 cases in the immunoglobulin M column. Abbreviations: IgG, immunoglobulin G, IgM, immunoglobulin M; n, number of patients with negative results .
Elevated levels of IgG or IgM anti-Histoplasma antibodies measured by EIA were present in 82% of cases. These results were not considered as the basis for diagnosis to avoid incorporation bias. Antibody was detected by EIA less often in immunocompromised than nonimmunocompromised patients (Table 3). The sensitivity for detection of antibodies by ID or CF (51%) was lower than by EIA (P = .0029). Histoplasma antigen or anti-Histoplasma antibodies by EIA were present in the CSF in 98% cases. The negative predictive value for combined antigen and antibody testing by EIA was 100% (Table 2). Quantitative antibody concentrations by EIA are shown in Figure 2.
Table 3.
Comparison of Diagnostic Testing in Immunocompromised and Nonimmunocompromised Patients With Central Nervous System Histoplasmosis
| Diagnostic test | Immunocompromised | Nonimmunocompromised | P Value |
|---|---|---|---|
| Culture | 5/23 (21.7) | 4/24 (16.7) | .9484 |
| Antigen | 22/25 (88) | 17/25 (64) | .0978 |
| ID or CF antibody | 8/21 (38.1) | 14/22 (63.6) | .0716 |
| EIA IgG or IgM antibody | 15/22 (68.2) | 22/23 (95.7) | .0430 |
| Antigen or antibody EIA | 23/25 (92) | 25/25 (100) | .4750 |
| Antigen or antibody ID or CF | 19/20 (95) | 20/22 (90.9) | .9326 |
Abbreviations: CF, complement fixation; EIA, enzyme immunoassay; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M.
IgG or IgM anti-Histoplasma antibodies were detected in the CSF in 7 of 9 confirmed cases (78%), 27 of 32 probable cases (84%), and 3 of 4 possible cases (75%). Testing was unable to be performed in 5 patients due to insufficient specimen. If the possible cases are excluded from the analysis, the sensitivity for detection of antibodies by EIA was 83%, compared to 82% if they are included.
The specificity for detection of antigen in the CSF was 97% (Table 2). Five controls had detectable antigen in the CSF. Diagnosis included Blastomyces meningitis and pulmonary or disseminated histoplasmosis without evidence of CNS involvement in 2 patients each (Supplementary Table 1). A fifth patient, who was receiving antibiotics for Helicobacter pylori gastritis, developed fever, altered mental status, rash, and eosinophilia. CSF leukocyte count was 4 cells/µL, the glucose was 55 mg/dL, and the protein was 28 mg/dL. No testing for fungal infection was ordered, but residual CSF was obtained as an “alternative diagnosis” control. Antigen was positive at 2.2 ng/mL but below the LOQ when repeated.
Specificity for detection of anti-Histoplasma IgG or IgM antibodies by EIA was 93% and the positive predictive value (PPV) was 52% (Table 2). Diagnoses in the controls with detectable antibodies (Supplementary Table 1) included cryptococcal or Blastomyces meningitis in 1 patient each; histoplasmosis not involving the brain or meninges in 3 patients; bacterial meningitis in 2 patients; and tuberculous meningitis, neurosarcoidosis, melanoma involving the brain, and migraine in 1 patient each. The specificity and PPV for detection of antibodies by ID were 100% (Table 2).
DISCUSSION
Information on the accuracy of the diagnostic tests for Histoplasma meningitis is meager, and much of what has been published predates currently available assays [1, 2]. The largest single-institution study evaluated 18 patients with CNS histoplasmosis and reviewed 86 additional cases reported in the literature [1]. In this population, antigen was detected in the CSF in 40%, antibody by ID or CF in 62%, and either antigen or antibody was detected in 67% of patients [1]. The antigen assay used in that study was an RIA developed in 1985 [12]. The antigen assay has evolved to a fourth-generation EIA [7] that incorporates EDTA treatment [11]. Using EDTA treatment, the sensitivity of antigen detection in the CSF was 85% in the current study. Similarly, the use of the newly developed antibody EIA improved the sensitivity for antibody detection from 51% by ID or CF to 82% by EIA. The combined sensitivity for Histoplasma antigen and anti-IgG or IgM antibody detection by EIA was 98% and the negative predictive value was 100%.
Incorporation bias caused by use of the antigen results for diagnosis of Histoplasma meningitis hampers assessment of its sensitivity. However, the sensitivity was the same (78%) in cases including antigen detection as the basis for diagnosis and in a subset in which antigen detection alone was excluded as laboratory basis for diagnosis. Also, no differences in sensitivity for any of the diagnostic tests were observed in the clinically suspected cases, which included all patients in whom antigen testing was ordered and the individual cases, in which enrollment bias was more likely (Supplementary Table 2). Inclusion of antigen and antibody results using the EIA as the basis for diagnosis reduced the proportion of cases categorized as possible CNS histoplasmosis from 33% in the earlier study [1] to 2% in this study (Table 2).
