Among young MSM on preexposure prophylaxis (PrEP), adherence declined dramatically over time. Self-report overestimated and Wisepill devices underestimated adherence. PrEP concentrations in hair and dried blood spots provided similar adherence information. Objective metrics should trigger adherence support for youth.
Keywords: adherence, adolescent, HIV prevention, preexposure prophylaxis
Abstract
Background
Young men-who-have-sex-with-men (MSM) are disproportionately impacted by human immunodeficiency virus (HIV). Preexposure prophylaxis (PrEP) could reduce HIV acquisition among youth, but suboptimal adherence threatens effectiveness. Optimal metrics of PrEP adherence among adolescents have remain undefined.
Methods
The Adolescent Trials Network 110/113 studies provided daily oral PrEP with tenofovir (TFV) disoproxil fumarate/emtricitabine over 48 weeks to a diverse population of MSM (aged 15–22 years). Self-reported adherence was assessed and PrEP drug concentrations measured from hair and dried blood spot (DBS) samples; 23% of participants received Wisepill electronic monitoring devices. The average number of PrEP doses per week taken was estimated, and concordance between measures assessed.
Results
Among 243 participants, hair samples were collected at 1186/1238 (96%) person-visits. The concordance of TFV levels in hair and TFV-diphosphate in DBS around thresholds consistent with taking ≥4 and 7 PrEP doses/week was high (76% and 80%). Hair and DBS concentrations correlated poorly with self-report and Wisepill metrics. Through week 12, 40%–60% of participants (by hair and DBS), ≤31% (Wisepill), and >85% (self-report) were estimated to have taken ≥4 PrEP doses/week (a threshold associated with protection among MSM). For all measures except self-report, adherence declined over time, with half of participants taking <2 doses/week by week 48.
Conclusions
Among youth on PrEP, adherence waned over time. Self-report overestimated adherence, and use of Wisepill was limited. Hair collection was highly acceptable and provided similar interpretations to DBS. Incorporation of either metric in future PrEP studies among youth could identify suboptimal adherence and trigger interventions.
Adolescents and young adults have among the highest human immunodeficiency virus (HIV) incidence rates in the United States and globally [1, 2]. In the United States, young black and Latino men who have sex with men (MSM) are disproportionately impacted by HIV [1]. Oral preexposure prophylaxis (PrEP) with tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC) is highly effective at preventing HIV acquisition if taken consistently [3–5]. However, in HIV treatment and prevention settings, adolescents and young adults have demonstrated suboptimal adherence [6–8], threatening to compromise the effectiveness of PrEP in this at-risk population.
Given the importance of adherence for PrEP effectiveness, strategies to assess adherence are needed. However, the optimal PrEP adherence metrics for adolescents have not yet been evaluated and may differ from those for adults due to differences in acceptability and feasibility of various measures. Self-report is subject to recall and social desirability biases and may overestimate adherence [9, 10]. Therefore, more objective measures such as electronic devices that track pill bottle openings (eg, Medication Event Monitoring System (MEMS) caps [11] and Wisepill [12]) and longer-term pharmacologic measures that estimate averaged adherence over weeks to months (eg, drug concentrations in small hair samples [13–15] and dried blood spots [DBS] [5, 16, 17]) are of interest. Hair has advantages in terms of collection in the field since it does not require phlebotomy, biohazardous precautions, or shipment/storage at cold temperatures. These objective adherence measures have been examined to a limited extent among young persons on PrEP [18, 19].
In this study, we examined 4 adherence measures (self-report, Wisepill, hair, and DBS) and changes in adherence over time among adolescent and young MSM enrolled in the Adolescent Trials Network (ATN) 110 [20] and 113 [21] studies. We compared these measures to each other and evaluated the acceptability of Wisepill use and hair collection in this at-risk population.
METHODS
Study Population
The ATN 110 and 113 studies were open-label demonstration projects and phase 2 safety studies of oral PrEP among MSM aged 18–22 and 15–17 years, respectively, in 12 US cities [20, 21]. Participants were HIV uninfected and reported HIV risk in the previous 6 months (eg, condomless anal intercourse, multiple partners, recent sexually transmitted infection). Participants were provided TDF/FTC 300/200 mg free of charge for 48 weeks of daily dosing. HIV testing, safety laboratory studies, and adherence assessments were performed at weeks 4, 8, and 12 and quarterly thereafter. All participants provided written informed consent, and each site’s institutional review board approved study procedures.
