Abstract
Interstitial lung disease (ILD) comprises many heterogeneous disease groups, the largest being CTD-associated and those labelled as idiopathic out of necessity. The mechanisms causing ILD are poorly understood, but most CTD- and idiopathic-ILD cases can respond to immunosuppression, clearly suggesting a pathological role for inflammation. By contrast, corticosteroid immunosuppression causes harm without benefit in the feared idiopathic pulmonary fibrosis, suggesting that inflammation plays little pathological role, and where ILD progresses rapidly to lethal outcome even with anti-fibrotic drug use. Given the treatment response differences apparent between ILD subgroups, and the dangers and costs of corticosteroid and anti-fibrotic drug use, respectively, it has become vital in every ILD patient to make an accurate subgroup diagnosis, to optimize treatment selections. This review discusses why differentiating CTD- and idiopathic-ILD subgroup cases remains so problematic, and why existing comprehensive CTD-specific serology would, if generally available, represent an ideal biomarker tool to enhance ILD subgroup diagnostic accuracy.
Keywords: interstitial lung disease, connective tissue disease, antibodies, biomarkers, serology, myositis, anti-synthetase syndrome
Rheumatology key messages
Widely differing interstitial lung disease treatment responses make an accurate subgroup diagnosis essential in every case.
CTD-specific autoantibodies represent ideal biomarkers to accurately assign CTD-interstitial lung disease subgroup diagnoses.
Existing, comprehensive, CTD-specific serology would, if made routinely available, clearly improve interstitial lung disease clinical care.
Introduction
The parenchymal tissues of the terminal airways comprise the alveolar epithelium and basement membrane, the peri-vascular/peri-lymphatic (i.e. the true) interstitial space and the capillary basement membrane and epithelium. Damage to any of these tissues could lead to diffuse parenchymal lung disease, although interstitial lung disease (ILD) is the preferred UK term [1]. The diffuse parenchymal lung disease pattern distinguishes ILD from other lung pathologies, including those affecting the pleura and larger airways. If ILD-associated parenchymal injury, and any associated inflammation, is not arrested to facilitate healing, irreversible pulmonary scarring and fibrosis may follow [2]. It is the combination of parenchymal injury, primary and/or secondary inflammation, pulmonary fibrosis and the resulting dyspnoea that physicians recognize overall as ILD. Impaired gas transfer can occur early, if inflammatory infiltrations are sufficiently severe, or only once fibrosis has become substantial, irrespective of cause. In individual cases the extent of parenchymal inflammation, relative to that of fibrosis, will depend on the mechanism causing injury, and also dictate the effectiveness of immunosuppressive therapies [3]. Patients accurately diagnosed and treated will normally fare better. Non-responsive or untreated patients usually progress from being dyspnoeic on exertion only to being dyspnoeic even at rest. Fatal outcomes ensue from respiratory failure, or its cardiac complications [4].
Why the difficulties in diagnosing CTD-associated ILD?
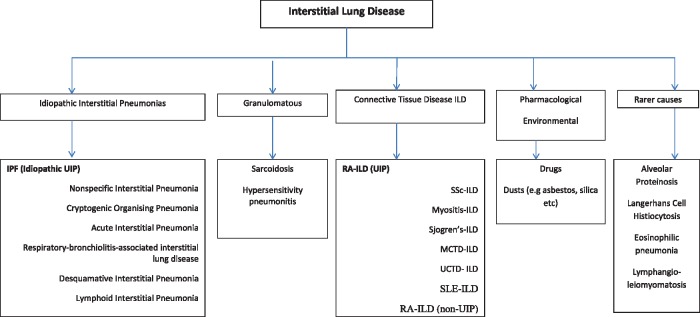
A representative classification of ILDs is shown in Fig. 1 [5]. Though apparently simple, this classification hides many potential pitfalls. The two largest ILD groups are the CTD-associated ILDs (CTD-ILDs) and the idiopathic-ILDs (also known as the idiopathic interstitial pneumonias, or IIPs). Both groups have many subgroups. Differentiating between subgroup cases can prove considerably difficult because their ILD features can overlap, as can be demonstrated on lung biopsy material where available, and on high resolution CT (HRCT) scans [6]. Non-specific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP) patterns on HRCT can be seen in CTD-ILD and idiopathic-ILD [7]. The NSIP pattern is characterized by ground glass changes typically present at the periphery and bases of the lungs, whereas a UIP pattern shows peripherally distributed reticular and fibrotic changes typically affecting the lung bases with honeycomb features, and the absence of significant ground glass changes [8]. The commonest ILD is idiopathic pulmonary fibrosis (IPF), where scans typically demonstrate the UIP pattern (IPF and idiopathic UIP are thus interchangeable terms). IPF/idiopathic UIP is the most feared of all ILDs, because it is so relatively common and is associated with a ∼50% mortality within only 3 years of ILD onset despite all treatments [4] (see later). When a physician accurately diagnoses a CTD in a patient with NSIP, that case would obviously be labelled as a CTD-ILD, and the detected CTD is assumed to be the underlying cause of the NSIP. If, on the other hand, no CTD signs were detectable, and no other cause for an NSIP pattern was apparent, that case would of necessity be assigned an idiopathic-ILD label, that is, idiopathic NSIP. In each instance, the final ILD diagnosis will have relied on use of the combined clinical, radiological, serological and (where available) lung histological features. However, this seemingly logical process can break down with CTDs.
