Abstract
BACKGROUND
Obesity is caused by an imbalance between energy intake, i.e. eating and energy expenditure (EE). Severe obesity is more prevalent in women than men worldwide, and obesity pathophysiology and the resultant obesity-related disease risks differ in women and men. The underlying mechanisms are largely unknown. Pre-clinical and clinical research indicate that ovarian hormones may play a major role.
OBJECTIVE AND RATIONALE
We systematically reviewed the clinical and pre-clinical literature on the effects of ovarian hormones on the physiology of adipose tissue (AT) and the regulation of AT mass by energy intake and EE.
SEARCH METHODS
Articles in English indexed in PubMed through January 2016 were searched using keywords related to: (i) reproductive hormones, (ii) weight regulation and (iii) central nervous system. We sought to identify emerging research foci with clinical translational potential rather than to provide a comprehensive review.
OUTCOMES
We find that estrogens play a leading role in the causes and consequences of female obesity. With respect to adiposity, estrogens synergize with AT genes to increase gluteofemoral subcutaneous AT mass and decrease central AT mass in reproductive-age women, which leads to protective cardiometabolic effects. Loss of estrogens after menopause, independent of aging, increases total AT mass and decreases lean body mass, so that there is little net effect on body weight. Menopause also partially reverses women's protective AT distribution. These effects can be counteracted by estrogen treatment. With respect to eating, increasing estrogen levels progressively decrease eating during the follicular and peri-ovulatory phases of the menstrual cycle. Progestin levels are associated with eating during the luteal phase, but there does not appear to be a causal relationship. Progestins may increase binge eating and eating stimulated by negative emotional states during the luteal phase. Pre-clinical research indicates that one mechanism for the pre-ovulatory decrease in eating is a central action of estrogens to increase the satiating potency of the gastrointestinal hormone cholecystokinin. Another mechanism involves a decrease in the preference for sweet foods during the follicular phase. Genetic defects in brain α-melanocycte-stimulating hormone–melanocortin receptor (melanocortin 4 receptor, MC4R) signaling lead to a syndrome of overeating and obesity that is particularly pronounced in women and in female animals. The syndrome appears around puberty in mice with genetic deletions of MC4R, suggesting a role of ovarian hormones. Emerging functional brain-imaging data indicates that fluctuations in ovarian hormones affect eating by influencing striatal dopaminergic processing of flavor hedonics and lateral prefrontal cortex processing of cognitive inhibitory controls of eating. There is a dearth of research on the neuroendocrine control of eating after menopause. There is also comparatively little research on the effects of ovarian hormones on EE, although changes in ovarian hormone levels during the menstrual cycle do affect resting EE.
WIDER IMPLICATIONS
The markedly greater obesity burden in women makes understanding the diverse effects of ovarian hormones on eating, EE and body adiposity urgent research challenges. A variety of research modalities can be used to investigate these effects in women, and most of the mechanisms reviewed are accessible in animal models. Therefore, human and translational research on the roles of ovarian hormones in women's obesity and its causes should be intensified to gain further mechanistic insights that may ultimately be translated into novel anti-obesity therapies and thereby improve women's health.
