Abstract
In mammals, insulin regulates blood glucose levels and plays a key regulatory role in appetite via the hypothalamus. In contrast, chickens are characterized by atypical glucose homeostasis, with relatively high blood glucose levels, reduced glucose sensitivity of pancreatic beta cells, and large resistance to exogenous insulin. The aim of the present study was to investigate in chickens the effects of 5 h fasting and 5 h insulin immuno-neutralization on hypothalamic mRNA levels of 23 genes associated with food intake, energy balance, and glucose metabolism. We observed that insulin immune-neutralization by administration of anti-porcine insulin guinea pig serum (AI) significantly decreased food intake and increased plasma glucose levels in chickens, while 5 h fasting produced a limited and non-significant reduction in plasma glucose. In addition, 5 h fasting increased levels of NPY, TAS1R1, DIO2, LEPR, GLUT1, GLUT3, GLUT8, and GCK mRNA. In contrast, AI had no impact on the levels of any selected mRNA. Therefore, our results demonstrate that in chickens, food intake inhibition or satiety mechanisms induced by insulin immuno-neutralization do not rely on hypothalamic abundance of the 23 transcripts analyzed. The hypothalamic transcripts that were increased in the fasted group are likely components of a mechanism of adaptation to fasting in chickens.
Keywords: insulin, food intake, metabolism, hypothalamus, gene expression
INTRODUCTION
The hypothalamus plays a pivotal role in feed intake and energy balance regulation in birds (Kuenzel, 1994; Kuenzel et al., 1999). With few exceptions, such as a lack of response to specific orexigenic peptides (Orexin A and B, galanin, motilin and melanin concentrating hormone) (Furuse, 2002), feed intake and energy homeostasis seem to be regulated in a similar way in chickens as in mammals (Furuse, 2002; Boswell, 2005; Kuenzel et al., 1999; Richards and Proszkowiec-Weglarz, 2007). Pro-opiomelanocortin (POMC), Agouty-related protein (AGRP), and neuropeptide Y (NPY) are synthesized in the arcuate nucleus of the hypothalamus, where they play opposing roles in feed intake regulation in mammals (Ollmann et al., 1997; Kalra et al., 1999; Schwartz et al., 2000; Mizuno et al., 2003). Through its receptor, insulin contributes to nutrient sensing, regulation of energy expenditure, food intake control (Nandi et al., 2004), and regulation of liver glucose production (Inoue, 2016). Foxo1 and Gpr17 have been recently proposed as the targets of the insulin receptor (IR) cascade in AGRP neurons of the arcuate nucleus to regulate peripheral metabolism and energy balance and insulin sensitivity (Ren et al., 2012; Ren et al., 2015; Scherer et al., 2016). In birds the infundibular nucleus plays a similar role to that of the mammalian arcuate nucleus (Wang et al., 2001).
Chickens exhibit several peculiarities in plasma glucose level control, glucose transporters, insulin release, and IR signaling (Hazelwood, 1986; Rideau, 1998; Simon et al., 2000; Dupont et al., 2012). Despite these peculiarities, a cross-talk exists in birds between insulin and the hypothalamus. Hyperphagia develops and glucose-stimulated insulin levels are enhanced following lesions of the ventromedial hypothalamus (VMH) in geese and chickens (Jaccoby et al., 1995). Intraventricular injection of insulin inhibits food intake in young chickens via the central melanocortin system (Shiraishi et al., 2008). Chicken brain IRs show specific glycosylation, enhanced affinity, and different tyrosine kinase activity in response to nutritional alterations vs. peripheral IRs (Simon and Leroith, 1986). Moreover, IRs have been localized by immunohistochemistry in neurons of several structures of hypothalamus (the paraventricular nucleus, ventromedial and lateral regions, and infundibular nucleus) and in the infundibulum, they co-localize with α-melanocyte stimulating hormone and NPY neurons (Shiraishi et al., 2011). Central insulin injection modified hypothalamic levels of several mRNAs. In one study, POMC, cocaine- and amphetamine-regulated transcript (CART) and corticotropin-releasing hormone (CRH) mRNAs were up-regulated, whereas NPY or AGRP mRNAs were unaltered (Honda et al., 2007). In another study, POMC mRNA increased and NPY mRNA decreased (Shiraishi et al., 2008). In contrast, intraperitoneal insulin injection did not modify NPY mRNA levels in the hypothalamus of selected high or selected low weight chickens at 90 days of age (Zhang et al., 2015). The lack of insulin effect on hypothalamic NPY mRNA in these lines following intraperitoneal injection (vs. central injection in other studies) may rely on the time course for insulin access and/or age of the chickens. In another study however, intraperitoneal insulin injection increased hypothalamic tryptophan hydroxylase 2 mRNA in 5 day-old chickens of selected high or selected low weight chickens (Rice et al., 2014). Hypothalamic NPY mRNA content was higher in selected high weight chickens than in selected low weight chickens (Zhang et al., 2015), though at a younger age opposite results were observed (Rice et al., 2014). Changes in hypothalamic NPY mRNA have also been observed in response to changes in nutritional status. NPY mRNA levels increased following feed restriction (Boswell et al., 1999a,b) or prolonged fasting, except in the lateral area (Zhou et al., 2005) and in day-old chickens unfed for 48 h (Higgins et al., 2010). In the latter model, a large number of genes (among which a large number of receptors) were differentially expressed in the hypothalamus between the various nutritional states (fed, unfed for 48 h, or refed).
The goal of the present study was to gain insight into insulin control of gene expression in the chicken hypothalamus, using 23 selected mRNAs and two experimental models. In the first model, insulin deficiency was induced in fed broiler chickens (16 or 17 days of age) by immune-insulin privation for 5 h. Insulin deprived chickens exhibited a large decrease in food intake, large increases in plasma glucose, non-esterified fatty acids (NEFA), amino acids (alpha-NH2-non protein), and glucagon, and a decrease in plasma T3. In 3 peripheral tissues of the same chickens used in the present study, transcriptome studies identified a rather large number of insulin-dependent genes: 1,573 in liver and 1,225 in muscle, but much less in abdominal adipose tissue following immune insulin privation (Ji et al., 2012; Simon et al., 2012). A short fasting period (5 h) was included as a second model, as fasting represents another status of “insulin privation”. Plasma insulin levels were not, however, measured in this experiment because of the presence of anti-insulin antibody in the insulin-deprived group. As compared to fed control chickens, 5 h fasting slightly decreased plasma glucose (though not significantly) and amino acid levels, increased plasma NEFA and glucagon levels, and decreased plasma T3. Therefore, several changes at the plasma level are shared by these two experimental models. The 23 mRNAs assessed included most of those discussed earlier. Others were chosen mainly from our study on effects of prolonged fasting in day-old chickens (48 h without feed) in the hypothalamus (Higgins et al., 2010) or had been identified as differentially expressed in the hypothalamus during development of genetically selected fat or lean chickens (Byerly et al., 2010). Fat and lean chickens differ in their glucose-insulin relationship, with a slightly higher insulin sensitivity in fat chickens (Simon and Leclercq, 1985). Finally, other selected mRNAs were identified as insulin-dependent in liver or muscle, after insulin privation (Dupont et al., 2008; Simon et al., 2012). As a whole, selected mRNAs are involved in feed intake regulation or neuronal, endocrine, or transcriptional control or glucose metabolism and glucose sensing.
