Abstract
The tiered aviary for laying hens includes a floor litter area to promote foraging and dust bathing. Data are needed on hens’ use of different litter substrates and effectiveness of substrates in removing excess feather lipids to ensure a suitable litter area. Bovans White hens were housed in commercial-style aviaries with access to one of 3 litter substrates (wood shavings, straw, or plastic turf mats—AstroTurf®, n = 4 aviary pens per substrate, 144 cage-reared hens populated per pen). Litter areas were videoed across 2 d each at 4 ages: immediately following first aviary opening (25 wk), then at 28, 50, and 68 weeks. Observations of hens throughout the d included percentages of all hens in each pen on the litter area, foraging and transitioning between the tiered enclosure and litter area. Percentages of hens dust bathing were observed from 11:00 to 15:00. Breast and back feather samples from 7 birds per pen at 28, 50, and 68 wk were analyzed for lipid content. Overall, fewer hens simultaneously accessed the AstroTurf® (P < 0.0001), but flocks showed relatively balanced transitions between the tiered enclosure and the litter area throughout the d, regardless of substrate. On average, less than 5% of all hens were observed dust bathing (peaks up to 15% of hens) with no differences among litter substrates or ages (P ≥ 0.18). On average, less than 2% of hens were observed foraging (peaks up to 4% of hens) with fewer hens foraging on AstroTurf® (P < 0.0001). Feather lipid differences among litter substrates (P < 0.0001) were inconsistent across sampling periods, possibly due to different birds sampled across time. At all ages, lipid levels were higher on the back over breast feathers (P < 0.0001) for hens housed with AstroTurf®. AstroTurf® may be suitable for nest boxes, but straw and shavings are more ideal litter substrates. Further study should investigate alternative substrates or regular substrate addition to encourage more foraging and dust bathing.
Keywords: cage-reared, laying hen, dust bathing, litter, lipids, foraging
INTRODUCTION
Consumer perceptions of how to house laying hens is driving producers, globally, to use non-cage housing systems over conventional cages. Within North America, tiered aviaries, which consist of internal enclosure-based resources (perches, nest boxes, feed, and water) and an open floor litter area, are being implemented as a system that encourages ethologically important behaviors, such as dust bathing and foraging (Cooper and Albentosa, 2003; Weeks and Nicol, 2006). The open litter area is unique to the aviary system compared to conventional and furnished cages.
In commercial aviaries or percheries (different from the aviary style in the current study), approximately 70% of sampled hens were observed using the litter area with each individual using approximately 50% of the available litter space (Carmichael et al., 1999), or approximately 24% of sampled hens were present on the littered floor (Channing et al., 2001). Use of the floor area increased with age (Channing et al., 2001). Examinations of the same-style commercial aviary as in the current study, showed the highest numbers of hens accessed the litter immediately after aviary doors opened, but hens transitioned between the enclosure and litter area throughout the d (Campbell et al., 2016a). There were periods of high numbers of hens on the litter area simultaneously, but overall, hens typically distributed themselves evenly throughout the litter area and the tiered enclosure (Campbell et al., 2016a). Location tracking of 35 individual hens from the same flock as the current study showed some birds spent up to approximately 50% of their observed time on the litter area, but not all hens were seen using this resource (Campbell et al., 2016b).
In semi-wild red junglefowl, foraging (ground-pecking) was observed to take up 60% of active time (20 min observed per h from sunrise to evening roosting), while ground scratching was performed in 34% of observed active time (Dawkins, 1989). However, domesticated hens may spend less time engaged in more energetically expensive behaviors, including foraging (defined as ground peck, ground scratch, explore, explore other objects, bill rake, taste, and drink, Schütz and Jensen, 2001). For example, while birds exhibit foraging in commercial aviary and perchery systems, foraging rates are lower than those of wild junglefowl. In the litter area, only 5.9% (Channing et al., 2001) up to an average of 8.4% (Carmichael et al., 1999) of sampled birds were observed foraging (defined as including scratching and ground pecking: Carmichael et al., 1999, or undefined: Channing et al., 2001).
Dust bathing is important for maintaining feather condition (van Liere, 1992) and is performed even in the absence of a suitable substrate (“sham dust bathing”: Cooper and Albentosa, 2003). Caged hens that have been deprived of a litter area show more dust bathing than aviary-housed hens (Colson et al., 2007). This indicates that the litter area enables birds to fulfill their behavioral need to dust bathe. Hens demonstrate motivation to dust bathe by pushing weighted doors to access substrate, both if previously deprived or non-deprived of substrate, suggesting hens may be dust bathing for pleasure rather than to reduce suffering (Widowski and Duncan, 2000). Thus, dust bathing might be a low behavioral need (Weeks and Nicol, 2006). In commercial aviaries and percheries (different from the aviary style of the current study), only 1.6% (Channing et al., 2001), or up to 3.9%, of sampled hens in the litter area have been recorded dust bathing (Carmichael et al., 1999). Alternatively, in a different floor-based system, higher percentages (∼15 to 20%) of hens that were on the litter area were observed dust bathing, but only when the litter was friable (Odén et al., 2002).
Maintenance of low feather lipid levels through dust bathing is important for optimal plumage condition, which enables effective thermoregulation by the hen (Sandilands et al., 2004). Feather lipid levels are affected by type of litter, and having access to litter or not. Compared to aviary-housed birds, hens housed in furnished cages with an Astroturf mat had higher lipid levels on their back feathers, although both furnished- and aviary-housed hens had lower chest lipid levels than conventional-housed hens (Blatchford et al., 2013). Comparisons of hens housed in furnished cages showed feather lipid levels on chest and body sides increased immediately after dust bathing in feed but decreased immediately after dust bathing in lignocellulose (Scholz et al., 2014).