Keywords: obesity, ovarian hormones, women, adipose tissue, weight regulation, central nervous system, estrogens, progestins, eating, energy expenditure
Introduction
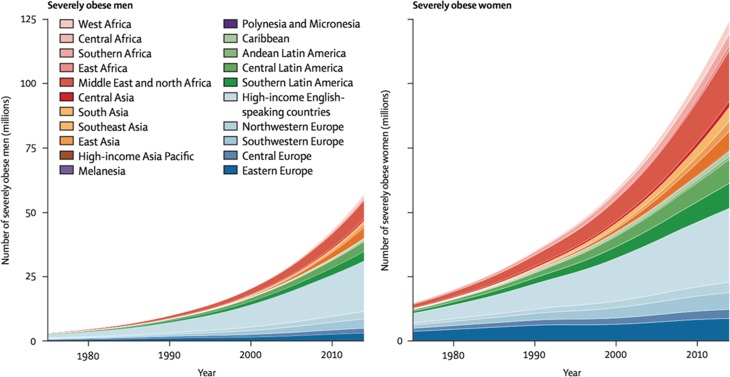
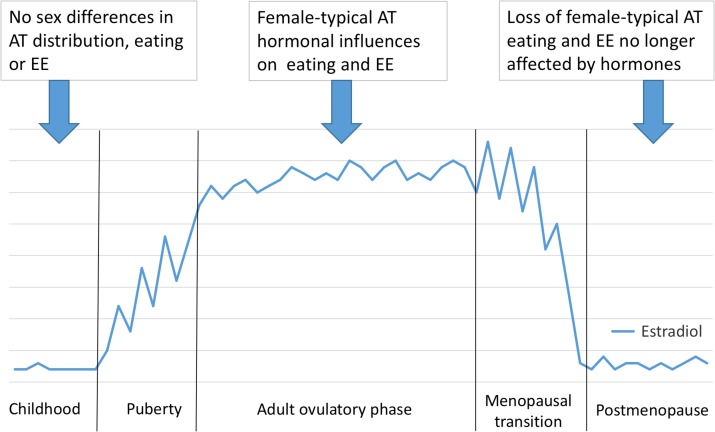
That obesity is pandemic is well known. (Obesity is typically defined as BMI [weight in kg/height in m2] ≥30.) In contrast, the importance of gender as a variable in obesity is less well appreciated. In fact, worldwide, about twice as many women as men suffer from severe obesity (i.e. grades 2 and 3 obesity, BMI ≥35 and 40 kg/m2, respectively) (Fig. 1). Although social factors, especially gender inequality, certainly contribute to male–female differences in obesity prevalence (Kautzky-Willer et al., 2016), the consistent worldwide disparity in prevalence of severe obesity strongly suggests that biological factors, i.e. physiological sex differences, also contribute. In support of this, pre-clinical and clinical studies have revealed women-specific factors in the two physiological determinants of obesity, the level of energy intake, which is to say eating, and the level of energy expenditure (EE). Furthermore, this work indicates that ovarian hormones, in particular estrogens, influence both eating and EE in women. Finally, data also implicate ovarian hormones in the metabolic function of adipose tissue (AT). In light of these considerations, the goal of this review is to provide a critical update on the roles of ovarian hormones on the principle components of obesity, i.e. eating and EE, and on AT physiology.
Figure 1.
Sex differences in prevalences of severe obesity (BMI ≥35 kg/m2) in representative population samples of adults aged ≥18 years in 200 countries worldwide. From NCD Risk Factor Collaboration, represented by Ezzati (2016), used with permission.
Methods
Articles in English indexed in PubMed through January 2016 were searched using the following keywords related to: (i) ‘reproductive hormones’: estrogen, estradiol, estrone, estriol, estrogen receptors 1 and 2 (ESR1 and ESR2 in humans, Esr1 and Esr2 in mice and rats; formerly known as ERα and ERβ, respectively), progesterone, progesterone receptor, androgen and androgen receptors, (ii) ‘weight regulation’: food intake, AT, adiposity, adipocyte, hunger, flavor hedonics, satiation, satiety, EE, physical activity, resting energy expenditure (REE), diet-induced EE, insulin, leptin, inflammation, ghrelin, cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1) and peptide YY(3-36) and (iii) ‘central nervous system (CNS)’: neuropeptide Y (NPY), α-melanocycte-stimulating hormone (α-MSH), dopamine, serotonin, melanocortin 3 or 4 receptor (MC3R, MC4R), agouti-related protein (AgRP), nucleus of the solitary tract (NTS), striatum, putamen, nucleus accumbens, caudate nucleus, frontal cortex and functional MRI (fMRI). Searches were of the form: body weight OR obesity AND (a ‘reproductive hormone’ set element) AND (a ‘weight regulation’ or ‘CNS’ set element), where capital letters indicate Boolean connectors. We sought to identify emerging research foci with clinical translational potential rather than to provide a comprehensive review.
