Summary
Cardiovascular disease (CVD) is an increasing cause of morbidity among persons living with human immunodeficiency virus (PLWH). CVD prevention strategies could offer important health benefits for PLWH and should be evaluated.
Keywords: HIV/AIDS, cardiovascular disease, lifetime risk
Abstract
Background
Cardiovascular disease (CVD) is an increasing cause of morbidity among persons living with human immunodeficiency virus (HIV; PLWH). We projected cumulative CVD risk in PLWH in care compared to the US general population and persons HIV-uninfected, but at high risk for HIV.
Methods
We used a mathematical model to project cumulative CVD incidence. We simulated a male and female cohort for each of 3 populations: US general population; HIV-uninfected, at high risk for HIV; and PLWH. We incorporated the higher smoking prevalence and increased CVD risk due to smoking into the HIV-infected and HIV-uninfected, at high risk for HIV populations. We incorporated HIV-attributable CVD risk, independent of smoking.
Results
For men, life expectancy ranged from 70.2 to 77.5 years and for women from 67.0 to 81.1 years (PLWH, US general population). Without antiretroviral therapy, lifetime CVD risk for HIV-infected males and females was 12.9% and 9.0%. For males, by age 60, cumulative CVD incidence was estimated at 20.5% in PLWH in care, 14.6% in HIV-uninfected high-risk persons, and 12.8% in the US general population. For females, cumulative CVD incidence was projected to be 13.8% in PLWH in care, 9.7% for high-risk HIV-uninfected persons, and 9.4% in the US general population. Lifetime CVD risk was 64.8% for HIV-infected males compared to 54.8% for males in the US general population, but similar among females.
Conclusions
CVD risks should be a part of treatment evaluation among PLWH. CVD prevention strategies could offer important health benefits for PLWH and should be evaluated.
Due to major advances in the treatment of human immunodeficiency virus (HIV) disease, the life expectancy of treatment-adherent people living with HIV (PLWH) is approaching that of the general population [1]. Aging PLWH and their healthcare providers now face new challenges related to prevention and treatment of common chronic conditions, including cardiovascular disease (CVD) [2].
While numerous studies have focused on the increased risk of CVD in PLWH [3–7], as evidenced by abnormal biomarkers of chronic inflammation and abnormal lipid metabolism, these studies have not estimated lifetime CVD risk at the population level [8]. Additionally, many studies have compared CVD risk between PLWH and the US general population, but these studies may have overestimated CVD risk attributable to HIV by not accounting for the higher prevalence of traditional CVD risk factors, such as smoking, among PLWH [9, 10].
Through simulation modeling, we estimated the lifetime CVD risk in treatment-adherent PLWH, considering competing mortality due to HIV. We compared this lifetime CVD risk to that in the general population, as well as to that in a “high-risk” population without HIV infection but with an increased prevalence of behavioral risk factors associated with HIV and CVD and their increased risk of competing mortality.
METHODS
Analytic Overview
We used the cost-effectiveness of preventing AIDS complications (CEPAC) model, a validated computer simulation of HIV disease that simulates both PLWH and HIV-uninfected persons [11–14]. We expanded CEPAC to incorporate age- and sex-specific CVD prevalence, incidence, and attributable mortality. We simulated male and female cohorts for each of the following 3 populations: the US general population; HIV-uninfected, at high risk for HIV (due to behavioral risk factors); and PLWH in care. The model followed each cohort from age 36 years until death [15]. We projected cumulative incidence of CVD in each cohort by ages 40, 50, and 60 years and over a lifetime, accounting for competing mortality from HIV and other causes.
We relied on multisite US data to inform input parameters regarding CVD risk. To approximate the increased CVD risk in those uninfected, at high risk for HIV, we assumed that they have the same traditional risk factors as PLWH. We used smoking as a proxy for traditional risk factors and incorporated data on smoking prevalence in PLWH and the increased CVD risk from smoking [16, 17]. We accounted for increased CVD risk due to HIV [18]. To account for higher all-cause mortality in those HIV-uninfected, at high risk for HIV, we used standardized mortality ratios (SMRs) [13, 19]. We separated non–CVD-attributable and CVD-attributable mortality to approximate the time at risk for CVD acquisition more accurately. We used CVD age- and sex-stratified prevalence to distinguish between age-related (non-CVD) and CVD-attributable mortality. We assumed that CVD-attributable mortality is the same for all persons with CVD.
Model Structure
Cost-Effectiveness of Preventing AIDS Complications Model
The CEPAC model is a validated computer simulation of HIV disease [11–14]. In the model, each simulated HIV-infected individual transitions between health states defined by CD4 count, HIV RNA, history of opportunistic infection (OI), and antiretroviral therapy (ART) use.
