Summary
Indoor residual spraying (IRS) can be effective for reducing the burden of malaria but is difficult to maintain. In an area of Uganda with historically high-transmission intensity, discontinuation of IRS was associated with a rapid increase in malaria morbidity despite universal LLIN distribution.
Keywords: IRS, malaria, resurgence, LLIN, Uganda.
Abstract
Background.
Indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) are the primary tools for malaria prevention in Africa. It is not known whether reductions in malaria can be sustained after IRS is discontinued. Our aim in this study was to assess changes in malaria morbidity in an area of Uganda with historically high transmission where IRS was discontinued after a 4-year period followed by universal LLIN distribution.
Methods.
Individual-level malaria surveillance data were collected from 1 outpatient department and 1 inpatient setting in Apac District, Uganda, from July 2009 through November 2015. Rounds of IRS were delivered approximately every 6 months from February 2010 through May 2014 followed by universal LLIN distribution in June 2014. Temporal changes in the malaria test positivity rate (TPR) were estimated during and after IRS using interrupted time series analyses, controlling for age, rainfall, and autocorrelation.
Results.
Data include 65 421 outpatient visits and 13 955 pediatric inpatient admissions for which a diagnostic test for malaria was performed. In outpatients aged <5 years, baseline TPR was 60%–80% followed by a rapid and then sustained decrease to 15%–30%. During the 4–18 months following discontinuation of IRS, absolute TPR values increased by an average of 3.29% per month (95% confidence interval, 2.01%–4.57%), returning to baseline levels. Similar trends were seen in outpatients aged ≥5 years and pediatric admissions.
Conclusions.
Discontinuation of IRS in an area with historically high transmission intensity was associated with a rapid increase in malaria morbidity to pre-IRS levels.
Malaria control activities in sub-Saharan Africa have increased approximately 20-fold over the last decade, resulting in substantial reductions in the burden of malaria [1–3]. Despite these advances, the malaria burden remains high, with an estimated 215 million cases and 438 000 deaths in 2015, of which 88% of cases and 90% of deaths occurred in sub-Saharan Africa [4]. The primary interventions for the prevention of malaria include long-lasting insecticidal nets (LLINs) and indoor residual spraying of insecticide (IRS). LLINs have been shown to reduce malaria morbidity and mortality across a range of epidemiological settings, and the World Health Organization (WHO) recommends universal coverage of at-risk populations [5, 6]. IRS has also been shown to be highly effective but it is more resource intensive to implement than distribution of LLINs and less than 10% of the at-risk population in sub-Saharan Africa is currently protected by IRS [4, 7, 8].
Uganda is emblematic of countries with the highest burden of malaria in sub-Saharan Africa, where progress in reducing malaria morbidity and mortality has been slowest. LLINs have been the primary intervention for the prevention of malaria in Uganda, and considerable effort has been made to achieving universal LLIN coverage, culminating in a universal LLIN distribution campaign that was conducted from 2013 to 2014. After decades of inactivity, IRS was adopted as a key component of Uganda’s malaria control strategy in 2006, with funding from the US President’s Malaria Initiative. Initial efforts focused on epidemic-prone areas in the southwestern part of the country. However, in 2008 the IRS program was moved to 10 districts in northern Uganda where transmission intensity was high. The IRS program in northern Uganda achieved coverage levels consistently above 95% and resulted in marked reductions in the malaria burden [9, 10]. In 2014, the IRS program was moved from the 10 districts in the north, with hopes that gains would mainly be sustained following the universal LLIN distribution campaign.
In this study, we used data from an enhanced health facility–based malaria surveillance program established in outpatient and inpatient settings in Apac District, one of the areas in northern Uganda where IRS was implemented and later withdrawn. The study spans a 77-month period from July 2009 through November 2015, covering 10 rounds of IRS before its discontinuation in May 2014, followed by universal distribution of LLINs in June 2014. Our primary objective was to evaluate trends in malaria morbidity before, during, and after the implementation of IRS.