MATERIALS AND METHODS
Animals and Experimental Protocol
The animals and experimental protocol used were described previously (Dupont et al., 2008). Briefly, male broiler chickens (ISA 915, Institute de Selection Animale, Saint Brieuc, France) were fed a conventional balanced diet ad libitum (3,050 kCal or 12.8 mJ/kg metabolizable energy, 22% proteins (N6.25), based on corn, wheat, peas, soybean meal, corn gluten, and rapeseed oil) and exposed to a 14L:10D light regimen. At 16 to 17 d of age, chickens of similar body weight were divided into five experimental groups (n = 7 birds each) as follows: fed groups that received a single intra-venous (i.v.) injection (1.5 mL/kg) of either normal guinea pig serum (NS, PromoCell, Heidelberg, Germany) or anti-porcine insulin guinea pig serum (AI) (C_1 and AI_1 groups, respectively), fed groups that received three i.v. injections (1.5 mL/kg each) at time 0, 2, and 4 h of either NS or AI (C_5 and AI_5 groups, respectively), and a fasted group that was fasted for 5 h and received three i.v. injections of NS at the times described above (F_5 group). The 5 h time point was based on availability of antiserum. Insufficient anti-serum was available to assess longer time points, while still administering the antiserum at 2 h intervals. Four replicates were performed at 16 d and three at 17 d of age to allow for collection of all samples within an hour on each day. Immune sera were prepared as described previously (Simon et al., 2000). Food intake was measured 1 h (C_1 and AI_1) or 5 h (C_5 and AI_5) after the first i.v. injection. At the same time, blood was sampled under EDTA, cooled on ice, and the plasma rapidly collected by centrifugation. Birds were killed by cervical dislocation after blood sample collection. Hypothalamic tissue samples were collected manually using a dissecting microscope as described previously (Byerly et al., 2009; Higgins et al., 2010) based on published information (Kuenzel and Masson, 1988) and stored at −80°C until RNA extraction. Initial incisions were made just anterior to the occulomotor nerve (nervus occulomotorius) and posterior to the tuberculum olfactorium. Next, lateral cuts were made 2 mm from the midline to yield a rectangular piece of tissue. This was placed on its side, and a final cut was made at a depth immediately below the subseptal organ (organum subseptale) and parallel to the basal surface of the hypothalamus. Plasma glucose levels were measured by the glucose oxidase method (Glucose Beckman Analyzer 2, Beckman, Palo Alto, CA). All procedures were approved by the Agricultural Agency and the Scientific Research Agency of INRA and conducted in accordance with the guidelines for Care and Use of Agricultural Animals in Agricultural Research and Teaching.
RNA Isolation and Reverse Transcription-Quantitative PCR
Total RNA was extracted using RNeasy Midi kits (Qiagen, Valencia, CA), according to the manufacturer's protocol and quantified using the RiboGreen Quantitation kit (Invitrogen, Carslbad, CA). Two-step reverse transcription-quantitative PCR (RT-qPCR) was performed to analyze hypothalamic mRNA levels. The RT reactions (20 μL) consisted of 1 μg of RNA, 50 units Superscript III reverse transcriptase (Invitrogen), 40 units of an RNase inhibitor (Invitrogen), 0.5 mM dNTPs, and 2.5 μM anchored oligo dT primers. A pool of all RNA (1 μg) from all treatment groups was used as a negative control for genomic DNA contamination and was processed as the other samples, but with omission of Superscript III enzyme. The RT reactions were diluted to 200 μL before being subjected to PCR. PCR was performed in 15 μL reactions containing 1 μL of diluted RT reaction, 400 nM of each gene-specific primer, 7.5 μL of 2 × PCR buffer (100 mM KCl, 20 mM Tris-HCl, pH 9, 0.2% Triton X-100), 3.8 mM MgCl2, 0.12 U/μL Taq polymerase, 400 nM dNTPs, 40 nM fluorescein (Invitrogen), and SYBR Green I Nucleic Acid Gel Stain (Invitrogen) diluted 1:10,000, and the MyIQ Single-Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Thermal cycling parameters were: 1 cycle at 95°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Dissociation curve analysis and gel electrophoresis were employed to ensure that a single PCR amplicon of appropriate size was amplified in each reaction. PCR products were sequenced to confirm their identities. Primer sequences, designed using Primer3 software (Rozen and Skaletsky, 2000), are listed in Table 1. The data obtained were normalized to the housekeeping gene β-actin (ACTB), and the data were transformed using the equation 2−Ct, where Ct represents the fractional cycle number when the amount of amplified product reached a fixed threshold for fluorescence. The data are presented as fold changes relative to the C_1 group for statistical analysis.
Table 1.
Gene-specific primers used for the analysis of mRNA levels using quantitative real-time RT-PCR.a
| Gene Nameb | GenBank Accession No.c | Forward primers (5’-3’) | Reverse primer (5’-3’) | PCR size (bp) |
|---|---|---|---|---|
| AGRP | NM_0,010,31457 | AAGTCTGGCCTGGGAAGAG | CCCCCTGCAGAAGATGAG | 122 |
| ACTB | X0082 | CAGGATGCAGAAGGAGATCACA | TAGAGCCTCCAATCCAGACAGAGTA | 101 |
| CHAT | NM_204,610 | TGATGAGGGCAGAGTTGACA | TGCGTCCTTAAATCTCTGCAT | 124 |
| CRH | NM_0,011,23031 | CACAGCAACAGGAAACTGATGGAAA | AAAGAGGTGACATCAGAGCAGCACTATG | 101 |
| DIO2 | NM_204,114 | ACTGTTTGAGGGCGCTAAACC | AAACACTAGCCCTCCAGAATACCTT | 110 |
| DPYSL2 | NM_204,494 | CTGCCAAGACCCACAACATA | TTCCCTTGGCTGATAACCAC | 91 |
| EGR1 | NM_204,136 | TCAGCACTTTCAGACATGACATCA | AGTACCAGTGGAAGAGGTGAATGC | 101 |
| ENO2 | NM_204,876 | GCCTCCTGCTCAAAGTCAAC | CATTCTCCTGGGCCAACTTA | 75 |
| GCK | AF525739 | ACGAAGCACCAGATGTACTCC | GGAGATGCACTCCGAGATGT | 86 |
| GLUT1 | NM_205,209 | GGGATCAATGCGGTTTTCTA | CGAAGAGCGAAACAACAGTG | 124 |
| GLUT2 | NM_207,178 | TATCGTCACAGGCATCCTCA | GAAGAACTGCAGCAGAGCAG | 118 |
| GLUT3 | NM_205,511 | CTCAACCAGCTGGGCATAGT | AGTGGCCAAAGTGCTTCAGT | 89 |
| GLUT8 | NM_204,375 | TGCAGGCAAATTGAAGCTAA | TGCCTGTTACTTGCTGGAGA | 124 |
| GPI | NM_0,010,06128 | CCATGCTGGGAGTGTGGTAT | GCTGGAAGTAGGCAGCAAAG | 99 |
| HK1 | NM_204,101 | CGTTACCTGCATTTCGGACT | CTTGCAAGGGAAGGAGAATG | 88 |
| LEP | LN794246d | GGCGATTCCAACGCCTACT | CCGCCATGGCTTGCA | 102 |
| LEPR | NM_204,323 | TTCCAAACCCCAAGAATTGCT | CAAATGACATTGCTTCAGGGTG | 102 |
| MC4R | NM_0,010,31514 | CGGGAGGCTGCTATGAACAA | AGCTGATGATGCCCAGAGTCA | 101 |
| NPY | NM_205,473 | GGGAAAGCACAGAAAACATTCC | AAATCCCATCACCACATCGAA | 101 |
| PGAM1 | NM_0,010,31556 | AGGCTCAGGTGAAGATCTGG | TGCTGATGGTGCTGAAGAAG | 87 |
| POMC | NM_0,010,31098 | CGCTACGGCGGCTTCA | TCTTGTAGGCGCTTTTGACGAT | 88 |
| TAS1R1 | XM_425,734 | GCGTGGCAGAAACACCAGAT | GCTTGACCAATATGCGCTTGT | 64 |
| TH | NM_204,805 | CGGACTGCTGTCATGAGCTA | CAGTTGCTCCCAGAGATGC | 102 |
| TRH | NM_0,010,30383 | TGGATGACATCCTGCAGAGATC | GGAAAGCCATTGTGGCAGA | 116 |
aAll primers used for expression analysis were designed using the Primer3 program (http://fakker.wi.mit.edu/primer3/input.htm; Rozen and Skaletsky, 2000).
