Abstract
Background
Human immunodeficiency virus (HIV)–exposed infants are disproportionately at risk of morbidity and mortality compared with their HIV-unexposed counterparts. The role of co-trimoxazole preventive therapy (CPT) in reducing leading causes of infectious morbidity is unclear.
Methods
We used data from the Breastfeeding, Antiretrovirals and Nutrition (BAN) clinical trial (conducted 2004–2010, Malawi) to assess the association of (1) CPT and (2) asymptomatic malaria parasitemia with respiratory and diarrheal morbidity in infants. In June 2006, all HIV-exposed infants in BAN began receiving CPT (240 mg) from 6 to 36 weeks of age, or until weaning occurred and HIV infection was ruled out. All HIV-exposed, uninfected infants (HEIs) at 8 weeks of age (n = 1984) were included when CPT was the exposure. A subset of HEIs (n = 471) were tested for malarial parasitemia using dried blood spots from 12, 24, and 36 weeks of age. Cox proportional hazards models for recurrent gap-time data were used to examine the association of time-varying exposures on morbidity.
Results
CPT was associated with a 36% reduction in respiratory morbidity (hazard ratio [HR], 0.64 [95% confidence interval {CI}, .60–.69]) and a 41% reduction in diarrheal morbidity (HR, 0.59 [95% CI, .54–.65]). Having asymptomatic malaria parasitemia was associated with a 40% increase in respiratory morbidity (HR, 1.40 [95% CI, 1.13–1.74]) and a 50% increase in diarrheal morbidity (HR, 1.50 [95% CI, 1.09–2.06]), after adjusting for CPT.
Conclusions
CPT may have an important role to play in reducing the leading global causes of morbidity and mortality in the growing population of HEIs in malaria-endemic resource-limited settings.
Keywords: co-trimoxazole, HIV, infant, infectious morbidity, malaria
Summary
Co-trimoxazole was associated with reduced infectious morbidity among HIV-exposed, uninfected infants in Malawi. Asymptomatic parasitemia was associated with increased infectious morbidity. Co-trimoxazole may play an important role in reducing morbidity among HIV-exposed, uninfected infants in malaria-endemic settings.
Diarrhea, respiratory infections, and malaria are among the leading causes of morbidity and mortality in children <5 years of age worldwide [1]. Human immunodeficiency virus (HIV)–exposed infants are disproportionately at risk of morbidity and mortality compared with their HIV-unexposed counterparts [2, 3]. HIV prevalence in pregnant women has been as high as 35% in some sub-Saharan Africa settings [4]. When adhering to antenatal, intrapartum, and postnatal World Health Organization (WHO) antiretroviral treatment recommendations, approximately 98% of HIV-infected mothers will not transmit HIV to their infants [5]. Therefore, the population of HIV-exposed, uninfected infants (HEIs) is growing as antiretroviral (ARV) programs roll out.
Effective interventions for preventing and treating diarrhea and respiratory infections exist [6–9]. However, ensuring timely access to proven life-saving interventions remains challenging [10]. Co-trimoxazole is a combined broad-spectrum antibiotic used to prevent and treat certain infections, including Pneumocystis jirovecii pneumonia, respiratory tract, and other infections. Co-trimoxazole also has antimalarial activity. To ensure that infants who do become HIV infected receive timely co-trimoxazole preventive therapy (CPT) to prevent often fatal opportunistic infections, WHO recommends that all HIV-exposed infants begin CPT between 4 and 6 weeks of age and continue CPT until complete cessation of breastfeeding and exclusion of HIV infection [11]. However, there has been conflicting evidence regarding the benefits of giving daily CPT to the growing population of HEIs. No difference in the rate of survival was observed in a recent randomized controlled trial that randomized HEIs to receive either CPT or a placebo from 2 to 4 weeks of age until 15 months of age in Botswana [12]. However, we have shown that CPT protected HEIs against severe (grade 3 or 4) infant morbidity and mortality in the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study in Malawi [13]. We have also shown that CPT protected HEI against asymptomatic malaria parasitemias, defined as polymerase chain reaction (PCR)–positive samples collected at non-ill routine visits, potentially reducing not only infant morbidity but also malaria transmission [14]. For this analysis, we explored (1) whether CPT protects HEI from 2 of the leading causes of childhood morbidity and mortality—respiratory and diarrheal infections—in a setting of high malaria endemicity and regardless of severity grade; and (2) whether presence of asymptomatic malaria parasitemia was associated with increased risk of respiratory and diarrheal infections.
