In a prospective, observational cohort, propensity-adjusted mortality was decreased in patients with carbapenem-resistant Enterobacteriaceae infections started on ceftazidime-avibactam versus colistin (absolute risk reduction 23% [95% CI, 9%–35%]; P = .001). Randomized controlled trials are needed to confirm findings.
Keywords: carbapenem-resistant Enterobacteriaceae, Klebsiella pneumoniae, colistin, ceftazidime-avibactam, benefit-risk
Abstract
Background
The efficacy of ceftazidime-avibactam—a cephalosporin–β-lactamase inhibitor combination with in vitro activity against Klebsiella pneumoniae carbapenemase–producing carbapenem-resistant Enterobacteriaceae (CRE)—compared with colistin remains unknown.
Methods
Patients initially treated with either ceftazidime-avibactam or colistin for CRE infections were selected from the Consortium on Resistance Against Carbapenems in Klebsiella and other Enterobacteriaceae (CRACKLE), a prospective, multicenter, observational study. Efficacy, safety, and benefit-risk analyses were performed using intent-to-treat analyses with partial credit and the desirability of outcome ranking approaches. The ordinal efficacy outcome was based on disposition at day 30 after starting treatment (home vs not home but not observed to die in the hospital vs hospital death). All analyses were adjusted for confounding using inverse probability of treatment weighting (IPTW).
Results
Thirty-eight patients were treated first with ceftazidime-avibactam and 99 with colistin. Most patients received additional anti-CRE agents as part of their treatment. Bloodstream (n = 63; 46%) and respiratory (n = 30; 22%) infections were most common. In patients treated with ceftazidime-avibactam versus colistin, IPTW-adjusted all-cause hospital mortality 30 days after starting treatment was 9% versus 32%, respectively (difference, 23%; 95% bootstrap confidence interval, 9%–35%; P = .001). In an analysis of disposition at 30 days, patients treated with ceftazidime-avibactam, compared with those treated within colistin, had an IPTW-adjusted probability of a better outcome of 64% (95% confidence interval, 57%-71%). Partial credit analyses indicated uniform superiority of ceftazidime-avibactam to colistin.
Conclusions
Ceftazidime-avibactam may be a reasonable alternative to colistin in the treatment of K. pneumoniae carbapenemase–producing CRE infections. These findings require confirmation in a randomized controlled trial.
The global spread of carbapenem-resistant Enterobacteriaceae (CRE) is an important threat to vulnerable patient populations worldwide [1–5]. Treatment options for CRE infections include polymyxins such as colistin and polymyxin B [6]. Concerns about polymyxins include toxicity, limited efficacy, dosing uncertainties and resistance, including worrisome mcr-1–mediated resistance [7–9]. Recently, ceftazidime-avibactam was approved for use by the Food and Drug Administration [10]. Avibactam is a non–β-lactam β-lactamase inhibitor that has activity against Ambler class A and class D serine carbapenemases, including Klebsiella pneumoniae carbapenemase (KPC) and OXA-48–like carbapenemases. In contrast, avibactam does not inhibit metallo-β-lactamase enzymes. Uncontrolled case series have shown variable outcomes in patients with CRE infections treated with ceftazidime-avibactam [11, 12]. Data comparing the use of ceftazidime-avibactam versus polymyxins in the treatment of CRE infections are limited.
The importance of patient-centered outcomes that go beyond mortality rates is increasingly recognized. Recently, several states of health were deemed by patients to be even worse than death, suggesting that patient-centered quality-of-life outcomes are important to measure [13]. Furthermore, when selecting an antibiotic strategy, issues of efficacy and safety should both be considered. In the current study, we analyzed the outcomes in patients initially treated with ceftazidime-avibactam versus colistin for CRE infections. We evaluated combined benefits and risks to estimate patient-level differences between ceftazidime-avibactam and colistin. The Consortium on Resistance Against Carbapenems in Klebsiella and Other Enterobacteriaceae (CRACKLE) study offered a unique opportunity to address these questions, because ceftazidime-avibactam was introduced into clinical practice while the study was ongoing.
METHODS
CRACKLE Study
CRACKLE is a prospective, observational study involving 18 hospitals that are a part of 8 healthcare systems predominantly located in the Great Lakes region of the United States, as described elsewhere (see Supplementary Table S1 for relative contributions of each hospital) [14–16]. All hospitalized patients who have a culture from which CRE is isolated are included. From 24 December 2011 until 1 January 2015, only data on patients with carbapenem-resistant Klebsiella pneumoniae were collected. From 1 January 2015 onward, all patients with any CRE were included. Clinical data on these patients were entered into a central, standardized database. Data collection methods did not change during the study period.
Patients and Clinical Data
In the study period from 24 December 2011 to 1 May 2016, all patients who started ceftazidime-avibactam or colistin treatment for a documented CRE infection were included (see Supplementary Table S2 for colistin dosing recommendations in place during the study period). Seven patients who started both colistin and ceftazidime-avibactam within a 24-hour window were excluded (See Supplementary Table S3). Standardized a priori definitions of infections were used, as described elsewhere [14]. Patients whose culture episode did not meet criteria for infection were excluded.