Hens’ use of areas in commercial aviaries and percheries and performance of specific behaviors can be influenced by system design (Odén et al., 2002), with the provided litter substrate a potential source of variation. A suitable litter substrate for both foraging and dust bathing needs to be friable and with adequate depth to assure small particles can be easily manipulated and tossed onto feathers (Moesta et al., 2008; Scholz et al., 2010). Hens vary in expression of dust bathing, and foraging depending on the type of substrate available (Scholz et al., 2010; Alvino et al., 2013). For example, hens spent more time dust bathing in lignocellulose (soft wood pellets) over wood shavings, food pellets, or Astroturf—artificial plastic turf (Scholz et al., 2010). Hens preferred to forage in food particles more than in wood shavings, lignocellulose, or Astroturf (Scholz et al., 2010). Complete dust bathing bouts occur more often in aviaries and barns with established litter substrates compared to daily provision of wood shavings in furnished cages (de Jong et al., 2005), which may also be related to differing space restrictions among the systems. Furthermore, individual tracking of a subset of birds from the same flock as the current study showed litter area use was dependent on the specific litter substrate available, with fewer birds accessing an AstroTurf® substrate (Campbell et al., 2016b). Within US commercial aviary systems, wood shavings and straw are most commonly used as initial floor substrates that then become deeper as manure and feathers accumulate. Astroturf pads are used in commercial aviary and furnished cage nest boxes and as scratch areas within furnished cages. Previous commercial studies showed Astroturf pads in nest boxes had very low microbial load (Jones et al., 2015), thus AstroTurf® pads were trialed in this study as potential new floor litter substrates.
The objectives of this study were to assess, within an aviary system, birds’ litter use by percentage of birds in the litter area, number of transitions between the system and litter area, and percentage of birds foraging (defined as ground scratching, typically followed by ground pecking) or dust bathing in one of 3 litter types: wood shavings, straw, or AstroTurf® across a lay cycle. Lipid levels of breast and back feathers were assessed at 28, 50, and 68 wk of age. The research hypothesis was that hen groups with different substrates would vary in their use of the litter areas and that more manipulable substrate (wood shavings and straw) would lower feather lipid content. Across the flock cycle, both differences within groups and fewer differences among groups were predicted as litter substrates became more uniform as a result of accumulating manure and feathers on top of the initial substrate.
MATERIALS AND METHODS
Ethics
All research was approved by the Michigan State University Institutional Animal Care and Use Committee prior to the start of data collection.
Hens and Housing
Cage-reared, beak-trimmed Bovans White pullets (n = 2304) were obtained from a commercial producer at 17 wk of age (December 2012). Pullets from an alternative rearing system that would provide birds with litter access during rearing were not available in the required numbers. Hens were placed in groups of 144 hens in one of 16 pens, each containing a NATURA60 Big Dutchman (Holland, MI) commercial-style aviary at the laying hen facility of Michigan State University (East Lansing, MI). Within the facility, there were 4 aviary rooms (each room 20 m L x 4.3 m W) that each held 4 aviary pens divided by wire mesh gates (343 cm L x 244 cm W x 230 cm H, Figure 1). There were also 8 rooms containing furnished cages (not part of this study) and thus the entire facility had 12 rooms housing laying hens (Figure 1). Each pen contained a tiered 3-level enclosure with feed troughs, water nipples, perches, nest boxes on the upper level, and access to an open litter area via a door on the lower level (Figure 2). Hens in each aviary pen were provided 1,131.88 cm2 of useable floor area per hen, comprising of 550.69 cm2/hen of tiered enclosure space (including both wire mesh flooring and metal ledges) and 305 cm2/hen of open litter area in front of the tiered enclosure. Hens did not have access to the area underneath the enclosure (Figure 2). Each hen had 5.08 cm of feeder space, water access at a density of 9 hens/nipple drinker, 13.55 cm of perch space, and 83.80 cm2 of nest box space. The aviary doors on the lower tier opened for the first time at 25 wk of age, providing birds access to the floor litter area. Subsequently, doors opened daily at approximately 10:00 (after which point the majority of eggs were expected to have been laid) and closed at 01:00. Mortality was recorded daily per individual pen, and no birds were replaced. Water was provided ad libitum, and hens were fed commercial diets (Webberville Feed & Grain Company, Webberville, MI). Dimmable LED lights (AgriShift PL 12 watt, ONCE, Inc., Plymouth, MN) were on from 04:00 until 19:30, with a 5-minute sunrise sequence and a 35-minute sunset sequence. The facility was tunnel ventilated and maintained at 21°C with twice-weekly manure removal from the tiered enclosures via belts under the wire-mesh flooring on each level. No manure removal occurred in the litter area.
Figure 1.
Representation of the facility that housed 4 aviary rooms each with 4 separate pens comprised of the tiered aviary enclosure and litter area with different litter substrates (AstroTurf®, straw, shavings, or concrete [not observed in this study] and 8 furnished cage rooms (shaded gray and not drawn to scale, they were of the same size as the aviary rooms and were not part of this study). Birds could not travel between the different aviary pens within each room.
Figure 2.
Representation of the aviary pens as seen from the end of the unit showing the open litter area, outer perch, aviary door for access to the litter area, inner perches and ledges on each tier, food, water, and location of the nest box within the enclosure. The shaded egg belt and underneath areas were inaccessible to hens.
Litter Substrates and Focal Enclosures
Prior to aviary doors opening for the first time, 3 different types of litter substrate (n = 4 pens/substrate) were placed on the concrete floor in a balanced design that accounted for room and pen location (relative to the door) within the room (Figure 1). Litter substrates were whole straw and wood shavings (both covering the floor at a depth of approximately 1 cm) and AstroTurf® NXT pads (GrassWorx LLC., St. Louis, MO). The litter areas of the remaining 4 pens were left as bare concrete. However, following aviary opening, hen movement on adjacent litter areas pushed some straw and shavings under the gates into the floor area of the bare concrete pens. Solid plastic panels were added across the bottom of the gates to prevent further substrate movement but the “bare concrete” litter treatment pens were no longer a true concrete-only comparison and were thus excluded from later analysis. Litter depth was assessed once at 69 wk of age in 3 locations across the litter area (in 2 front quarters, and once in the center back). For all substrates, litter remained dry and friable even as feathers and manure accumulated on the original substrate. Non-parametric Kruskall-Wallis test with post-hoc Wilcoxon each-pair tests (Bonferroni correction applied) showed significant differences in litter depth between substrate types (H = 9.85, DF = 2, P = 0.007). AstroTurf® had the shallowest depth and shavings the deepest (means ± SEM: AstroTurf® 0.58 ± 0.07 cm, straw 1.63 ± 0.07 cm, shavings 2.08 ± 0.07 cm). Litter substrate was observed trapped underneath the intersecting AstroTurf® mats within each pen, creating an uneven floor surface.