bAbbreviation of the gene names are defined in Table 2 and text.
cReference chicken gene sequences that contain the corresponding PCR products list.
dFrom Seroussi et al. (2016).
Statistical Analysis
All data were analyzed by one-way ANOVA using the general linear models (GLM) procedure of the Statistical Analysis System (SAS) software (SAS Institute, Cary, NC, USA). The treatment means for food intake and plasma glucose level were compared by Fisher's test or Student t test, while the Duncan's multiple range test option of the GLM procedure for SAS was used to determine significance of mean differences for gene expression analysis. Significance was set at P < 0.05.
RESULTS
Insulin immune-neutralization for 1 (AI_1) or 5 h (AI_5) significantly (P < 0.008 and P < 0.0001, respectively) decreased feed intake in comparison to corresponding control groups (Figure 1). Plasma glucose levels were significantly increased after insulin immune-neutralization at both time points, while chicks fasted for 5 h had similar (P > 0.05) plasma glucose levels as chicks fed ad libitum (Figure 1). These findings indicate that AI depressed insulin activity which led to increased glucose levels and decreased feed intake. Despite the large and multiple effects observed following insulin immuno-neutralization at the level of plasma parameters, liver or muscle IR signaling, and liver or muscle transcriptome (Dupont et al., 2008; Simon et al., 2012), levels of none of the selected mRNAs were modified in the hypothalamus at either 1 h or 5 h insulin privation, as compared to the respective fed controls (Table 2). EGR1 mRNA, which decreased both in liver and leg muscle after insulin-neutralization (Dupont et al., 2008; Simon et al., 2012), was lower in the hypothalamus in the 5 h insulin neutralization group compared to the 5 h fasted group but not to the 5 h fed group. A similar situation was observed for CRH mRNA (Table 2). Origin of the opposite changes in EGR1 and CRH in response to insulin-neutralization and fasting are unknown.
Figure 1.
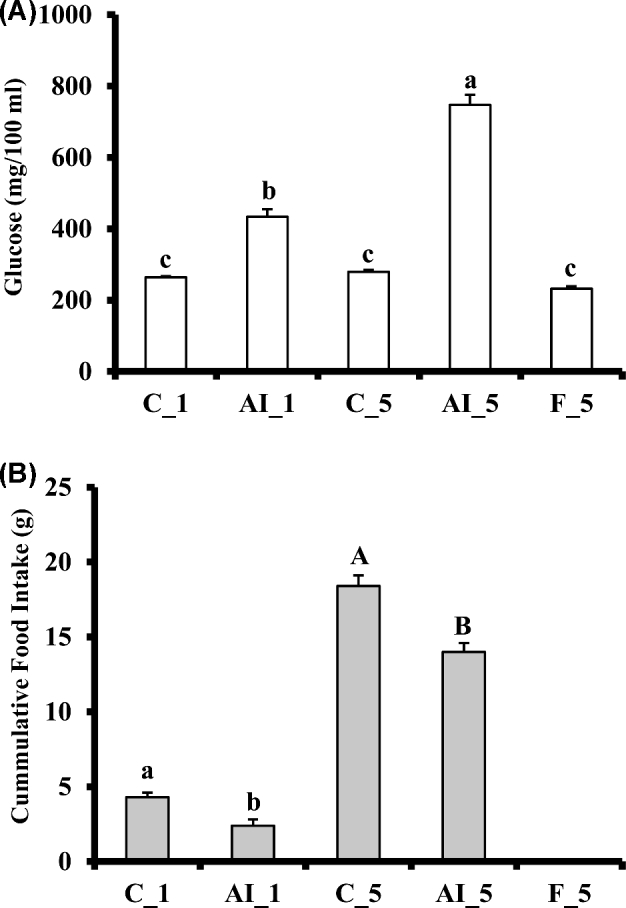
Effects of insulin immune-neutralization and fasting on (A) cumulative feed intake and (B) plasma glucose levels. At 16 to 17 d of age, chickens at similar body weight were divided into five experimental groups (n = 7 birds each) as follows: fed groups that received a single i.v. injection (1.5 mL/kg) of either normal guinea pig serum (NS, PromoCell, Heidelberg, Germany) or anti-porcine insulin guinea pig serum (AI) (C_1 and AI_1 groups, respectively), fed groups that received three i.v. injections (1.5 mL/kg each) at time 0, 2, and 4 h of either NS or AIS (C_5 and AI_5 groups, respectively), and a fasted group that was fasted for 5 h and received three i.v. injections of NS at the times described above (F_5 group). Each value represents the mean ± SE of seven birds (n = 7). Due to the large differences in feed intake between 1 h (C_1 and AI_1) and 5 h (C_5 and AI_5) after the first i.v. injection, results for feed intake at 1 h and 5 h were analyzed separately in order to test for differences due specifically to AI. Different letters denote statistically significant differences for mean comparisons (P < 0.05).
Table 2.
Effect of insulin immuno-neutralization and fasting on mRNA expression levels in the hypothalamus of chickens.