METHODS
Study Site and Vector Control Interventions
The study was conducted in Apac District, an area with historically high-transmission intensity; in 2002 the entomological inoculation rate was estimated to be more than 1500 infectious bites per person per year [11]. Malaria transmission is perennial in this area, with peaks following the 2 annual rainy seasons. IRS was first implemented in Apac District in May 2008 with a single round of DDT followed by a second round conducted in February 2010 using the pyrethroid alpha-cypermethrin. Due to concerns around the emergence of pyrethroid resistance [10], IRS was switched to the carbamate bendiocarb in August 2010, with repeated rounds of spraying approximately every 6 months through May 2014. For each round of IRS, the proportion of houses sprayed and population protected was >90% (Table 1). A series of targeted LLIN distribution campaigns were conducted in the area between 2006 and 2010, followed by a universal coverage campaign carried out in June 2014 as part of a national program. According to a malaria indicator survey conducted in December 2014, 94% of households reported owning at least 1 LLIN and 77% of persons reported sleeping under an LLIN the prior evening in the mid-north region of Uganda, which includes Apac District [12].
Table 1.
Details of Indoor Residual Spraying of Insecticide in Apac District
| Formulation of Insecticide | Dates of Spraying | Households Sprayed, % | Population Protected, % |
|---|---|---|---|
| DDT | March 2008–May 2008 | 92.4 | 91.0 |
| Alpha-cypermethrin | 23 February 2010–31 March 2010 | 99.9 | 99.9 |
| Bendiocarb | 23August2010–21 September 2010 | 99.5 | 99.5 |
| Bendiocarb | 5 January 2011–30 January 2011 | 99.6 | 99.6 |
| Bendiocarb | 23 May 2011–20 June 2011 | 97.6 | 97.8 |
| Bendiocarb | 9 November 2011–10 December 2011 | 93.1 | 94.0 |
| Bendiocarb | 23 April 2012–2 June 2012 | 90.3 | 90.4 |
| Bendiocarb | 22 October 2012–30 November 2012 | 92.3 | 93.2 |
| Bendiocarb | 2 April 2013–25 May 2013 | 97.5 | 96.6 |
| Bendiocarb | 4 November 2013–7 December 2013 | 92.7 | 93.5 |
| Bendiocarb | 22 April 2014–23 May 2014 | 92.6 | 91.3 |
Health Facility-based Surveillance
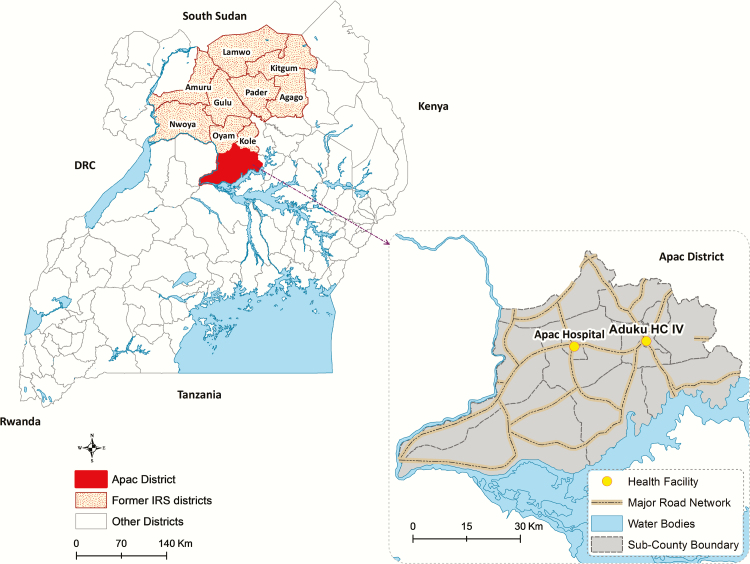
Enhanced malaria surveillance was conducted at 1 outpatient facility (Aduku Health Center) and 1 inpatient facility; this included children aged <14 years (Apac Hospital) as previously described (Figure 1) [13, 14]. Briefly, data were collected on demographics, whether malaria was suspected (outpatient facility only), whether a laboratory test for malaria was performed, the type of laboratory test performed (microscopy or rapid diagnostic test [RDT]), and the laboratory test result. Additional training and support were provided to maximize the proportion of patients with suspected malaria who underwent diagnostic testing at the outpatient facility and among all children admitted at the inpatient facility. For inpatients, additional data were collected on disease severity. Patients with severe malaria were defined as having a positive diagnostic test and any of the following (based on available data): severe anemia (hemoglobin <5 g/dL), coma, jaundice, or death during hospitalization. Patients with complicated malaria were defined as having a positive diagnostic test and any of the following: any of the above criteria for severe malaria, inability to breastfeed or drink, convulsions, lethargy, or inability to sit up or to stand.