Video Recording and Decoding
Ceiling-mounted high-resolution digital video cameras (VF450, Clinton Electronics, Loves Park, IL) were used to capture the open litter area of each pen from aviary opening until lights off for 2 consecutive d at each of the 4 ages across the lay cycle: first aviary opening (25 wk of age), 28 wk, 50 wk, and 68 wk of age. Video footage was analyzed from 5 min after opening of the aviary system up to lights off. Every 20 min, the number of hens in each litter area was counted. The number of birds dust bathing or foraging was counted by continuous sampling during each first 2 min of every 20 min from 11:00 to 15:00 for dust bathing (a time period determined to capture most dust bathing activity, Campbell et al., 2016c) and from 5 min after aviary opening until lights off for foraging (no peak period was identified prior to analyses). Only one d of video at each age point was observed for foraging, as across all age points, the majority of observations were “zero hens foraging.” Foraging was defined as scratching feet backwards, typically followed by pecking on the ground (easily distinguished from the top-down camera view) but did not include hens walking around and pecking, as this could not be reliably distinguished from the top-down view. Transitions were defined as birds moving between the tiered enclosure and the open litter area via the outer perch (previous observations in commercial aviaries showed almost all movement onto the litter area and back involved the outer perch (Campbell et al., 2016a and see Figure 2). The number of birds in transition was counted across a 5-minute observation period, with 6 consecutive observation periods (30 min of video) immediately following aviary opening, 6 consecutive observation periods (30 min of video) immediately prior to lights off, and 3 consecutive observation periods (15 min of continuous video) every h throughout the day.
Inter-observer reliability of observers was minimal of 90% (assessed by both correlation and visual inspection of data). Individual observers watched hens in pens from each of the litter substrate groups. Observers were not specifically informed of substrate type or the objectives of the study; litter substrates could be distinguished from the video at 25 and 28 wk but not at 50 and 68 wk.
Feather Lipid Level Assessment
At 28, 50, and 68 wk of age, 1 g of breast and back feathers were cut at the base of the rachis from 7 birds per pen (n = 28 birds per litter substrate) on the same d to assess feather surface lipid levels. Both breast and back feathers were sampled, as substrates can differentially affect lipid levels in these 2 areas, depending on availability of particles to be tossed onto the back area (Blatchford et al., 2013). Birds were randomly selected from each enclosure but were not included if they were severely feather pecked, as adequate feather samples could not be obtained. Dirty feathers were avoided. The same individual birds were not deliberately used at each sampling period, as it was not possible to locate tagged individuals without removing all hens from the enclosures. Each individual hen's 2 feather samples (breast and back) were stored after collection in separate zip-lock bags at −20°C until lipid analysis.
In preparation for feather lipid analysis, feather samples were allowed to thaw at room temperature. Feathers were cut in small pieces of approximately 1 cm with ethanol-rinsed scissors and forceps. One gram of feathers was measured out using an analytical balance (Ohaus Explorer, E11140, Ohaus Corporation, Parsippany, NJ), wrapped into (7 × 7 cm) filter paper (Whatman® glass microfiber filters, Grade GF/C sheets, Sigma-Aldrich, St Louis, MO) using clean forceps, and closed with a metal ring. Gloves were used when handling the filter papers and individual samples to prevent any transfer of oils from the researcher's hands. The forceps were cleaned with ethanol between each sample.
The pre-extraction weight (W1) of each sample was recorded, and then samples were placed in a 100° C drying oven for 2 h and weighed for a second time to calculate the dry matter weight of each feather sample (W2). Feather lipids were then extracted by the Soxhlet extraction method using 2 extraction units. A total of 25 samples was inserted in each extraction chamber at each time, and extraction was performed using ether anhydrous (Fisher Scientific, Fair Lawn, NJ 0,7410; Cat. No. E138–20; Lot No. 124,158 & 132,032) for 16 hours. The feather samples were transferred to a desiccator for 30 min to cool, then left for 8 h to ensure full evaporation of the ether. Feather samples were then weighed for the third time (W3) in order to calculate the amount of feather lipid by disappearance from the dried pre-extraction sample weight (W2).
Data and Statistical Analyses
The number of hens in the litter, foraging, or dust bathing or in transition were converted to a proportion of all hens in the pen per sampling age (calculations took cumulative enclosure mortality into account). The difference between the proportion of hens exiting and entering the tiered enclosure was calculated (positive values indicate more hens were exiting at that specific time point). All calculated proportions were averaged across the d each for the 2 sampling d (only one sampling d for foraging) for each age (n = 96 in the final model, 4 enclosures x 3 substrates x 2 d x 4 ages). Data were logit transformed (Warton and Hui, 2011) and analyzed using General Linear Mixed Models in JMP® 12.1.0 (SAS Institute, Cary, NC) with α set at 0.05 and using restricted maximum likelihood estimation methods. The effects of substrate, age, and their interaction were included as fixed effects with observation d and pen classed as random effects. Where significant differences were present, Student's t tests were performed on the least squares means with Bonferroni correction applied for multiple post-hoc testing if more than 3 comparisons were made. Comparisons among substrates for feather lipid content (back and breast feathers compared separately) were made using separate General Linear Models (GLM) at each age as different birds were sampled across time. The difference in lipid content between back and breast feathers was also calculated (positive values indicate higher feather lipid levels on the back feathers) and compared among substrates using separate GLM at each age. As there was virtually no difference between the raw and back-transformed means, the raw percentage means ± SEM or total observed percentage raw values are presented in the tables and figures.