Fig. 1.
A classification of ILD into groups and subgroups
The IPF and RA-ILD (UIP) subgroups are formatted left in each box, and made bold, to highlight that these ILDs exhibit similar fibrotic HRCT patterns (UIP) and are similarly treatment-resistant, with rapid progression to lethal outcomes within only 3–5 years of ILD onset. In contrast, and formatted to the right in each box, the other IIP and CTD-ILD subgroups, the latter including RA-ILD (non-UIP), show varying degrees of cellularity (i.e ground glass) on HRCT, and are usually responsive to immunosuppression to some extent. IPF: Idiopathic Pulmonary Fibrosis, ILD: Interstitial Lung Disease, UIP: Usual Interstitial Pneumonia, HRCT: High Resolution Computed Tomography, IIP: Idiopathic Interstitial Pneumonia. (Adapted from Ryerson CJ, Collard HR. Update on the diagnosis and classification of ILD. Curr Opin Pulm Med 2013;19:453–9).
This is because ILD may be the presenting sign of a CTD, that is, where extra-pulmonary CTD features have yet to appear [9–11]. ILD thus represents a forme fruste CTD. Furthermore, CTD symptoms may be present but of only very subtle character, and so easily missed in a busy respiratory clinic. For example, in the anti-synthetase syndrome the classic CTD features other than ILD (i.e. Raynaud’s syndrome, myositis, mechanic’s hands, fever, rash, inflammatory arthritis) may be absent or very subtle only [12]. Moreover, if ILD is successfully treated early, the immunosuppressive agents used may well preclude the development of CTD signs other than ILD. Physicians should employ serology to screen for a CTD in every ILD case, though chest physicians may employ serology less rigorously than would perhaps be expected of a rheumatologist [13]. False positive serology and the existence of sero-negative CTDs would also cause difficulties. A low suspicion for the presence of a CTD may mean that some CTD-ILD patients never see a rheumatologist.
The diagnostic limitations of HRCT
HRCT is an important assessment tool to differentiate between fibrosis and inflammation in ILD patients. Where clinical history and examination fail to confirm a clear ILD diagnosis, and no CTD symptoms or signs are apparent, clinicians must then rely on HRCT, if serology is diagnostically inadequate or unhelpful. However, HRCT has poor utility as a stand-alone diagnostic tool. For instance, HRCT appearances have no proven associations with any defined disease processes. Also, not only do the same HRCT patterns occur in different ILD subgroups, but different HRCT patterns can occur in the same disease subgroup [14]. Furthermore, similar HRCT patterns can be associated with differential outcomes. For example, the most common HRCT appearances in SSc are those of NSIP or UIP, the latter thus mimicking IPF. However, in contrast to IPF, SSc-ILD patients with UIP can respond to immunosuppression [15–17]. Honeycombing is a recognized HRCT feature of IPF, but honeycombing can also occur in myositis-ILD, thus again mimicking IPF, yet myositis-UIP cases can respond to immunosuppression [18]. In the absence of comprehensive CTD serology, inaccurate diagnostic assignments by HRCT will have occurred previously, and likely will continue to occur. That fibrotic HRCT changes respond differently in IPF than in CTD-ILDs with predominantly fibrotic HRCT changes may reflect differential disease mechanisms. Such a range and variability of HRCT appearances, clinical associations and treatment responses highlights the problems of diagnostic uncertainty when CTD-specific serology is inadequate. In addition, there are the difficulties that HRCT patterns may overlap, and that inter-observer variability of image analysis also occurs [19, 20].