Modeled PLWH initiate ART according to US guidelines at diagnosis and linkage to care [20]. ART decreases HIV RNA, increases CD4 count, and decreases OI incidence and HIV-associated mortality. Virologic failure leads to switching ART regimens. PLWH have additional HIV-attributable mortality that depends on CD4 count, history of OI, and ART complications.
The CEPAC model tracks survival in those without HIV whose survival is determined by age- and sex-stratified mortality derived from the US life tables for the general population and SMR-adjusted mortality for those at high risk for HIV acquisition. Details on the SMR adjustment and other model details are provided in the Supplementary Appendix.
Incorporating Cardiovascular Disease into the Cost-effectiveness of Preventing AIDS Complications Model
To extend the CEPAC model to include cardiovascular morbidity and mortality, each simulated person is assigned a CVD status at model initiation based on age- and sex-specific prevalence. Prevalent cases and those who develop CVD based on age- and sex-stratified CVD incidence are subject to additional, CVD-attributable mortality over their remaining life span. Modeled CVD endpoints include any of the following: myocardial infarction (MI), stroke, angina, or coronary heart disease.
We derived CVD-attributable mortality from National Center for Health Statistics data, which report deaths due to diseases of the heart and cerebrovascular diseases, stratified by age and sex [21]. We divided the number of deaths due to “diseases of the heart” and “cerebrovascular diseases” by the number of persons with CVD in each age and sex group. The number of persons with CVD was estimated based on CVD prevalence and the US population size in 2008 (Table 1) [21, 22].
Table 1.
Model Input Parameters for Analysis of Cardiovascular Disease Risk in People Living With Human Immunodeficiency Virus in the United States
| Variable | Base Case Value | Reference | |||
|---|---|---|---|---|---|
| Cohort characteristics | |||||
| Age, y (SD) | 36 (0) | [15] | |||
| Distribution of initial CD4 (mean cells/µL) ± SD | 751 (267) | [30] | |||
| HIV RNA distribution after acute infection, % | |||||
| >100000 copies/mL | 25 | Derived from [33] | |||
| 30001–100000 copies/mL | 42 | ||||
| 10001–30000 copies/mL | 21 | ||||
| 3001–10000 copies/mL | 12 | ||||
| Overall first-line antiretroviral therapy efficacy | |||||
| HIV RNA suppressed at 6 months, % | 91 | [32] | |||
| Smoking prevalence, % | |||||
| Age | US General Population (M/F) | Derived from [17] | |||
| ≤50 years | 27.7/21.4 | ||||
| >50 years | 17.6/14.9 | ||||
| HIV-Uninfected, at High Risk for HIV and PLWH (M/F) | |||||
| ≤50 years | 43.0/36.3 | ||||
| >50 years | 37.2/31.6 | ||||
| CVD-attributable mortality, % monthly M/F | |||||
| Age (y) | All cohorts | Derived from [21, 22] | |||
| 20–29 | 0.11/0.07 | ||||
| 30–39 | 0.15/0.09 | ||||
| 40–49 | 0.24/0.13 | ||||
| 50–59 | 0.21/0.14 | ||||
| 60–69 | 0.24/0.22 | ||||
| 70+ | 0.46/0.65 | ||||
| CVD prevalence, % at initiation (M/F) | |||||
| Age (y) | General Population a | HIV-Uninfected, at High Risk for HIV b | PLWHb | ||
| 20–29 | 0.6/0.5 | 0.7/0.6 | 1.2/1.0 | ||
| 30–39 | 1.5/1.3 | 1.7/1.4 | 2.9/2.4 | ||
| 40–49 | 3.2/2.7 | 3.5/2.9 | 6.1/4.9 | ||
| 50–59 | 8.4/5.9 | 10.1/6.7 | 17.2/11.1 | ||
| 60–69 | 15.8/8.9 | 19.0/10.1 | 28.1/14.4 | ||
| 70+ | 28.4/17.6 | 34.1/20.0 | 56.2/30.1 | ||
| Estimated CVD incidence, cases per 10000 people per month (M/F) | |||||
| Age (y) | General Population | HIV-Uninfected, at High Risk for HIV | PLWH | ||
| 20–29 | 0.5/0.4 | 0.6/0.5 | 1.1/0.8 | ||
| 30–39 | 1.4/1.1 | 1.5/1.2 | 2.7/2.1 | ||
| 40–49 | 3.0/2.4 | 3.4/2.6 | 5.9/4.6 | ||
| 50–59 | 8.1/5.5 | 9.8/6.2 | 17.2/10.9 | ||
| 60–69 | 15.1/8.4 | 18.1/9.6 | 31.9/16.9 | ||
| 70+ | 36.4/22.9 | 43.7/26.0 | 76.8/45.7 | ||
All values are reported as male/female.