RESULTS
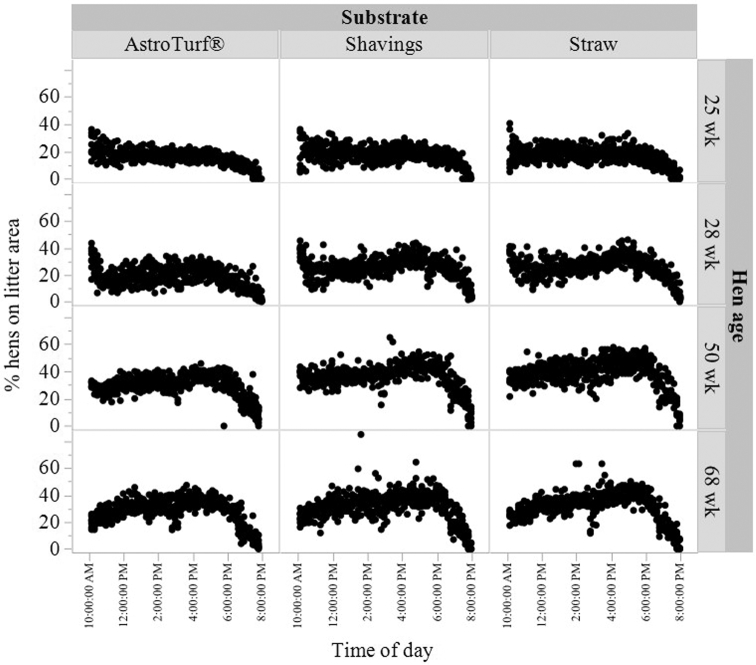
Upon the aviary first opening (25 wk), on average across all substrates, less than 20% of all hens were recorded on the litter area simultaneously. Overall litter occupancy increased up to 40% at 50 weeks. Examination of raw individual observation values revealed that up to 85% of hens were observed on the litter simultaneously in the shavings substrate at 68 wk of age. Irrespective of litter type, similar percentages of hens on the litter were typically recorded over the d (Figure 3). There was an interaction between age and substrate on the proportion of hens present on the litter area (F(6,74) = 3.87, P = 0.002) with the lowest proportion of hens on the AstroTurf® substrate within the 28 and 50 wk ages (Figure 4). There was also a main effect of substrate (F(2,9) = 11.29, P < 0.004) with the fewest hens on the AstroTurf® substrate (Figure 4) and an effect of age (F(3,74) = 233.99, P < 0.0001). The lowest proportion of hens were on the litter at 25 wk, and the highest proportion of hens on the litter at 50 wk (Figure 4).
Figure 3.
Raw percentages of total hens in each aviary pen observed on the litter area across the d (hens counted every 20 min), with access to different litter substrates (AstroTurf®, shavings, or straw; n = 4 pens per substrate). Observations were made across 2 d each at 4 ages (25, 28, 50, and 68 wk).
Figure 4.
Mean percentage ± SEM (raw values) of all hens in each aviary pen on the litter area (hens counted every 20 min) with access to different litter substrates (AstroTurf®, shavings, or straw; n = 4 pens per substrate type) observed across 4 ages (25, 28, 50, and 68 wk). Two d were observed at each age. Values without a common letter indicate significant differences among substrates across ages.
Transitions between the litter area and tiered enclosure were highest immediately after the aviary doors opened in the morning, but otherwise were relatively evenly distributed across the d at all ages (Figure 5). Transitions did not differ among litter types, ages, or their interaction (all P ≥ 0.17).
Figure 5.
Raw percentage difference between total hens in each aviary pen exiting and entering the tiered enclosure (positive values indicate more hens exited) throughout the d for pens with different litter substrates (AstroTurf®, shavings, or straw; n = 4 pens per substrate type) sampled at 4 ages (25, 28, 50, and 68 wk). Five-minute observation periods occurred throughout the d, and 2 d were observed at each age.
On average, less than 2% of all hens in the pen were seen foraging with some peaks of approximately 4% of total hens in the enclosure foraging (Figure 6). There was an interaction between age and substrate (F(6,27) = 13.76, P < 0.0001) where foraging was initially highest for straw and shavings then decreased over time, while the low levels observed on AstroTurf® remained relatively constant (Figure 6). There was a main effect of litter substrate (F(2,9) = 19.15, P < 0.0006) with the fewest hens observed foraging on the AstroTurf® (Figure 6, Table 1) and an effect of age (F(3,27) = 26.33, P < 0.0001) with the most hens observed foraging at 25 wk following first aviary opening (Figure 6, Table 1).
Figure 6.
Raw percentages of all hens in each aviary pen observed foraging (ground scratching followed by ground pecking) and dust bathing on the litter area across the d with access to different litter substrates (AstroTurf®, shavings, or straw; n = 4 enclosures per substrate). Observations across 4 ages (25, 28, 50, and 68 wk) were made for 2 min every 20 min from aviary opening until lights off for one d each per age for foraging and for 2 min every 20 min from 11:00 until 15:00 for 2 d each per age for dust bathing.
Table 1.
Percentages of all hens in each aviary pen foraging (ground scratching typically followed by ground pecking) with access to one of 3 litter substrates.*,†
| AstroTurf® | Shavings | Straw | |
|---|---|---|---|
| 25 wk | 0.17 ± 0.09D,E | 1.06 ± 0.09A,B | 1.59 ± 0.09A |
| 28 wk | 0.11 ± 0.09E | 0.22 ± 0.09D | 0.56 ± 0.09B,C |
| 50 wk | 0.24 ± 0.09D | 0.29 ± 0.09C,D | 0.22 ± 0.09D |
| 68 wk | 0.25 ± 0.09D | 0.25 ± 0.08C,D | 0.25 ± 0.09D |
*Values are the least squares means (±SEM) with n = 4 pens per substrate type of AstroTurf®, shavings, or straw.