Adherence Measurements
Self-reported adherence was measured based on recall of pill-taking over the preceding 30 days using the timeline follow-back method [22]. Adherence was operationalized as the proportion of pills taken divided by the number expected over 30 days preceding each study visit.
Study sites were randomly selected to provide participants with the Wisepill wireless adherence monitoring device (Wisepill Technologies, Cape Town, South Africa), which was offered consecutively to up to 60 participants in ATN 110 and 40 in ATN 113. Each device held approximately a 2-week supply of TDF/FTC. Participants were responsible for refilling the device between visits. Each time the Wisepill is opened, the date and time are wirelessly transmitted to a server. Adherence was measured based on the number of device openings and the number expected over 30 days preceding each visit. Device openings >1/day were coded as 1/day.
Small hair samples (50–100 strands) were collected by trained study staff from the occipital scalp, although the protocol allowed for opting-out of collection. Hair samples were stored at ambient temperature and shipped to the University of California San Francisco Hair Analytical Laboratory. Drug concentrations in the 1.5 cm of hair closest to the scalp, representing drug exposure over the preceding 6 weeks, were used as the metric in this study, although segmental analysis can allow for determination of adherence over shorter periods of time [23]. Hair levels are measured via validated liquid chromatography/tandem mass spectrometry (LC/MS-MS)–based methods [15]. The assays to measure hair TFV and FTC concentrations have been peer reviewed and approved by the Division of AIDS’ Clinical Pharmacology and Quality Assurance (CPQA) program [24] and are validated from 0.002–0.4 ng TFV/mg hair and 0.02–4.0 ng FTC/mg hair.
Whole blood was collected via phlebotomy and spotted onto filter paper to create DBS, which were stored at –20°C or –80°C and shipped to the Antiviral Pharmacology Laboratory at the University of Colorado. A 3-mm DBS punch sample was extracted in 70:30 methanol, and the lysed cellular matrix was analyzed for tenofovir-diphosphate (TFV-DP), representing longer-term exposure (approximately 17-day half-life), and emtricitabine-triphosphate (FTC-TP), representing shorter-term exposure (approximately 1.5-day half-life) [16]. Our methods are CPQA approved [17, 25] and use LC/MS-MS with dynamic ranges of 2.5–2000 fmol/sample (TFV-DP) and 0.1–200 pmol/sample (FTC-TP).
Measurement of Acceptability of Hair Collection and Use of Wisepill
At each follow-up visit, participants were asked to provide a hair sample. Acceptability was measured based on the number of samples collected of the total number expected. A convenience sample of participants who received Wisepill devices took a quantitative, self-completed survey that included questions about the likelihood of future use. Given that DBS collection requires phlebotomy (a standard procedure for laboratory monitoring), we did not separately evaluate its acceptability.
Statistical Analyses
The concordance of drug detection of TFV-DP and FTC-TP in DBS and of TFV and FTC in hair was tabulated after pooling visits through week 48. Week 4 was excluded from analyses because hair samples were cut to 1.5 cm to reflect drug exposure over the preceding 6 weeks and thus could not be used to measure adherence at 4 weeks. Spearman correlation coefficients were estimated to assess the relationship between pharmacologic (hair and DBS) and nonpharmacologic (self-report and Wisepill) measures.
The average number of tablets/week taken was estimated for each adherence measure and categorized as 0 or below the limit of quantitation (BLQ) of the assay, <2, 2–3, 4–6, and ≥7 tablets/week. These categories were selected based on analyses that suggested that taking ≥4 TDF/FTC tablets/week provides high levels of protection against HIV among MSM [5]. For self-report and Wisepill, these categories corresponded to 0%, >0% to <21.4%, 21.4% to <50%, 50% to <93%, and ≥93% of expected doses taken, with the bounds of the intervals based on 1.5, 3.5, and 6.5 tablets/week. For hair, the number of tablets/week was estimated [13] based on a directly observed dosing study of TDF [15]. Dosing categories for TFV concentrations in hair were BLQ, lower limit of quantitation (LLQ) to <0.0096, 0.0096 to <0.0206, 0.0206 to <0.0370, and ≥0.0370 ng/mg. For DBS, dosing categories were BLQ, LLQ to 349, 350–699, 700–1259, and ≥1250 fmol/sample based on a pharmacokinetic model [17] and confirmed with a directly observed dosing study [26]. Adequate adherence consistent with taking ≥4 doses/week has been defined as ≥700 fmol/punch for TFV-DP in DBS [5] and ≥0.023 ng/mg for TFV in hair [15].