The 2011 American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines for the diagnosis and management of IPF suggested that an accurate ILD diagnosis is optimally secured by the combination of a thorough medical history (to detect causative CTDs or occupational or environmental exposures, etc.), a careful clinical examination (to detect CTD or granulomatous signs, etc.), serological screening, an HRCT scan and lung histology where feasible, though co-morbidities dictate that only a minority of UK cases are sufficiently fit for lung biopsy [21]. A definite occupational, iatrogenic, granulomatous, CTD, etc. history and/or detection of clinical signs would likely secure an underlying ILD diagnosis, and so preclude the need for lung biopsy. However, when diagnostic doubts remain, final diagnostic decisions are increasingly made in the UK by expert multidisciplinary teams in tertiary ILD clinics, with input from pulmonary clinicians, radiologists, pathologists and increasingly rheumatologists with an interest in ILD. Tertiary ILD service developments have in part been driven by the advent of newer drugs for IPF, a particularly challenging ILD that is preceded by little or no inflammation, and that is therefore non-responsive to immunosuppression [22, 23].
The diagnostic use of serology in the absence of ILD-specific biomarkers
A factor that has to date critically limited ILD subgroup diagnostic capability has been the lack of reliable, ILD-specific biomarkers. UK physicians, including rheumatologists, have thus struggled diagnostically with idiopathic ILD and with CTD-ILD when CTD signs other than ILD are absent, though this situation is set to improve. IPF can only be diagnosed once a CTD has been definitively excluded, but the 2011 and 2013 ATS/ERS guidelines gave only very limited advice regarding the stringency of serology required to interrogate for a CTD [21, 24]. Thus, and even in most tertiary UK ILD clinics, CTD exclusion continues to rely on routine immunology, that is, immunology testing for rheumatoid factor, anti-CCP antibodies, ANA and extractable nuclear antigens, the latter usually limited to anti-Ro/-La/-Jo/-Sm/-RNP/-Scl-70. A recent ERS/ATS research statement on interstitial pneumonia with autoimmune features suggests, for the first time, that classification criteria should contain a serological domain [25]. This statement suggests use of serology more extensive than previously advised in any ATS/ERS guidelines [8, 21, 24], though no details are given regarding how to use this newly advocated serology, or regarding its diagnostic utility [25]. Moreover, for many of the newer antibody specificities listed in this update, and especially for some of those associated with myositis, serological detection systems are unfortunately not currently available in routine UK clinical practice. Thus, in myositis-ILD, a growing number of myositis-specific autoantibodies (MSAs) and myositis-associated autoantibodies (MAAs) are now detectable (Tables 1 and 2) [26]. MSA/MAAs accurately predict the presence of myositis clinical features, including the likelihood of developing an associated ILD, so these antibodies represent surrogate ILD biomarkers, and hence their inclusion in the most recent ERS/ATS update [25]. Currently, however, many MSA/MAAs are only detectable by expensive immunoprecipitation techniques [26], although immunoblot technology for a number of these antibodies is now available in some National Health Service hospitals. Even when these are available, issues of diagnostic accuracy and nomenclature still arise. For instance, in the case of anti-PL-7 and anti-PL-12, these antibodies often associate with ILD in the absence of myositis [11, 27], that is, in amyopathic ILD. Affected patients may not initially or ever exhibit CTD features other than their ILD [11, 12]. Reliable detection of an ILD-only MSA/MAA here would presumably secure a CTD-ILD diagnosis, and it could be argued that detected ILD-only MSA/MAAs could be appropriately renamed as ILD-specific/associated autoantibodies (i.e. ISA/IAA), or as CTD-ILD-specific/associated autoantibodies, though the latter appears somewhat cumbersome, especially regarding the issue of an acronym. As the intracellular targets of many MSA/MAAs, and of all eight of the known anti-synthetases, are cytoplasmic rather than nuclear, a negative ANA on routine screening does not of itself exclude a CTD-ILD diagnosis. However, the staining patterns observed when ANA screening is undertaken by indirect immunofluorescence testing on HEp-2 cells will potentially disclose the presence of a CTD [28, 29]. The HEp-2 technique is rapid and inexpensive, so if available its use for screening ILD patients for an underlying CTD is recommended.
Table 1.
