Abstract
Fecal microbiota transplantation (FMT) may be a novel approach to eliminate multidrug-resistant bacteria from the gut and to prevent future infections. Using whole metagenome sequencing data from 8 FMT donor–recipient pairs, we identified 37 and 95 antimicrobial resistance genes that were acquired by or removed from FMT recipients, respectively.
Keywords: fecal microbiota transplantation, antimicrobial resistance, multidrug resistance, metagenomics, Clostridium difficile infection
Globally, the incidence of antimicrobial resistant infections is growing [1]. The cupboard of available and efficacious antimicrobial agents is becoming increasingly bare, few new agents exist in the developmental pipeline, and the time lag to drug accessibility is long. There is an urgent need to consider novel interventions, including nonpharmacologic approaches to preventing colonization and possibly treating infection from multiply drug-resistant bacteria.
Fecal microbiota transplantation (FMT) is a nonpharmacologic treatment for recurrent Clostridium difficile infection (CDI) in hospitalized patients with antibiotic-associated diarrhea. FMT involves the infusion of healthy donor feces into the intestinal tract of patients with recurrent CDI via a nasojejunal/nasoduodenal tube or via colonoscope or retention enema. FMT has been shown to be highly effective for treating CDI [2, 3] and has led to the elimination of C. difficile from the intestinal tract of infected patients. Engraftment of the donor’s intestinal microbiota and microbiota recovery in the recipient have been demonstrated [4]. Following treatment of recurrent CDI with FMT, investigators began to notice that other highly drug-resistant bacteria were also disappearing along with C. difficile. Initial case reports indicated that several types of multidrug-resistant opportunistic organisms such as carbapenemase- and extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae, vancomycin-resistant Enterococci, and methicillin-resistant Staphylococcus aureus were being eliminated in patients undergoing FMT for recurrent CDI [5]. Shortly thereafter, case reports began emerging using FMT to directly decolonize non-CDI patients with resistant infections or patients at risk of infection [5, 6]. A recent study showed complete decolonization of various organisms in 15 of 20 (70%) patients [7]. Finally, a study by Millan et al showed that FMT may contribute to the reduction of antimicrobial-resistant genes in FMT recipients [8]. In this study, we sought to replicate these findings and to ask whether FMT recipients could also acquire antimicrobial resistance genes as the result of FMT. We sought to characterize changes to microbiota composition and antimicrobial resistance gene carriage as the result of FMT.
MATERIALS AND METHODS
Patients who had multiply recurrent CDI (defined as a primary episode followed by at least 2 recurrences) were eligible and enrolled (Jewish General Hospital protocol number 10–050) following informed consent for both the FMT and the metagenomic analyses. An episode of CDI (primary or recurrence) was defined as the presence of a positive C. difficile toxin assay (enzyme immunoassay or nucleic acid amplification assay) or clinicopathologic symptoms/signs of CDI (typical endoscopic or pathologic changes) and at least 3 diarrheal bowel movements in 24 hours. A CDI recurrence was defined as an episode of CDI within 60 days following the end of treatment for a previous CDI episode. FMT donors were identified from family, household members, or friends of CDI patients, and selected donors were enrolled following informed consent for donor screening procedures, stool donation, and metagenomic analyses. Screening of donor candidates included stool culture for bacterial enteric pathogens; C. difficile cytotoxin assay; ova and parasite enzyme-linked immunosorbent assay (Giardia lamblia, Entamoeba histolytica, and Cryptosporidium species); and serology for human immunodeficiency virus, syphilis, hepatitis A virus (hepatitis A immunoglobulin M), hepatitis B virus (hepatitis B surface antigen), hepatitis C virus (hepatitis C total antibody) and human T-lymphotropic virus types 1/2 (antibody). Donors also reported no acute illness, no diarrhea, no functional bowel disorders, no receipt of antibiotics within 1 month, and no immunosuppressive therapy within 3 months of stool donation.