Abbreviations: CVD, cardiovascular disease; HIV; human immunodeficiency virus; PLWH; people living with HIV; SD, standard deviation.
aDerived from National Health Interview Survey using positive predictive values (see Methods and Supplementary Appendix).
bDerived using CEPAC model as described in the Methods.
Cohorts Modeled
We simulated male and female cohorts from the following 3 distinct populations: US general population; HIV-uninfected, at high risk for HIV; and PLWH. Each cohort differed in terms of CVD prevalence, CVD incidence over time, and age-related (non–CVD-related) mortality (Table 1). We included the HIV-uninfected, at high risk for HIV cohort (assuming they never acquire HIV throughout their lifetime) to allow for comparison of their lifetime CVD risk to that of PLWH, thereby explicitly demonstrating the effect of HIV on CVD lifetime risk alone in the face of competing HIV-attributable mortality.
1) General population
Cardiovascular disease prevalence (Table 1): We derived age- and sex-stratified US general population CVD prevalence from the 2010 National Health Interview Survey (NHIS), adjusting for overreporting in self-report data [23, 24]. Details on CVD prevalence derivation are in the Supplementary Appendix.
Cardiovascular disease incidence: We estimated age- and sex-stratified CVD incidence using the following basic epidemiologic principal: prevalence = incidence × duration of disease, estimating incidence as the ratio of prevalence to disease duration. To estimate CVD duration, we used CEPAC to ensure consistency in life expectancy estimates across all cohorts. We simulated HIV-uninfected individuals by “turning off” all HIV-related parameters. We assumed that, once diagnosed, CVD persists for life. The life expectancy of individuals in each 10-year age and sex stratum therefore approximates CVD disease duration for that decade of life. Additional details and validation are in the Supplementary Appendix.
Age-related (non-cardiovascular disease) mortality: We derived age- and sex-specific mortality from 2008 US life tables and removed CVD-attributable mortality (Supplementary Appendix). We assumed that among persons with CVD, mortality consists of non–CVD-attributable and CVD-attributable components. Based on this assumption, we derived non–CVD mortality by subtracting CVD-attributable mortality multiplied by the CVD prevalence from total mortality for each age/sex group:
2) Human immunodeficiency virus-uninfected, at high risk for human immunodeficiency virus population
Cardiovascular disease prevalence: The increased CVD prevalence in this cohort was attributed to higher smoking prevalence [16, 17]. We assumed that smoking prevalence in this cohort would be the same as among PLWH in the United States, which is twice that of the general population, and stratified by age and sex (Table 1) [17]. From the Framingham Heart Study [16], we used a relative risk of CVD compared to nonsmokers of 1.9 and 1.7 for male and female smokers. We calculated overall age- and sex-stratified CVD prevalence in this cohort as the weighted average of CVD prevalence among smokers and nonsmokers (Table 1).
Cardiovascular disease incidence: We derived CVD incidence from the age, sex, and smoking status-stratified CVD incidence in the US general population (described above), taking into consideration the higher smoking prevalence and assuming that smoking behavior does not change upon HIV diagnosis (Table 1; Supplementary Appendix).
Age-related (non-cardiovascular disease) mortality: Persons at high risk for HIV may exhibit behaviors that subject them to higher mortality, including smoking and alcohol use [2, 13, 19, 25–29]. To adjust for excess mortality from these behavioral risk factors, we derived sex-specific SMRs that quantified the relative change in age-related (non-CVD and non–HIV-related) mortality for persons at high risk for HIV compared to the US general population; these SMRs also capture increased non-CVD and non-HIV mortality due to behavioral risk factors (eg, mortality associated with HCV). We derived these SMRs as the average of published risk group–specific SMRs, weighted by the distribution of risk groups among US PLWH in care [13, 15, 19]. The SMRs differed for males (1.2) and females (2.9) due to different risk group composition (Supplementary Appendix).
3) Persons living with human immunodeficiency virus
We derived HIV-related parameters, including demographic and clinical characteristics and treatment efficacy, from published literature (Table 1). We used published data on the average age (36 years) and mean CD4 count (751 cells/µL) at seroconversion [15, 30]. PLWH in the model present to care at a mean CD4 count of 351 cells/µL [31]. We focused on PLWH who are treatment adherent.
Cardiovascular disease prevalence: We estimated the prevalence of CVD among PLWH from CVD prevalence in the HIV-uninfected, at high risk for HIV cohort, but further increased to account for the additional CVD risk associated with HIV infection per se (risk ratio [RR] = 1.76) based on multisite cohort data [18]. This increased risk was independent of smoking.