Clinical associations of the known myositis-specific autoantibodies and the likelihood of antibody-positive patients developing an associated interstitial lung disease
| MSA | Prevalence in myositis (%) | Clinical associations | Likelihood of developing ILD |
|---|---|---|---|
| Anti-synthetases: Anti-Jo-1 (histidyl) | 15–20 |
|
∼70% develop ILD [30] |
| Anti-PL-12 (alanyl) | <5 | ∼90% develop ILD, myositis often mild [11] | |
| Anti-PL-7 (threonyl) | <5 | ∼90% develop ILD, often prior to myositis [31] | |
| Anti-KS (asparaginyl) | <5 | ∼90% develop ILD, myositis rare [32] | |
| Anti-OJ (isoleucyl) | <5 | Up to 100% develop ILD [33] | |
| Anti-EJ (glycyl) | <5 | Up to 100% develop ILD [34] | |
| Anti-Zo (phenylalanyl) | <1 | ILD-associated [35] | |
| Anti-Ha (tyrosyl) | <1 | No literature | |
| Anti-MDA5 | Associated with clinically amyopathic DM | Rapidly progressive ILD, especially in Japanese and Chinese patients | 50–70% develop rapidly progressive ILD in Japanese/Chinese ethnicity [36, 37], ∼50% in Caucasian [38] |
The following MSAs are rarely if ever associated with likelihood of developing an ILD: anti-Mi-2, anti-NXP2, anti-TIF1-γ, anti-SAE and anti-SRP. ILD: interstitial lung disease; MDA5: melanoma differentiation-associated gene 5; MSA: myositis-specific autoantibody. (Table adapted from Betteridge ZE et al. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther 2011;13:209.)
Table 2.
Clinical associations of various SSc-associated antibodies and myositis-associated antibodies and the likelihood of antibody-positive patients developing interstitial lung disease
| SSc-associated antibody or MAA | Prevalence of antibody | Disease associations | Likelihood of developing ILD |
|---|---|---|---|
| Anti-topoisomerase (Scl70) | ∼30% of SSc patients [39] | dcSSc [40] | ∼60% of patients develop ILD [41] |
| Anti-Th/To | ∼5% of SSc patients [40] | lcSSc [42] | ∼50% of patients develop ILD [43] |
| Anti-U3 RNP | ∼8% of patients with SSc [44] | Occurs in SSc. Associated with skeletal muscle involvement and pulmonary arterial hypertension [45] | ∼40% of patients have ILD [45] |
| Anti-U11/U12 RNP | ∼3% of patients with SSc [46] | Occurs in SSc | ∼80% of patients develop ILD which is often severe [46] |
| Anti-RuvBL1/2 | 1–2% of SSc patients [47] | Associated with myositis overlap and diffuse skin thickening [47] | Over 50% of patients will develop ILD [47] |
| Anti-EIF2B | ∼1% of patients with SSc/SSc overlap [48] | Associated with SSc/SSc overlap syndrome | Up to 100% of patients will develop ILD [48] |
| Anti-PM-Scl | Occurs in up to 17% of patients with overlap myositis [49], 3–6% of patients with SSc [50] | Associated with scleromyositis | ∼50% of patients will develop ILD [49, 51] |
| Anti-Ku | Occurs in 13% of patients with overlap myositis [49] | Associated with myositis overlap syndrome | ∼30% of patients will develop ILD [49] |
| Anti-U1 RNP | 5–35% of patients presenting with SSc or overlap syndromes [50] | Associated with MCTD | ∼35% show HRCT abnormalities associated with ILD, ∼20% classified as severe [52] |
| Anti-Ro-52/60 | ∼40% of IIM patients have anti-Ro-52/60 alongside their MSA or MAA [51] | Frequently occur with an MSA, especially with the anti-synthetases, or with various MAAs | ∼40% of patients develop ILD, however it is unlikely that these antibodies are responsible for the ILD risk, which is instead due to the MSA/MAA detected [51] |
The following SSc-associated antibodies are less frequently associated with ILD: anti-centromere antibody and anti-RNA polymerase III. Table compiled from the cited references. HRCT: high resolution CT; IIM: idiopathic inflammatory myopathy; ILD: interstitial lung disease; MAA: myositis-associated antibody; MCTD, mixed connective tissue disease; MSA: myositis-specific antibody.
The recently described anti-melanoma differentiation-associated gene 5 (MDA5) antibody occurs in DM patients who are clinically amyopathic, but who may suffer a very aggressive ILD, that is, one proving lethal within months or only weeks of ILD onset, despite all immunosuppressive interventions [36]. Anti-MDA5 could thus also be termed an ISA. Fortunately this antibody and its associated ILD are rare in the UK [26]. In other CTDs with a potential to develop ILD with or without myositis, for example, in mixed CTD, many patients will also possess one or more MAAs. In SSc there are many SSc-specific antibodies that are also strongly associated with ILD development (see Table 2). Thus, MSAs, MAAs and SSc-associated antibodies should all be regarded as surrogate biomarkers of ILD, or when appropriate even as ISA/IAAs. The recent ERS/ATS update recognizes the growing evidence suggesting that serology should be utilized more readily to enhance CTD-ILD diagnosis. However, this update also points out that detailed prospective studies are now required to validate the newly proposed classification criteria for interstitial pneumonia with autoimmune features [25]. In view of the current inadequacies of routine serology, and given that respiratory physicians may lack expertise in assessing extra-pulmonary CTD clinical features, and that these may anyway be absent or of only subtle degree at ILD onset, it seems likely that at least some CTD patients with HRCT appearances suggestive of UIP will be misdiagnosed as IPF. Similarly, some patients may be assigned an idiopathic-ILD label when they actually have a CTD-ILD that remains immunologically undisclosed.