Patients with bacteremia were analyzed as such regardless of the primary source. Each patient was included once at the time of the most recent treated CRE infection. The Pitt bacteremia score based on the day of the index culture was calculated, and patients with a score ≥4 were considered critically ill [17]. The Charlson comorbidity index was determined at admission [18]. Renal failure was defined as a serum creatinine level ≥2 mg/dL and/or the use of renal replacement therapy. The study was approved by the institutional review boards of all study sites. Because CRACKLE is an observational study, treatment of CRE infections was at the discretion of the treating physician. Only patients who were treated with colistin or ceftazidime-avibactam were included in the study. Before the approval date of ceftazidime-avibactam, 81 patients (82% of the total colistin-first cohort) were included in the colistin-first group. After the approval date, an additional 18 patients (18% of the total colistin-first cohort) were included in the colistin-first group. Of note, no significant difference in unadjusted 30-day mortality was observed between these 2 groups (25 of 81 [31%] vs 8 of 18 [44%]; P = .28).
Microbiology
Guidelines from the Clinical and Laboratory Standards Institute were used to define CRE [19]. Bacterial identification and routine antimicrobial susceptibility testing was performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (bioMerieux), supplemented by GN4F Sensititre tray (Thermo Fisher) or Etest (bioMerieux), as indicated. On available isolates, detection of carbapenemase genes and repetitive extragenic palindromic (rep)–polymerase chain reaction (PCR) strain typing was performed as described elsewhere [14]. Briefly, PCR amplification of blaKPC, blaNDM, blaVIM, blaIMP, and blaOXA-48 genes was conducted using established primers and methods; amplicons were sequenced at a commercial sequencing facility (MCLAB), and analyzed [20, 21]. rep-PCR was performed using the DiversiLab Strain typing system (Bacterial BarCodes; bioMerieux). Isolates with ≥ 95% similarity were considered of the same rep-PCR type.
Statistical Methods
Intention-to-treat analyses were used to compare strategies of initiating ceftazidime-avibactam versus colistin for the initial treatment of CRE infection, in the presence of routine additional clinical care and consequential downstream adjustments to therapy. There were 3 analysis foci: efficacy, safety, and benefit-risk. Efficacy analyses were conducted using the efficacy analysis set (n = 137). Safety and benefit-risk analyses were conducted using the safety/benefit-risk analysis set consisting of the patients in the efficacy analysis without renal failure at treatment initiation (n = 72), because patients with renal failure at treatment initiation were not at risk for the major safety outcome of incident renal failure.
Analyses focused on ordinal outcomes (Table 1) constructed from benefits and harms experienced during the “patient journey” that have an important impact on patients. Ordinal outcomes have pragmatic advantages compared with separate analyses of the different outcomes [22]. Adjustment for confounding by indication was performed using inverse probability of treatment weighting (IPTW) [23, 24]. Covariates included in the model for the decision between ceftazidime-avibactam and colistin were Pitt score (dichotomized as <4 or ≥4), type of infection (bloodstream infection vs urinary tract infection vs other), and (in the main sensitivity analysis) creatinine level ≥2 mg/dL at the time of first positive culture.
Table 1.
Ordinal Outcomes for Efficacy, Safety, and Benefit-Risk Analyses With Categories in Ascending Order of Desirability
| Analysis | Outcomes |
|---|---|
| Efficacy | 1. Hospital death 2. Alive in hospital or discharged not to home 3. Discharged home |
| Safety | 1. Hospital death 2. Not observed to die, with incident renal failure 3. Not observed to die, without incident renal failure |
| Benefit-risk | 1. Hospital death 2. Alive in hospital or discharged not to home, incident renal failure 3. Alive in hospital or discharged not to home, no incident renal failure 4. Discharged home |
The primary efficacy analysis was an IPTW-adjusted disposition plot displaying the probability of disposition outcomes over time. The probability of hospital mortality in the first 30 days was compared between treatment groups adjusting for potential confounding by indication through IPTW. This analysis relies on correct specification of the treatment initiation model. In a sensitivity analysis, the primary outcome was evaluated by using the standardized risks, conditioning on the same confounders using a logistic regression model for the probabilities of the different outcome categories. This sensitivity analysis relies on correct specification of the logistic regression models for the outcomes in both treatment groups. In another sensitivity analysis for the primary outcome, we added Charlson score, age, and race to the list of confounders in the treatment initiation model.
Efficacy, safety, and benefit-risk were also analyzed using the desirability of outcome ranking (DOOR), resulting in estimates of the probability that a randomly selected patient initially treated with ceftazidime-avibactam would have a better overall outcome than a randomly selected patient initially treated with colistin (with half credit given for tied ranks) [22, 25]. Estimates were adjusted using IPTW.
The ordinal outcomes for safety and benefit-risk were also analyzed using the partial credit strategy, which provides a score of 1 (100%) to the most desirable category of the ordinal outcome, 0 (0%) to the least desirable, and partial credit to the intermediate categories [26]. For safety, the following scoring was implemented: the category “not observed to die, no renal failure” was scored as 1, the category “not observed to die, with renal failure” was given a partial credit of x, and the category “died in the hospital” was scored as 0. Analyses display the contrast between ceftazidime-avibactam and colistin as x varies, allowing for personalized patient-clinician team decision making.