Adipose tissue physiology
Measurement of body adiposity
Anthropomorphic adiposity measures, such as BMI, waist circumference (WC) and waist–hip ratio (WHR) are simple and inexpensive, but relatively imprecise measures of body adiposity. Errors are introduced by sex, age, ethnicity and individual differences in body composition, such as muscle mass. For example, in one study, BMI misclassified the adiposity of one-third of young adult female athletes (Ode et al., 2007). Furthermore, BMI systematically over-estimates the adiposity of shorter people and under-estimates the adiposity of taller people. Although both BMI and WC are better predictors of total-visceral AT (defined below) than WHR (Pouliot et al., 1994; Kamel et al., 2000; Koren et al., 2013), in women, WC and WHR predict cardiovascular disease better than BMI (Goh et al., 2014). With respect to clinical practice, however, an expert panel (Klein et al., 2007) concluded that additional anthropomorphic measures are unlikely to affect clinical management if BMI and standard cardiometabolic risk factors are considered (Kiernan and Winkleby, 2000).
Numerous other methods more accurately detect whole-body adiposity. Some, including air displacement plethysmography and hydrostatic weighing (Silver et al., 2010; Moon et al., 2011; Fields et al., 2015), are limited in their ability to measure regional AT differences. Dual-energy X-ray absorptiometry (DEXA) can detect regional fat and bone-mineral density and with the aid of a special algorithm can distinguish intra-abdominal and subcutaneous fat (Kaul et al., 2012), but DEXA does not measure AT volume or distinguish water and other lean-tissue mass (Santen et al., 2010). MRI and computed tomography (CT) are the best available methods to measure AT volume and regional distribution in vivo. Whole-body imaging (i.e. axial images every few cm) is most accurate. Single-level, cross-sectional images are more common, but which abdominal level best estimates central subcutaneous and total-visceral AT is controversial (Kuk et al., 2006; Shen et al., 2012).
Histological or plasma assays may soon provide suitably accurate and clinically practical measures of specific AT depots. For example, Lê et al. (2011) found that the degree of macrophage infiltration in subcutaneous AT biopsies predicted total-visceral AT volume, intrahepatic lipid content and several plasma markers of cardiometabolic-disease risk. Therefore, such methods may provide safe and cost effective ways to measure AT compartments relevant for health in the future.
General aspects of AT
Energy is stored for long periods as intracellular droplets of triacylglycerol within adipocytes, which are mostly organized in discrete AT depots (Rosen and Spiegelman, 2014). Preadipocytes are pluripotent cells that can differentiate into white or brown adipocytes, macrophages, or muscle and bone progenitors (Tchkonia et al., 2013). The differentiation of adipocytes from preadipocytes is controlled by insulin-like growth factor, glucocorticoids, and other growth factors and hormones (Cristancho and Lazar, 2011; Tang and Lane, 2012). Although the roles of gonadal hormones in this process remain obscure, the important role of estrogens is indicated by the effect of estrogenic endocrine disruptors, such as bisphenol, which disrupt normal pre-adipocyte differentiation in vitro and by their association with obesity (Vom Saal et al., 2012; Boucher et al., 2014; Ohlstein et al., 2014).
The traditional view was that AT is a passive storage depot. AT energy storage is indeed long term; the half-life of individual triacylglycerol molecules in the subcutaneous AT of healthy humans is 1.6 years, which is orders of magnitude more than that of any other energy metabolite (Arner et al., 2011). Nevertheless, contemporary research clearly indicates, first, that AT is a dynamic organ that contributes to metabolic homeostasis in multiple ways, and, second, that in obesity, AT becomes dysfunctional and contributes to whole-body pathophysiology and health risk. These pathophysiological processes are mediated both by free fatty acids (FFA) and other metabolites released by AT and by signaling molecules secreted by adipocytes, called adipokines, which have local (paracrine) and endocrine signaling functions (Lee et al., 2013; Tchkonia et al., 2013). Additional signaling molecules are secreted by AT endothelial cells and AT-resident immune cells.