Figure 1.
Map of Uganda showing study district (solid red) and other 9 districts where indoor residual spraying of insecticide was discontinued (red dots). Expanded view of study district showing location of outpatient (Aduku HC IV) and inpatient (Apac Hospital) health facilities where surveillance data were collected. Abbreviations: HC IV, Health Center Level 4; IRS, indoor residual spraying of insecticide.
Statistical Analyses
Data were entered using Microsoft Access (Microsoft Corporation, Redmond, Washington) and analyzed using Stata (StataCorp, College Station, Texas). The primary outcome was the test positivity rate (TPR), defined as the proportion of patients tested for malaria (denominator) who tested positive (numerator). The period of observation extended from July 2009 through November 2015 for outpatient surveillance and from June 2011 through November 2015 for inpatient surveillance. The exposure variable of interest was time, evaluated as a categorical variable in relation to the timing of IRS, including a baseline period (July 2009–August 2010); an initial period of effective IRS (September 2010–February 2011); a sustained period of effective IRS (March 2011–August 2014), which included the first 3 months after the last round of IRS was completed; and the 4–18 month period following IRS discontinuation (September 2014–November 2015) when a resurgence of malaria was observed. Temporal changes in the monthly TPR over time periods of interest were estimated by interrupted time series using ordinary least-squares regression with Newey-West standard errors adjusted for estimates of monthly rainfall with a 1-month lag [15], method of laboratory testing (microscopy vs RDT), and autocorrelation. Outpatient surveillance data were stratified for patients aged <5 years and ≥5 years. Among inpatients with laboratory-confirmed malaria, the probabilities of having complicated malaria, severe malaria, or death with malaria were compared between the periods of effective IRS and 4–18 months after IRS was discontinued using logistic regression controlling for age, monthly rainfall with a 1-month lag, and autocorrelation by including a quadratic term for the day of observation.
RESULTS
Characteristics of the Study Population
Over the 77-month observation period, there were 126 260 outpatient encounters, of which 53.6% were suspected of having malaria. Among patients with suspected malaria, 96.7% underwent laboratory testing. Laboratory testing was exclusively based on microscopy until 2012 when RDTs became available at the health center. Initially RDT use was low but increased to 29.4% during the 4–18 month period after IRS was discontinued. Following the implementation of IRS, there was a shift to an older age range among patients suspected and tested for malaria that then returned to baseline after IRS was discontinued (Table 2).
Table 2.