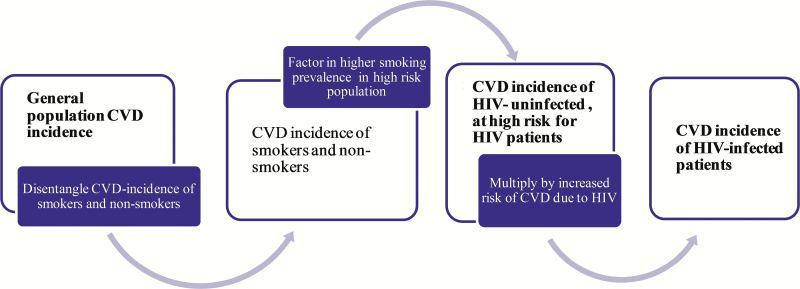
Cardiovascular disease incidence: We derived CVD incidence in PLWH using the estimated incidence of CVD in the HIV-uninfected, at high risk for HIV population, with additional adjustments for HIV-specific risk (RR = 1.76; Figure 1) [18]. The HIV-specific risk factors also account for differences in the prevalence of some traditional risk factors among PLWH and uninfected populations, such as glucose intolerance and dyslipidemia.
Figure 1.
Schematic of step-by-step estimation of cardiovascular disease incidence for cohorts of human immunodeficiency virus (HIV)–uninfected, at high risk for HIV and people living with HIV. Abbreviations: CVD, cardiovascular disease; HIV, human immunodeficiency virus.
Age-related mortality (non-cardiovascular disease, non- human immunodeficiency virus): Mortality in PLWH could occur due to 1 of 3 categories: HIV-attributable, CVD-attributable, or “age-related.” We derived age-related mortality in PLWH using the method described above for HIV-uninfected persons, at high risk for HIV. This age-related mortality did not include HIV-attributable mortality, which was added separately and accounts for mortality associated with chronic HIV infection (such as renal disease or anemia).
Sensitivity Analysis
We varied inputs that affected CVD risk including CD4 count at presentation to HIV care (173–516 cells/µL), smoking prevalence (35.6/30.4–43.7/37.3% M/F), Framingham-based relative risk of CVD due to smoking (1.7/1.4–2.2/2.1 M/F), increase in risk for CVD due to HIV (1.0–2.1), SMR adjustments (1.0/1.1–2.1/10.6 M/F), and age at HIV infection (26–41 years) [15–19, 31].
We also estimated the lifetime CVD risk among PLWH without ART. Combining the most influential parameters in 1-way sensitivity analyses, we created “lowest” and “highest” scenarios for CVD cumulative risk for PLWH. The lowest risk scenario included low CD4 count at presentation (173 cells/µL), lowest relative risk of CVD due to HIV (1.0), and high SMR (2.1/10.6 M/F). The highest risk scenario included high CD4 count at presentation to care (516 cells/µL), highest relative risk due to HIV (2.1), and low SMR (1.0/1.1 M/F). For the population of HIV-uninfected, at high risk for HIV, we varied SMRs to create similar lowest and highest CVD risk scenarios (1.0/1.1–2.1/10.6 M/F).
RESULTS
Base Case
Survival
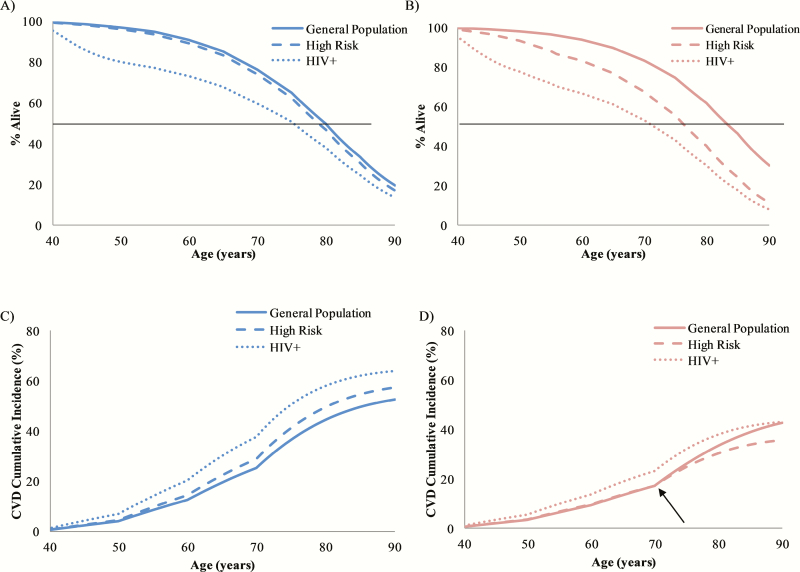
For men, life expectancy ranged from 70.2 to 77.5 years and for females from 67.0 to 81.1 years (Table 2; Figure 2A and 2B).