| Gene Name | Treatment | ||||
|---|---|---|---|---|---|
| C_1 | AI_1 | C_5 | AI_5 | F_5 | |
| POMC | 100.00 ± 12.28 | 97.57 ± 16.44 | 86.76 ± 17.87 | 92.48 ± 22.36 | 114.46 ± 30.34 |
| MC4R | 100.00 ± 7.27 | 90.11 ± 7.74 | 93.76 ± 4.60 | 85.92 ± 7.79 | 107.97 ± 10.46 |
| EGR1 | 100.00 ± 5.98a | 97.68 ± 8.78a | 92.64 ± 4.92a,b | 76.07 ± 5.13b | 102.96 ± 9.26a |
| CRH | 100.00 ± 15.19a,b | 82.32 ± 7.95a,b | 84.01 ± 12.21a,b | 74.38 ± 9.60b | 116.17 ± 18.16a |
| TRH | 100.00 ± 6.80 | 95.50 ± 8.08 | 94.39 ± 4.77 | 86.01 ± 7.76 | 108.61 ± 10.47 |
| ENO2 | 100.00 ± 4.97 | 99.28 ± 1.88 | 98.05 ± 1.82 | 97.07 ± 7.60 | 108.34 ± 7.25 |
| PGAM1 | 100.00 ± 11.84 | 83.22 ± 3.40 | 80.06 ± 6.88 | 85.88 ± 10.78 | 92.19 ± 14.14 |
| GPI | 100.00 ± 4.55 | 98.51 ± 3.44 | 99.29 ± 1.48 | 94.82 ± 8.30 | 109.99 ± 7.47 |
| DPYSL2 | 100.00 ± 5.60 | 98.65 ± 5.27 | 97.12 ± 2.94 | 96.52 ± 7.45 | 104.13 ± 9.36 |
| HK1 | 100.00 ± 4.92 | 97.63 ± 2.69 | 102.17 ± 4.67 | 96.79 ± 6.78 | 102.50 ± 6.02 |
Reverse transcription quantitative PCR (RT-qPCR) was used to quantify mRNA levels for pro-opiomelanocortin (POMC), melanocortin 4 receptor (MC4R), early response gene (EGR1), corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), neural enolase 2 (ENO2), phosphoglycerate mutase 1 (PGAM1), glucose phosphate isomerase (GPI), dihydropyrimidinase like protein (PRYSL2), and hexokinase 1 (HK1) in hypothalami collected from ad libitum fed chickens 1 h after one normal serum (NS) injection (C_1) or one anti-insulin serum (AI) injection (AI_1) and after 5 h during which three injection of NS (C_5) or AI (AI_5) were administered. Birds fasted for 5 h (F_5) were also analyzed for comparison. Each value represents the mean ± SE of seven birds (n = 7).
a,bDifferent letters denote statistically significant differences for mean comparisons (P < 0.05).
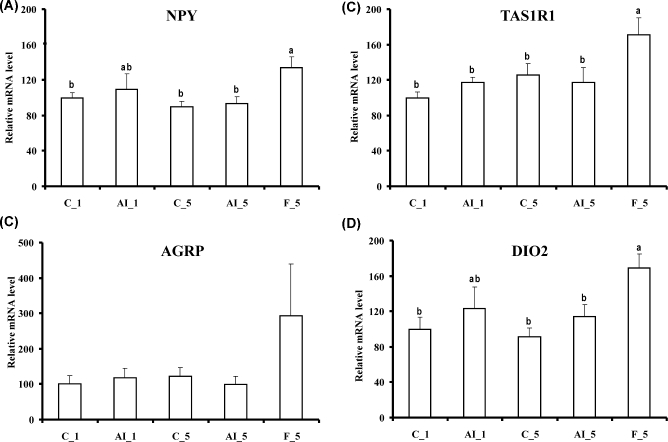
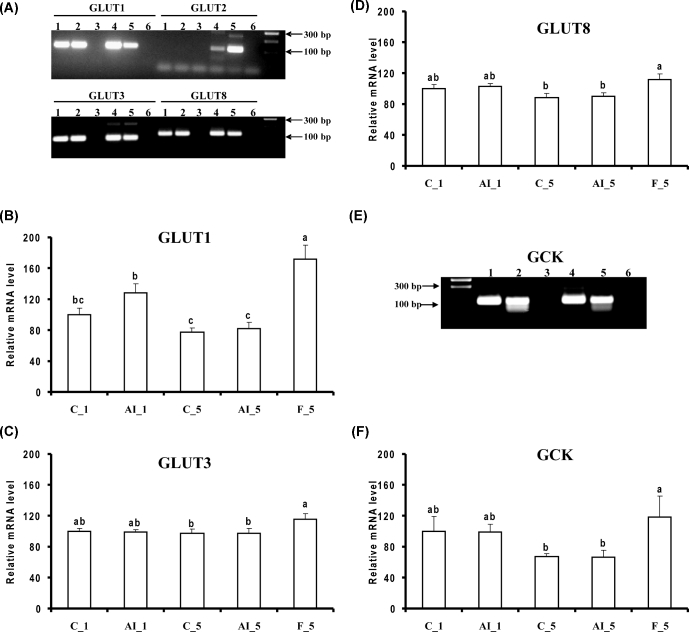
In contrast, 5 h fasting altered the levels of several mRNAs. NPY, sweet taste receptor 1 (TAS1R1), and iodothyronine deiodinase 2 (DIO2) mRNA levels increased as compared to both fed controls or 5 h insulin deprived fed chickens (Figure 2), and LEPR mRNA levels were increased by 5 h fasting as compared to fed controls (Figure 3). LEP messenger levels were unchanged (Figure 3). Fasting also increased mRNAs for several glucose transporters (GLUT1, GLUT3, and GLUT8) and GCK, also as compared to both fed controls or 5 h insulin deprived chickens (Figure 4). Choline O-acetyltransferase (CHAT) and tyrosine hydroxylase (TH) mRNA levels were unchanged (Figure 5), suggesting a lack of activation of cholinergic and dopaminergic systems in the hypothalamus, at the mRNA level, in any of the present conditions. Finally, levels of eight other mRNAs were unaffected by any of the treatments (see Table 2).
Figure 2.
Effects of insulin immune-neutralization and fasting on hypothalamic levels of mRNA for (A) neuropeptide Y (NPY), (B) agouty gene related protein (AGRP), (C) sweet taste receptor 1 (TAS1R1), and (D) iodothyronine deiodinase 2 (DIO2). See legend to Figure 1 for further details.
Figure 3.
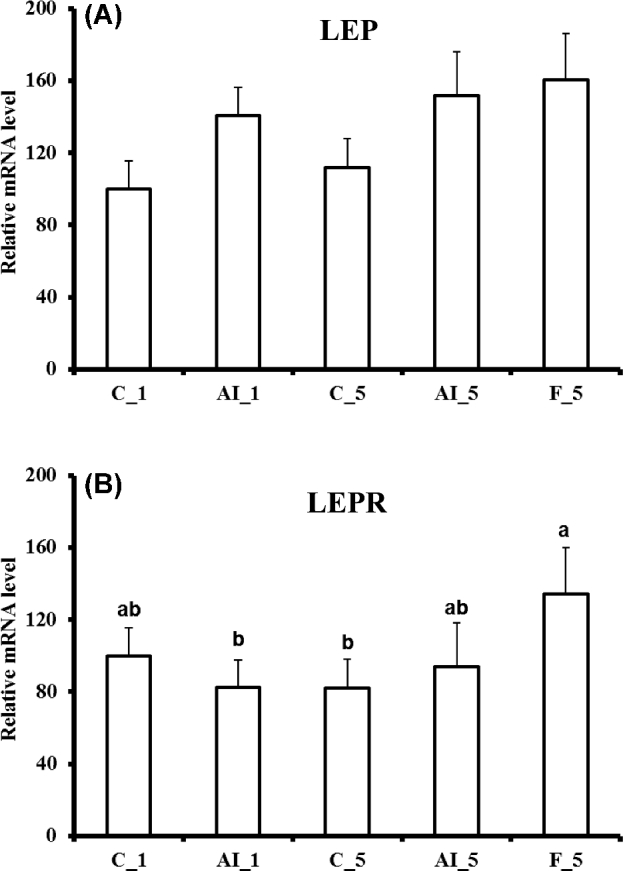
Effects of insulin immune-neutralization and fasting on hypothalamic levels of mRNA for (A) leptin (LEP) and (B) leptin receptor (LEPR). See legend to Figure 1 for further details.