Differential outcomes in CTD-ILDs
In the past, CTD- and idiopathic-ILD patients with similar HRCT patterns would have been treated with similar immunosuppressive regimes, so diagnostic labels then were therapeutically of lesser importance. However, recent mechanistic research and clinical trials in IPF have dramatically altered the treatment landscape. Making an accurate ILD diagnosis, to differentiate a CTD-ILD from an idiopathic-ILD, has thus become crucial, as treatments may differ markedly, and especially between IPF and most other ILD subgroups. Following the publication of the PANTHER study, which examined the impact of treating IPF patients with high-dose prednisolone co-prescribed with AZA, this immuno-suppressive regime was deemed contra-indicated because of its thus proven iatrogenic dangers [23]. New anti-fibrotic agents such as pirfenidone and nintedanib are now licensed and available for use to slow IPF disease progression [53–55]. These agents are, however, considerably expensive, so their use in England can only be recommended when an IPF diagnosis is deemed robust, that is, when made in a designated tertiary ILD centre, and according to the 2011–13 ATS/ERS guidelines [21, 24].
Most CTD-ILD cases have a capacity to respond to immunosuppression. If a degree of fibrosis has already occurred, suppressing residual inflammation would likely still act to limit fibrotic progression, thus stabilizing dyspnoea and associated disability. Such treatment responsiveness supports the notion that in most CTD-ILD cases, including myositis-ILD, ground glass changes reflect a suppressible inflammation component [56]. That treatment responses are also seen where fibrotic HRCT changes predominate in some CTD-ILD cases is poorly understood. A generic and suppressible inflammatory component could be assumed in all CTD-ILD cases, yet treatment outcomes are not always good. For instance, when RA patients develop an associated ILD (RA-ILD) with a UIP pattern on HRCT, that is, RA-ILD (UIP), such cases are notoriously non-responsive to immunosuppression [57]. RA-ILD (UIP) is associated with a lethal outcome within only 3 years of ILD onset, thus clearly mimicking IPF [57, 58]. Moreover, the RA-ILD (UIP) pattern on HRCT is identical to that of IPF. Therefore, although RA-ILD (UIP) is much rarer than IPF, it is a diagnosis just as feared as IPF. No research to date has reported on whether anti-fibrotic agents have a therapeutic role to play in RA-ILD (UIP). Some RA-ILD patients may develop organising pneumonia changes on HRCT or NSIP changes on HRCT, rather than UIP, and these cases do by contrast usually respond to immunosuppression [57]. Clearly, if a CTD-ILD other than RA-ILD (UIP) was misdiagnosed as IPF, and immunotherapy thus withheld, an opportunity to stabilize disease progression could be missed. The similarity of outcomes for IPF and RA-ILD (UIP), the treatment response differences apparent between IPF and the other idiopathic-ILDs and between RA-ILD (UIP) and the other CTD-ILDs, illustrate the degree of heterogeneity that is apparent within the disease spectrum that ILD represents.