For the benefit-risk analysis, the following scoring was implemented: the category “died in the hospital” was scored as 0; the category “alive in the hospital or discharged not to home with renal failure” was given a partial credit of x1, with 0 ≤ x1 ≤ 1; the category “alive in the hospital or discharged not to home without renal failure” was given a partial credit of x2 with x1 ≤ x2 ≤1; and the category “discharged home” was scored as 1. Analyses display the contrast between ceftazidime-avibactam and colistin as x1 and x2 vary. Estimates were adjusted using IPTW. The nonparametric bootstrap with 5000 replicates was implemented to obtain all confidence intervals (CIs) using Efron’s percentile method, and CIs were inverted to obtain P values.
RESULTS
Baseline Characteristics
During the study period, 137 patients within the CRACKLE study met criteria for CRE infection and started on treatment with ceftazidime-avibactam or colistin. Of the 137 patients, 38 (28%) received ceftazidime-avibactam first, and 99 (72%) colistin first. The baseline characteristics are summarized in Table 2 (IPTW-adjusted characteristics are in Supplementary Table S4). Patients tended to be both chronically and acutely ill, with a median Charlson comorbidity score of 3 (interquartile range [IQR], 1–5) and a median Pitt bacteremia score of 4 (2–6).
Table 2.
Baseline Characteristics
| Characteristic | Patients, No. (%)a | P Value | ||
|---|---|---|---|---|
| Ceftazidime-Avibactam (n = 38) | Colistin (n = 99) | All (N = 137) | ||
| Female sex | 15 (39) | 57 (58) | 72 (53) | .06b |
| Age, median (IQR), y | 57 (45–64) | 63 (54–76) | 61 (50–73) | .03c |
| Race/ethnicity | .71b | |||
| Black | 14 (37) | 42 (42) | 56 (41) | |
| White | 21 (55) | 47 (47) | 68 (50) | |
| Other | 3 (8) | 10 (10) | 13 (9) | |
| Charlson comorbidity index, median (IQR) | 2 (1–5) | 3 (2–5) | 3 (1–5) | .15c |
| Diabetes mellitus | 18 (47) | 42 (42) | 60 (44) | .60b |
| COPD | 5 (13) | 27 (27) | 32 (23) | .08b |
| History of malignancy | 7 (18) | 11 (11) | 18 (13) | .24b |
| Immunosuppressed | 11 (29) | 14 (14) | 25 (18) | .04b |
| Renal failure at admission | 8 (21) | 36 (36) | 44 (32) | .09b |
| Renal failure at time of culture | 11 (29) | 44 (44) | 55 (40) | .10b |
| Heart disease | 14 (37) | 50 (51) | 64 (47) | .15b |
| Critical illness at time of cultured | 7 (18) | 40 (40) | 47 (34) | .02b |
| Location at time of culture | .23b | |||
| Emergency department | 6 (16) | 20 (20) | 26 (19) | |
| Intensive care unit | 20 (53) | 61 (62) | 81 (59) | |
| Ward | 12 (32) | 18 (18) | 30 (22) | |
| Time to culture, median (IQR), de | 3 (0–8) | 2 (0–13) | 2 (0–12) | >.99c |
| Origin | .40b | |||
| Home | 18 (47) | 36 (36) | 54 (39) | |
| Hospital transfer | 7 (18) | 15 (15) | 22 (16) | |
| Skilled nursing facility | 11 (29) | 35 (35) | 46 (34) | |
| Long-term acute care | 2 (5) | 13 (13) | 15 (11) | |
| Type of infection | .59b | |||
| Bloodstream | 15 (39) | 48 (48) | 63 (46) | |
| Pneumonia | 9 (24) | 21 (21) | 30 (22) | |
| Urinary tract | 6 (16) | 13 (13) | 19 (14) | |
| Wound | 6 (16) | 8 (8) | 14 (10) | |
| Other | 2 (5) | 9 (9) | 11 (8) | |
| Type of CRE | >.99f | |||
| Klebsiella pneumoniae | 37 (97) | 96 (97) | 133 (97) | |
| Enterobacter sp. | 1 (3) | 3 (3) | 4 (3) | |
| Susceptibility (susceptible/tested) | ||||
| Colistin | 23/30 (77) | 63/68 (93) | 86/98 (88) | .04f |
| Ceftazidime-avibactam | 18/19 (95) | 5/5 (100) | 23/24 (96) | >.99f |
Abbreviations: COPD chronic obstructive pulmonary disease; CRE, carbapenem-resistant Enterobacteriaceae; IQR, interquartile range.
aData represent No. (%) unless otherwise specified.
bDetermined with χ2 test.
cDetermined with Wilcoxon rank-sum test.
dCritical illness defined as Pitt bacteremia score ≥4.
eTime from admission to culture.
fDetermined with Fisher exact test.