The possible category includes 5 patients in who histoplasmosis was identified but CNS involvement was not established by culture, cytopathology, or detection of antigen or antibody in the CSF. A similar approach was used previously in CNS histoplasmosis [1], blastomycosis [9], and coccidioidomycosis [10]. Exclusion of the possible cases did not impact the sensitivity of antigen or antibody detection for diagnosis of CNS histoplasmosis. Noteworthy is that the possible category used in this study differs from that described in the guidelines for diagnosis of invasive mycoses, in which possible cases had no mycologic evidence for diagnosis [13].
Schestatsky et al identified 17 cases of meningitis among 217 patients with disseminated histoplasmosis [2]. They reviewed the findings in 11 nonimmunocompromised patients with isolated CNS disease. All had chronic meningitis complicated by hydrocephalus. Detection of antibodies in the CSF by ID was the most sensitive method for diagnosis, positive in 7 of 8 (88%) patients. A single case with a negative antibody and culture was diagnosed by biopsy of a spinal cord mass. The authors concluded that the detection of antibody in CSF by ID is a sensitive method for diagnosis of Histoplasma meningitis. In contrast, in our study CSF antibody testing by CF and ID was only positive in about half of all cases, and in less than two-thirds of immunocompetent patients.
Immunocompromise increases fungal burden, which was reflected by a trend toward higher antigen concentration in immunocompromised than nonimmunocompromised patients. Immunocompromise also impairs antibody production [7], which was confirmed in this study. However, using the EIA assay, antibody was detected in the CSF in 68% of immunocompromised patients, including one in whom the antigen test was negative.
The specificity for detection of antigen in CSF was 97%. Cross-reactions were not observed in patients with meningitis caused by Cryptococcus, Aspergillus, Candida, or Mycobacterium tuberculosis. Prior studies confirmed lack of cross-reactivity in cryptococcal meningitis [14]. Antigen was detected in the CSF in patients with meningitis caused by Blastomyces, which contains cross-reactive antigens [7]. Antigen was detected in the CSF in 2 patients with extra-CNS histoplasmosis. Contamination of the CSF with serum antigens caused by a traumatic lumbar puncture or passive diffusion across an inflamed blood–brain barrier may have caused these positive results.
No cause for a false-positive antigen result at 2.2 ng/mL was apparent in the patient with a serum sickness–like allergic reaction to antibiotic treatment for Helicobacter gastritis. However, the result was below the LOQ upon repeat testing. The result would not have been reported as positive in clinical testing at MiraVista Diagnostics because of excessive variability between the 2 results.
The specificity for detection of intrathecal antibody production was 93%. Elevated levels of IgG antibodies were noted in the CSF in 3 of 12 (25%) patients with histoplasmosis without meningitis (Supplementary Table 1). These may be caused by contamination with antibodies present in the patient’s blood. Similar findings were noted in our earlier study of antibody detection by RIA [6]. One patient had cavitary histoplasmosis and a CF titer in serum of 1:16. The second patient presented with an intracranial hemorrhage complicated by Staphylococcus epidermidis meningitis caused by an infected intracranial pressure monitor and had a CF titer in serum of 1:8 [6]. Cross-reactions were noted in the CSF of patients with meningitis caused by blastomycosis or cryptococcosis.
Causes for positive antibody results in several patients with other conditions, including a case of tuberculous meningitis and of neurosarcoidosis are unknown. Histoplasmosis should be rigorously excluded before beginning immunosuppressive therapy for presumed neurosarcoidosis as failure to do so can be catastrophic. The specificity of antibody detection by ID was 100% but was performed in only 22 patients. Antibody was falsely positive by CF in 7% of patients, supporting earlier findings [6].
A major limitation of this study is its retrospective design and incomplete clinical and laboratory information. As CNS histoplasmosis is rare, a prospective study is not feasible. Another important limitation is incorporation bias caused by reliance on the use of MiraVista antigen EIA for diagnosis. However, excluding patients in which antigen results were used for diagnosis, representing one-third of cases, would limit the ability of the study to determine the role for antibody detection using the new EIA. Furthermore, the proportion of patients with positive antigen results was the same (78%) in all cases and in the subset excluding detection of antigen as the sole basis of diagnosis. More information is needed to determine the specificity of these methods in patients with neurosarcoidosis, tuberculosis, and other types of fungal meningitis.