†Observations were made across 2 min every 20 min throughout one observation d at 25, 28, 50, and 68 wk of age.
A–EDifferent superscript letters indicate differences among substrates across all ages.
On average, less than 5% of all hens in the pen dust bathed simultaneously in the litter area (Table 2). At maximum, 15% of all hens were observed dust bathing on straw and shavings substrates across most ages (Figure 6). There was no effect of substrate, age, or their interaction on the average proportions of hens dust bathing (all P ≥ 0.18).
Table 2.
Percentages of total hens in each aviary pen observed dust bathing with access to one of 3 litter substrates.*,†
| AstroTurf® | Shavings | Straw | |
|---|---|---|---|
| 25 wk | 1.74 ± 0.60 | 4.56 ± 0.60 | 3.86 ± 0.60 |
| 28 wk | 3.38 ± 0.60 | 3.32 ± 0.60 | 4.15 ± 0.60 |
| 50 wk | 3.12 ± 0.60 | 3.73 ± 0.60 | 3.03 ± 0.60 |
| 68 wk | 4.16 ± 0.60 | 3.64 ± 0.60 | 3.44 ± 0.60 |
*Values are the least squares means (±SEM) with n = 4 pens per substrate type of AstroTurf®, shavings, or straw.
†Observations were made across 2 min every 20 min from 11:00 to 15:00 throughout 2 observation d at 25, 28, 50, and 68 wk of age.
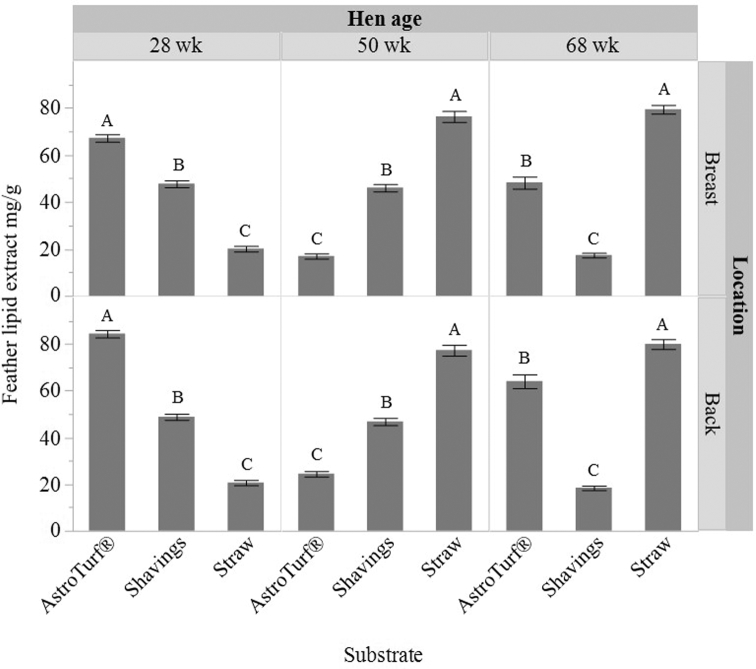
At 28 wk, hens in the straw group had the lowest lipid content of the breast feathers, and hens in the AstroTurf® group the highest (F(2,83) = 271.68, P < 0.0001) with the same pattern found for the back feathers (F(2,81) = 548.66, P < 0.0001, Figure 7). At 50 wk, hens in the AstroTurf® group had the lowest lipid content of the breast feathers and hens in the straw group the highest (F(2,82) = 287.64, P < 0.0001), with the same pattern found for the back feathers (F(2,82) = 232.26, P < 0.0001, Figure 7). Finally, at 68 wk, hens in the shavings group had the lowest lipid content of the breast feathers and hens in the straw group the highest (F(2,82) = 270.13, P < 0.0001), with the same pattern found for the back feathers (F(2,82) = 222.83, P < 0.0001, Figure 7). At all ages, there were higher feather lipid levels on the back feathers from hens housed with access to AstroTurf® (F(2,82) = 31.95 to 115.71, P < 0.0001, Table 3).
Figure 7.
Mean ± SEM feather lipid extract (mg/g) of feathers sampled from the breast and back of hens with access to one of 3 litter substrates (AstroTurf®, shavings, or straw; n = 4 enclosures per substrate, n = 7 hens per pen) with feathers taken at 3 ages (28, 50, and 68 wk). Different superscript letters indicate significant differences among substrates within each separate feather location and age.
Table 3.
Difference in feather lipid extract (mg/g) among feathers sampled from the back and breast of hens with access to one of 3 litter substrates.*,†
| AstroTurf® | Shavings | Straw | |
|---|---|---|---|
| 28 wk | 17.14 ± 0.88B | 1.05 ± 0.87A | 0.55 ± 0.87A |
| 50 wk | 7.41 ± 0.68B | 0.69 ± 0.68A | 0.94 ± 0.66A |
| 68 wk | 15.78 ± 0.82B | 1.05 ± 0.81A | 0.47 ± 0.82A |
*n = 7 hens sampled per pen, n = 4 pens per substrate type of AstroTurf®, shavings, or straw with feathers taken at 28, 50, and 68 wk of age.
†Positive values are the least squares means (±SEM), indicating higher lipid content was always present on the back feathers.
A,BDifferent superscript letters indicate significant differences among substrates within each age.
DISCUSSION
The aim of this study was to compare hens’ use of 3 different types of litter substrates as assessed by proportion of all birds in the litter, proportion of all birds foraging or dust bathing in this area, and transitions between the tiered enclosure and litter area across a flock cycle. Fewer hens were seen in the AstroTurf® litter area compared with hens in litter areas with straw or shavings. Moreover, the proportions of hens seen foraging in the AstroTurf® area were lower compared to straw or shavings early in the flock cycle. Hens with access to AstroTurf® consistently had higher feather lipids on their back feathers (relative to breast feather levels), suggesting this substrate did not enable hens to maintain cleanliness of the back area.