Almost half of patients (n = 63; 46%) presented with CRE bloodstream infection (sources are summarized in Supplementary Table S5). Other common infection types included respiratory tract infections in 30 patient (22%) and urinary tract infection in 19 (14%) (Table 2). Almost all patients (n = 133; 97%) were infected with K. pneumoniae; the other 4 (3%) were infected with Enterobacter spp. A total of 98 CRE isolates were tested to determine colistin susceptibility. Patients treated first with ceftazidime-avibactam were less likely to have colistin-susceptible isolates (23 of 30; 77% of tested) than patients treated with colistin first (63 of 68; 93% of tested; P = .04). Of the 24 isolates on which ceftazidime-avibactam susceptibility testing was performed, 23 (96%) were reported as susceptible. The single isolate that tested resistant was in the ceftazidime-avibactam–first treatment group. In 54 carbapenem-resistant K. pneumoniae isolates, the presence or absence of carbapenemase genes was tested; 28 (52%) and 24 (44%) were positive for blaKPC-2 and blaKPC-3, respectively. In 2 of 54 isolates (4%), no carbapenemase genes were found. None of the tested isolates was positive for blaNDM, blaVIM, blaIMP, or blaOXA-48. ST258A (18 of 54; 33%) and ST258B (23 of 54; 43%) were the most commonly encountered clades of carbapenem-resistant K. pneumoniae.
Efficacy, Safety, and Benefit-Risk
Efficacy
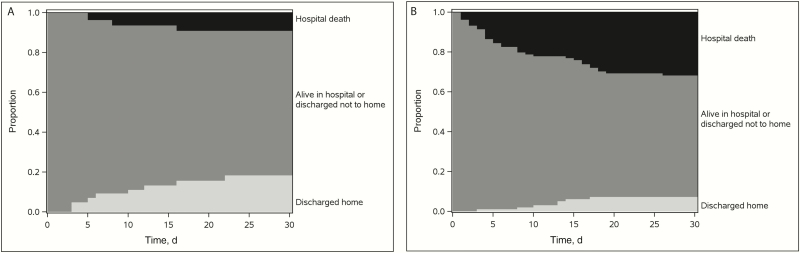
Efficacy was evaluated using the efficacy analysis set (n = 137). All-cause in-hospital mortality 30 days after the start of treatment was 3 of 38 (8%) in the ceftazidime-avibactam group versus 33 of 99 (33%) in the colistin group. After IPTW adjustment, the estimated adjusted percentages were 9% and 32%, respectively, resulting in a difference of 23% (95% CI, 9%–35%; P = .001; Table 3). Figure 1 displays the IPTW-adjusted disposition over time for patients initially treated with ceftazidime-avibactam (Figure 1A) versus those initially treated with colistin (Figure 1B). Patients treated with ceftazidime-avibactam were less likely to die and more likely to be discharged home during the first 30 days after starting treatment. DOOR analyses indicated that the IPTW-adjusted probability of a better outcome on ceftazidime-avibactam compared with colistin is 64% (95% CI, 57%–71%; Table 3).
Table 3.
Ordinal Outcomes and DOOR Estimates of Probability for Efficacy, Safety and Benefit-risk Outcomes in the First 30 Days of Treatmenta
| Outcome | Ceftazidime-Avibactam First | Colistin First | DOOR: IPTW-Adjusted Probability Estimate (95% CI) |
|||
|---|---|---|---|---|---|---|
| No. (%) | IPTW-Adjusted % (95% CI) | No. (%) | IPTW-adjusted % (95% CI) | IPTW-Adjusted Cumulative Difference for Colistin Minus Ceftazidime- Avibactam, % (95% CI) | ||
| Efficacy | ||||||
| disposition (n = 137) | n = 38 | n = 99 | 0.64 (.57– .71) | |||
| Hospital death | 3 (8) | 9 (3–20) | 33 (33) | 32 (23–41) | 23 (9–35) | |
| Alive in hospital or discharged not to home | 27 (71) | 72 (57–86) | 59 (60) | 61 (51–70) | 11 (−1 to 23) | |
| Discharged home | 8 (21) | 18 (8–31) | 7 (7) | 7 (3–13) | ||
| Safety | ||||||
| death and incident renal failure (n = 72) | n = 26 | n = 46 | 0.62 (.52–.72) | |||
| Hospital death | 2 (8) | 9 (3–24) | 12 (26) | 25 (13–38) | 16 (−2 to 32) | |
| Not observed to die, with incident renal failure | 1 (4) | 5 (3–19) | 6 (13) | 13 (4–24) | 24 (4–43) | |
| Not observed to die, without incident renal failure | 23 (88) | 86 (69–100) | 28 (61) | 62 (47–76) | ||
| Benefit-risk | ||||||
| analysis for death, discharge and incident renal failure (n = 72) | n = 26 | n = 46 | 0.64 (.53–.75) | |||
| Hospital death | 2 (8) | 9 (3–24) | 12 (26) | 25 (13–38) | 16 (−2 to 32) | |
| Alive in hospital or discharged not to home, incident renal failure | 1 (4) | 5 (3–19) | 5 (11) | 11 (3–21) | 22 (2–41) | |
| Alive in hospital or discharged not to home, no incident renal failure | 17 (65) | 65 (44–84) | 25 (54) | 56 (42–70) | 13 (−4 to 31) | |
| Discharged home | 6 (23) | 20 (7–38) | 4 (9) | 8 (2–16) | ||
Abbreviations: CI, confidence interval; DOOR, desirability of outcome ranking; IPTW, inverse probability of treatment weighting.
aThe DOOR estimates represent the probability that a randomly chosen patient from the study population has a more desirable outcome when starting ceftazidime-avibactam treatment than when starting colistin treatment.
Figure 1.
Inverse probability of treatment weighting (IPTW)–adjusted efficacy: disposition over time (n = 137; IPTW-adjusted probability estimates of hospital mortality and discharge status). A, Ceftazidime-avibactam group (n = 38). B, Colistin group (n = 99).