FMT was performed by V. L. and M. M. in Montréal, Québec, between 2010 and 2012. In brief, FMT preparation included cessation of the chronic suppressive oral vancomycin 2 days prior to FMT, a clear fluid diet for 24 hours prior to FMT, and a self-administered rectal enema on the morning of the FMT with no additional bowel preparation. The FMT protocol included a rectal enema infusion of 150 g of filtered and homogenized donor stools, blended in 750 mL of nonbacteriostatic normal saline. The stool suspension was delivered via the enema tube with the patient in the lateral decubitus position and was retained for 2 hours with the patient changing position laterally every 30 minutes. The study subject was assessed on days 3, 7, 14, 30, 60, and 90 post-FMT, where stool was collected and C. difficile culture and cytotoxin assay were performed. For this study, treatment failure was defined as onset of a CDI episode ≤60 days post‐FMT if no antibiotics had been taken during the post-FMT period. Treatment success was defined by absence of a CDI episode within ≤60 days post‐FMT if no antibiotics had been taken during the period.
We performed whole metagenome DNA sequencing from stool specimens from donors and from recipients prior to FMT, and at the 6 visits following FMT. Total microbial DNA was extracted from 200 mg of stool using the QIAamp DNA stool mini kit. Library preparation was performed using a Cold Spring Harbor Protocol for highly multiplexed sample sequencing [9]. Whole metagenome sequencing was performed on the Illumina 2500 HiSeq platform at the McGill University Genome Québec Innovation Centre. Sequencing read datasets were quality filtered and then annotated using MetaPhlAn [10] for taxonomic composition and the Comprehensive Antimicrobial Resistance Database (CARD) [11] for antimicrobial gene identification. Sequencing reads were normalized and aligned to CARD using Bowtie2; positive genes exhibited >95% homology over 80% of CARD gene length. Log-based changes in relative abundance of microbial taxa in the FMT recipients were investigated. We defined acquisition of an antimicrobial resistance gene by the recipient as a resistance gene present in the donor at the FMT visit, absent in the recipient at or before the FMT visit and then present in the recipient at any subsequent visit. Antimicrobial resistance gene depletion was defined as a resistance gene absent in the donor at the FMT visit, present in the recipient at the FMT visit, and then absent in the recipient at all subsequent visits. All analyses were performed in R (version 3.3).
RESULTS
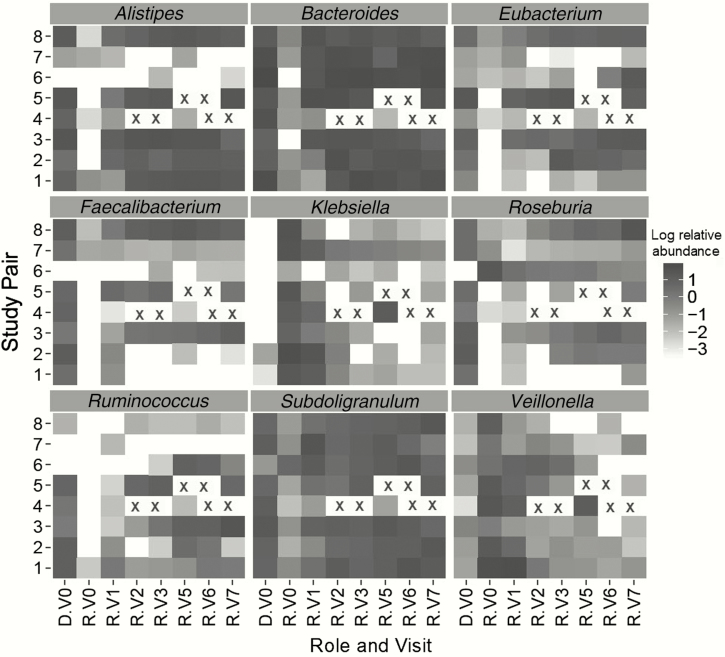
Eight FMT donor and recipient pairs were enrolled in the study. All were treatment successes except for FMT recipient number 4, who was a documented treatment failure. No adverse events were reported. A total of 64 metagenomic datasets from the 8 FMT recipients and 8 donors were available. Significant changes in the intestinal microbiota of FMT recipients were observed (Figure 1). As expected, normal commensal bacteria increased in abundance (eg, Bacteroides, Eubacterium) in recipients, whereas opportunistic pathogens decreased (eg, Klebsiella) (Figure 1). At baseline, prior to FMT, quinolone resistance genes (eg, gyrA, gyrB, and parC) were enriched in the recipients, tetracycline resistance genes (eg, tetQ) were enriched in the donors, and aminoglycoside resistance genes (eg, variants of APH) were present at similar levels in donors and recipients. The donors in this study were excluded if they had received antibiotics in the 30 days prior to donation. This exclusion criterion has been widened to 6–12 months in recent FMT procedures, thereby reducing the possible selection of resistance genes in donor stool. A total of 37 genes met the criterion for FMT gene acquisition; there were 93 pair–gene combinations for which there was evidence of acquisition. Of these 93 