When hens first gained access to the floor area at 25 wk, hens were present on all substrates in equal but low numbers. This was the time when litter substrates would have been most distinct from one another, before hens contributed material to the litter buildup, and thus we might have expected the greatest differences in use. However, the motivation for cage-housed birds to explore this area likely played a role here. After 3 wk of litter access (28 wk), fewer hens were seen on AstroTurf® compared to straw and shavings. This indicates both of these manipulable substrates were more appealing to hens than the plastic AstroTurf® mats. Over time, litter continued to accumulate as feces and feathers built up, with no litter substrate differences observed at 68 wk. However, some of this accumulated litter was observed trapped under the AstroTurf® mats, with the result that the manipulable substrate available to hens had the lowest depth in the AstroTurf® enclosures by the end of the flock cycle. Hens may have made better use of the AstroTurf® areas earlier in the flock cycle if the mats were placed in a manner that kept more manipulable substrate on top of the mats as it accumulated.
Astroturf has been used as a nest box substrate in aviary designs and as a scratchpad and nest box substrate in furnished cages. Among litter treatments no difference in floor eggs were found (Regmi et al., unpublished data), indicating that hens did not associate the litter areas with a nest. Hens coming into lay with no prior experience with plastic turf preferred this substrate over wire floor for laying. However, when birds had an established location preference for laying, they did not prefer plastic turf for laying or for other behaviors, except for a tendency to prefer plastic turf for dust bathing (Hughes, 1993). In contrast, adult hens did readily switch laying location preferences if a manipulable substrate was available to choose over wire floor (Hughes, 1993). Recent research did find hens changed their nest box preferences if the Astroturf mat was removed from their preferred nest box, exposing the wire mesh floor underneath (Riber and Nielsen, 2013). Thus, AstroTurf® in alternative housing systems may be preferred in contrast to wire floor, but in the present study, it did not encourage hens to use additional floor litter area space in the aviary to the same extent as friable substrates.
In addition to providing hens with more space, the litter area is designed to promote behaviors such as dust bathing and foraging that hens prioritize or need to perform (Weeks and Nicol, 2006). Unexpectedly, no differences were found in dust bathing among the substrates, in contrast to studies that show hens prefer to scratch and dust bathe in friable litter substrates more than on turf mats (Alvino et al., 2013; Guinebretière et al., 2014). Differences in substrate composition were likely minimized by the buildup of litter across time from depositions by the birds.
Overall, the total average number of birds dust bathing was less than 5% of hens in each pen. These numbers are similar to some previous observations in a commercial perchery system (Carmichael et al., 1999), but much lower than recent observations in a different aviary style, which found an average of up to 35.7% of all hens (at mid lay) dust bathing in the litter area (straw pellets on concrete floor; Louton et al., 2016). The suitability of different substrates for complete dust bathing sequences may have been found in the present study if observations had been made of dust bathing bout durations, disturbances, and bout terminations (Alvino et al., 2013; Louton et al., 2016). In this study we used cage-reared birds that may have developed sham dust bathing behavior on wire. They may have continued to perform this behavior on wire even in the presence of a more suitable dust bathing substrate (Louton et al., 2016; Olsson et al., 2002). Specific timing of substrate exposure during rearing can affect acceptance of new substrates for dust bathing as adults (Nicol et al., 2001). Birds in the Louton et al. (2016) study were also cage-reared, but in general, matching the rearing system with layer housing system is recommended for optimal behavior and welfare of adult hens (Janczak and Riber, 2015). Any sham dust bathing within the caged part of the enclosure was not measured in this study and warrants further investigation, including comparing the behavior of floor- or aviary-reared pullets.
A lower proportion of hens was seen foraging (scratching typically followed by ground pecking) on the AstroTurf® compared to straw and shavings. On all substrates, less than 1% of hens on average were observed engaged in this type of foraging. In a commercial perchery, approximately 6 to 9% of observed ISA Brown hens were foraging in a friable litter substrate (though the exact litter substrate was not specified, Carmichael et al., 1999). Their foraging description was similar to ours; however, the unreported rearing background of pullets in that study potentially contributed to their observation of more foraging. For example, in deep-litter housing with floor-reared birds, higher proportions of hens foraging (ground scratching and pecking) have been recorded (percentage represented within a pie chart, exact number not specified: Appleby et al., 1989). The most foraging was observed in the straw and shavings immediately following aviary opening, suggesting both novelty and the manipulable substrates promoted scratching, but this effect dissipated within 3 wk of first litter access. A similar study of hens in commercial percheries found birds spent high proportions of their time standing idle and feeding compared to the proportions seen foraging and dust bathing (Channing et al., 2001). Previous research observing inter-bird distances and performance of different behaviors suggests the provided litter area space of 305 cm2/hen was sufficient for ground pecking (Keeling, 1994), but further kinematic analysis of individual birds within groups in these systems would be needed to confirm this. It is also possible that different substrates may encourage foraging behaviors for a longer period of time (e.g., wheat bran promoted pecking and scratching in an experimental setting, Guinebretière et al., 2014). Alternatively, regular replacement, top dressing with fresh litter substrate, or manure removal throughout the flock cycle may encourage performance of foraging behavior long-term.
Birds that were floor-reared with litter access may also exhibit more foraging behavior as adults compared to cage-reared birds as used in this study (Huber-Eicher and Sebö, 2001). Feather pecking is proposed to be re-directed foraging behavior (Blokhuis, 1986), with rearing environment influencing the development of foraging and feather-pecking behavior (Huber-Eicher and Wechsler, 1997). Hens in these aviary systems did suffer from injurious pecking and feather loss (Regmi et al. unpublished data). By the end of the flock cycle, approximately 12% of sampled birds across all substrates had moderate feather wear on their heads and necks, based on the Welfare Quality® scoring system (Welfare Quality®, 2009, Regmi et al. unpublished data). Thus, the low levels of foraging may have been replaced by feather pecking behavior. Rearing birds with substrate access may help with the transition to alternative housing systems as layers. Promoting positive foraging behavior in favor of abnormal, negative feather pecking behavior would improve the welfare of birds housed in large group systems and contribute to the successful implementation of alternative housing.