RESULTS
Characteristics of Study Participants and Summary of Adherence Measures
This analysis included participants with data on at least 1 adherence measure from at least 1 follow-up visit: 176/200 ATN 110 participants and 67/79 ATN 113 participants. In this subset, median age was 19 years (range, 15–22); 32% self-identified as Hispanic/Latino. Among non-Hispanic/Latino participants, 67.9% identified as black/African American, 26.5% as white, 4.3% as American Indian, and 1.2% as Asian/Pacific Islander. Sixty-five participants had Wisepill monitoring data and were similar in age to those not provided the Wisepill but more likely to be Hispanic/Latino (46% vs 27%, P = .013) and less likely to be black/African American (43% vs 58%, P = .066). Table 1 summarizes median adherence levels across all-person visits for the 4 measures.
Table 1.
Summary Statistics for Each Adherence Measure Across All Person-Visits in the Adolescent Trials Network 110 and 113 Studies
| Measure | N | Median | Interquartile Range | Range |
|---|---|---|---|---|
| TFV in hair, ng/mg | 768 | 0.013 | 0.003, 0.030 | 0.002, 0.32 |
| FTC in hair, ng/mg | 761 | 0.16 | 0.02, 0.45 | 0.02, 2.84 |
| TFV-DP in DBS, fmol/sample | 993 | 592.9 | 90.4, 1073.6 | 0, 3171.5 |
| FTC-TP in DBS, pmol/sample | 993 | 0.14 | 0, 0.25 | 0, 0.60 |
| Self-reported adherence, % | 992 | 90 | 70.0, 100 | 0, 100 |
| Wisepill bottle openings, % of expected | 254 | 3 | 0, 35.5 | 0, 100 |
Due to study close-out, not all hair samples that were collected were analyzed.
Abbreviations: DBS, dried blood spot; FTC, emtricitabine; FTC-TP, emtricitabine-triphosphate; TFV, tenofovir; TFV-DP, tenofovir-diphosphate.
Acceptability of Hair Collection and Wisepill Usage
Hair samples were collected at 1186/1238 (95.7%) of expected person-visits. The main reasons for noncollection were lack of time or inability of the participant to provide a sample. Due to close-out of the study, 418 (35.7%) of collected hair samples were not analyzed. In a survey administered to 18 participants who used the Wisepill, 10 (55.6%) said they would be “not at all likely,” 5 (27.8%) “somewhat likely,” and only 3 (16.7%) “very likely” to use the device in the future if available outside the study.