Table 2.
Life Expectancy and Cumulative Risk of Cardiovascular Disease
| Cohort | Life Expectancy (y) | Cardiovascular Disease Cumulative Risk, % (lowest–highest) | |||||
|---|---|---|---|---|---|---|---|
| Males, age (y) | 40 | 50 | 60 | 70 | 80 | Lifetime | |
| US general population | 77.5 | 0.7 | 4.2 | 12.8 | 25.6 | 44.6 | 54.8 |
| HIV-uninfected, at high risk for HIV | 76.4 | 0.8 (0.7–0.8) | 4.6 (4.5–4.7) | 14.6 (14.0–14.8) | 29.1 (27.5–29.6) | 49.5 (46.2–50.7) | 59.1 (55.3–61.0) |
| PLWH | 70.2 | 1.3 (1.1–1.5) | 7.1 (5.9–8.8) | 20.5 (16.7–26.2) | 37.9 (30.8–47.8) | 57.9 (47.9–70.0) | 64.8 (54.5–76.3) |
| Females, age (y) | |||||||
| US general population | 81.1 | 0.6 | 3.4 | 9.4 | 17.3 | 33.5 | 46.1 |
| HIV-uninfected, at high risk for HIV | 73.4 | 0.6 (0.6–0.6) | 3.5 (3.4–3.6) | 9.7 (8.0–10.3) | 17.3 (12.3–19.2) | 30.4 (16.3–36.8) | 36.7 (16.7–49.9) |
| PLWH | 67.0 | 1.0 (0.9–1.2) | 5.5 (4.2–7.0) | 13.8 (8.8–18.7) | 23.3 (12.7–32.7) | 38.0 (16.0–55.2) | 43.8 (16.3–66.8) |
Abbreviations: HIV; human immunodeficiency virus; PLWH; people living with HIV.
Figure 2.
Model projected survival (A, B) and cardiovascular disease (CVD) cumulative incidence curves (C, D). Males are shown in blue (A, C) and females in red (B, D). The median survival for the US general population; the human immunodeficiency virus (HIV)–uninfected, at high risk for HIV population; and the people living with HIV (PLWH) population was 77.5, 76.4, and 70.2 years for males. For females, life expectancy was 81.1, 73.4, and 67.0 years. Cumulative CVD risk for males was 54.8%, 59.1%, and 64.8% for the US general; HIV-uninfected, at high risk for HIV; and PLWH populations. Cumulative CVD risk for females was 46.1%, 36.7%, and 43.8% for the US general; HIV-uninfected, at high risk for HIV; and PLWH populations. Kinks in the cumulative incidence curves are due to limitations in age stratification from our source data. Around age 70 years in females, the cumulative incidence in the US general population begins to exceed that of the HIV-uninfected, at high risk for HIV population (panel D, arrow). Abbreviations: CVD, cardiovascular disease; HIV, human immunodeficiency virus.
Cardiovascular Disease Cumulative Incidence
By age 60 years, CVD risk was the lowest for males and females in the US general population (12.8% for males and 9.4% for females), higher in the HIV-uninfected, at high risk for HIV cohort (14.6% for males and 9.7% for females), and highest among PLWH (20.5% for males and 13.8% for females). For males this ranking continued through their lifetimes, with projected lifetime CVD risk at 54.8% for the US general population, 59.1% for the HIV-uninfected, at high risk for HIV cohort, and 64.8% for PLWH. For females, however, after age 70 years, the cumulative CVD risk of the US general population began to exceed that of the HIV-uninfected, at high risk for HIV cohort. The projected lifetime CVD risk for US general population females was 46.1%, 36.7% for the HIV-uninfected, at high risk for HIV cohort, and 43.8% for the PLWH cohort (Table 2 and Figure 2C and 2D).