Safety
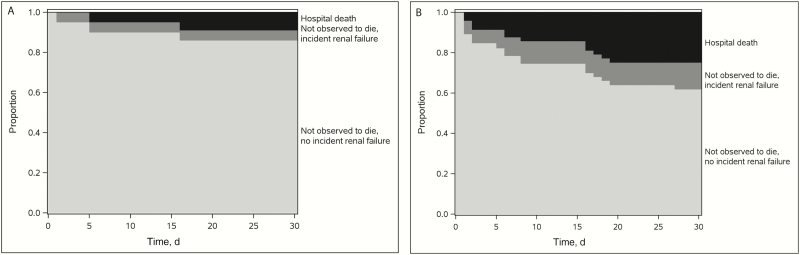
Safety was evaluated using the safety/benefit-risk analysis set (n = 72; 26 initially treated with ceftazidime-avibactam and 46 initially treated with colistin; see Supplementary Table S6 for IPTW-adjusted characteristics), which excludes the patients with renal failure at treatment initiation, that is, those not at risk for incident renal failure. Figure 2 displays the IPTW-adjusted safety outcome over time for patients initially treated with ceftazidime-avibactam (Figure 2A) versus those initially treated with colistin (Figure 2B). The IPTW-adjusted estimates for (1) hospital death, (2) not observed to die with incident renal failure, and (3) not observed to die without incident renal failure were 9%, 5%, and 86% for ceftazidime-avibactam, respectively, and 25%, 13%, and 62% for colistin (Table 3). DOOR analyses indicated that the IPTW-adjusted probability of a better outcome with ceftazidime-avibactam than with colistin is 62% (95% CI, 52%– 72%; Table 3).
Figure 2.
Inverse probability of treatment weighting (IPTW)–adjusted safety over time: renal failure (n = 72; restricted to patients at risk for incident renal failure, without renal failure at treatment initiation). A, Ceftazidime-avibactam group (n = 26). B, Colistin group (n = 46).
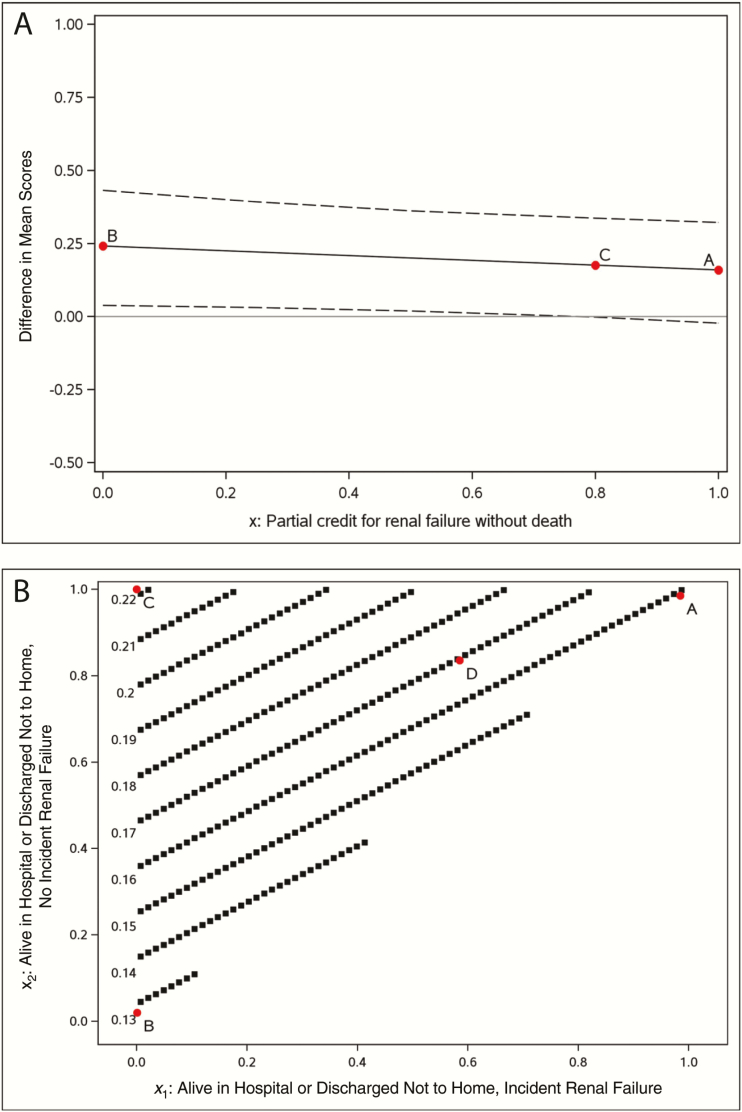
The estimated IPTW-adjusted between-treatment difference (ceftazidime-avibactam minus colistin) in mean scores (and associated 95% CIs) is displayed as a function of the partial credit assigned to those not observed to die with renal failure in Figure 3A. Analyses are based on patients without renal failure at treatment initiation. Being not observed to die without renal failure is assigned a credit of 1 and hospital death a credit of 0. Partial credit for being not observed to die with renal failure is assigned x (on the horizontal axis), with 0 ≤ x ≤ 1. Results are plotted as the partial credit x varies to allow visualization of the impact on the estimated treatment effect.