Change in Adherence Over Time by Pharmacologic and Nonpharmacologic Measures
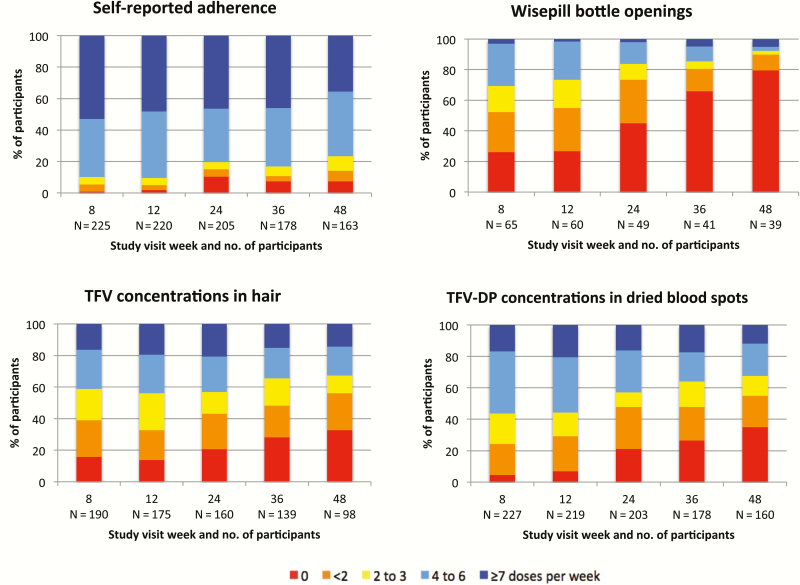
Figure 1 demonstrates PrEP adherence over time as assessed using the 4 measures. For all measures, adherence declined over time, particularly following week 12. Despite this finding, self-reported adherence remained relatively high, with more than 75% of participants reporting that they took ≥4 PrEP doses/week through 48 weeks. Fewer than one third of participants had recorded Wisepill openings consistent with taking ≥4 doses/week at any visit. For hair and DBS measures, adherence rates were similar, with 40%–60% of participants estimated to have taken ≥4 doses/week at weeks 8 and 12. However, adherence declined over time per both pharmacologic measures; by week 48, half of participants were taking <2 doses/week based on either hair or DBS concentrations. Per the pharmacologic measures (although self-reported adherence was higher), fewer than 20% of participants were taking PrEP on a daily basis (7 doses/week) at any visit.
Figure 1.
Levels of adherence by study week for 4 adherence measures: self-report, Wisepill openings, tenofovir concentrations in hair, and tenofovir-diphosphate concentrations in dried blood spots. Estimated number of preexposure prophylaxis doses taken per week are displayed. Abbreviations: TFV, tenofovir; TFV-DP, tenofovir-diphosphate.
Levels of Adherence Among Human Immunodeficiency Virus Seroconverters
Seven participants were diagnosed with HIV during the 48 weeks of follow-up. In samples collected at the visit closest to the date of seroconversion, all 7 participants had TFV hair concentrations and TFV-DP concentrations in DBS consistent with taking <2 doses/week. Six of the 7 participants had TFV hair concentrations at or below the assay’s LLQ. Mean self-reported adherence (assessed at the seroconversion visit for 4 participants) was 83%. One participant received a Wisepill device and had zero device openings recorded in the 30 days prior to the seroconversion visit.
Concordance of Drug Detection in Hair and Dried Blood Spots
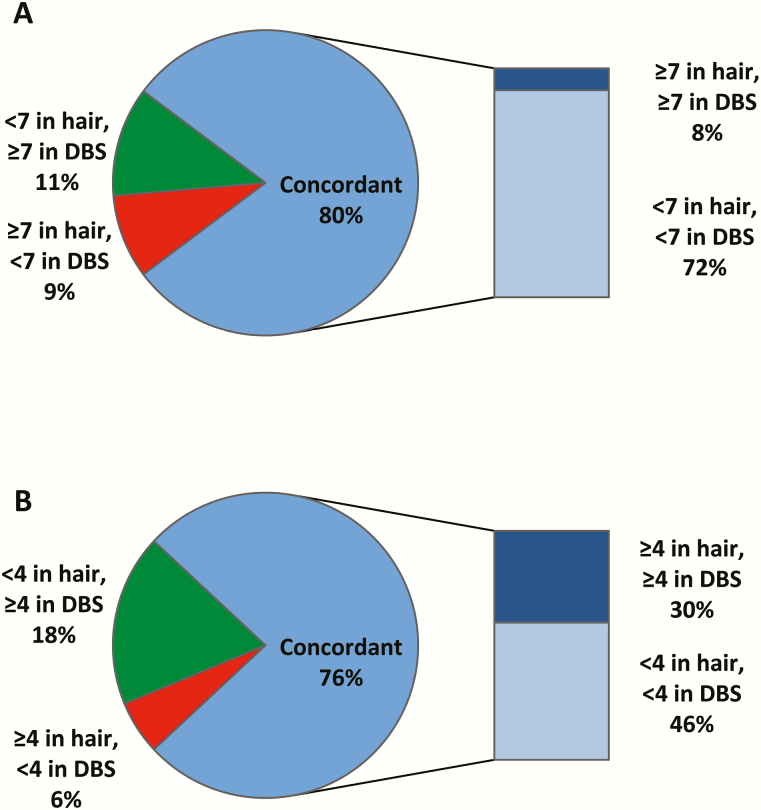
The concordance of levels of TFV in hair and TFV-DP in DBS consistent with taking ≥4 and 7 PrEP doses/week was high (76% and 80%, respectively; Figure 2). The concordance of drug detection and nondetection between hair and DBS measures was high across study visits (88.6% for TFV in hair and TFV-DP in DBS; Table 2). Concordance was also high for TFV and FTC detection and nondetection in hair samples (84%). Concordance was lower between both hair measures and FTC-TP in DBS and between TFV-DP and FTC-TP in DBS (Table 2).