Characteristics of Study Populations from Outpatient and Inpatient Surveillance
| Characteristic | Time Category | |||
|---|---|---|---|---|
| Baseline, | Initial Period of Effective IRS, | Sustained Period of Effective IRS, | 4–18 Months After IRS Discontinued, | |
| July 2009– August 2010 | September 2010– February 2011 | March 2011– August 2014 | September 2014– November 2015 | |
| Outpatient surveillance (Aduku Health Center Level 4) | ||||
| Total number of patient encountersa | 25 945 | 8840 | 67 575 | 23 900 |
| Malaria suspected (% total) | 14 718 (56.7) | 5034 (57.0) | 37 003 (54.8) | 10 879 (45.5) |
| Malaria laboratory testing done (% suspected) | 14 104 (95.8) | 4994 (99.2) | 36 888 (99.7) | 9435 (86.7) |
| Microscopy performed (% total tested)b | 14 104 (100) | 4994 (100) | 35 373 (95.9) | 6656 (70.6) |
| Mean age (years) if tested (SD) | 15.6 (18.0) | 21.3 (19.0) | 19.9 (18.7) | 15.8 (17.6) |
| Age <5 years (% total tested) | 6577 (46.6) | 1370 (27.4) | 11 223 (30.4) | 3430 (36.4) |
| Tested positive for malaria (% total tested) | 7899 (56.0) | 1643 (32.9) | 8418 (22.8) | 4569 (48.4) |
| Inpatient surveillance (Apac Hospital)c | ||||
| Total number of children aged ≤ 13 years admitteda | Prior to implementation of inpatient surveillance |
8604 | 5991 | |
| Malaria laboratory testing done (% total admitted) | 8345 (97.0) | 5610 (93.6) | ||
| Microscopy performed (% total tested)b | 8210 (98.4) | 3756 (67.0) | ||
| Mean age (years) if tested (SD) | 3.2 (2.8) | 3.6 (2.9) | ||
| Tested positive for parasites (% total tested) | 4650 (55.7) | 4683 (83.5) | ||
| Complicated malaria (% laboratory confirmed cases) | 1264 (27.2) | 1146 (24.5) | ||
| Severe malaria (% laboratory confirmed cases) | 261 (5.6) | 90 (1.9) | ||
| Death with malaria (% laboratory confirmed cases) | 42 (0.5) | 17 (0.3) | ||
Abbreviations: IRS, indoor residual spraying of insecticide; SD, standard deviation.
aExcludes patients with no age recorded.
bRapid diagnostic test performed if microscopy not done.
cInpatient surveillance began June 2011.
Over the 54-month inpatient observation period, 14 595 children were admitted to the hospital, of which 95.6% underwent diagnostic testing for malaria. Among children tested for malaria, the proportion of testing based on RDTs was only 1.6% during the period of effective IRS, increasing to 33.0% during the 4–18 month period after IRS was discontinued. The age distribution of children admitted to the hospital was similar during the period before and after IRS was discontinued (Table 2).
Temporal Changes in Malaria Morbidity in Relation to IRS
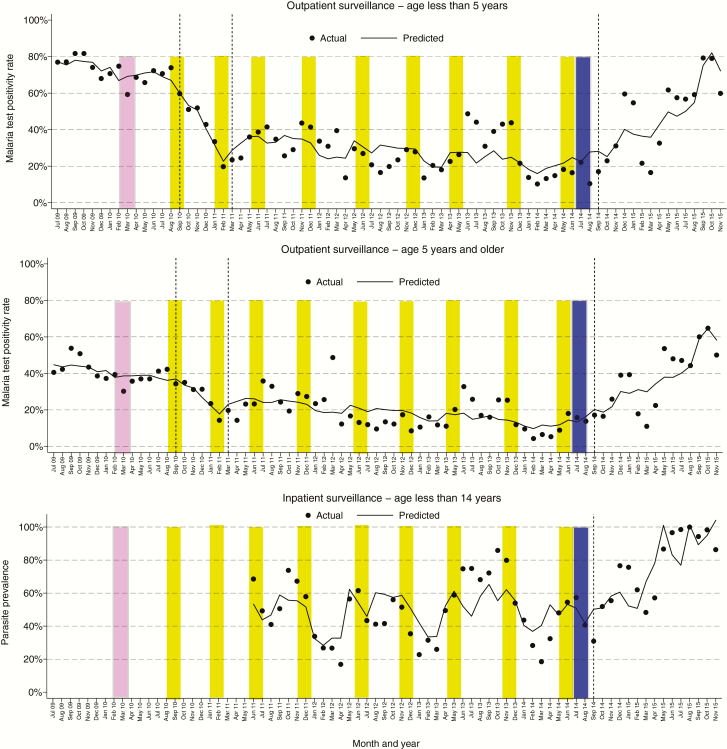
For the outpatient surveillance data, the baseline period, which included the 1 round of IRS with alpha-cypermethrin, was not associated with a significant change in the TPR. During this period, monthly TPRs ranged from 59% to 82% in patients aged <5 years and from 30% to 54% for patients aged ≥5 years (Figure 2). Following the first round of IRS with bendiocarb, there was a marked decrease in the TPR. From September 2010 to February 2011, there was an absolute decrease in the TPR of 5.84% per month (P < .0001), reaching 20% in patients aged <5 years, and 3.06% per month (P = .001), reaching 14% in patients aged ≥5 years (Figure 2, Table 3). Rounds of IRS with bendiocarb were