Sensitivity Analyses
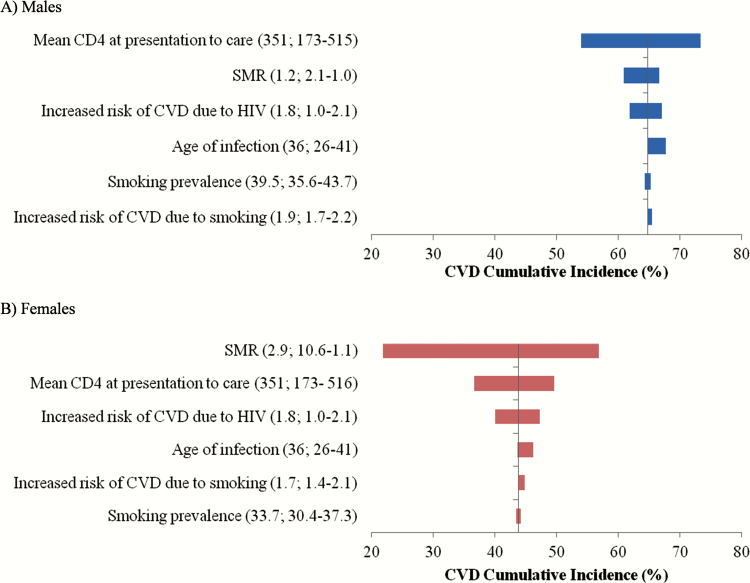
The parameters that had the greatest effect on lifetime CVD risk for PLWH included SMRs, the CVD relative risk multiplier due to HIV, and CD4 count at presentation to care (Figure 3, Supplementary Appendix). Sensitivity analyses in which we varied age at seroconversion, rates of chronic AIDS death, CVD prevalence, and smoking prevalence across the 95% confidence interval (CI) of reported values (35.6/30.4–43.7/37.3% M/F) did not have a substantial impact on cumulative CVD risk. In the absence of ART, lifetime CVD risk for PLWH was 12.9% for males and 9.0% for females.
Figure 3.
Tornado diagrams summarizing sensitivity of the cardiovascular disease lifetime risk among people living with human immunodeficiency virus to variation in key input parameters. Males (A) are in blue and females (B) are in red. Input base case values and ranges are in parentheses. Abbreviations: CVD, cardiovascular disease; HIV, human immunodeficiency virus; SMR, standardized mortality ratio.
Standardized Mortality Ratios
Projected lifetime CVD risks for PLWH were most sensitive to SMRs. With higher SMRs (2.1/10.6 M/F), lifetime CVD risk decreased to 21.8% for HIV-infected females and to 60.9% for HIV-infected males. When SMRs were reduced (1.0/1.1 M/F), lifetime CVD risk increased to 56.9% and 66.7% for HIV-infected females and males.
Human Immunodeficiency Virus-Related Cardiovascular Disease Relative Risk Multiplier
Decreasing the relative risk of CVD due to HIV to 1.0 resulted in lower lifetime CVD risk in PLWH: 31.3% in females and 53.0% in males. When we used the upper 95% CI value (RR = 2.1), lifetime CVD risk increased to 47.3% in females and to 67.1% in males [18].
Lowest and Highest Cardiovascular Disease Risk Scenarios for the Human Immunodeficiency Virus-Infected Population
In the lowest CVD risk scenario, the cumulative CVD risk by age 60 was 16.7% for males and 8.8% for females; and 54.5% and 16.3% over a lifetime. In the highest CVD risk scenario, the cumulative CVD risk by age 60 years was 26.2% for males and 18.7% for females; and 76.3% and 66.8% over a lifetime.
DISCUSSION
Our model-based evaluation shows that treatment-adherent PLWH in the United States have a greater risk of CVD at younger ages (age <60 years) compared to both the US general population and the HIV-uninfected, at high risk for HIV population. HIV-infected men remain at higher risk for CVD over their lifetimes, whereas HIV-infected women have lower lifetime CVD risk compared to the US general population. Because SMRs for both HIV-infected women and women at high risk for HIV were substantially higher than those for men, women had lower projected survival than men. This is in contrast to data for the general population where women’s life expectancy is generally longer than that of men. The higher SMR in women is explained by the fact that most women with or at risk for HIV have additional risk factors such as drug or alcohol abuse, which puts them at risk for lower life expectancy.
The increased relative CVD risk from HIV is similar to the increased CVD risk due to diabetes (HIV, 1.75; diabetes, 2.1/2.0, M/F) [18, 34]. The projected cumulative lifetime CVD risks for PLWH (ages 50–75) that we report are also similar to the lifetime CVD risk in diabetic patients, despite the competing mortality of HIV disease (diabetes, 67.1/57.3%, M/F; HIV, 64.8/43.8%, M/F) [35]. A large body of evidence supports the benefits of CVD prophylaxis for diabetes mellitus, and diabetes is explicitly incorporated into CVD prevention guidelines [36]. If HIV carries similar cumulative lifetime CVD risk, it is important to investigate the benefit of CVD prevention approaches among PLWH [37, 38]. While new evidence suggests that in PLWH only 50% of MIs are of the traditional type (type 1, from plaque instability), the other 50% are type 2 MI, or secondary to ischemia, due to either increased oxygen demand or decreased supply. This type of MI is more common among persons who inject drugs, who are also less likely to receive the full benefits from ART due to suboptimal adherence [39]. A large, ongoing, randomized controlled trial will help illuminate the specific role, if any, of statins as CVD prophylaxis in PLWH given this heterogeneity in MI type [40].