Academic issues
Given that the 2011 ATS/ERS guidelines for IPF [21] were constructed without reference to comprehensive serology to test for MSA or MAA and SSc-associated autoantibodies, a question naturally arises regarding the robustness of an IPF diagnosis when made without such serology. Such a stricture may well have blighted previous mechanistic IPF research. For instance, in the few research studies in which relatively comprehensive myositis serology testing was undertaken in IPF cases, who would by definition have required IPF-consistent HRCT changes for study inclusion (i.e. UIP, or fibrotic NSIP), the results demonstrated such serology to be positive in a significant number of cases [9, 59]. As MSAs have highly significant HLA associations [60], these results could imply that IPF also has strong HLA associations, yet genetic studies using genome-wide association scan technology in IPF have failed to demonstrate significant HLA associations [61, 62]. Moreover, many of the MSA-positive cases in these studies were younger females, an observation more in keeping with a CTD-ILD rather than an IPF phenotype. Some of the MSA-positive IPF cases were presumably myositis-ILD cases, but where the CTD diagnosis had remained covert until the research myositis serology was undertaken. In contrast, in a Mexican study, highly significant HLA associations were found in IPF [63]. In this study the IPF diagnoses were based on the 2002 ATS/ERS IIP diagnostic guidelines, which gave no guidance on use of serology to interrogate for the presence of a CTD [8]. The highly significant HLA associations detected in this study, with odds ratios >10, could again imply that their IPF case cohort was in fact contaminated by many covert CTD-ILD cases, such as those with MSA/MAA. The contradictory nature of these genetic results suggests that, even in a phenotype as apparently robust as IPF diagnosed strictly in a specialized ILD clinic setting, case stratification errors have likely still occurred. To optimize accuracy of case stratifications to guarantee homogeneous cohorts for future IPF genetic studies will clearly require modern and comprehensive serology to be used to definitively exclude all CTD cases. Amyopathic ILD patients without a rash, and especially those with an anti-synthetase other than anti-Jo-1, would clearly cause confusion in any ILD study where comprehensive serology was not available. If all patients diagnosed as IPF in a tertiary UK ILD clinic were interrogated by immunoprecipitation, would a substantial cohort prove positive for CTD-associated antibodies? If so, then undertaking comprehensive serology on all incident ILD cases without obvious CTD signs would seem justifiable, at least until this question has been addressed.
Conclusions
The relative paucity of aetiopathological insights so far gained in ILD reflects the difficulties of accurately assigning ILD subgroup diagnoses without comprehensive CTD serology, and that case-stratification errors have likely occurred in previous research. This may have contributed to the apparent confusion in the literature regarding diagnostic labels, mechanistic issues and the apparent contradictory nature of genetic research outputs. These insight deficits also reflect the rarity of ILD, and that the invasiveness of lung biopsy procedures has severely hampered investigations of the ILD organ target. To facilitate accurate future clinical ILD care, so as to optimizie outcomes, it will be vital that comprehensive CTD-serotyping becomes standard in routine practice, though substantial technological developments will be required to achieve this. Until then, serology by immunoprecipitation will continue to be crucial, and especially in the ILD research setting. Given the extreme rarity of some ILD subgroups, and the sample size problems that would arise for instance during between-subgroup comparative research, it is essential that multicentre collaborative efforts develop to recruit the large ILD subgroup cohorts required to facilitate such research. Such cohorts would make it possible to prospectively correlate ILD clinical phenotypes with serotypes and HRCT-generated radiological phenotypes for all ILD subgroups. Examining the validity of HRCT to assign idiopathic-ILD subgroup diagnoses would also become possible. It is feasible that some idiopathic subgroups, such as idiopathic NSIP and COP, will become smaller or even disappear, as more individual idiopathic-ILD cases are appropriately reassigned into CTD-ILD subgroups. Serologically better defined ILD subgroups will ensure more meaningful between-subgroup evaluations, for instance in future immunogenetic comparisons. The use of larger, more homogeneous subgroup cohorts will clearly facilitate ILD research, and thus ultimately improve quality of patient care.
Acknowledgements