Figure 2.
Concordance of tenofovir concentrations in hair and tenofovir-diphosphate concentrations in dried blood spots based on drug concentrations consistent with taking 7 preexposure prophylaxis doses per week (A) and 4 PrEP doses per week (B). Abbreviations: DBS, dried blood spot.
Table 2.
Concordance of Detection of Tenofovir, Emtricitabine, and Their Metabolites in Dried Blood Spot and Hair Samples Across All Person-Visits, Excluding Study Week 4
| Adherence Measure | Concordant | Discordant | |
|---|---|---|---|
| % | Detectable/ Undetectable, % |
Undetectable/ Detectable, % |
|
| TFV in hair, TFV-DP in DBS | 88.6 | 2.9 | 8.5 |
| FTC in hair, TFV-DP in DBS | 88.9 | 2.6 | 11.2 |
| TFV in hair, FTC-TP in DBS | 73.7 | 24.4 | 2.0 |
| FTC in hair, FTC-TP in DBS | 76.5 | 21.6 | 2.0 |
| TFV-DP in DBS, FTC-TP in DBS | 71.2 | 28.6 | 0.2 |
| TFV in hair, FTC in hair | 84.0 | 6.0 | 3.2 |
Abbreviations: DBS, dried blood spot; FTC, emtricitabine; FTC-TP, emtricitabine-triphosphate; TFV, tenofovir; TFV-DP, tenofovir-diphosphate.
Correlations between Pharmacologic and Nonpharmacologic Measures
Hair and DBS concentrations of TDF and FTC metabolites were poorly correlated with self-report (all r < 0.40, P < .001; Table 3). Adherence estimates for both pharmacologic measures were also poorly correlated with Wisepill openings.
Table 3.
Spearman Correlation Coefficients for Pharmacologic and Nonpharmacologic Adherence Measurements Across All Person-Visits, Excluding Study Week 4
| Adherence metric | Hair TFV | Hair FTC | DBS TFV-DP | DBS FTC-TP | Self-report |
|---|---|---|---|---|---|
| Self-report | 0.28a | 0.29a | 0.39a | 0.40a | |
| Wisepill | 0.40a | 0.36a | 0.58a | 0.36a | 0.25a |
Abbreviations: DBS, dried blood spot; FTC, emtricitabine; FTC-TP, emtricitabine-triphosphate; TFV, tenofovir; TFV-DP, tenofovir-diphosphate.
a P < .001 for all correlations.
DISCUSSION
In this study of primarily racial/ethnic minority adolescent and young MSM in urban centers in the United States, we performed the first comprehensive analysis and comparison of 4 metrics that are increasingly used to assess adherence in PrEP studies and programs: self-report, Wisepill device openings, and the quantification of PrEP drug concentrations in hair and DBS. With all 4 metrics, we found higher rates of initial adherence to PrEP, with waning adherence over time and inadequate adherence by 48 weeks. As in prior studies [27], self-report overestimated adherence compared to pharmacologic measures. In contrast, Wisepill openings underestimated adherence relative to pharmacologic metrics, likely because the devices were not being used. We found hair and DBS measures to be concordant in terms of estimating number of PrEP doses taken per week and found high acceptability (>95%) of hair collection. Therefore, future investigators may elect to use either DBS or hair, with the decision on which metric to use based on feasibility and acceptability in the particular study context and population. In all 7 participants who seroconverted, levels of PrEP drugs in hair and DBS were low, although self-reported adherence was high. Overall, our findings underscore that self-report to PrEP overestimates actual adherence in young people, that either hair or DBS are acceptable adherence metrics in this group, and that the waning of adherence over time is particularly dramatic among youth.