Figure 4.
Effects of insulin immune-neutralization and fasting on hypothalamic levels of mRNA for genes involved in glucose transport and metabolism. (A) PCR products for glucose transporters 1, 2, 3, and 8 (GLUT1, GLUT 2, GLUT3, and GLUT8, respectively). Hypothalamic levels of mRNA for (B) GLUT1, (C) GLUT2, (D) GLUT3, and (F) GCK were determined by reverse transcription-quantitative PCR. (E) PCR products for glucokinase (GCK). Numbers 1 to 6 in panels A and E refer to different tissues. Samples are identified as follows: 1, C_5; 2, AI_5; 3, no RT sample; 4, brain sample from 3 wk old chicken; 5 liver sample from embryonic day 18 and 6, blank. See legend to Figure 1 for further details.
Figure 5.
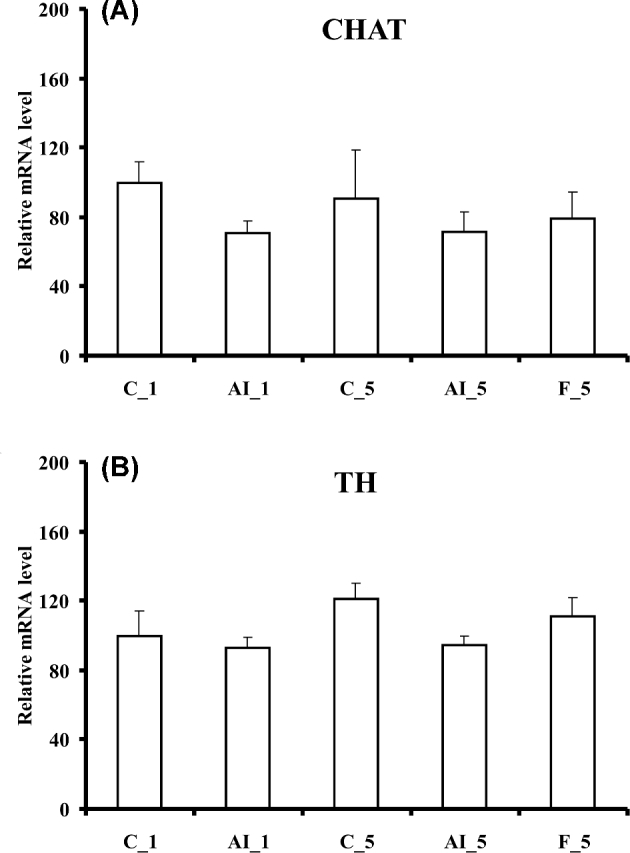
Effects of insulin immune-neutralization and fasting on hypothalamic levels of mRNA for (A) choline O-acetyltransferase (CHAT) and (B) tyrosine hydroxylase (TH). See legend to Figure 1 for further details.
DISCUSSION
In the present study, 5 h insulin immune neutralization in fed chickens had no effect on hypothalamic levels of the mRNAs investigated (only EGR1 tended to decrease). This was highly surprising, considering the inhibition of food intake, changes in levels of numerous mRNAs in peripheral tissues, known effects of central insulin injection on the chicken hypothalamus, and the multiple and major effects of insulin in the mammalian hypothalamus. Though, to our knowledge, insulin has not been measured in chicken cerebrospinal fluid (csf), it would be very unlikely if both csf insulin and glucose levels had not been altered during the 5 h insulin neutralization, as the half-life of plasma insulin is about 5 min in chickens and about 7 min in turkeys (Langslow, 1976; McMurtry et al., 1987). Uptake of plasma glucose and insulin through blood-brain barrier and relationships between plasma glucose and insulin levels and csf glucose and insulin levels have been investigated in mammals. Changes in csf are tempered in comparison with peripheral variations of glucose or insulin, but do exist, showing some delay and variations of lower magnitude. The adjustment of csf insulin is disturbed in some pathologies (Begg, 2015). In contrast, changes in csf glucose level have been observed in chickens in various conditions: a decrease in response to peripheral hypoglycemia (induced by intravenous tolbutamide or insulin injection) with a delay of about 20 to 40 minutes (Anderson and Hazelwood, 1969; Simon and Leclercq, 1985) or in response to overnight fasting of 16 h (Simon and Leclercq, 1985). Since no changes were observed for any of hypothalamic mRNAs presently investigated despite the rather extreme experimental conditions, the inhibition of food intake following insulin neutralization must rely on mechanisms other than control at the transcription level in the hypothalamus within the period of time investigated. Effects on neurotransmitter release within the hypothalamus are a likely possibility. A change in glucose sensing or metabolism and/or a decrease in insulin signaling is another possibility. Up to now, major steps of the insulin-IR cascade have not been characterized in the chicken brain. Only IR tyrosine kinase activity has been measured and shown to be refractory to extreme nutritional changes (Simon and Leroith, 1986). In peripheral tissues, the early steps of insulin receptor signaling appear to be responsive to insulin in the liver but refractory in muscle and adipose tissue (Dupont et al., 2004; Dupont et al., 2012). So far, only the phosphorylation of Src homology 2 domain containing transforming protein 1 adaptor protein (Shc), an IR substrate, appeared insulin sensitive in muscle (Dupont et al., 2004).
Plasma glucose levels were not significantly different between 5 h fasting and fed control chickens. A catabolic state had likely already developed during the 5 h fast, as suggested by the significant increases in plasma NEFA and glucagon levels and significant decrease in amino acid index reported previously (Dupont et al., 2008). The 5 h fast certainly represents a transition step during which a hunger state develops and orexigenic mechanisms are stimulated. Though some orexigenic gene mRNA levels did not change in the 5 h fasting group (e.g., POMC, MC4R), expression of 8 genes was altered out of the 23 measured. These genes are involved in the control of food intake, endocrine control of metabolism, or glucose metabolism.
Hypothalamic NPY and DIO2 mRNAs were increased by 5 h fasting. Similar increases were previously observed for NPY and DIO2 mRNA following fasting in growing chickens or in 48 h non-fed, day-old chickens (Boswell et al., 1999a,b; Zhou et al., 2005; Higgins et al., 2010). In liver, DIO2 mRNA was, in contrast, down-regulated by both 5 h fasting and 5 h insulin deprivation (Dupont et al., 2008). Expression of two other genes of the endocrine system was changed in the hypothalamus following fasting. CRH (as compared to insulin-immuno-neutralization) and LEPR (as compared to fed controls) mRNA levels were increased following fasting. CRH has been previously shown to decrease feed intake in chickens (Denbow et al., 1999), and differential CRH signaling may influence appetite regulatory mechanisms in chickens with different body weight and feed consumption (Cline et al., 2009). Moreover, CRH acts as a mediator for a number of anorexic peptides in chickens (Honda et al., 2007; Tachibana et al., 2004; Tachibana et al., 2006). An increase in CRH mRNA was previously observed when insulin was administered in chickens (Honda et al., 2007). Alternatively, the increase in CRH mRNA levels following fasting might be a result of fasting-induced stress. The increase in LEPR mRNA is consistent with our previous results (Higgins et al., 2010). The chicken LEP gene, which has been identified only very recently, is expressed in the hypothalamus (Seroussi et al., 2016). In the present study, hypothalamic LEP mRNA levels were unchanged by fasting.