In conclusion, CNS histoplasmosis should be included in the differential diagnosis in patients with subacute or chronic meningitis. Testing CSF for antigen and anti-Histoplasma antibodies by EIA provides the highest diagnostic yield with a sensitivity of 98% and a specificity of 90%. False-positive results, while uncommon, may occur with extra-CNS histoplasmosis, certain other fungal diseases, and unknown causes. Antibody testing by ID is also recommended because of its higher specificity and PPV (100%), but its lower sensitivity (44%) must be recognized. While the sensitivity for culture is low, cultivation remains the gold standard for confirming the diagnosis of CNS histoplasmosis, and may be the only basis for diagnosis in some cases. Culture should be performed by plating at least 10 mL of CSF using media suitable for isolation of Histoplasma and methods capable of detecting slow-growing organisms [15]. Repeat CSF analysis and brain or meningeal biopsy should be considered in patients with persistent findings if the initial tests are nondiagnostic, particularly if immunosuppressive therapy is considered or the patient is severely ill and diagnosis cannot be delayed [15].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the physicians who also contributed cases to the study: David Bamberger, University of Missouri, Kansas City; Vanja Douglas, University of California, San Francisco; Amy Stuart, Binford, Berkshire, United Kingdom; Bruce A. Hamilton, Cincinnati, Ohio; Nicolas Cortes-Penfield, Baylor College of Medicine, Houston, Texas; Paul H. Edelstein, Perelman School of Medicine, University of Pennsylvania, Philadelphia; Curtis J. FitzSimmons, University of Missouri, Kansas City School of Medicine; Randall T. Hayden, St Jude’s Children’s Research Hospital, Memphis, Tennessee; Scott Homman, University of Illinois College of Medicine, Rockfield; Bradford S. McGwire, Ohio State University, Columbus; Rana M. Nasser, Marshfield Clinic Health System, Marshfield, Wisconsin; Anne H. Rowley, Northwestern University Medical Center, Chicago, Illinois; Rebecca D. Shadowen, Commonwealth Medical Plaza, Bowling Green, Kentucky; Frederick T. Steiner, Indiana University Health Ball Memorial Hospital, Muncie; Donald F. Storey, Irving, Texas; Christine Tang, Community Health Network, Indianapolis, Indiana; Mallory D. Witt, Harbor-UCLA Medical Center, Torrance, California. We also thank Wesley Keown, MiraVista Diagnostics, Indianapolis, Indiana, for assisting with the statistical analysis and creation of the figures.
Potential conflicts of interest. L. J. W. is a medical director and part owner of MiraVista Diagnostics and A. A. is an employee of MiraVista Diagnostics, a company that offers the described tests commercially. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system. A clinical review. Medicine (Baltimore) 1990; 69:244–60. [DOI] [PubMed] [Google Scholar]
- 2. Schestatsky P, Chedid MF, Amaral OB, Unis G, Oliveira FM, Severo LC. Isolated central nervous system histoplasmosis in immunocompetent hosts: a series of 11 cases. Scand J Infect Dis 2006; 38:43–8. [DOI] [PubMed] [Google Scholar]
- 3. Gelfand JA, Bennett JE. Active Histoplasma meningitis of 22 years’ duration. JAMA 1975; 233:1294–5. [PubMed] [Google Scholar]
- 4. Wheat LJ, Freifeld AG, Kleiman MB et al. Infectious Diseases Society of America Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 5. Wheat LJ, Kohler RB, Tewari RP, Garten M, French ML. Significance of Histoplasma antigen in the cerebrospinal fluid of patients with meningitis. Arch Intern Med 1989; 149:302–4. [PubMed] [Google Scholar]
- 6. Wheat J, French M, Batteiger B, Kohler R. Cerebrospinal fluid Histoplasma antibodies in central nervous system histoplasmosis. Arch Intern Med 1985; 145:1237–40. [PubMed] [Google Scholar]
- 7. Hage CA, Ribes JA, Wengenack NL et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53:448–54. [DOI] [PubMed] [Google Scholar]
- 8. Richer SM, Smedema ML, Durkin MM et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 2016; 62:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bariola JR, Perry P, Pappas PG et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 2010; 50:797–804. [DOI] [PubMed] [Google Scholar]
- 10. Kassis C, Zaidi S, Kuberski T et al. Role of coccidioides antigen testing in the cerebrospinal fluid for the diagnosis of coccidioidal meningitis. Clin Infect Dis 2015; 61:1521–6. [DOI] [PubMed] [Google Scholar]
- 11. Swartzentruber S, LeMonte A, Witt J et al. Improved detection of Histoplasma antigenemia following dissociation of immune complexes. Clin Vaccine Immunol 2009; 16:320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wheat LJ, Kohler RB, Tewari RP. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N Engl J Med 1986; 314:83–8. [DOI] [PubMed] [Google Scholar]
- 13. De Pauw B, Walsh TJ, Donnelly JP et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhuang D, Hage C, De Jesus M et al. Cryptococcal glucoxylomannan does not exhibit cross-reactivity in the MVista Histoplasma antigen enzyme immunoassay. Clin Vaccine Immunol 2008; 15:392–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.