Figure 3.
Inverse probability of treatment weighting (IPTW)–adjusted partial credit analysis. A, Safety: estimated between-treatment difference (ceftazidime-avibactam minus colistin) in mean scores and associated 95% confidence bands, as a function of the partial credit assigned to those not observed to die with renal failure (more details in Section 5.25–5.45). B, Benefit-risk: estimated between-treatment difference (ceftazidime-avibactam minus colistin) in mean scores as a function of the partial credits assigned to those alive in the hospital or discharged not to home, with or without incident renal failure (more details in Section 5.85–6.45).
Positive differences indicate favorable results for ceftazidime-avibactam. For example, point A assigns a partial credit of 1 to patients not observed to die with renal failure, implying that renal failure is irrelevant and only survival matters, resulting in an estimated difference of 0.16 (95% CI, −.02 to .32), equivalent to a binary end point of hospital death. As another extreme example, point B assigns a partial credit of 0 to patients not observed to die with renal failure implying that incident renal failure is as poor an outcome as death, resulting in an estimated difference of 0.24 (95% CI, .04–.43). This is equivalent to a binary end point of not observed to die without renal failure. Point C assigns a partial credit of 0.8 (selected by the authors) to patients not observed to die with renal failure, resulting in an estimated difference of 0.18 (95% CI, 0–.34).
Benefit-Risk Analysis
Benefit-risk was evaluated using the safety/benefit-risk analysis set (n = 72). The IPTW-adjusted estimates for (1) hospital death, (2) alive in the hospital or discharged not to home with incident renal failure, (3) alive in the hospital or discharged not to home without incident renal failure, and (4) discharged home were 9%, 5%, 65%, and 20% for ceftazidime-avibactam respectively, and 25%, 11%, 56%, and 8% for colistin (Table 3). DOOR analyses indicated that the IPTW-adjusted probability of a better outcome with ceftazidime-avibactam than with colistin is 64% (95% CI, 53%–75%; Table 3).
The estimated between-treatment difference (ceftazidime-avibactam minus colistin) is displayed as a function of the partial credits assigned to (1) being alive in the hospital or discharged not to home with incident renal failure and (2) alive in the hospital or discharged not to home without incident renal failure, in Figure 3B. Estimates are adjusted using IPTW. Analyses are based on patients without renal failure at treatment initiation. Hospital death is assigned a credit of 0, and being discharged home a credit of 1. Partial credit is given for (1) being alive in the hospital or discharged not to home with incident renal failure (x1, with 0 ≤ x1 ≤ 1) and (2) being alive in the hospital or discharged not to home without incident renal failure (x2, with x1 ≤ x2 ≤ 1). Results are plotted as partial credits vary to allow visualization of the impact on the estimated treatment effect.
Positive differences indicate favorable results for ceftazidime-avibactam. For example, point A in Figure 3B is the extreme example of both x1 and x2 being assigned a partial credit of 1. This is equivalent to analysis of a hospital death resulting in an estimated difference of 0.16 (95% CI, −.02 to .32). Point B is another extreme example of both x1 and x2 being assigned a partial credit of 0. This is equivalent to analysis of being discharged home resulting in an estimated difference of 0.13 (95% CI, −.04 to .31]). Point C is another extreme example where x1 is assigned a partial credit of 0 and x2 a partial credit of 1. This is equivalent to analysis of a binary end point (being discharged home or alive in hospital or discharged not to home without incident renal failure versus other), resulting in an estimated difference of 0.22 (95% CI, .02–.41). As an example of a value judgment in which both disposition and development of renal failure are thought to be important, point D on the graph indicates assigned values of 0.6 for x1 and 0.8 for x2, resulting in an estimated difference of 0.17 (95% CI, .02–.30).
Antimicrobial Treatment
Anti-CRE directed treatment characteristics are outlined in Table 4 and Supplementary Table S7. The median time from collection of the index culture until starting ceftazidime-avibactam or colistin was similar in the 2 groups: 3 (IQR, 2–4) versus 2 (1–4) days, respectively, in the ceftazidime-avibactam and colistin groups (P = .22). The median durations of treatment were also similar: 10 (IQR, 5–26) versus 10 (4–18) days, respectively. The use of other antibiotics directed against CRE was common and included tigecycline (n = 72; 53%), aminoglycosides (n = 60; 44%), and carbapenems (n = 70; 51%), among others (Table 4). Of note, fewer patients (n = 24; 63%) in the ceftazidime-avibactam–first group received additional CRE-active antibiotics than in the colistin-first group (n = 93; 94%), P < .001. One patient in the ceftazidime-avibactam–first group received colistin later and 5 patients in the colistin-first group received ceftazidime-avibactam later in their treatment course.
Table 4.