Over the first 3 months of the study, approximately half of youth achieved hair and DBS drug concentrations consistent with taking ≥4 doses/week, estimated to provide protection from HIV acquisition among MSM [5]. However, adherence decreased over time, and by week 48, only one third of participants had drug concentrations in hair or DBS consistent with taking ≥4 doses/week. Of note, declining levels of adherence over time have been demonstrated in other clinical trials [28, 29] that used subjective measures, with younger participants at greater risk. However, the decline in adherence seen in our study is more profound than has been noted in other trials, likely reflecting the increased accuracy of objective measures to demonstrate this phenomenon over nonobjective measures.
Since adherence to PrEP is critical to its effectiveness [27], strategies to both monitor and promote adherence over time will be essential to the success of PrEP among youth. Incorporation of adherence measures that are accurate, acceptable to participants, and feasible to collect will be important as PrEP is rolled out in both domestic and global settings. This study used the timeline follow-back method to assess self-reported adherence, a validated tool [30] that prompts participants to remember key life events that may have impacted pill-taking in the preceding 30 days [22]. Yet, even with this enhanced assessment tool, self-report appeared to vastly overestimate pill consumption compared to pharmacologic measures in this study. Although positive provider–patient interactions can reduce the social desirability bias that plagues self-reported adherence [31, 32], multiple studies have demonstrated at this point that incorporation of some form of objective adherence monitoring into PrEP assessment is useful.
Wisepill devices send a signal to a server each time the device is opened, leading to interest in using them for both real-time adherence monitoring and to trigger feedback or reminders if missed doses are detected [12]. In the subset of participants in this study who were provided with the Wisepill device, 84% said they were only somewhat or not at all likely to use it in the future. Moreover, many participants who had evidence of drug ingestion based on hair and DBS concentrations had no Wisepill openings recorded, suggesting that participants did not use the devices even when provided. The relatively bulky and conspicuous nature of the device, which limited acceptability in other studies [33], may not be appealing to US-based adolescents. The low number of device openings could also have resulted from participants taking out multiple pills at once to store elsewhere (“pocket doses”) or not refilling the devices between study visits. Our findings therefore raise concerns about the accuracy and acceptability of Wisepill as an adherence metric among young people on PrEP.
Because pharmacologic measures of adherence (mainly plasma) were critical to the interpretation of PrEP trial results and because hair and DBS concentrations can estimate average drug intake over longer time periods than plasma, there has been increasing interest in using hair and DBS metrics to monitor PrEP adherence. In the iPrEx open-label extension study, DBS measures were associated with protective efficacy, [5] and hair and DBS drug concentrations were highly correlated [19]. The current study is the largest to date to compare hair and DBS concentrations among adolescents. We found concentrations of TFV-DP in DBS and TFV and FTC in hair (all relatively long-term measures of exposure) to be concordant (88.6% and 88.9%, respectively; Table 2). FTC-TP levels in DBS represent drug exposure over shorter time frames [16], which is likely responsible for the lower concordance rates between FTC-TP in DBS and TFV concentrations in hair (73.7%), FTC levels in hair (76.5%), or TFV-DP levels in DBS (71.2%). The high rates of acceptability of hair collection in this study (which used an opt-out approach to sampling) were similar to acceptability rates (95%) for hair sampling in studies in Africa and Asia [34–36]. Together, these data suggest that in future studies, investigators may choose to include either hair or DBS measurements, depending on the feasibility and acceptability of sample collection and storage capabilities in their setting. A limitation of both long-term measures is that drug-taking is generally assessed over the preceding 4–6 weeks. Thus, if hair or DBS samples are collected quarterly or less frequently, these measures may not reflect dosing >6 weeks prior to sample collection. Moreover, both hair and DBS levels assess averaged dosing but cannot discern patterns of pill consumption, for example, in relation to sexual activity.