METHODS
An observational cohort study was conducted using data and specimens from the BAN study. Details of the BAN study have been reported previously [15]. In brief, the BAN study was conducted in Lilongwe, Malawi, from 2004 to 2010, to assess the benefit and safety of maternal or infant ARVs to prevent HIV transmission during breastfeeding. HIV-positive pregnant women were recruited at antenatal clinics and randomized after delivery via a factorial design to receive, or not receive, a lipid-based nutrient supplement throughout breastfeeding and to receive 1 of the following postpartum prevention of mother-to-child HIV transmission regimens: (1) 28 weeks of maternal triple ARVs; (2) 28 weeks of daily infant nevirapine (NVP); or (3) no further ARVs postpartum (enhanced control). All mothers and infants received 1 dose of NVP at delivery or birth and 7 days of postpartum zidovudine and lamivudine. Mothers were counseled to exclusively breastfeed for 24 weeks and wean from 24 to 28 weeks postpartum. Mothers with CD4+ T-cell count >200–250 cells/μL and infants weighing at least 2000 g at birth were enrolled in the BAN study. Ethical approval for the BAN study was obtained from the Malawi National Health Science Research Committee and the institutional review boards at the University of North Carolina at Chapel Hill and the US Centers for Disease Control and Prevention.
All infants enrolled in BAN who remained HIV uninfected at 8 weeks of age (n = 1984) were included in analyses of CPT and both diarrheal and respiratory morbidity. Infants who became HIV infected after 8 weeks of age were censored at the time of their last HIV-negative test. Infant HIV status was determined by Roche Amplicor 1.5 DNA PCR (Roche Molecular Systems, Pleasanton, California) at 2, 12, 28, and 48 weeks. PCR-positive results were confirmed by testing an additional blood specimen. The window of infection was then narrowed by testing infant dried blood spot specimens collected at 4, 6, 8, 18, 24, 32, and 36 weeks.
Analyses of asymptomatic malaria parasitemias and infectious morbidity included a subset of infants (n = 471) who remained HIV uninfected through 48 weeks of age and were tested for asymptomatic parasitemias using stored dried blood spots from 12, 24, and 36 weeks of age. Selection of infants for malaria parasitemia testing has been previously described [14]. In brief, infants were chosen based on birthdate to mitigate the impact of calendar time on malaria prevalence. In addition, only specimens collected during peak malaria season (October–April) were used to increase the likelihood of detecting asymptomatic parasitemias.
Molecular analysis to determine asymptomatic malaria parasitemias was conducted using real-time PCR to detect the single-copy gene Plasmodium falciparum lactate dehydrogenase (pfldh) as previously described [16]. Samples testing positive for malaria parasites by 2 previously described real-time PCR assays were considered positive [17]. Samples positive with only 1 assay were considered indeterminate and not used in analyses. Asymptomatic malaria infection was treated as a dichotomous time-varying variable (detectable vs undetectable parasitemia).
All HIV-exposed infants began receiving CPT as part of the BAN study on 13 June 2006, in accordance with WHO and Malawi Ministry of Health guidelines. CPT (240 mg once daily) was initiated at 6 weeks of age and continued through 36 weeks of age or until weaning occurred and HIV infection was ruled out. CPT was treated as a time-varying exposure, with infants attending BAN study visits prior to 13 June 2006 considered CPT unexposed and infants attending BAN study visits after 13 June 2006 considered CPT exposed.
Infants with a documented case of diarrhea or respiratory infection, regardless of severity grade, from 8 to 48 weeks of age on any 1 of the following routine study visit forms were considered to have the outcome of interest: infant’s physical examination form, infant symptom form, and the infant’s concomitant medication log. Diarrhea or respiratory events occurring >2 weeks apart were treated as separate events. In analyses of asymptomatic parasitemias and infectious morbidity, only diarrhea or respiratory events occurring from 12 to 48 weeks were used to ensure that the outcome did not occur prior to the first sample tested. To estimate associations, asymptomatic parasitemia was defined using the most recent sample collected before the morbidity outcome.