Treatment Characteristics
| Characteristic | Patients, No. (%)a | P Value | ||
|---|---|---|---|---|
| Ceftazidime- Avibactam (n = 38) | Colistin (n = 99) | All (N = 137) | ||
| Time to treatment, median (IQR), db | 3 (2–4) | 2 (1–4) | 3 (1–4) | .22c |
| Duration of treatment, median (IQR), d | 10 (5–26) | 10 (4–18) | 10 (5–19) | .52d |
| Additional antibiotics | ||||
| None | 14 (37) | 6 (6) | 20 (15) | <.001e |
| Tigecycline | 12 (32) | 60 (61) | 72 (53) | .002e |
| Amikacin | 6 (16) | 23 (23) | 29 (21) | .34e |
| Gentamicin | 12 (32) | 14 (14) | 26 (19) | .02e |
| TMP/SMX | 4 (11) | 12 (12) | 16 (12) | .80e |
| Carbapenem | 11 (29) | 59 (60) | 70 (51) | .001e |
| Fosfomycin | 1 (3) | 3 (3) | 4 (3) | >.99c |
Abbreviations: IQR, interquartile range; TMP/SMX, trimethoprim/sulfamethoxazole.
aData represent No. (%) of patients, unless otherwise specified.
bDays from index culture until first dose of colistin or ceftazidime-avibactam.
cDetermined with Fisher exact test.
dDetermined with Wilcoxon rank-sum test.
eDetermined with χ2 test.
DISCUSSION
We compared the use of colistin versus ceftazidime-avibactam in the treatment of CRE infections. Clinical outcomes were better in the patients who were treated first with ceftazidime-avibactam rather than colistin. Specifically, all-cause 30-day hospital mortality was substantially decreased in the ceftazidime-avibactam group. A previous recent report of 3 cases of CRE bloodstream infection treated with ceftazidime-avibactam similarly reported good outcomes in these patients [11]. A larger retrospective study of 37 patients with CRE infections reported a 30-day survival of 76%, and clinical success in 59% [12]. However, of concern was the observed resistance to ceftazidime-avibactam that occurred in 3 of 10 patients with microbiologic failure [12]. In addition to treatment-emergent resistance, another important unanswered question is the impact of combination therapy in CRE infections when ceftazidime-avibactam is used. In the INCREMENT study, which did not include any patients treated with ceftazidime-avibactam, combination therapy tended to have the most benefit in more severely ill patients [27]. However, whether the same effect will be observed in patients treated with regimens containing ceftazidime-avibactam is unclear. In our cohort, ceftazidime-avibactam was used as monotherapy in 37% of patients.
Based on randomized controlled trials (RCTs), ceftazidime-avibactam was recently approved for complicated urinary tract infection and complicated intra-abdominal infections [10]. Another trial focused on ceftazidime-resistant pathogens in an open-label comparison with best-available therapy [28]. Nonetheless, an important unanswered question is how ceftazidime-avibactam performs overall in the treatment of CRE infections. Ideally, this question will be answered through an RCT. Unfortunately, results from any potential CRE-specific RCT are not imminently expected for ceftazidime-avibactam, although several carbapenem-resistant pathogen-specific RCT evaluating other novel therapeutic agents with in vitro activity against CRE are currently ongoing; examples include studies involving imipenem-relebactam (NCT02452047), meropenem-vaborbactam (NCT02168946), and cefiderocol (S-649266, NCT02714595).
The introduction of ceftazidime-avibactam during the study period of CRACKLE posed a unique opportunity to compare outcomes in patients treated with ceftazidime-avibactam versus colistin. Although important sources of bias remained, all data were collected in the same standardized, prospective manner. The relatively short study period decreases the likelihood that supportive, nonantibiotic treatment measures changed dramatically over the course of this study. For these reasons, this observational study represents a reasonable opportunity to inform the medical community about the relative efficacy and toxicity of ceftazidime-avibactam compared with colistin, while awaiting definitive RCT data.
Novel statistical methods such as DOOR and partial credit analyses were applied using ordinal outcomes. Ordinal outcomes allow for a more synthesized evaluation of benefits and harms, providing information on the overall effects on patients aligning with needs of clinicians when making treatment decisions and the priorities of patients. This is in contrast to segmented evaluation of treatment effects on outcomes where associations between component outcomes can be missed and competing risks can confound interpretation. This is particularly important when treatments have toxic effects that affect patient function and quality of life. Partial credit analyses allow for individual patient-clinician teams to apply their own values or preferences (ie, their own partial credit) regarding outcomes to obtain personalized estimates of treatment effects to guide decisions. Here, we observed that for all possible values of partial credit, ceftazidime-avibactam performed better than colistin. However, we included only disposition and renal failure in these analyses. Other potential adverse events associated with antibiotics, such as Clostridium difficile infection, rash, or hypersensitivity reactions, should be included in future studies.
Our study has several limitations. First, it was an observational study in which treatment was not randomly assigned. Confounding by indication is therefore a potential issue [29]. We have addressed this issue by applying IPTW. However, any confounding adjustment method can adjust only for measured variables and relies on correct specification of the adjustment models. Unmeasured variables associated with the outcomes of interest may have been unevenly distributed between patient groups, and, given the available sample, we could not adjust for all measured potential confounders. An indicator of lack of randomization of treatment allocation is the higher proportion of colistin-resistant CRE in the ceftazidime-avibactam group. Nonetheless, sensitivity analyses led to findings very similar to the ones presented here. Second, detailed data on dosing of colistin were not available for these patients. It is possible that the observed differences may in part be a consequence of relative underdosing of colistin. Third, the number of patients included in this study is relatively small. Nonetheless, this is the largest comparative study to date to address this question.