Our study is also the first to assess concordance between TFV and FTC concentrations in hair samples among participants on PrEP. We found that TFV and FTC concentrations in hair were also concordant. Thus, if hair samples are collected to assess adherence to TDF/FTC, either TFV or FTC can be measured, rather than both. The concordance between TFV and FTC levels also has implications for the use of coformulated FTC and tenofovir alafenamide (TAF), which is increasingly replacing TDF/FTC for treatment in the United States and is being studied for PrEP among MSM and transwomen. Thus, if clinical trials find that TAF/FTC is effective for PrEP in these populations, FTC hair concentrations could be used to measure long-term drug exposure in future studies.
This study is subject to several limitations. Not all participants were provided with Wisepill devices, although sites that provided Wisepill devices to participants were randomly selected. Reasons for Wisepill nonopenings (eg, pocketed doses) were not collected, and survey participants were not asked why they would be likely/unlikely to use Wisepill in the future. Participants were also responsible for refilling the device between visits. In addition, due to attrition during the parent studies, not all adherence measures were available at each time point. Finally, due to study close-out, some collected hair samples were not analyzed.
In this study of TDF/FTC-based PrEP, we found that hair and DBS drug concentrations provided similar estimates of adherence and were highly acceptable among racially/ethnically diverse adolescent and young MSM. Although some surveyed participants stated the Wisepill was acceptable, this device appeared not to be used by many participants, and most did not envision using it in the future, raising concerns about its ultimate utility among adolescents and young adults. Given that levels of adherence to PrEP waned over time, strategies to bolster adherence such as text message reminders, financial incentives, and peer support will be needed to ensure that young people on PrEP consistently achieve adequate levels of protection from HIV (eg, ≥4 doses per week among MSM [5]). As PrEP use expands among the populations at highest risk for HIV infection, including young people in the United States and globally, measuring and supporting adherence to this powerful prevention method will be essential to its success.
Notes
Acknowledgments. The authors thank the participants in the ATN 110 and 113 studies and the dedicated study staff. We also thank the staff of the Hair Analytical Laboratory at the University of California San Francisco and the Colorado Antiviral Pharmacology Laboratory at the University of Colorado for their work on the hair and dried blood spot assays, respectively.
Financial support. This work was supported by the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01 HD040533 and U01 HD040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (B. Kapogiannis and S. Lee), with supplemental funding from the National Institute on Drug Abuse (K. Davenny and S. Kahana) and the National Institute of Mental Health (P. Brouwers and S. Allison). Gilead Sciences donated the study drug and provided funding for testing some of the dried blood spots and a portion of overall study costs. The study was scientifically reviewed by the ATN’s Community Prevention Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson and C. Partlow) at the University of Alabama Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat (J. Korelitz and B. Driver). The hair assays for this study were supported by a grant from the National Institute of Allergy and Infectious Diseases (2R01 AI098472 to M. G.). This work was also supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD052163 to C. Brindis, N. Adler to support C. A. K.]; the National Institute of Mental Health (K23 MH097649 to P. S.); and the National Center for Advancing Translational Sciences (UCSF-CTSI UL1 TR000004 for performing statistical analyses).
Potential conflicts of interest. Gilead Sciences donated TDF/FTC as study drug and provided funding for the testing of some dried blood spots and a portion of overall study costs, but had no other role in the design or conduct of the study or the analysis or interpretation of data. C. A. K., S. G. H., P. B., A. L., K. K., and M. G. report that grant support from the National Institutes of Health has been paid to their institutions. P. L. A. has received grant support from Gilead Sciences paid to his institution. A. Y. L. reports that Gilead has donated study drug for other studies he has led. All other authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. HIV surveillance report, 2015; vol. 27 Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 12 May 2017.
- 2. UNAIDS. Global AIDS update Available at: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 3 June 2016.