Our results provide important insights into the potential impact of smoking cessation on CVD lifetime risk in PLWH. One recent study suggests that smoking cessation can dramatically improve life expectancy in PLWH [41].
This study has several limitations. We explicitly modeled smoking as the major CVD risk factor among persons HIV-uninfected, at high risk for HIV and among PLWH. Other risk factors were modeled implicitly; as a result, we have limited ability to examine differences in CVD risk due to other individual risk factors such as hypertension, impaired glucose tolerance, or dyslipidemia explicitly. Our estimates for lifetime CVD risk in the non-HIV population were consistent with the results of population-based studies [42]. Since there is no similar independent data source for PLWH, we made every attempt to inform our estimates using data from population-based studies among PLWH. Additionally, we did not distinguish between mortality from acute and chronic CVD and used aggregated mortality rates from population-based surveys. While we recognize that different subcategories of CVD (eg, MI, stroke, peripheral vascular disease) may have different short- and long-term mortality [43], use of aggregated data allowed us to focus on overall population-based impact. We also did not explicitly investigate the influence of racial and ethnic differences, smoking duration, duration of HIV infection, or means of HIV acquisition, all of which could influence CVD risk [44, 45]. Last, we did not assess the economic impact of CVD care and potential prevention efforts due to the relatively low cost of primary CVD prevention in relation to the cost of ART. A comprehensive analysis of cost related to acute CVD events among PLWH is beyond the scope of this analysis.
These results have important implications for the care of PLWH in the United States. While CVD is increasingly recognized as a common cause of death in treated PLWH, additional attention and guidance should be paid to CVD screening and risk factor counseling for PLWH. These results can be used in clinical practice to facilitate discussion between PLWH and clinicians, providing quantitative evidence to guide discussion around CVD risk reduction in PLWH who receive HIV care. Further, to the extent that PLWH in Europe are also living longer, are at risk for CVD mortality, and have higher smoking rates than those living in the United States [46], the overall findings of this study may be generalized to these settings.
Given that the projected CVD risk among PLWH was similar to those with diabetes, we believe that HIV should be considered a major risk factor for CVD and that PLWH could benefit from preventive strategies similar to persons with diabetes mellitus. It is critical to test the effectiveness of CVD primary prevention therapies for PLWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The funding sources had no role in the design, analysis, or interpretation of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). E. L. had access to all of the data and takes responsibility for the integrity and accuracy of the data analysis.
Financial support. This research was funded by the NIH (R01 AI042006, T32 AI007433, K01 HL123349, R01 DA031059, P30 DA040500, P30 AI069354, and P30 AI042853) and by the Steve and Deborah Gorlin MGH Research Scholars Award (Executive Committee on Research to R. P. W.).
Potential conflicts of interest. P. E. S. has served as a consultant to AbbVie, Janssen, Bristol-Myers Squibb, Gilead, GlaxoSmithKline/ViiV, and Merck and has received grants from Bristol-Myers Squibb, GlaxoSmithKline/ViiV, and Gilead. M. C. W. has served as a consultant for OptumInsight on topics unrelated to HIV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Samji H, Cescon A, Hogg RS et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R et al. ; D:A:D Study Group Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 3. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS 2014; 28:2573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Subramanian S, Tawakol A, Burdo TH et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freiberg MS, Chang CC, Kuller LH et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silverberg MJ, Leyden WA, Xu L et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014; 65:160–6. [DOI] [PubMed] [Google Scholar]
- 8. Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS 2016; 11:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang S, Mary-Krause M, Cotte L et al. ; Clinical Epidemiology Group of the French Hospital Database on HIV Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010; 170:1228–38. [DOI] [PubMed] [Google Scholar]
- 10. Phillips AN, Carr A, Neuhaus J et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008; 13:177–87. [DOI] [PubMed] [Google Scholar]
- 11. Freedberg KA, Losina E, Weinstein MC et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001; 344:824–31. [DOI] [PubMed] [Google Scholar]
- 12. Ross EL, Weinstein MC, Schackman BR et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis 2015;60:1102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Losina E, Schackman BR, Sadownik SN et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis 2009; 49:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schackman BR, Fleishman JA, Su AE et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care 2015; 53:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prejean J, Song R, Hernandez A et al. ; HIV Incidence Surveillance Group Estimated HIV incidence in the United States, 2006–2009. PLoS One 2011; 6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Agostino RB Sr, Vasan RS, Pencina MJ et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 17. Mdodo R, Frazier EL, Dube SR et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 18. Althoff KN, McGinnis KA, Wyatt CM et al. ; Veterans Aging Cohort Study Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seage GR 3rd, Holte SE, Metzger D et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol 2001; 153:619–27. [DOI] [PubMed] [Google Scholar]
- 20. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents- Developed by the HHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC). 2012. [Google Scholar]
- 21. National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville, Maryland: 2012. [PubMed] [Google Scholar]
- 22. US Census Bureau. 2008. Age and Sex Composition in the United States: 2008 Available at: http://www.census.gov/population/age/data/2008comp.html. Accessed 1 June 2012.