repeated approximately every 6 months through May 2014, with a sustained reduction in TPR up to 3 months following the discontinuation of IRS. However, even during this sustained reduction, there were unexplained short periods where the TPRs “spiked” above what were predicted (eg, July 2013–November 2013). From March 2011 to August 2014, there was an absolute decrease in the TPR of 0.40% per month (P = .002), reaching as low as 10% in patients aged <5 years, and 0.41% per month (P < .0001), reaching 4% in patients aged ≥5 years (Figure 2, Table 3). A universal LLIN distribution campaign was conducted the month following the last round of IRS. During the 4–18 months after IRS was discontinued, there was a significant increase in the TPR, returning to baseline levels. From September 2014 to November 2015, there was an absolute increase in the TPR of 3.29% per month (P < .0001), reaching as high as 79% in patients aged <5 years, and 2.78% per month (P < .0001), reaching 65% in patients aged ≥5 years (Figure 2, Table 3). Of note, monthly estimates of rainfall with a 1-month lag time were controlled for in all analyses, and there was no significant difference in average monthly rainfall in 2015 (mean, 106.1 mm) compared to each of the proceeding 4 years (range, 97.6–111.6 mm; P > .74 for all pair-wise comparisons).
Figure 2.
Temporal changes in malaria test positivity rates in relation to indoor residual spraying of insecticide (IRS). Vertical dashed lines separate the study period into the following 4 categories: a baseline period (July 2009–August 2010); an initial period of effective IRS (September 2010–February 2011); a sustained period of effective IRS (March 2011–August 2014), which included the first 3 months after the last round of IRS was completed; and the 4–18 month period following IRS discontinuation (September 2014–November 2015) when a resurgence of malaria was observed. Pink bars show the timing of IRS with a single round of alpha-cypermethrin, yellow bars indicate the timing of IRS with 9 rounds of bendiocarb, and blue bars show the mass deployment of long-lasting insecticidal nets.
Table 3.
Temporal Changes in Malaria Test Positivity Rates in Relation to Indoor Residual Spraying of Insecticide
| Patient Population | Age Group | Estimated Average Monthly Change in Absolute Value of TPR | |||||
|---|---|---|---|---|---|---|---|
| Initial Period of Effective IRS, | Sustained Period of Effective IRS, | 4–18 Months After IRS Discontinued, | |||||
| September 2010–February 2011 | March 2011August 2014 | September 2014–November 2015 | |||||
| Δ TPR (95% CI)a | P Value | Δ TPR (95% CI)a | P Value | Δ TPR (95% CI)a | P Value | ||
| Outpatient surveillance | <5 years | –5.84% (–7.44% to –4.24%) | <.001 | –0.40% (–0.64% to –0.16%) | .002 | 3.29% (2.01% to 4.57%) | <.001 |
| ≥5 years | –3.06% (–4.89% to –1.24%) | .001 | –0.41% (–0.61% to –0.21%) | <.001 | 2.78% (2.01% to 3.54%) | <.001 | |
| Inpatient surveillanceb | ≤13 years | N/A | N/A | 5.66% (3.22% to 8.10%) | <.001 | ||
Abbreviations: CI, confidence interval; IRS, indoor residual spraying of insecticide; N/A, not applicable; TPR, test positivity rate.
aAdjusted for rainfall with a 1-month lag and proportion of laboratory testing based on microscopy vs rapid diagnostic test.
bInpatient surveillance began June 2011.