- 3. Baeten JM, Donnell D, Ndase P et al. ; Partners PrEP Study Team Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grant RM, Lama JR, Anderson PL et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant RM, Anderson PL, McMahan V et al. ; iPrEx study team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS 2014; 28:1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosek SG, Siberry G, Bell M et al. ; Adolescent Trials Network for HIVAIDS Interventions The acceptability and feasibility of an HIV preexposure prophylaxis (PrEP) trial with young men who have sex with men. J Acquir Immune Defic Syndr 2013; 62:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nachega JB, Hislop M, Nguyen H et al. . Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr 2009; 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care 2012; 24:1448–52. [DOI] [PubMed] [Google Scholar]
- 10. Nieuwkerk PT, de Boer-van der Kolk IM, Prins JM, Locadia M, Sprangers MA. Self-reported adherence is more predictive of virological treatment response among patients with a lower tendency towards socially desirable responding. Antivir Ther 2010; 15:913–6. [DOI] [PubMed] [Google Scholar]
- 11. Baxi SM, Liu A, Bacchetti P et al. . Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 2015; 68:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haberer JE, Musiimenta A, Atukunda EC et al. . Short message service (SMS) reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS 2016; 30:1295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi M, Glidden DV, Mayer K et al. . Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016; 3:e521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koss CA, Bacchetti P, Hillier SL et al. . Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017; doi: 10.1089/aid.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu AY, Yang Q, Huang Y et al. . Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castillo-Mancilla J, Seifert S, Campbell K et al. . Emtricitabine-triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother 2016; 60:6692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo-Mancilla JR, Zheng JH, Rower JE et al. . Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bekker LG, Hughes J, Amico R et al. . HPTN 067/ADAPT: background and methods and Cape Town results. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment & Prevention Vancouver, Canada, 2015. [Google Scholar]
- 19. Gandhi M, Glidden DV, Liu A et al. ; iPrEx Study Team Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx open label extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis 2015; 212:1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hosek SG, Rudy B, Landovitz R et al. ; Adolescent Trials Network for HIVAIDS Interventions An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr 2017; 74:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosek S, Landovitz R, Rudy B et al. . An HIV pre-exposure prophylaxis demonstration project and safety study for adolescent MSM ages 15–17 in the US (ATN 113). International AIDS Conference Durban, South Africa, 2016. [Google Scholar]
- 22. Sobell L, Sobell M. Timeline follow-back. In: Litten R, Allen J. Measuring Alcohol Consumption. Totowa, New Jersey: Humana Press, 1992:41–72. [Google Scholar]
- 23. Jakobsson G, Kronstrand R. Segmental analysis of amphetamines in hair using a sensitive UHPLC-MS/MS method. Drug Test Anal 2014; 6(Suppl 1):22–9. [DOI] [PubMed] [Google Scholar]
- 24. DiFrancesco R, Taylor CR, Rosenkranz SL et al. . Adding value to antiretroviral proficiency testing. Bioanalysis 2014; 6:2721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng JH, Rower C, McAllister K et al. . Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson P, Liu A, Castillo-Mancilla J et al. . TFV-DP in dried blood spots (DBS) following directly observed therapy: DOT-DBS study. In: Conference on Retroviruses and Opportunistic Infections. Seattle, WA, 2017. [Google Scholar]
- 27. Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS 2016; 11:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haberer JE, Baeten JM, Campbell J et al. . Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med 2013; 10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gengiah TN, Moosa A, Naidoo A, Mansoor LE. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm 2014; 36:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braithwaite RS, McGinnis KA, Conigliaro J et al. . A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res 2005; 29:1190–7. [DOI] [PubMed] [Google Scholar]
- 31. Do HM, Dunne MP, Kato M, Pham CV, Nguyen KV. Factors associated with suboptimal adherence to antiretroviral therapy in Viet Nam: a cross-sectional study using audio computer-assisted self-interview (ACASI). BMC Infect Dis 2013; 13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson MO, Chesney MA, Goldstein RB et al. ; NIMH Healthy Living Project Team Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: a mediation model. AIDS Patient Care STDS 2006; 20:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bachman Desilva M, Gifford AL, Keyi X et al. . Feasibility and acceptability of a real-time adherence device among HIV-positive IDU patients in China. AIDS Res Treat 2013; 2013:957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prasitsuebsai W, Kerr SJ, Truong KH et al. . Using lopinavir concentrations in hair samples to assess treatment outcomes on second-line regimens among Asian children. AIDS Res Hum Retroviruses 2015; 31:1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hickey MD, Salmen CR, Tessler RA et al. . Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014; 66:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koss CA, Natureeba P, Mwesigwa J et al. . Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015; 29:825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]