Similar to previous observations in a commercial aviary of the same style (Campbell et al., 2016a), hens showed transitions between the litter area and the tiered enclosure throughout the d, with a peak in hens exiting the enclosure immediately following the aviary door opening. Regardless of litter substrate type, hens appeared to regulate their distribution between the enclosure and the litter area, with the number of hens exiting equal to the numbers of hens entering, but litter usage was not evaluated at the individual level in the present study. Observations of individual painted hens from the same flock as the current study (though not all pens were observed) showed that some hens visited the litter area throughout the d, while others were never observed out of the enclosure (Campbell et al., 2016b). These findings suggest either that some hens prefer to stay in the enclosure or that they are prevented from accessing the litter by more dominant hens. These individual differences may also contribute to the inconsistent differences in feather lipid levels between substrates across ages. As different birds were sampled at each age, wide variation in use of the litter area (potentially including birds that never visited the litter area) and dust bathing behavior (dust bathing on litter vs. sham dust bathing on wire) may all have influenced feather lipid content. However, the AstroTurf® substrate was consistently less effective at removing feather lipids from the back feathers. This result is similar to that of a previous comparison between commercial aviary- and furnished-cage systems, which supported the importance of a friable substrate that can be tossed onto the back at removing lipids from that area of the body (Blatchford et al., 2013).
Overall, hens in these aviaries visited the litter area across the flock cycle, but fewer hens visited the AstroTurf® substrate. Small percentages of hens were observed dust bathing and foraging, with the fewest hens foraging on AstroTurf®, and the most foraging observed immediately following aviary opening when substrates were fresh with minimal contribution by the birds. The AstroTurf® substrate was also least effective at removing feather lipids from the back feathers of individual hens. Further studies could examine the addition of fresh litter to the litter area, removal and replacement of soiled litter with fresh substrate, or use of slightly friable wood and oyster shell blocks (for example) to determine whether these management practices might promote more foraging (and potentially reduce feather pecking). The rearing background of the pullets should also be examined for its influence on development of foraging and dust bathing.
Acknowledgements
Thank you to Hassan Albeer, Meredith Anderson, Rebecca Bronstein, Courtney Daigle, Samantha Dorey, Marisa Erasmus, Nichole Fairfield, Shelby Goodwin, Alexis Hinson, Elizabeth Kim, Dave Main, Nicholas Newsome, Cara Robison, Prafulla Regmi, Eileen Stefansky, Sam Terzichs, Marissa Tetzlaff, Robert Van Wyhe, Silvia Villanueva, Diondra Voishich, and Jesse Whitfield for assistance with video and on-farm data collection and laboratory assistance, all from Michigan State University (East Lansing, MI). We also thank Laura Warin and Hélène Pecourt from AgroParisTech (Paris, France) and CSIRO (Armidale, NSW, Australia), Elodie Revilliod from Isara-Lyon (Lyon, France) and CSIRO (Armidale, NSW), and Richard Blatchford (University of California at Davis, CA). Appreciation is expressed towards Angelo Napolitano, Joseph Leszcz and the staff at the Michigan State University Poultry Research and Teaching Center (East Lansing, MI). This work was supported in part by the NationalInstitute of Food and Agriculture, U.S. Department of Agriculture, Hatch projects #1002990 and #1010765. Funding support was also provided by the Department of Animal Science and by AgBioResearch at Michigan State University through the Michigan Alliance for Animal Agriculture. We thank the anonymous reviewers for their comments that improved earlier versions of this manuscript.
REFERENCES
- Alvino G. M., Tucker C. B., Archer G. S., Mench J. A.. 2013. Astroturf as a dustbathing substrate for laying hens. Appl. Anim. Behav. Sci. 146:88–95. [Google Scholar]
- Appleby M. C., Hughes B. O., Hogarth G. S.. 1989. Behaviour of laying hens in a deep litter house. Br. Poult. Sci. 30:545–553. [DOI] [PubMed] [Google Scholar]
- Blatchford R. A., Mench J. A., De Luz M. A., Siegford J. M., Makagon M. M., Campbell D. L. M., Swanson J. C.. 2013. The effectiveness of dust bathing substrates in enriched colony and aviary laying hen housing systems. Proc. Poult. Sci. Assoc. Ann. Mtg. 92:93. (Abstr.). [Google Scholar]
- Blokhuis H. J. 1986. Feather-pecking in poultry: Its relation with ground-pecking. Appl. Anim. Behav. Sci. 16:63–67. [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M.. 2016a. Laying hen movement in a commercial aviary: Enclosure to floor and back again. Poult. Sci. 95:176–187. [DOI] [PubMed] [Google Scholar]
- Campbell D. L. M., Karcher D. M., Siegford J. M.. 2016b. Location tracking of individual laying hens housed in aviaries with different litter substrates. Appl. Anim. Behav. Sci. 184:74–79. [Google Scholar]
- Campbell D. L. M., Makagon M. M., Swanson J. C., Siegford J. M.. 2016c. Litter use by laying hens in a commercial aviary: Dust bathing and piling. Poult. Sci. 95:164–175. [DOI] [PubMed] [Google Scholar]
- Carmichael N. L., Walker A. W., Hughes B. O.. 1999. Laying hens in large flocks in a perchery system: Influence of stocking density on location, use of resources and behaviour. Br. Poult. Sci. 40:165–176. [DOI] [PubMed] [Google Scholar]
- Channing C. E., Hughes B. O., Walker A. W.. 2001. Spatial distribution and behaviour of laying hens housed in an alternative system. Appl. Anim. Behav. Sci. 72:335–345. [DOI] [PubMed] [Google Scholar]
- Colson S., Arnould C., Michel V.. 2007. Motivation to dust-bathe of laying hens housed in cages and aviaries. Animal 1:433–437. [DOI] [PubMed] [Google Scholar]
- Cooper J. J., Albentosa M. J.. 2003. Behavioural priorities of laying hens. Av. Poult. Biol. Rev. 14:127–149. [Google Scholar]
- Dawkins M. S. 1989. Time budgets in Red Junglefowl as a baseline for the assessment of welfare in domestic fowl. Appl. Anim. Behav. Sci. 24:77–80. [Google Scholar]
- de Jong I. C., Reuvekamp B., Fiks T.. 2005. Evaluation of substrate quality in two different housing systems (barn systems and furnished cages) for laying hens with respect to dustbathing and foraging behaviour. In: LayWel—Welfare Implications of Changes in Production Systems for Laying Hens. Work package 4, Behaviour. http://www.laywel.eu/web/pdf/deliverable%2045-2.pdf, visited on October 5, 2016.