A change-in-estimate approach was used to assess for confounding. Potential confounding variables consisted of rainy season (November to March), BAN randomization arm, and co-trimoxazole status (for association between asymptomatic parasitemias and both diarrheal and respiratory morbidity). Potential confounders that did not change the point estimate by >10% were dropped to create a more parsimonious model. Cox proportional hazards models for recurrent gap-time data with robust variance estimators [18] were used to estimate associations between (1) CPT and diarrheal or respiratory morbidity from 8 to 48 weeks of age, and (2) asymptomatic parasitemias and diarrheal or respiratory morbidity from 12 to 48 weeks of age, allowing for repeated events of diarrhea or respiratory infection. All data analyses were conducted using SAS version 9.3 software (SAS Institute, Cary, North Carolina).
RESULTS
A total of 1984 infants contributed 1414 person-years (PY) of follow-up to analyses of CPT and infectious morbidity (CPT unexposed: 260 PY, CPT exposed: 1154 PY). The median infant birth weight was 3.0 kg (interquartile range [IQR], 2.7–3.3). Most mothers were married (92%) and reported at least 1 previous live birth (88%) (Table 1). Fifty percent of mother–infant pairs were randomized to receive a maternal lipid-rich nutrient supplement. Overall, 35% of mother–infant pairs were randomized to the maternal ARV arm, 37% to the infant NVP arm, and 27% to the enhanced control arm of the BAN study. Mothers had a median baseline CD4+ count of 440 cells/μL (IQR, 332–582 cells/μL) and 60% had a baseline plasma HIV RNA load >10000 copies/mL of blood. The median maternal age was 26 years (IQR, 23–29 years).
Table 1.
Baseline Characteristics of 1984 Mother–Infant Pairs
| Characteristic | Totala | |
|---|---|---|
| No. | (%) | |
| Antiretroviral randomization | ||
| Maternal antiretroviral | 701 | (35) |
| Infant nevirapine | 739 | (37) |
| Enhanced controlb | 544 | (27) |
| Nutritional randomization | ||
| Received supplement | 992 | (50) |
| Mothers | ||
| Age, y | ||
| 15–25 | 945 | (48) |
| 26–35 | 936 | (47) |
| 36–45 | 98 | (5) |
| Primary school only | 1286 | (65) |
| Married | 1833 | (92) |
| Parity ≥1 | 1733 | (88) |
| CD4+ count, cells/μL | ||
| 200–350 | 573 | (29) |
| 351–500 | 663 | (33) |
| >500 | 748 | (38) |
| Plasma viral load, copies/mL | ||
| ≤1000 | 204 | (10) |
| 1001–10000 | 586 | (30) |
| >10000 | 1189 | (60) |
| Hemoglobin <11 g/dL | 1038 | (52) |
| Infants | ||
| Female | 1022 | (52) |
| Birth weight <2.5 kg | 136 | (7) |
aMaternal age and baseline viral load are missing for 5 mothers, education is missing for 2 mothers, and parity is missing for 6 mothers.
bMothers and infants randomized to the enhanced control arm received single-dose nevirapine peripartum plus twice-daily zidovudine and lamivudine during labor and for 7 days postpartum.
CPT-exposed infants experienced 5.32 respiratory events and 2.87 diarrhea events per 100 person-weeks between 8 and 48 weeks of age. The incidence rate of respiratory and diarrheal morbidity among CPT-unexposed infants was 8.09 and 4.39 per 100 person-weeks, respectively. CPT was associated with a 36% relative reduction in respiratory morbidity (hazard ratio [HR], 0.64 [95% confidence interval {CI}, .60–.69]) and a 41% relative reduction in diarrheal morbidity (HR, 0.59 [95% CI, .54–.65]). Adjustment for rainy season and randomization arm resulted in similar findings (respiratory HR, 0.65 [95% CI, .60–.69]; diarrheal HR, 0.59 [95% CI, .54–.65]) (Table 2).
Table 2.