TAS1R1, which has been proposed in mice as a hypothalamic sensor of glucose (Ren et al., 2009), is expressed at a higher level in chickens selected for increased body fat than in their counterparts selected for leanness (Byerly et al., 2010). In the present study, hypothalamic TAS1R1 showed no changes when glucose levels largely increased following insulin neutralization but did increase following the 5 h fasting period, most likely in response to transient alterations in plasma glucose levels. Since glucose is the energetic fuel in brain, the increase in hypothalamic TAS1R1 mRNA may signal a quick adaptation to transient decreases in glucose levels during fasting. A similar adaptation is also suggested by the changes observed at the level of mRNAs coding for GLUT1, GLUT3, GLUT8, and GCK.
In mammals, a large family of glucose transporters facilitates cell glucose uptake. Among these, GLUT4, which largely accounts for insulin sensitive glucose uptake in muscle and adipocytes, is also present in the hypothalamus, where it appears to play a critical role in glucose uptake (Ren et al., 2015). The GLUT4 gene is not present in the yet incompletely sequenced chicken genome. Recently, GLUT12 appeared to act as an insulin-sensitive glucose transporter in chicken muscles and may play a similar role in this species as GLUT4 in mammals (Coudert et al., 2015). To our knowledge, the presence of GLUT12 in chicken hypothalamus has not been shown. Herein, other GLUT mRNAs were investigated. GLUT1 is expressed ubiquitously and facilitates basal glucose uptake. GLUT2 has a low affinity to glucose, and its expression is limited to the basolateral membrane of hepatocytes, kidney, small intestine, and pancreatic beta-cells. In mammals GLUT2 is also expressed in the hypothalamus, contributing to the control of food intake (Stolarczyk et al., 2010). GLUT3 is a high affinity transporter responsible for glucose uptake in tissues and cells where extracellular substrate concentration is low (e.g., neuronal cells) (Kono et al., 2005), while GLUT8 has been shown to be insulin responsive in the blastocyst of mammals (Carayannopoulos et al., 2000). The presence of GLUT1, GLUT3, and GLUT8 mRNAs was confirmed by PCR in brain and liver tissues of chickens. In contrast, mRNA expression of GLUT2 was not detectable in the chicken hypothalamus in the present study (data not shown). Similar expression patterns were observed by Kono et al. (2005) in chicken tissues. The 5 h fasting treatment increased hypothalamic mRNA levels for GLUT1, GLUT3, and GLUT8 (Figure 4), with GLUT1 demonstrating the largest effect.
Mammalian hexokinase IV (glucokinase, GCK) plays a crucial role in glucose homeostasis (Postic et al., 2001; Matschinsky, 2002) by phosphorylating hexose into hexose-6-phosphate in the first step of glycolysis. GCK is predominantly located in pancreatic beta-cells and in the liver, but also is present in glucose-sensitive tissues including the hypothalamus (Magnuson, 1992; Schuit et al., 2001; Penicaud et al., 2002). Within the hypothalamus of mammals, GCK has been shown to be expressed in the arcuate nucleus, lateral nucleus, dorsomedial nucleus, ventromedial nucleus, and paraventricular nucleus (Lynch et al., 2000; Maekawa et al., 2000; Li et al., 2003). Even though the chicken GCK gene has not been identified in the chicken genome, a 1,326 bp liver cDNA fragment that shows high homology with human GCK cDNA has been sequenced (Berradi et al., 2005). Chicken GCK has been shown to be insulin dependent in fed chickens (Dupont et al., 2008), GCK mRNA increased in liver in response to a meal (Rideau et al., 2008) and protein levels decreased during fasting (Dupont et al., 2008; Rideau et al., 2008). Furthermore, the use of a specific activator of mammalian GCK supported the existence a functional and potent GCK in chicken. This glucokinase activator induced hypoglycemia and inhibited food intake without recruiting insulin, suggesting that liver glucokinase activation by itself accounted for the hypoglycemia (Rideau et al., 2010). We detected GCK mRNA in the chicken hypothalamus (Figure 4E). To our knowledge, this is the first report showing the presence of the mRNA for this enzyme in the avian hypothalamus. Previously, Berradi et al. (2005) were not able to detect chicken GCK in chicken brain at the protein level. It is, however, possible that the protein level of GCK in whole brain is too low to be detected. In mammals, GCK is recognized as a specific marker for glucose sensing neurons (Yang et al., 1999; Dunn-Meynell et al., 2002; Kang et al., 2004, 2006).
In conclusion, 5 h insulin immuno-neutralization in fed chickens was without any effect on the various mRNAs presently investigated in the hypothalamus, despite an early decrease in food intake. A change in glucose and/or insulin signaling are likely to be involved in this “satiety” mechanism. The fact that all the mRNAs studied remained at a “normal” fed level is largely unexpected. By contrast, 5 h of fasting altered mRNA levels for 8 genes involved in food intake, glucose transport, and glucose metabolism. This short period of fasting was clearly perceived as a signal of the development of a glucose shortage event. Therefore, these “fast responder” genes are likely components of a mechanism of emergency adaptation to food deprivation in the chicken.