instances, 72 pair–gene combinations of resistance gene acquisition occurred at visit 1 (3 days post-FMT) and another 13 occurred at visit 2 (7 days post-FMT), suggesting transfer from the donor to recipient. Of the genes possibly acquired, only a few were clinically relevant (eg, ESBL [TEM-33 and OXA-137] and quinolone resistance [gyrB]) (Table 1). A total of 95 resistance genes met the criterion for FMT gene depletion. There was evidence for depletion in 127 pair–gene combinations. Multiple clinically relevant quinolone, β-lactamase, ESBL, and vancomycin resistance genes were depleted in the FMT recipients (Table 1 ).
Figure 1.
Changes in selected genera in microbiota composition after fecal microbiota transplantation (FMT). Each box corresponds to specific bacterial genera; only those genera exhibiting a change of 1 log in relative abundance are presented. Shading corresponds to the log relative abundance of each genera in the donor and in the recipient following FMT (darker = more abundance; lighter or white less abundant or absent). An X indicates a visit with missing data. Rows correspond to FMT donor–recipient pairs. Columns represent study role (donor [D] and recipient [R]) and study visit as follows: D.V0, infusion; R.V0 at FMT; R.V1, 3 days post-FMT; R.V2, 7 days post-FMT; R.V3, 14 days post-FMT; R.V5, 30 days post-FMT; R.V6, 60 days post-FMT; and R.V7, 90 days post-FMT. V4 (not shown) corresponds to a single specimen for 1 recipient, collected outside of the normal study visit schedule.
Table 1.
Evidence for Antimicrobial Resistance Gene Acquisition Following Fecal Microbiota Transplantation for Clostridium difficile Infection
| Resistance Gene Class | Genes | Donor–Recipient Pair: Acquisition by Visit and Pair | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Selected genes acquired following FMT by visit and pair | |||||||||
| Aminoglycoside | AAC; APH; AAD | V5 | V5 | V1 | V3 | ||||
| Chloramphenicol | cat; catS | V4 | V5, V7 | V2 | |||||
| β-lactamase | cblA-1; cepA; cfxA-cfxA6 | V2, V3 | V3 | V1, V2 | V1 | V1, V2 | V1 | V1 | |
| Trimethoprim | dfrF | V3 | V1 | V1 | V1 | ||||
| Macrolide | ermB; ermF; ermG; ermQ; ermT | V2, V3 | V2 | V1, V2 | V1, V2 | V1 | V1 | V6 | |
| Quinolone | gyrB | V1 | |||||||
| Mulidrug efflux | mdtA; mdtB; mdtC; mdtM; mdtN; mdtP; pmrA | V1 | V1 | ||||||
| Macrolide efflux | mefA; mel | V6 | V1 | V1, V2 | V1, V2 | V1 | |||
| Sulfonamide | sul2 | V5 | |||||||
| ESBL | OXA-347; TEM-33 | V1 | V1 | ||||||
| Tetracycline | tet32; tet40; tetO; tetQ; tetW; tetX | V2, V4, V5 | V1, V3 | V1 | V1 | V1 | V1 | V1 | V1 |
| Donor–Recipient Pair: Depletion by Pair | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Selected genes depleted following FMT by pair | |||||||||
| β-lactamase | CMY-43; CMY-98; OXY-1, OXY-2; OXY-3-1; OXY-5-1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| ESBL | CTX-M-40; CTX-M-8; SHV-100; SHV-106 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Fosfomycin | Any FosA | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| Polymyxin | PmrC-F | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Quinolone | QnrB11 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vancomycin | vanRA; vanXA | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
Only selected genes are shown. Resistance gene acquisition was defined as a gene present in the donor at the FMT visit; absent in the recipient at or before the FMT visit, and then present in the recipient at any subsequent visit. For each pair, the visit for which the resistance gene was acquired is shown (eg, V1 = visit 1 [2–3 days post-FMT] and V2 = visit 2 [7 days post-FMT]). Resistance gene depletion was defined as a gene absent in the donor at the FMT visit, present in the recipient at the FMT visit, and then absent in the recipient at all subsequent visits. Resistance gene depletion is indicated for each pair by the number 1 in the table.