Hazard Ratiosa for the Association Between Co-trimoxazole Preventive Therapy From 6 to 36 Weeks of age and Infant Diarrhea or Respiratory Infection From 8 to 48 Weeks of Age
| Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | |
| Diarrhea | ||||
| Routine CPT vs no routine CPT | 0.59 | (.54–.65) | 0.59 | (.54–.65) |
| Respiratory infection | ||||
| Routine CPT vs no routine CPT | 0.64 | (.60–.69) | 0.65 | (.60–.69) |
Abbreviations: CI, confidence interval; CPT, co-trimoxazole preventive therapy; HR, hazard ratio.
aAdjusted for rainy season (November–March) and Breastfeeding, Antiretrovirals and Nutrition study antiretroviral and nutrition randomization arm.
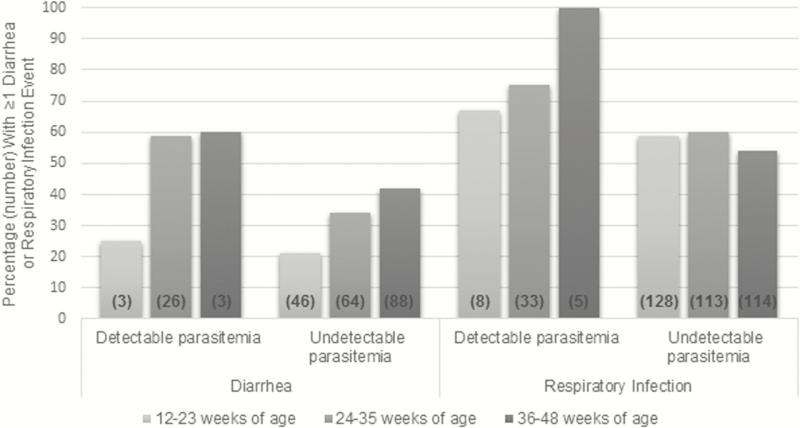
A total of 676 dried blood spots from 471 infants were tested for asymptomatic malaria parasitemias at 12, 24, and 36 weeks of age (230 specimens at 12 weeks, 231 at 24 weeks, and 215 at 36 weeks). As previously reported, a total of 61 (9%) specimens had detectable parasitemias during peak malaria season [14]. Specifically, 12 (5%) infants experienced asymptomatic malaria infection at 12 weeks of age, 44 (19%) at 24 weeks, and 5 (2%) at 36 weeks of age. A greater proportion of infants with detectable parasitemias had at least 1 episode of diarrhea and respiratory infection between 12 and 23 weeks of age, 24 and 35 weeks of age, and 36 and 48 weeks of age, compared with infants with no detectable parasitemias (Figure 1). Having asymptomatic parasitemias was associated with a 50% relative increase in diarrheal morbidity (HR, 1.50 [95% CI, 1.09–2.06]) and a 40% relative increase in respiratory morbidity (HR, 1.40 [95% CI, 1.13–1.74]) after adjusting for CPT status, rainy season (November–March), and BAN antiretroviral and nutrition randomization arms (Table 3).
Figure 1.
Percentage and number of human immunodeficiency virus–exposed, uninfected infants with ≥1 diarrhea or respiratory infection event, by malaria parasitemia status and weeks of age.
Table 3.
Hazard Ratiosa for the Association Between Asymptomatic Malaria at 12, 24, and 36 Weeks of Infant Age and Infant Diarrhea or Respiratory Infection From 12 to 48 Weeks of Age
| Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | |
| Diarrhea | ||||
| Asymptomatic parasitemias vs no parasitemias | 1.93 | (1.45–2.57) | 1.50 | (1.09–2.06) |
| Respiratory infection | ||||
| Asymptomatic parasitemias vs no parasitemias | 1.59 | (1.31–1.94) | 1.40 | (1.13–1.74) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted for co-trimoxazole status, rainy season (November–March) and Breastfeeding, Antiretrovirals and Nutrition study antiretroviral and nutrition randomization arm.
DISCUSSION
CPT was associated with a significant reduction in respiratory and diarrheal morbidity in HEIs. In contrast, asymptomatic malaria infection was associated with a significant increase in respiratory and diarrheal morbidity, after adjusting for CPT status. This adds to our previous findings of CPT being associated with reduced risk of both clinical and asymptomatic malaria [14], as well as severe infectious morbidity and mortality in HEIs in Malawi [13].