The authors of this review are co-ordinating a UK-wide multicentre collaborative recruitment of ILD cases, initially called: identifying disease susceptibility genes and autoantibodies associated with the development and clinical characteristics of interstitial lung disease (ILD) in patients with and without proven connective tissue diseases (CTDs), Version 1.0 18 December 2010. This was subsequently renamed: the UK Biomarkers in Interstitial Lung Disease (UK-BILD) Study. This is NIHR-approved (UKCRN ID: 15775), so case recruitment is eligible for CRN network support (http://public/ukcrn.org.uk/search/). Centres interested in participating in UK-BILD should contact Mr Paul New, who will facilitate the local ethical approval process (paul.new@liverpool.ac.uk). CC received Phd funding from the the Institute of Ageing and Chronic Disease as well as Arrowe Park Endowment Funds.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Wells AU, Hirani N on behalf of the British Thoracic Society Interstitial Lung Disease Group. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63:v1–v58. [DOI] [PubMed] [Google Scholar]
- 2. Wells AU, Denton CP.. Interstitial lung disease in connective tissue disease-mechanisms and management. Nat Rev Rheumatol 2014;10:728–39. [DOI] [PubMed] [Google Scholar]
- 3. Kim DS, Collard HR, King TE Jr.. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. King TE Jr, Pardo A, Selman M.. Idiopathic pulmonary fibrosis. Lancet 2011;378:1949–61. [DOI] [PubMed] [Google Scholar]
- 5. Ryerson CJ, Collard HR.. Update on the diagnosis and classification of ILD. Curr Opin Pulm Med 2013;19:453–9. [DOI] [PubMed] [Google Scholar]
- 6. Corte TJ, Copley SJ, Desai SR. et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J 2012;39:661–8. [DOI] [PubMed] [Google Scholar]
- 7. Dixon S, Benamore R.. The idiopathic interstitial pneumonias: understanding key radiological features. Clin Radiol 2010;65:823–31. [DOI] [PubMed] [Google Scholar]
- 8. American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe K, Handa T, Tanizawa K. et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 2011;105:1238–47. [DOI] [PubMed] [Google Scholar]
- 10. Fischer A, Swigris JJ, du Bois RM. et al. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med 2009;103:1719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalluri M, Sahn SA, Oddis CV. et al. Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest 2009;135:1550–6. [DOI] [PubMed] [Google Scholar]
- 12. Takato H, Waseda Y, Watanabe S. et al. Pulmonary manifestations of anti-ARS antibody positive interstitial pneumonia – with or without PM/DM. Respir Med 2013;107:128–33. [DOI] [PubMed] [Google Scholar]
- 13. Castelino FV, Goldberg H, Dellaripa PF.. The impact of rheumatological evaluation in the management of patients with interstitial lung disease. Rheumatology 2011;50:489–93. [DOI] [PubMed] [Google Scholar]
- 14. Song JW, Do KH, Kim MY. et al. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest 2009;136:23–30. [DOI] [PubMed] [Google Scholar]
- 15. Desai SR, Veeraraghavan S, Hansell DM. et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004;232:560–7. [DOI] [PubMed] [Google Scholar]
- 16. Goldin JG, Lynch DA, Strollo DC. et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358–67. [DOI] [PubMed] [Google Scholar]
- 17. Park JH, Kim DS, Park IN. et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705–11. [DOI] [PubMed] [Google Scholar]
- 18. Bonnefoy O, Ferretti G, Calaque O. et al. Serial chest CT findings in interstitial lung disease associated with polymyositis-dermatomyositis. Eur J Radiol 2004;49:235–44. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt SL, Sundaram B, Flaherty KR.. Diagnosing fibrotic lung disease: when is high-resolution computed tomography sufficient to make a diagnosis of idiopathic pulmonary fibrosis? Respirology 2009;14:934–9. [DOI] [PubMed] [Google Scholar]
- 20. Thomeer M, Demedts M, Behr J. et al. Multidisciplinary interobserver agreement in the diagnosis of idiopathic pulmonary fibrosis. Eur Respir J 2008;31:585–91. [DOI] [PubMed] [Google Scholar]
- 21. Raghu G, Collard HR, Egan JJ. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coward WR, Saini G, Jenkins G.. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 2010;4:367–88. [DOI] [PubMed] [Google Scholar]
- 23. Raghu G, Anstrom KJ, King TE Jr. et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Travis WD, Costabel U, Hansell DM. et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer A, Antoniou KM, Brown KK. et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976–87. [DOI] [PubMed] [Google Scholar]
- 26. Betteridge ZE, Gunawardena H, McHugh NJ.. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther 2011;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomonaga M, Sakamoto N, Ishimatsu Y. et al. Comparison of pulmonary involvement between patients expressing Anti-PL-7 and Anti-Jo-1 antibodies. Lung 2015;193:79–83. [DOI] [PubMed] [Google Scholar]
- 28. Chan EK, Damoiseaux J, Carballo OG. et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014-2015. Front Immunol 2015;6:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mariz HA, Sato EI, Barbosa SH. et al. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum 2011;63:191–200. [DOI] [PubMed] [Google Scholar]
- 30. Marie I, Josse S, Hatron PY. et al. Interstitial lung disease in anti-Jo-1 patients with antisynthetase syndrome. Arthritis Care Res 2013;65:800–8. [DOI] [PubMed] [Google Scholar]
- 31. Marie I, Josse S, Decaux O. et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med 2013;24:474–9. [DOI] [PubMed] [Google Scholar]
- 32. Hirakata M, Suwa A, Takada T. et al. Clinical and immunogenetic features of patients with autoantibodies to asparaginyl-transfer RNA synthetase. Arthritis Rheum 2007;56:1295–303. [DOI] [PubMed] [Google Scholar]