- Guinebretière M., Beyer H., Arnould C., Michel V.. 2014. The choice of litter material to promote pecking, scratching and dustbathing behaviours in laying hens housed in furnished cages. Appl. Anim. Behav. Sci. 155:56–65. [Google Scholar]
- Huber-Eicher B., Sebö F.. 2001. The prevalence of feather pecking and development in commercial flocks of laying hens. Appl. Anim. Behav. Sci. 74:223–231. [Google Scholar]
- Huber-Eicher B., Wechsler B.. 1997. Feather pecking in domestic chicks: its relation to dust bathing and foraging. Anim. Behav. 54:757–768. [DOI] [PubMed] [Google Scholar]
- Hughes B. O. 1993. Choice between artificial turf and wire floor as nest sites in individually caged laying hens. Appl. Anim. Behav. Sci. 36:327–335. [Google Scholar]
- Janczak A. M., Riber A. B.. 2015. Review of rearing-related factors affecting the welfare of laying hens. Poult. Sci. 94:1454–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. R., Cox N. A., Guard J., Fedorka-Cray P. J., Buhr R. J., Gast R. K., Abdo Z., Rigsby L. L., Plumbee J. R., Karcher D. M., Robison C. I., Blatchford R. A., Makagon M. M.. 2015. Microbiological impact of three commercial laying hen housing systems. Poult. Sci. 94:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling L. J. 1994. Inter-bird distances and behavioural priorities in laying hens: The effect of spatial restriction. Appl. Anim. Behav. Sci. 39:131–140. [Google Scholar]
- Louton H., Bergmann S., Reese S., Erhard M H., Rauch E.. 2016. Dust-bathing behavior of laying hens in enriched colony housing systems and an aviary system. Poult. Sci. 95:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesta A., Knierim U., Briese A., Hartung J.. 2008. The effect of litter condition and depth on the suitability of wood shavings for dustbathing behaviour. Appl. Anim. Behav. Sci. 115:160–170. [Google Scholar]
- Nicol C. J., Lindberg A. C., Phillips A. J., Pope S. J., Wilkins L. J., Green L. E.. 2001. Influence of prior exposure to wood shavings on feather pecking, dustbathing and foraging in adult laying hens. 73:141–155. [DOI] [PubMed] [Google Scholar]
- Odén K., Keeling L. J., Algers B.. 2002. Behaviour of laying hens in two types of aviary systems on 25 commercial farms in Sweden. Br. Poult. Sci. 43:169–181. [DOI] [PubMed] [Google Scholar]
- Olsson I. A. S., Keeling L. J., Duncan I. J. H.. 2002. Why do hens sham dustbathe when they have litter? Appl. Anim. Behav. Sci. 76:53–64. [Google Scholar]
- Riber A. B., Nielsen B. L.. 2013. Changes in position and quality of preferred nest box: Effects on nest box use by laying hens. Appl. Anim. Behav. Sci. 148:185–191. [Google Scholar]
- Sandilands V., Savory J., Powell K.. 2004. Preen gland function in layer fowls: Factors affecting morphology and feather lipid levels. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 137:217–225. [DOI] [PubMed] [Google Scholar]
- Scholz B., Urselmans S., Kjaer J. B., Schrader L.. 2010. Food, wood, or plastic as substrates for dustbathing and foraging in laying hens: A preference test. Poult. Sci. 89:1584–1589. [DOI] [PubMed] [Google Scholar]
- Scholz B., Kjaer J. B., Petow S., Schrader L.. 2014. Dustbathing in food particles does not remove feather lipids. Poult. Sci. 93:1877–1882. [DOI] [PubMed] [Google Scholar]
- Schütz K. E., Jensen P.. 2001. Effects of resource allocation on behavioural strategies: A comparison of Red Junglefowl (Gallus gallus) and two domesticated breeds of poultry. Ethology 107:753–765. [Google Scholar]
- van Liere D. W. 1992. Dustbathing as related to proximal and distal feather lipids in laying hens. Behav. Proc. 26:177–188. [DOI] [PubMed] [Google Scholar]
- Warton D. L., Hui F. K. C.. 2011. The arcsine is asinine: The analysis of proportions in ecology. Ecology 92:3–10. [DOI] [PubMed] [Google Scholar]
- Weeks C. A., Nicol C. J.. 2006. Behavioural needs, priorities and preferences of laying hens. Worlds Poult. Sci. J. 62:296–307. [Google Scholar]
- Welfare Quality® 2009. Welfare Quality® assessment protocol for poultry (broilers, laying hens). Welfare Quality® Consortium, Lelystad, Netherlands. [Google Scholar]
- Widowski T. M., Duncan I. J. H.. 2000. Working for a dustbath: Are hens increasing pleasure rather than reducing suffering? Appl. Anim. Behav. Sci. 68:39–53. [DOI] [PubMed] [Google Scholar]