Similar incidence of diarrhea and pneumonia was seen among HIV-exposed, uninfected children in Uganda randomized to either discontinue co-trimoxazole after complete cessation of breastfeeding or to continue co-trimoxazole until 2 years of age [19]. However, only outcomes occurring after cessation of breastfeeding were assessed; as younger infants suffer generally higher rates and more severe morbidity (and mortality) of infections, the effects of any intervention need to be examined in each age group specifically.
The association between asymptomatic malarial infection and increasing infant morbidity from other causes has biological plausibility. It is possible that asymptomatic malaria infection may make infants more susceptible to other infections by increasing immune activation, decreasing effective immune responsiveness, or by predisposing to anemia and increase metabolic demands. Notably, helminthic infections are known to compromise vaccine responses and shift immunity to Th2-type response that can impair the control of replication of viral and other infections [20], suggesting that prevention of parasitic infections, including malaria, in childhood needs to be a global health priority. Of interest, and concurrent with our findings, an association between malaria parasitemia and Salmonella septicemia was previously noted in young children residing in a malaria-endemic area [21].
In Botswana, similar mortality was seen between HIV-exposed, uninfected children randomized to receive CPT and those randomized to placebo from 14 to 34 days of life until age 15 months [12]. In contrast, we previously found lower mortality among infants who received CPT in BAN compared with infants who did not [13]. There are several potential reasons for the difference in findings. First, CPT was not randomized in BAN, creating the potential for confounding by unmeasured variables (eg, bed net or artemisinin combination therapy use). Second, unlike Malawi, Botswana is not a malaria-endemic country. Third, Botswana has a lower overall infant mortality rate than Malawi. In addition, pneumococcal and rotavirus vaccines were introduced into the national immunization schedule in Botswana in 2012, resulting in significant declines in hospitalizations and death due to gastroenteritis [22] and, potentially, respiratory infection. Pneumococcal and rotavirus vaccines are not part of the immunization schedule in Malawi. A similar randomized controlled trial of co-trimoxazole prophylaxis in breastfed HEIs is planned in South Africa [23]. However, only 10% of the South African population lives in malaria-endemic areas [24], potentially limiting the generalizability of findings to resource-limited malaria-endemic countries.
Our study has some limitations. Misclassification of CPT exposure or infectious morbidity status may have occurred. CPT status was based on calendar time. However, a prior random review of pharmacy records in the BAN study indicated good adherence to CPT guidelines [25]. Diarrhea and respiratory events were determined by retrospective review of clinical forms, including concomitant medication logs. Patient symptoms, as well as medications prescribed for diarrhea and respiratory infection, are not always unique to these conditions. Therefore, some outcome misclassification may have occurred. However, our definition of outcome represents clinical practice in resource-limited settings where laboratory capacity to confirm diagnoses may be limited. Unmeasured variables that could affect the incidence of respiratory or diarrheal morbidity over the included time period could also have confounded our results. Selection bias may have occurred if infants tested or not tested for asymptomatic malaria parasitemia differed by unmeasured variables, such as multiple antibiotic use. However, infants did not differ by measured baseline covariates collected in BAN [14]. Testing results for asymptomatic parasitemia were only available for the 12-, 24-, and 36-week study visits, requiring reliance on prior study visits to define this exposure for many morbidity outcomes. This may have led to some misclassification of exposure.
We have shown CPT to be associated with reduced infectious morbidity and reduced asymptomatic and clinical malaria in HIV-exposed, uninfected infants in a malaria-endemic setting. In addition, our finding that asymptomatic malarial infection is associated with increased infectious morbidity from other causes in infants suggests another possible mechanism by which CPT may decrease infectious morbidity and improve the health of infants in malaria-endemic settings. This builds on previous work that demonstrated reduced mortality in HEIs in this population. CPT may have an important role to play in reducing the leading global causes of morbidity and mortality in the growing population of HEIs in malaria-endemic, resource-limited settings. Randomized controlled trials to directly study the effects of CPT in improving infant and young child health in malaria-endemic, resource-limited settings are needed to inform policy and practice.