- 33. Sato S, Kuwana M, Hirakata M.. Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology 2007;46:842–5. [DOI] [PubMed] [Google Scholar]
- 34. Hamaguchi Y, Fujimoto M, Matsushita T. et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PloS One 2013;8:e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N.. Anti-synthetase syndrome: a new autoantibody to phenylalanyl transfer RNA synthetase (anti-Zo) associated with polymyositis and interstitial pneumonia. Rheumatology 2007;46:1005–8. [DOI] [PubMed] [Google Scholar]
- 36. Sato S, Hirakata M, Kuwana M. et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005;52:1571–6. [DOI] [PubMed] [Google Scholar]
- 37. Koga T, Fujikawa K, Horai Y. et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 2012;51:1278–84. [DOI] [PubMed] [Google Scholar]
- 38. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R.. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res 2016;68:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mierau R, Moinzadeh P, Riemekasten G. et al. Frequency of disease-associated and other nuclear autoantibodies in patients of the German Network for Systemic Scleroderma: correlation with characteristic clinical features. Arthritis Res Ther 2011;13:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum 2005;35:35–42. [DOI] [PubMed] [Google Scholar]
- 41. Walker UA, Tyndall A, Czirjak L. et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis 2007;66:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol 2010;37:42–53. [DOI] [PubMed] [Google Scholar]
- 43. Mitri GM, Lucas M, Fertig N, Steen VD, Medsger TA Jr.. A comparison between anti-Th/To- and anticentromere antibody-positive systemic sclerosis patients with limited cutaneous involvement. Arthritis Rheum 2003;48:203–9. [DOI] [PubMed] [Google Scholar]
- 44. Arnett FC, Reveille JD, Goldstein R. et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum 1996;39:1151–60. [DOI] [PubMed] [Google Scholar]
- 45. Aggarwal R, Lucas M, Fertig N, Oddis CV, Medsger TA Jr.. Anti-U3 RNP autoantibodies in systemic sclerosis. Arthritis Rheum 2009;60:1112–8. [DOI] [PubMed] [Google Scholar]
- 46. Fertig N, Domsic RT, Rodriguez-Reyna T. et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum 2009;61:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaji K, Fertig N, Medsger TA Jr. et al. Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosis-related antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res 2014;66:575–84. [DOI] [PubMed] [Google Scholar]
- 48. Betteridge ZWF, Bunn C, Denton CD. et al. Anti-EIF2B: a novel interstitial lung disease associated autoantibody in patients with systemic sclerosis [Abstract]. Arthritis Rheum 2012;64:854.21989653 [Google Scholar]
- 49. Lega JC, Fabien N, Reynaud Q. et al. The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome. Autoimmun Rev 2014;13:883–91. [DOI] [PubMed] [Google Scholar]
- 50. Denton CP. Advances in pathogenesis and treatment of systemic sclerosis. Clin Med 2016;16:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Selva-O'Callaghan A, Labrador-Horrillo M, Solans-Laque R. et al. Myositis-specific and myositis-associated antibodies in a series of eighty-eight Mediterranean patients with idiopathic inflammatory myopathy. Arthritis Rheum 2006;55:791–8. [DOI] [PubMed] [Google Scholar]
- 52. Gunnarsson R, Aalokken TM, Molberg O. et al. Prevalence and severity of interstitial lung disease in mixed connective tissue disease: a nationwide, cross-sectional study. Ann Rheum Dis 2012;71:1966–72. [DOI] [PubMed] [Google Scholar]
- 53. King TE Jr, Bradford WZ, Castro-Bernardini S. et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. New Engl J Med 2014;370:2083–92. [DOI] [PubMed] [Google Scholar]
- 54. Richeldi L, du Bois RM, Raghu G. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. New Engl J Med 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- 55. Noble PW, Albera C, Bradford WZ. et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760–9. [DOI] [PubMed] [Google Scholar]
- 56. Debray MP, Borie R, Revel MP. et al. Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol 2015;84:516–23. [DOI] [PubMed] [Google Scholar]
- 57. Kim EJ, Elicker BM, Maldonado F. et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322–8. [DOI] [PubMed] [Google Scholar]
- 58. Bongartz T, Nannini C, Medina-Velasquez YF. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takahashi T, Wada I, Ohtsuka Y. et al. Autoantibody to alanyl-tRNA synthetase in patients with idiopathic pulmonary fibrosis. Respirology 2007;12:642–53. [DOI] [PubMed] [Google Scholar]
- 60. Chinoy H, Salway F, Fertig N. et al. In adult onset myositis, the presence of interstitial lung disease and myositis specific/associated antibodies are governed by HLA class II haplotype, rather than by myositis subtype. Arthritis Res Ther 2006;8:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fingerlin TE, Murphy E, Zhang W. et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 2013;45:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Noth I, Zhang Y, Ma SF. et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med 2013;1:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Falfan-Valencia R, Camarena A, Juarez A. et al. Major histocompatibility complex and alveolar epithelial apoptosis in idiopathic pulmonary fibrosis. Hum Genet 2005;118:235–44. [DOI] [PubMed] [Google Scholar]