Inpatient surveillance began in June 2011, when the third round of IRS with bendiocarb was being conducted. During the sustained period of effective IRS, monthly TPRs among all children admitted to the hospital ranged from 17% to 86%, with an average of 56%. During the 4–18 months after IRS was discontinued, there was an absolute increase in the TPR of 5.66% per month (P < .0001), reaching 100% in August 2015 (Figure 2, Table 3). Despite the marked increase in TPR following discontinuation of IRS, there was no evidence for worsening of disease severity. Comparing the 4–18 month period after IRS was discontinued to the sustained period of effective IRS, the probabilities of having complicated malaria (24.5% vs 27.2%) and severe malaria (1.9% vs 5.6%) were lower (P < .0001 for both comparisons), and there was no significant change in the probability of death with malaria during hospitalization (0.3% vs 0.5%, P = .42).
DISCUSSION
In a district of Uganda with historically high malaria transmission intensity, the implementation of IRS with the carbamate bendiocarb was associated with a rapid decline in the malaria TPR among outpatients, followed by a sustained decline over a 4-year period. Immediately following the discontinuation of IRS, a universal LLIN distribution campaign was conducted with the hopes that gains achieved by IRS would be maintained. However, TPRs began to rise 4 months after IRS was discontinued, reaching pre-IRS levels within 18 months. Similar trends among children admitted to the district hospital were seen following discontinuation of IRS, although there was no evidence of worsening in disease severity.
LLINs and IRS are the most widely used interventions for the prevention of malaria in Africa, and the WHO recommends universal access to either of these measures [4]. The benefits of LLINs have been well established in randomized controlled trials, with use of LLINs associated with reductions in the incidence of malaria of 50% and child mortality of 20% [5]. It has been estimated that the incidence of malaria decreased by 40% across sub-Saharan Africa between 2000 and 2015 and that LLINs were responsible for 68% of cases averted [1]. In Uganda, the proportion of households with at least 1 LLIN increased from 47% to 90% between 2009 and 2014, and the average number of LLINs per household increased from 0.8 to 2.5 [12]. Historically, IRS has played a major role in the elimination of malaria in several countries outside of Africa and in greatly reducing the burden of malaria in parts of Africa with low or seasonal transmission [16, 17]. Although the effectiveness of IRS has been well established through historical and operational evidence, high-quality data from randomized, controlled trials on the impact of IRS in stable transmission settings are limited [18]. Recent cluster randomized trials from Africa that compared IRS combined with LLINs vs either intervention alone have provided mixed results [19–21]. Adding IRS to the use of LLINs appears to be most effective in areas where LLIN coverage is low, pyrethroid resistance is high, and/or when using IRS with nonpyrethroid-based insecticides [22].
In our study, the benefits of IRS with bendiocarb were clear, as evidenced by the dramatic decline in malaria morbidity after its initiation followed by an equally dramatic increase shortly after IRS was discontinued. The resurgence of malaria occurred despite universal LLIN distribution immediately following discontinuation of IRS. These findings raise questions about whether the LLINs were used properly or if insecticide resistance may have limited their effectiveness. The recent spread of resistance to pyrethroid, the only class of insecticides currently available for LLINs, is of concern. The emergence of high-level pyrethroid resistance among the primary vectors, An. gambiae s.s. and An. arabiensis, has been reported in Uganda and other parts of Africa [23, 24]. Also of concern are putative changes in vector behavior and shifts in the relative abundance of vector species, which may increase exposure risk during the early evening hours when people are outside of their bed nets and unprotected by LLINs [25]. One reassuring finding from our study was the lack of an increased risk of severe or complicated malaria among pediatric inpatients during the resurgence of malaria following discontinuation of IRS. However, following the discontinuation of IRS, the TPR among outpatients aged ≥5 years increased to levels higher than those observed before the initiation of effective IRS, suggesting a loss of some immunity against uncomplicated malaria.