A rough categorization of human AT includes five major depots (Shen et al., 2003). (i) Subcutaneous AT can be found from the head to foot in obese individuals. Subcutaneous AT includes superficial and deep sections separated by a fascial plane (Scarpa's fascia) in the lower trunk and gluteofemoral areas. As described below, the different components of subcutaneous AT are separately regulated. (ii) Visceral or intraperitoneal AT includes the omental (attached to the stomach), mesenteric (attached to the small intestine) and epiploic (attached to the large intestine) depots. These depots contain numerous lymph nodes and are especially prone to infiltration by macrophages during hypertrophy (Gabrielsson et al., 2003; Lee et al., 2013; Tchkonia et al., 2013). In addition, mesenteric AT is exposed to absorbed lipids as they drain through the lymphatics. Finally, the vasculature of visceral AT drains into the hepatic-portal vein, exposing the liver to increased levels of the FFA, adipokines and immune mediators that it releases. (iii) Retroperitoneal and pelvic AT are separate depots, but are often categorized together with visceral AT because they cannot be easily distinguished with imaging; we refer to this sum as ‘total-visceral AT’. (iv) Intra- and extra-pericardial AT are found around the heart and great vessels. (v) Intramuscular AT lies between the muscle fascicles. Subcutaneous and visceral AT have received the most research attention and are the focus of this review. Visceral fat is associated with impaired health, while other AT compartments contribute to a lower degree or may even have a protective effect (Després and Lemieux, 2006).
Vague (1947) first noted that, compared with men, women tend to have relatively more gluteofemoral AT and relatively less centrally located AT, i.e. abdominal subcutaneous and total-visceral AT. More recent studies have confirmed this notion and indicate that these effects are evident across a wide range of BMI (Gallagher et al., 2005). Nevertheless, there is substantial individual variation in obesity habitus. Indeed, some obese women have a male-typical, central AT distribution (Karpe and Pinnick, 2015).
Female-typical adiposity
Puberty and young adulthood
The typical female pattern of regional AT distribution emerges during puberty (Shen et al., 2009; Taylor et al., 2010). In healthy weight females, the greater absolute AT mass becomes obvious only after puberty (healthy weight is BMI 18.5–24.9 kg/m2). As a result, a normative young adult woman in the USA (height 1.6 m, BMI 22.5 kg/m2) has ~18 kg body fat (~30% of body weight), of which only ~5% is total-visceral AT (Gallagher et al., 1996; Shen et al., 2009; Camhi et al., 2011), whereas a normative male (height 1.8 m) has ~12 kg body fat (~15% body weight), of which ~11% is total-visceral AT.
Female-typical AT results from differences among the AT depots in the balance of uptake and release of FFA (Santosa and Jensen, 2015). FFA uptake and triacylglyceride synthesis are greater in women's glutoefemoral than abdominal subcutaneous AT, and lipolysis rates are lower, thus selectively increasing the relative size of the gluteofemoral depot. These processes are controlled by complex interactions of genes and ovarian hormones that are only beginning to be understood, for example, through recent studies of differential AT gene expression (Gesta et al., 2006; Fried et al., 2015; Friedl et al., 2015; Karpe and Pinnick, 2015). One study (Karastergiou et al., 2013) found that of 284 genes that were differentially expressed in gluteofemoral compared with abdominal subcutaneous AT in overweight (mean BMI, 27 kg/m2) adults, 159 were differentially expressed only in women. Furthermore, many of the genes were homeobox-family (HOX) genes, which are involved in cell differentiation. Thus, a parsimonious hypothesis is that increased levels of estrogens or other reproductive hormones during puberty differentially activate HOX and other genes to determine regional AT distribution and physiology.