REFERENCES
- Anderson D. K., Hazelwood R. L.. 1969. Chicken cerebrospinal fluid: normal composition and response to insulin administration. J. Physiol. 202:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg D. P. 2015. Insulin transport into the brain and cerebrospinal fluid. Vitam. Horm. 98:229–248. [DOI] [PubMed] [Google Scholar]
- Berradi H., Taouis M., Cassy S., Rideau N.. 2005. Glucokinase in chicken (Gallus gallus). Partial cDNA cloning, immunodetection and activity determination. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 141:129–139. [DOI] [PubMed] [Google Scholar]
- Boswell T. 2005. Regulation of energy balance in birds by the neuroendocrine hypothalamus. J. Poult. Sci. 42:161–181. [Google Scholar]
- Boswell T., Dunn I. C., Corr S. A.. 1999a. Hypothalamic neuropeptide Y mRNA is increased after feed restriction in growing broilers. Poult. Sci. 78:1203–1207. [DOI] [PubMed] [Google Scholar]
- Boswell T., Dunn I. C., Corr S. A.. 1999b. Neuropeptide Y gene expression in the brain is stimulated by fasting and food restriction in chickens. Br. Poult. Sci. 40Suppl:S42–43. [DOI] [PubMed] [Google Scholar]
- Byerly M. S., Simon J., Cogburn L. A., Le Bihan-Duval E., Duclos M. J., Aggrey S. E., Porter T. E.. 2010. Transcriptional profiling of hypothalamus during development of adiposity in genetically selected fat and lean chickens. Physiol. Genomics 42:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly M. S., Simon J., Lebihan-Duval E., Duclos M. J., Cogburn L. A., Porter T. E.. 2009. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296:R1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayannopoulos M. O., Chi M. M., Cui Y., Pingsterhaus J. M., McKnight R. A., Mueckler M., Devaskar S. U., Moley K. H.. 2000. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc. Natl. Acad. Sci. U S A 97:7313–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. A., Kuo A. Y., Smith M. L., Nandar W., Prall B. C., Siegel P. B., Denbow D. M.. 2009. Differential feed intake responses to central corticotrophin releasing factor in lines of chickens divergently selected for low or high body weight. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 152:130–134. [DOI] [PubMed] [Google Scholar]
- Coudert E., Pascal G., Dupont J., Simon J., Cailleau-Audouin E., Crochet S., Duclos M. J., Tesseraud S., Metayer-Coustard S.. 2015. Phylogenesis and Biological Characterization of a New Glucose Transporter in the Chicken (Gallus gallus), GLUT12. PLoS One 10:e0139517 doi: 10.1371/journal.pone.0139517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denbow D. M., Snapir N., Furuse M.. 1999. Inhibition of food intake by CRF in chickens. Physiol. Behav. 66:645–649. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell A. A., Routh V. H., Kang L., Gaspers L., Levin B. E.. 2002. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 51:2056–2065. [DOI] [PubMed] [Google Scholar]
- Dupont J., Dagou C., Derouet M., Simon J., Taouis M.. 2004. Early steps of insulin receptor signaling in chicken and rat: apparent refractoriness in chicken muscle. Domest. Anim. Endocrinol. 26:127–142. [DOI] [PubMed] [Google Scholar]
- Dupont J., Metayer-Coustard S., Ji B., Rame C., Gespach C., Voy B., Simon J.. 2012. Characterization of major elements of insulin signaling cascade in chicken adipose tissue: apparent insulin refractoriness. Gen. Comp. Endocrinol. 176:86–93. [DOI] [PubMed] [Google Scholar]
- Dupont J., Tesseraud S., Derouet M., Collin A., Rideau N., Crochet S., Godet E., Cailleau-Audouin E., Metayer-Coustard S., Duclos M. J., Gespach C., Porter T. E., Cogburn L. A., Simon J.. 2008. Insulin immuno-neutralization in chicken: effects on insulin signaling and gene expression in liver and muscle. J. Endocrinol. 197:531–542. [DOI] [PubMed] [Google Scholar]
- Furuse M. 2002. Central regulation of food intake in the neonatal chick. Anim. Sci. J. 73:83–94. [Google Scholar]
- Hazelwood R. L. 1986. Carbohydrate metabolism. Pages 303–325 in Avian Physiology. Sturkie P. D. ed. Cornell University Press, New York. [Google Scholar]
- Higgins S. E., Ellestad L. E., Trakooljul N., McCarthy F., Saliba J., Cogburn L. A., Porter T. E.. 2010. Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genomics 11:162 doi: 10.1186/1471-2164-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Kamisoyama H., Saneyasu T., Sugahara K., Hasegawa S.. 2007. Central administration of insulin suppresses food intake in chicks. Neurosci. Lett. 423:153–157. [DOI] [PubMed] [Google Scholar]
- Inoue H. 2016. Central insulin-mediated regulation of hepatic glucose production [Review]. Endocr. J. 63:1–7. [DOI] [PubMed] [Google Scholar]
- Jaccoby S., Arnon E., Snapir N., Robinzon B.. 1995. Effects of estradiol and tamoxifen on feeding, fattiness, and some endocrine criteria in hypothalamic obese hens. Pharmacol. Biochem. Behav. 50:55–63. [DOI] [PubMed] [Google Scholar]
- Ji B., Ernest B., Gooding J. R., Das S., Saxton A. M., Simon J., Dupont J., Metayer-Coustard S., Campagna S. R., Voy B. H.. 2012. Transcriptomic and metabolomic profiling of chicken adipose tissue in response to insulin neutralization and fasting. BMC Genomics 13:441 doi: 10.1186/1471-2164-13-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S. P., Dube M. G., Pu S., Xu B., Horvath T. L., Kalra P. S.. 1999. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20:68–100. [DOI] [PubMed] [Google Scholar]
- Kang L., Dunn-Meynell A. A., Routh V. H., Gaspers L. D., Nagata Y., Nishimura T., Eiki J., Zhang B. B., Levin B. E.. 2006. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 55:412–420. [DOI] [PubMed] [Google Scholar]
- Kang L., Routh V. H., Kuzhikandathil E. V., Gaspers L. D., Levin B. E.. 2004. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 53:549–559. [DOI] [PubMed] [Google Scholar]
- Kono T., Nishida M., Nishiki Y., Seki Y., Sato K., Akiba Y.. 2005. Characterisation of glucose transporter (GLUT) gene expression in broiler chickens. Br. Poult. Sci. 46:510–515. [DOI] [PubMed] [Google Scholar]
- Kuenzel W., Beck M. M., Teruyama R.. 1999. Neural sites and pathways regulating food intake in birds: a comparative analysis to mammalian systems. J. Exp. Zool. 283:348–364. [DOI] [PubMed] [Google Scholar]
- Kuenzel W. J. 1994. Central neuroanatomical systems involved in the regulation of food intake in birds and mammals. J. Nutr. 124:1355S–1370S. [PubMed] [Google Scholar]
- Kuenzel W. J., Masson M.. 1988. A stereotaxic atlas of the brain of the chick (Gallus domesticus). The Johns Hopkins University Press, Baltimore. [Google Scholar]
- Langslow D. R. 1976. The half-life of bovine and chicken insulin in chicken plasma. Gen. Comp. Endocrinol. 29:423–425. [DOI] [PubMed] [Google Scholar]
- Li B., Xi X., Roane D. S., Ryan D. H., Martin R. J.. 2003. Distribution of glucokinase, glucose transporter GLUT2, sulfonylurea receptor-1, glucagon-like peptide-1 receptor and neuropeptide Y messenger RNAs in rat brain by quantitative real time RT-PCR. Brain. Res. Mol. Brain. Res. 113:139–142. [DOI] [PubMed] [Google Scholar]
- Lynch R. M., Tompkins L. S., Brooks H. L., Dunn-Meynell A. A., Levin B. E.. 2000. Localization of glucokinase gene expression in the rat brain. Diabetes 49:693–700. [DOI] [PubMed] [Google Scholar]
- Maekawa F., Toyoda Y., Torii N., Miwa I., Thompson R. C., Foster D. L., Tsukahara S., Tsukamura H., Maeda K.. 2000. Localization of glucokinase-like immunoreactivity in the rat lower brain stem: for possible location of brain glucose-sensing mechanisms. Endocrinology 141:375–384. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A. 1992. Tissue-specific regulation of glucokinase gene expression. J. Cell Biochem. 48:115–121. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M. 2002. Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes. 51Suppl 3:S394–404. [DOI] [PubMed] [Google Scholar]