In the last decade, the WHO has reaffirmed the importance of IRS as a primary intervention for reducing malaria transmission in Africa. IRS coverage increased from <2% in 2006 to 11% in 2010. However, the spread of pyrethroid resistance has led many control programs to replace pyrethroids with more expensive alternatives such as carbamates or organophosphates, leading to downscaling of IRS programs. This has resulted in a reported 53% decrease in the number of houses sprayed between years of peak coverage and 2015 across 18 countries in Africa supported by the US President’s Malaria Initiative [26]. Historically most malaria resurgences have been linked to weakening of control programs. In a systematic review of 75 malaria resurgence events in 61 countries that occurred from the 1930s through the 2000s, withdrawal of IRS was a major contributing factor, but this was felt to be primarily due to resource constraints or complacency rather than insecticide resistance [27]. In a cross-case study review, premature reductions of coverage or withdrawal of IRS were linked to malaria resurgence in Cape Verde, Sri Lanka, and Turkey [28]. Contemporary data from Africa on the impact of discontinuing IRS are limited given that this has been a recent phenomenon. In the high-transmission region of northeastern Zambia, but not in the rest of the country where transmission is lower, a resurgence of malaria cases was reported following disruption of IRS [29]. In 2 districts of Tanzania, an increase in parasite prevalence was reported following the discontinuation of IRS after 4 years despite distribution of LLINs [26]; in Benin an increase in entomological measures of transmission was reported following the discontinuation of IRS after 3 years despite distribution of LLINs [30]. In contrast, following a 3-year experimental program of IRS in South Pare, Tanzania, which ended in 1959, the expected resurgence of malaria was delayed for several years. This was attributed to changes brought about in the original vector mosquito populations and a program to increase the use of antimalarial drugs [31].
Our study had several limitations. Most importantly were the observational study design and the lack of a control group. Thus, we are unable to exclude the possibility that factors other than the discontinuation of IRS contributed to the resurgence of malaria seen. For example, adherence to use of LLINs was not measured, therefore although LLIN coverage was reportedly high, it is unknown whether there were sufficient numbers of LLINs and they were being used properly. In addition, the availability of artemisinin combination therapies (ACTs) was not measured and stock-outs could have contributed to the resurgence. Another potential limitation was use of the TPR as our outcome measure, which is a surrogate measure of malaria incidence and can be influenced by factors such as health facility attendance, selection bias for those referred for testing, and the accuracy of laboratory testing. Despite these limitations, it is likely that the observed changes in malaria morbidity were related to starting and stopping effective IRS given the longitudinal nature of the data, the magnitude of changes, and the consistency with which data were collected over an extended period of time. It should also be pointed out that our study area bordered several districts where IRS was not implemented (Figure 1), which may have contributed to the rapid resurgence of malaria.
Uganda and other countries in Africa are now facing difficult decisions about the role of IRS when demands exceed the availability of resources. Current funding levels are insufficient to achieve full IRS coverage in Uganda, and current gains in malaria reductions may not be sustained if control measures are withdrawn. In addition, the emergence of pyrethroid resistance has required countries to consider alternative classes of insecticide, which can be considerably more expensive. There is an urgent need to better define when IRS should be maintained, changed to different formulations, or can be safely discontinued or scaled back. This will require effective surveillance systems to monitor trends in disease burden and insecticide resistance. In this area of historically high transmission, the reductions in malaria achieved through effective IRS could not be maintained by a LLIN distribution campaign alone. Additional interventions are needed to supplement LLINs, ensure LLINs are being used properly, and prevent stock-outs of ACTs when IRS is withdrawn from areas where malaria transmission was historically high.
Notes
Acknowledgments. We thank the staff at the Aduku Health Center and the Apac Hospital.
Disclaimer. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors had the final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Centers for Disease Control and Prevention (U01GH000076), the Doris Duke Charitable Foundation, and the National Institutes of Health as part of the International Centers of Excellence in Malaria Research program (U19AI089674).