Mouse models provide further evidence that abdominal and gluteofemoral subcutaneous adipocytes differentiate in a sex-dependent, cell-autonomous fashion (Fried et al., 2015). For example, Esr1 was necessary for establishing the identity of mouse white-adipocyte progenitor cells (Lapid et al., 2014), and female transgenic mice lacking Esr1 selectively in adipocytes became obese due to expansion of gonadal AT, whereas additional knockout of Esr2 had no effect (Davis et al., 2013). Although this result cannot be translated simply to humans because humans do not have gonadal AT depots, the similar gene-expression patterns of mouse gonadal AT and human omental AT (Gesta et al., 2006) encourages the view that estrogen-ESR1 signaling contributes similarly to the development of human visceral AT. Finally, when the testes-determining Sry gene was transplanted from the Y chromosome to an autosome in order to yield XX or XY mice with either male or female gonads, XX mice had twice as much AT as XY mice independent of gonadal status, indicating an important contribution of sex-linked genes to the sex difference in total body fat (Chen et al., 2012). Ongoing research is translating these kinds of data into human AT physiology (Karastergiou et al., 2013).
The search for genes mediating the high heritability of human obesity and regional AT distribution has not yet been successful. The failure of most studies to take into account the age of onset of obesity, in particular pubertal development, may contribute to this lack of success. A recent large (n = 224 459) genome-wide association study identified 49 single-nucleotide DNA polymorphisms (SNP) related to WHR phenotypes, with 19 expressed more in women (Shungin et al., 2015). Overall, most SNP were in or near genes expressed by adipocytes or related to insulin resistance, suggesting that their analysis might lead to a better understanding of regional fat distribution and related pathophysiology. None was apparently related to ESR1, ESR2, androgen receptor or synthesis of estrogens or androgens, which might be expected to be especially important in the development of obesity during puberty. Interestingly, however, SNPs associated with fat distribution had little overlap with those found in a similar analysis to be associated with BMI, which were predominately expressed in the brain (Locke et al., 2015). This suggests that different biological processes underlie the accumulation and distribution of excess body fat (Lee and Mattson, 2014; Fu et al., 2015). Moreover, epigenetic regulatory mechanisms, which are not revealed in SNP analyses, are also involved in the development of obesity (Dalgaard et al., 2016).
Other methods have revealed some genes apparently involved in the estrogenic regulation of total adiposity. A study of SNP rs7757956 in intron 4 of ESR1 indicated that an activational effect of estrogen signaling via ESR1 influences the development of obesity in pubertal girls (Tobias et al., 2007). Girls, but not boys, on average 12 years old, who were in Tanner stages 3–5 and bore a TT genotype at rs7757956 had 9% more body fat, as measured by DEXA, than TA- or AA-genotype girls. They also had 18% more height-adjusted body fat than TT-genotype girls in Tanner stages 1–2. As the TT genotype is common (75%), this may be an important contributor to female obesity. The influence of this SNP at later ages remains to be investigated.
Studies of hormonal contraception have failed to reveal significant effects on body weight. Gallo et al. (2014) screened 734 English-language reports of effects of combination oral hormonal contraceptives or combination skin patches on body weight in healthy reproductive-age women and found 49 that met their quality criteria (inclusion of at least three cycles, inclusion of effect means and variabilities, etc.). These failed to show any consistent effect of combination contraceptive use or its discontinuation on body weight, although the amount and quality of data were not judged to be of the highest quality. For example, only four studies included placebo-treated or no-intervention groups. A similar review of the effects of progestin-only contraceptives on body weight identified only 15 studies, most of moderate to low quality (Lopez et al., 2013). Of these, 12 failed to detect weight changes, but in three studies there were weight gains of ~2 kg/y. Percent body AT was also increased in two studies in which body composition was measured. Thus, progestin-only contraception may slightly increase body weight and adiposity, but the available evidence does not establish this unambiguously.
Gonadotropin-releasing hormone (GnRH) agonists are often used to treat early or precocious puberty. Studies of the effect of GnRH-agonist treatment on weight gain are mixed, with some studies suggesting a small increase (Aguiar et al., 2006; Wolters et al., 2012), some showing a decrease (van der Sluis et al., 2002) and others indicating no effect (Głab et al., 2009; Ko et al., 2011). Interestingly, weight increase seems to occur only in girls who are healthy-weight when initiating treatment, but not in girls who are already overweight (Wolters et al., 2012). GnRH agonists are also used in the treatment of endometriosis, but this is rarely done for more than 6 months in adult women due to deleterious effects on bone metabolism. Unfortunately, to the best of our knowledge, none of the studies investigating the use of GnRH agonists in women with endometriosis have described effects on body weight.