- 23. Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10 2012;1–207. [PubMed] [Google Scholar]
- 24. Machón M, Arriola L, Larrañaga N et al. Validity of self-reported prevalent cases of stroke and acute myocardial infarction in the Spanish cohort of the EPIC study. J Epidemiol Community Health 2013; 67:71–5. [DOI] [PubMed] [Google Scholar]
- 25. Shirley DK, Kaner RJ, Glesby MJ. Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clin Infect Dis 2013; 57:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. High KP, Brennan-Ing M, Clifford DB et al. ; OAR Working Group on HIV and Aging HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60(Suppl 1):S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutton HE, McCaul ME, Chander G et al. Alcohol use, anal sex, and other risky sexual behaviors among HIV-infected women and men. AIDS Behav 2013; 17:1694–704. [DOI] [PubMed] [Google Scholar]
- 28. Justice AC, McGinnis KA, Tate JP et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016; 161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ingle SM, May MT, Gill MJ et al. ; Antiretroviral Therapy Cohort Collaboration Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis 2014; 59:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Multicenter AIDS Cohort Study Public Dataset: Release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 31. Althoff KN, Gange SJ, Klein MB et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis 2010; 50:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sax PE, DeJesus E, Mills A et al. ; GS-US-236-0102 Study Team Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379:2439–48. [DOI] [PubMed] [Google Scholar]
- 33. Daar ES, Tierney C, Fischl MA et al. ; AIDS Clinical Trials Group Study A5202 Team Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham Study. Diabetes Care 1979; 2:120–6. [DOI] [PubMed] [Google Scholar]
- 35. Lloyd-Jones DM, Leip EP, Larson MG et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006; 113:791–8. [DOI] [PubMed] [Google Scholar]
- 36. Goff DC Jr, Lloyd-Jones DM, Bennett G et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longenecker CT, Eckard AR, McComsey GA. Statins to improve cardiovascular outcomes in treated HIV infection. Curr Opin Infect Dis 2016; 29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gili S, Grosso Marra W, D’Ascenzo F et al. Comparative safety and efficacy of statins for primary prevention in human immunodeficiency virus-positive patients: a systematic review and meta-analysis. Eur Heart J 2016; 37:3600–9. [DOI] [PubMed] [Google Scholar]
- 39. Crane HM, Paramsothy P, Drozd DR et al. ; Centers for AIDS Research Network of Integrated Clinical Systems Cohort Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol 2017; 2:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. AIDS Clinical Trials Group. A5332: Randomized trial to prevent vascular events in HIV (REPRIEVE). In: actgnetwork.org [internet]. Bethesda (MD): ACTG [cited 11 January 2016]. Available at: https://actgnetwork.org/study/a5332-randomized-trial-prevent-vascular-events-hiv-reprieve.
- 41. Reddy KP, Parker RA, Losina E et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US-based modeling study. J Infect Dis 2016; 214:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes 2010; 3:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inglis SC, Bebchuk J, Al-Suhaim SA et al. Peripheral artery disease and outcomes after myocardial infarction: an individual-patient meta-analysis of 28,771 patients in CAPRICORN, EPEHESUS, OPTIMAAL and VALIANT. Int J Cardiol 2013; 168:1094–101. [DOI] [PubMed] [Google Scholar]
- 44. Safford MM, Brown TM, Muntner PM et al. ; REGARDS Investigators Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012; 308:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grinspoon SK, Grunfeld C, Kotler DP et al. State of the Science Conference: Initiative to Decrease Cardiovascular Risk and Increase Quality of Care for Patients Living with HIV/AIDS: executive summary. Circulation 2008;118: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trickey A, May MT, Vehrenschild J et al. ; for the Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies [epub ahead of print]. Lancet HIV 2017. Available at: http://www.thelancet.com/journals/lanhiv/article/PIIS2352-3018(17)30066–8/fulltext?elsca1=tlpr. Accessed 12 May 2017. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.