- McMurtry J. P., Rosebrough R. W., Steele N. C.. 1987. Insulin metabolism and its effect on blood electrolytes and glucose in the turkey hen. Comp. Biochem. Physiol. A Comp. Physiol. 86:309–313. [DOI] [PubMed] [Google Scholar]
- Mizuno T. M., Makimura H., Mobbs C. V.. 2003. The physiological function of the agouti-related peptide gene: the control of weight and metabolic rate. Ann. Med. 35:425–433. [DOI] [PubMed] [Google Scholar]
- Nandi A., Kitamura Y., Kahn C. R., Accili D.. 2004. Mouse models of insulin resistance. Physiol. Rev. 84:623–647. [DOI] [PubMed] [Google Scholar]
- Ollmann M. M., Wilson B. D., Yang Y. K., Kerns J. A., Chen Y., Gantz I., Barsh G. S.. 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 278:135–138. [DOI] [PubMed] [Google Scholar]
- Penicaud L., Leloup C., Lorsignol A., Alquier T., Guillod E.. 2002. Brain glucose sensing mechanism and glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 5:539–543. [DOI] [PubMed] [Google Scholar]
- Postic C., Shiota M., Magnuson M. A.. 2001. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog. Horm. Res. 56:195–217. [DOI] [PubMed] [Google Scholar]
- Ren H., Cook J. R., Kon N., Accili D.. 2015. Gpr17 in AgRP Neurons Regulates Feeding and Sensitivity to Insulin and Leptin. Diabetes 64:3670–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Orozco I. J., Su Y., Suyama S., Gutierrez-Juarez R., Horvath T. L., Wardlaw S. L., Plum L., Arancio O., Accili D.. 2012. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 149:1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Zhou L., Terwilliger R., Newton S. S., de Araujo I. E.. 2009. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr. Neurosci. 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice B. B., Zhang W., Bai S., Siegel P. B., Cline M. A., Gilbert E. R.. 2014. Insulin-induced hypoglycemia associations with gene expression changes in liver and hypothalamus of chickens from lines selected for low or high body weight. Gen. Comp. Endocrinol. 208:1–4. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Proszkowiec-Weglarz M.. 2007. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult. Sci. 86:1478–1490. [DOI] [PubMed] [Google Scholar]
- Rideau N. 1998. Peculiarities of insulin secretion in chickens. Ann. N Y Acad. Sci. 839:162–165. [DOI] [PubMed] [Google Scholar]
- Rideau N., Berradi H., Skiba-Cassy S., Panserat S., Cailleau-Audouin E., Dupont J.. 2008. Induction of glucokinase in chicken liver by dietary carbohydrates. Gen. Comp. Endocrinol. 158:173–177. [DOI] [PubMed] [Google Scholar]
- Rideau N., Derouet M., Grimsby J., Simon J.. 2010. Glucokinase activation induces potent hypoglycemia without recruiting insulin and inhibits food intake in chicken. Gen. Comp. Endocrinol. 169:276–283. [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H.. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365–386. [DOI] [PubMed] [Google Scholar]
- Scherer T., Lindtner C., O’Hare J., Hackl M., Zielinski E., Freudenthaler A., Baumgartner-Parzer S., Todter K., Heeren J., Krssak M., Scheja L., Furnsinn C., Buettner C.. 2016. Insulin Regulates Hepatic Triglyceride Secretion and Lipid Content via Signaling in the Brain. Diabetes. 65:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit F. C., Huypens P., Heimberg H., Pipeleers D. G.. 2001. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 50:1–11. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Woods S. C., Porte D. Jr, Seeley R. J., Baskin D. G.. 2000. Central nervous system control of food intake. Nature. 404:661–671. [DOI] [PubMed] [Google Scholar]
- Seroussi E., Cinnamon Y., Yosefi S., Genin O., Smith J. G., Rafati N., Bornelov S., Andersson L., Friedman-Einat M.. 2016. Identification of the Long-Sought Leptin in Chicken and Duck: Expression Pattern of the Highly GC-Rich Avian leptin Fits an Autocrine/Paracrine Rather Than Endocrine Function. Endocrinology. 157:737–751. [DOI] [PubMed] [Google Scholar]
- Shiraishi J., Tanizawa H., Fujita M., Kawakami S., Bungo T.. 2011. Localization of hypothalamic insulin receptor in neonatal chicks: evidence for insulinergic system control of feeding behavior. Neurosci. Lett. 491:177–180. [DOI] [PubMed] [Google Scholar]
- Shiraishi J., Yanagita K., Fujita M., Bungo T.. 2008. Central insulin suppresses feeding behavior via melanocortins in chicks. Domest. Anim. Endocrinol. 34:223–228. [DOI] [PubMed] [Google Scholar]
- Simon J., Guillaumin S., Chevalier B., Derouet M., Guy G., Marche G., Ricard F. H., Leclercq B.. 2000. Plasma glucose-insulin relationship in chicken lines selected for high or low fasting glycaemia. Br. Poult. Sci. 41:424–429. [DOI] [PubMed] [Google Scholar]
- Simon J., Leclercq B.. 1985. Fat and lean chickens: prefattening period and in vivo sensitivity to insulin, atropine, and propranolol. Am. J. Physiol. 249:R393–401. [DOI] [PubMed] [Google Scholar]
- Simon J., Leroith D.. 1986. Insulin receptors of chicken liver and brain. Characterization of alpha and beta subunit properties Eur. J. Biochem. 158:125–132. [DOI] [PubMed] [Google Scholar]
- Simon J., Milenkovic D., Godet E., Cabau C., Collin A., Metayer-Coustard S., Rideau N., Tesseraud S., Derouet M., Crochet S., Cailleau-Audouin E., Hennequet-Antier C., Gespach C., Porter T. E., Duclos M. J., Dupont J., Cogburn L. A.. 2012. Insulin immuno-neutralization in fed chickens: effects on liver and muscle transcriptome. Physiol. Genomics. 44:283–292. [DOI] [PubMed] [Google Scholar]
- Stolarczyk E., Guissard C., Michau A., Even P. C., Grosfeld A., Serradas P., Lorsignol A., Penicaud L., Brot-Laroche E., Leturque A., Le Gall M.. 2010. Detection of extracellular glucose by GLUT2 contributes to hypothalamic control of food intake. Am. J. Physiol. Endocrinol. Metab. 298:E1078–1087. [DOI] [PubMed] [Google Scholar]
- Tachibana T., Saito E. S., Takahashi H., Saito S., Tomonaga S., Boswell T., Furuse M.. 2004. Anorexigenic effects of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide in the chick brain are mediated by corticotrophin-releasing factor. Regul. Pept. 120:99–105. [DOI] [PubMed] [Google Scholar]
- Tachibana T., Sato M., Oikawa D., Furuse M.. 2006. Involvement of CRF on the anorexic effect of GLP-1 in layer chicks. Comp Biochem. Physiol. A Mol .Integr. Physiol. 143:112–117. [DOI] [PubMed] [Google Scholar]
- Wang X., Day J. R., Vasilatos-Younken R.. 2001. The distribution of neuropeptide Y gene expression in the chicken brain. Mol. Cell Endocrinol. 174:129–136. [DOI] [PubMed] [Google Scholar]
- Yang X. J., Kow L. M., Funabashi T., Mobbs C. V.. 1999. Hypothalamic glucose sensor: similarities to and differences from pancreatic beta-cell mechanisms. Diabetes. 48:1763–1772. [DOI] [PubMed] [Google Scholar]
- Zhang W., Kim S., Settlage R., McMahon W., Sumners L. H., Siegel P. B., Dorshorst B. J., Cline M. A., Gilbert E. R.. 2015. Hypothalamic differences in expression of genes involved in monoamine synthesis and signaling pathways after insulin injection in chickens from lines selected for high and low body weight. Neurogenetics. 16:133–144. [DOI] [PubMed] [Google Scholar]
- Zhou W., Murakami M., Hasegawa S., Yoshizawa F., Sugahara K.. 2005. Neuropeptide Y content in the hypothalamic paraventricular nucleus responds to fasting and refeeding in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141:146–152. [DOI] [PubMed] [Google Scholar]