Menopause and hormone therapy
Aging per se increases adiposity, which complicates the estimation of the effects of menopause. Age and menopause are best segregated with multiple regression or similar statistical analyses, and therefore we consider only such studies. Table I shows data from five such cross-sectional studies, four in which DEXA was used to estimate whole-body lean and fat masses (Ley et al., 1992; Svendsen et al., 1995; Panotopoulos et al., 1996; Trémollieres et al., 1996) and one in which whole-body MRI was used (Phillips et al., 2008). Strikingly, these studies indicate that in both healthy-weight and mildly obese women, menopause increases body fat by ~5% of body weight and decreases fat-free body mass by a slightly smaller amount. These opposing changes explain why menopause has no marked effect on body weight or BMI in most studies. The obvious clinical implication of these data is that women should be advised to lose several kilograms of body fat during the menopausal transition in order to maintain cardiometabolic health.
Table I.
Menopause-associated increases in adiposity, measured with DEXA or CT and dissociated from the effect of aging with multiple-regression analysis.
| Study | Lean mass (kg) | Fat mass (kg) | % Fat | |||
|---|---|---|---|---|---|---|
| Menopausal status: | Pre- | Post- | Pre- | Post- | Pre- | Post- |
| Panotopoulos et al. (1996) | 43 | 41* | 30 | 31* | ~41 | ~44* |
| Ley et al. (1992) | 38 ± 3 | 38 ± 3 | 19 ± 6 | 23 ± 5* | 32 | 36* |
| Trémollieres et al. (1996) | 36 ± 3 | 36 ± 3a | 18 ± 5 | 18 ± 5a | 32 | 32a |
| 35 ± 3b* | 20 ± 5b* | 35b* | ||||
| Phillips et al. (2008) | 41 | 37 | 22 ± 1 | 32 ± 2* | ~35 | ~46 |
| Svendsen et al. (1995) | Δ = −4.0* | Δ = 3.1* | Δ = 5.4 ± 1.6* | |||
Data are means ± SEM; lean mass does not include bone; ~ indicates estimated from data given. Subject characteristics (mean ± SD) were: Panotopoulos et al. (1996): French women, 26 premenopausal, aged 43 ± 4 y, BMI 31 ± 3 kg/m2, and 73 postmenopausal, aged 54 ± 4 y, BMI 31 ± 4 kg/m2; data are sums of arms, trunk and legs, not whole body; variabilities of sums not given; Ley et al. (1992): British women, 61 premenopausal, aged 32 ± 6 y, BMI 22 ± 2 kg/m2, and 70 postmenopausal, aged 53 ± 5 y, BMI 24 ± 2 kg/m2; (Trémollieres et al., 1996): French women, 68 premenopausal, aged 49 ± 3 y, BMI 22 ± 2 kg/m2; (Phillips et al., 2008): US American women, 58 premenopausal, aged 39 ± 1 y, BMI 24 ± 1 kg/m2, and 20 postmenopausal, aged 61 ± 2 y, BMI 28 ± 1 kg/m2, not all variability or statistics reported; (Svendsen et al., 1995): Swedish women collated by age decade; overall n = 407, age range 18–80 y, mean BMI per decade, ~22–25 kg/m2; data are estimated menopause effect (Δ). *Significant menopause effect. DEXA, dual-energy X-ray absorptiometry; CT, computerized tomography.
aIn total, 100 younger postmenopausal, aged 54 ± 3 y, BMI 22 ± 2 kg/m2.
bIn total, 37 older postmenopausal, aged 64 ± 4 y, BMI 23 ± 2 kg/m2.