In summary, we report here evidence for superiority of ceftazidime-avibactam over colistin in the initial treatment of infections caused by K. pneumoniae carbapenemase–producing CRE. The use of ceftazidime-avibactam was associated with improved clinical outcomes, especially decreased all-cause hospital mortality rate and improved benefit-risk outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy And Infectious Diseases, National Institutes of Health (NIH; awards UM1AI104681 and R21AI114508 [D. v. D. and R. A. B.], and R01AI100560, R01AI063517, and R01AI072219 [R. A. B.]; and Division of Microbiology and Infectious Diseases protocol 10–0065 and award R01AI119446-01 to K. S. K.); the Clinical and Translational Science Collaborative of Cleveland (D. v. D. and F. P.); the National Center for Advancing Translational Sciences component of the NIH and the NIH Roadmap for Medical Research (grant UL1TR000439); the NIH (Mid-Career Mentoring Award K24-AI093969 to V. G. F. and research awards R01AI104895 and R21AI107302 to Y. D.); the Cleveland Department of Veterans Affairs (award 1I01BX001974 to R. A. B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10); the Research Program Committees of the Cleveland Clinic (D. v. D.); and the STERIS Corporation (unrestricted research grant to D. v. D.).
Potential conflicts of interest. D. v. D. has served on advisory boards for Allergan, Achaogen, Shionogi, Tetraphase, Sanofi-Pasteur, MedImmune, and Astellas and received research funding from Steris and Scynexis. S. S. R has received research support from bioMerieux, BD Diagnostics, BioFire, OpGen, Forest Laboratories, Achaogen, Nanosphere, and Pocared, and an honorarium from bioMerieux. R. R. W. has received grant support from Allergan. Y. D. has received grant support from The Medicines Company and the NIH and has served on advisory boards for Meiji, Tetraphase, and Achaogen. K. S. K. has served as a consultant and grant investigator and on the speakers’ bureau for Allergan, from which he has also received a consulting fee, a grant, and a speaker honorarium; has received a grant from and served as a consultant for Merck; and has served as a consultant for Xellia and Achaogen. R. A. B. has served as a grant investigator and received grants from AstraZeneca, Merck, Melinta, Steris, the NIH, and VA Merit Review Award Program. V. G. F. has received grant or research support from Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, and Theravance; has served as a paid consultant for Affinium, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, The Medicines Company, MedImmune, Pfizer, Theravance, and Trius; has received honoraria from Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, and Vertex Pharmaceuticals; and has served as a Merck V710 Vaccine cochair. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
Presented in part: European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Amsterdam, the Netherlands, 10 April 2016.
References
- 1. Munoz-Price LS, Poirel L, Bonomo RA et al. . Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tumbarello M, Trecarichi EM, De Rosa FG et al. ; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–43. [DOI] [PubMed] [Google Scholar]
- 3. Gomez-Simmonds A, Nelson B, Eiras DP et al. . Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2016; 60:3601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falcone M, Russo A, Iacovelli A et al. . Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 2016; 22:444–50. [DOI] [PubMed] [Google Scholar]
- 5. Hauck C, Cober E, Richter SS et al. ; Antibacterial Resistance Leadership Group Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 2016; 22:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 2013; 75:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao X, Doi Y, Zeng L, Lv L, Liu JH. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect Dis 2016; 16:288–9. [DOI] [PubMed] [Google Scholar]
- 8. Liu YY, Wang Y, Walsh TR et al. . Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16:161–8. [DOI] [PubMed] [Google Scholar]
- 9. Rojas LJ, Salim M, Cober E et al. ; Antibacterial Resistance Leadership Group Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 2017; 64:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu G, Abraham T, Lee S. Ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 2016; 63:1147–8. [DOI] [PubMed] [Google Scholar]
- 12. Shields RK, Potoski BA, Haidar G et al. . Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med 2016; 176:1557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Duin D, Perez F, Rudin SD et al. . Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Messina JA, Cober E, Richter SS et al. . Hospital readmissions in patients with carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2016; 37:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Duin D, Cober ED, Richter SS et al. . Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin Microbiol Infect 2014; 20:O1117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 1999; 11:7–12. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 19. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Wayne, PA: CLSI; 2014. [Google Scholar]
- 20. Lascols C, Hackel M, Marshall SH et al. . Increasing prevalence and dissemination of NDM-1 metallo-beta-lactamase in India: data from the SMART study (2009). J Antimicrob Chemother 2011; 66:1992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viau RA, Hujer AM, Marshall SH et al. . “Silent” dissemination of Klebsiella pneumoniae isolates bearing K. pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio. Clin Infect Dis 2012; 54:1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans SR, Rubin D, Follmann D et al. . Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015; 61:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 24. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 25. Evans SR, Follmann D. Comment: fundamentals and innovation in antibiotic trials. Stat Biopharm Res 2015; 7:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans SR, Follmann D. Using outcomes to analyze patients rather than patients to analyze outcomes: a step toward pragmatism in benefit:risk evaluation. Stat Biopharm Res 2016; 8:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M et al. ; REIPI/ESGBIS/INCREMENT Investigators Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17:726–34. [DOI] [PubMed] [Google Scholar]
- 28. Carmeli Y, Armstrong J, Laud PJ et al. . Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016; 16:661–73. [DOI] [PubMed] [Google Scholar]
- 29. Evans SR, Harris AD. Methods and issues in studies of CRE. Virulence 2017; 8:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.