Abbreviations: ESBL, extended-spectrum β-lactamase; FMT, fecal microbiota transplantation.
DISCUSSION
This study confirms changes in recipient microbiota composition in response to FMT [12]. These changes in taxonomic composition may contribute to the acquisition and depletion of antimicrobial resistance genes in FMT recipients. We show that antimicrobial resistance gene acquisition from FMT donor stool is likely. Some resistance genes are endogenous to commensal bacteria, so some level of resistance gene exchange is expected. However, several clinically consequential resistance genes emerged in the recipient immediately after FMT; this observation is important for the selection and screening of healthy FMT stool donors and argues for the development of defined microbial communities, low in antimicrobial resistance genes. Information about spontaneous resistance gene acquisition and depletion in healthy subjects would also be useful for understanding the potential risk of resistance gene transmission in FMT. We observed a large depletion of antimicrobial resistance genes as the result of FMT, specifically the loss of multiple clinically relevant antimicrobial resistance genes, including genes belonging to the ESBL, glycopeptide, and quinolone antimicrobial classes. This concurs with other published studies [5, 8]. By changing the composition of the intestinal microbiota, FMT changes the distribution of antimicrobial-resistant gene-carrying organisms. FMT may be a nonpharmacologic approach to managing patients colonized with or at risk from infections due to multidrug-resistant bacteria. It is still not clear how effective or durable this approach could be, but it seems promising.
Notes
Financial support. This work was supported by a Blue Sky Grant from the British Columbia Centre for Disease Control Foundation (to A. R. M.).
Potential conflicts of interest. A. R. M. has received research funding from a 2011 Pfizer Aspire Antibacterial Research Award. M. M. has been an employee of bioMérieux since October 2012. C. V. received a studentship from the Research Institute of the McGill University Health Centre, as well as a Frederick Banting and Charles Best Canada Graduate Scholarship (Doctoral Award) from the Canadian Institutes of Health Research (GSD-113375). C. V. has been an employee of Roche Diagnostics since 2016. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. 2016 review on antimicrobial resistance. London: Wellcome Trust and UK Government, 2016. [Google Scholar]
- 2. van Nood E, Vrieze A, Nieuwdorp M et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]
- 3. Lee CH, Steiner T, Petrof EO et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016; 315:142–9. [DOI] [PubMed] [Google Scholar]
- 4. Seekatz AM, Aas J, Gessert CE et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio 2014; 5:e00893–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manges AR, Steiner TS, Wright AJ. Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect Dis (Lond) 2016; 48:587–92. [DOI] [PubMed] [Google Scholar]
- 6. Laffin M, Millan B, Madsen KL. Fecal microbial transplantation as a therapeutic option in patients colonized with antibiotic resistant organisms. Gut Microbes 2017; 976:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilinski J, Grzesiowski P, Sorensen N et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Infect Dis 2017; 65:364–70. [DOI] [PubMed] [Google Scholar]
- 8. Millan B, Park H, Hotte N et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin Infect Dis 2016; 62:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb Protoc 2010; 2010:pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 10. Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods 2012; 9:811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McArthur AG, Waglechner N, Nizam F et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 2013; 57:3348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weingarden A, González A, Vázquez-Baeza Y et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015; 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]