Notes
Acknowledgments. We are especially grateful to all the women and infants who participated in the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study. The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. The BAN study was supported by grants from the Prevention Research Centers Special Interest Project of the CDC (SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01); the National Institute of Allergy and Infectious Diseases (R03 AI100694, R56 AI091547, and U01 AI068632); the University of North Carolina Center for AIDS Research (P30 AI050410); the National Institutes of Health (NIH) Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 TW01039-06); the Fogarty International Clinical Research Scholars Program (R24 TW007988); the American Recovery and Reinvestment Act); and the Infectious Disease Epidemiology Training Grant (5T32 AI070114). The Call to Action prevention of mother-to-child HIV transmission program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the US Agency for International Development.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the Breastfeeding, Antiretrovirals and Nutrition (BAN) Study Team:
Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki, David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki, Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba
References
- 1. Liu L, Oza S, Hogan D et al. . Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 2. Brennan AT, Bonawitz R, Gill CJ et al. . A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016; 30:2351–60. [DOI] [PubMed] [Google Scholar]
- 3. Koyanagi A, Humphrey JH, Ntozini R et al. ; ZVITAMBO Study Group Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J 2011; 30:45–51. [DOI] [PubMed] [Google Scholar]
- 4. Eaton JW, Rehle TM, Jooste S et al. . Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS 2014; 28(suppl 4):S507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect 2012; 88(Suppl 2):i44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health 2008; 98:1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clasen TF, Alexander KT, Sinclair D et al. . Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2015. doi:10.1002/14651858.CD004794.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munos MK, Walker CL, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol 2010; 39(suppl 1):i75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simoes EA, Cherian T, Chow J, Shahid-Salles SA, Laxminarayan R, John TJ. Acute respiratory infections in children. In: Jamison DT, Breman JG, Measham AR. et al. eds. Disease Control Priorities in Developing Countries. 2nd ed New York: Oxford University Press, 2006. [Google Scholar]
- 10. Carvajal-Vélez L, Amouzou A, Perin J et al. . Diarrhea management in children under five in sub-Saharan Africa: does the source of care matter? A countdown analysis. BMC Public Health 2016; 16:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 12. Lockman S, Hughes M, Powis K, et al. . Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu study): a double-blind, randomised, placebo-controlled trial. Lancet Glob Health. 2017; 5(5):e491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kourtis AP, Wiener J, Kayira D et al. . Health outcomes of HIV-exposed uninfected African infants. AIDS 2013; 27:749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis NL, Barnett EJ, Miller WC et al. . Impact of daily cotrimoxazole on clinical malaria and asymptomatic parasitemias in HIV-exposed, uninfected infants. Clin Infect Dis 2015; 61:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Horst C, Chasela C, Ahmed Y et al. ; Breastfeeding, Antiretroviral, and Nutrition Study Team Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the breastfeeding, antiretroviral, and nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials 2009; 30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pickard AL, Wongsrichanalai C, Purfield A et al. . Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother 2003; 47:2418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor SM, Juliano JJ, Trottman PA et al. . High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol 2010; 48:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika 1981; 68:373–9. [Google Scholar]
- 19. Sandison TG, Homsy J, Arinaitwe E et al. . Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ 2011; 342:d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lohman-Payne B, Slyker J, Rowland-Jones SL. Immune-based approaches to the prevention of mother-to-child transmission of HIV-1: active and passive immunization. Clin Perinatol 2010; 37:787–805, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 1987; 155:1319–21. [DOI] [PubMed] [Google Scholar]
- 22. Enane LA, Gastañaduy PA, Goldfarb DM et al. . Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 2016; 62(suppl 2):S168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coutsoudis A, Daniels B, Moodley-Govender E et al. . Randomised controlled trial testing the effect of cotrimoxazole prophylaxis on morbidity and mortality outcomes in breastfed HIV-exposed uninfected infants: study protocol. BMJ Open 2016; 6:e010656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moonasar D, Nuthulaganti T, Kruger PS et al. . Malaria control in South Africa 2000-2010: beyond MDG6. Malar J 2012; 11:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dow A, Kayira D, Hudgens M et al. . Effects of cotrimoxazole prophylactic treatment on adverse health outcomes among HIV-exposed, uninfected infants. Pediatr Infect Dis J 2012; 31:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]