Potential conflict of interest: All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Weiss DJ, Cameron E et al. . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noor AM, Kinyoki DK, Mundia CW et al. . The changing risk of Plasmodium falciparum malaria infection in Africa: 2000-10: a spatial and temporal analysis of transmission intensity. Lancet 2014; 383:1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis 2010; 10:545–55. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. World Malaria Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 5. Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev 2004; CD000363. [DOI] [PubMed] [Google Scholar]
- 6. Lim SS, Fullman N, Stokes A et al. . Net benefits: a multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PLoS Med 2011; 8:e1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination. Geneva: World Health Organization, 2006. [Google Scholar]
- 8. West PA, Protopopoff N, Wright A et al. . Enhanced protection against malaria by indoor residual spraying in addition to insecticide treated nets: is it dependent on transmission intensity or net usage? PLoS One 2015; 10:e0115661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kigozi R, Baxi SM, Gasasira A et al. . Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One 2012; 7:e42857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinhardt LC, Yeka A, Nasr S et al. . The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda. Am J Trop Med Hyg 2013; 88:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okello PE, Van Bortel W, Byaruhanga AM et al. . Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006; 75:219–25. [PubMed] [Google Scholar]
- 12. Uganda Bureau of Statistics (UBOS), ICF International. Uganda Malaria indicator survey 2014–15: key indicators. Kampala, Uganda, and Rockville, Maryland, USA: UBOS and ICF International, 2015. [Google Scholar]
- 13. Sserwanga A, Harris JC, Kigozi R et al. . Improved malaria case management through the implementation of a health facility-based sentinel site surveillance system in Uganda. PLoS One 2011; 6:e16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sserwanga A, Sears D, Kapella BK et al. . Anti-malarial prescription practices among children admitted to six public hospitals in Uganda from 2011 to 2013. Malar J 2015; 14:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarnavsky E, Grimes D, Maidment R et al. . Extension of the TAMSAT satellite-based rainfall monitoring over Africa and from 1983 to present. J Appl Meteorol Clim 2014; 53:2805–22. [Google Scholar]
- 16. Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health 2004; 9:846–56. [DOI] [PubMed] [Google Scholar]
- 17. Najera JA, Gonzalez-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med 2011; 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev 2010; CD006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbel V, Akogbeto M, Damien GB et al. . Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis 2012; 12:617–26. [DOI] [PubMed] [Google Scholar]
- 20. Pinder M, Jawara M, Jarju LB et al. . Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet 2015; 385:1436–46. [DOI] [PubMed] [Google Scholar]
- 21. West PA, Protopopoff N, Wright A et al. . Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med 2014; 11:e1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lines J, Kleinschmidt I. Is malaria control better with both treated nets and spraying? Lancet 2015; 385:1375–7. [DOI] [PubMed] [Google Scholar]
- 23. Quiñones ML, Norris DE, Conn JE et al. . Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: a challenge for malaria control and elimination. Am J Trop Med Hyg 2015; 93:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranson H, N’guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 2011; 27:91–8. [DOI] [PubMed] [Google Scholar]
- 25. Gatton ML, Chitnis N, Churcher T et al. . The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 2013; 67:1218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oxborough RM. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): urgent need for affordable, long-lasting insecticides. Malar J 2016; 15: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen JM, Smith DL, Cotter C et al. . Malaria resurgence: a systematic review and assessment of its causes. Malar J 2012; 11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith Gueye C, Newby G, Gosling RD et al. . Strategies and approaches to vector control in nine malaria-eliminating countries: a cross-case study analysis. Malar J 2016; 15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masaninga F, Chanda E, Chanda-Kapata P et al. . Review of the malaria epidemiology and trends in Zambia. Asian Pac J Trop Biomed 2013; 3:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ossè RA, Aïkpon R, Gbédjissi GL et al. . A shift from indoor residual spraying (IRS) with bendiocarb to long-lasting insecticidal (mosquito) nets (LLINs) associated with changes in malaria transmission indicators in pyrethroid resistance areas in Benin. Parasit Vectors 2013; 6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pringle G. Malaria in the Pare area of Tanzania. 3. The course of malaria transmission since the suspension of an experimental programme of residual insecticide spraying. Trans R Soc Trop Med Hyg 1967; 61:69–79. [DOI] [PubMed] [Google Scholar]