Menopause appears to preferentially increase total-visceral AT, although the magnitude of the effect is uncertain. In two studies (Kanaley et al., 2001; Franklin et al., 2009) in which several axial MRI scans were made between the head of the femur and the kidneys in white US American women, both abdominal subcutaneous and total-visceral AT increased after menopause, with a slightly greater relative increase in total-visceral AT. In another MRI study (Phillips et al., 2008), however, the relative increase in total-visceral AT was twice that of total AT. Several studies using DEXA confirmed a correlation between postmenopausal status and increased total-visceral AT (Svendsen et al., 1995; Trémollieres et al., 1996; Gambacciani et al., 1999; Lovejoy et al., 2008). Even in non-obese women, significant increases in total-visceral fat (Abdulnour et al., 2012) and percent body lipid (Ho et al., 2010) occurred during the menopausal transition. Moreover, in BMI-matched (~25 kg/m2) pre- and postmenopausal women, percent visceral AT was significantly lower in the premenopausal group, whereas no association of age and total-visceral AT was detected (Kanaley et al., 2001).
We are not aware of imaging studies on the effects of surgical menopause by ovariectomy on AT. Although results have to be interpreted with caution due to methodological limitations, such as lack of control for the indications for surgery or for hysterectomy versus ovariectomy, the available data support an association between surgical menopause and increased weight gain (Sowers et al., 1996; Matthews et al., 2001; Tom et al., 2012), in line with the importance of ovarian hormones for obesity.
In younger postmenopausal women (age 50–59 y), estrogenic hormone therapy (HT) reduces fat mass, improves bone-mineral density, appears to preserve fat-free mass (FFM), reduces the risk of type-2 diabetes mellitus, retards atherosclerosis and reduces all-cause mortality (Santen et al., 2010; Manson et al., 2013; Manson and Kaunitz, 2016). At least in the case of atherosclerosis, benefits occur only if estrogenic HT is begun within less than ~6 y of menopause (Hodis et al., 2016). In addition, estradiol treatment, but not progestin or testosterone treatment, was shown to lower plasma very low-density lipoptrotein-triglyceride concentrations by ~30% in healthy postmenopausal women by increasing their plasma clearance (Smith et al., 2014).
Santen and colleagues reviewed 13 placebo-controlled DEXA or CT studies in which unopposed estrogen HT or estrogen plus progestin HT reduced body fat. HT with progestins alone increased adiposity (Clark et al., 2005; Dal'Ava et al., 2014), indicating that the effects of HT on adiposity loss are purely estrogenic. Santen et al. (2010) also reviewed eight studies in which estrogenic HT reduced the tendency of postmenopausal women to develop more central obesity, versus two in which this was not the case, consistent with the data reviewed above indicating that estrogens contribute to the determination of regional adiposity distribution.
That observation that estrogen levels are higher in non-obese than obese premenopausal women also supports a role of estrogens in restraining AT. Age-corrected early-follicular estradiol levels were 40 pg/ml in healthy-weight women versus 33 pg/ml in obese women, with no overlap in 95% confidence intervals (95% CI) (Freeman et al., 2010). In contrast, after menopause estradiol levels were lower overall, although higher in obese than in non-obese women (21 vs 12 pg/ml, no overlap in 95% CI). After menopause, estradiol originates mainly in the AT and appears to have no endocrine function.
It is important to emphasize that as a result of the opposite effects of HT on adiposity and FFM, HT leads to little or no change in total body weight. According to a Cochrane meta-analysis (Norman et al., 2000), unopposed estrogen treatment had no significant effect on total weight (nine randomized controlled trials [RCTs], mean difference vs no HT, 0.0 kg, 95% CI: −0.6 to 0.7 kg) or BMI (two RCTs, mean difference −0.1 kg, 95% CI: −0.4 to 0.1 kg); similarly, estrogen plus progestin treatment had no significant effect on weight (10 RCTs, mean difference, 0.0 kg, 95% CI: −0.4 to 0.5 kg) or BMI (10 RCTs, mean difference −0.1 kg, 95% CI: −0.3 to 0.1 kg). Thus, the fear of some women that estrogenic HT leads to weight gain (Légaré et al., 2000) is unfounded. Rather, the likelihood of beneficial changes in body composition and metabolic health with estrogenic HT should be considered by women deciding on postmenopausal HT.
Obesity pathophysiology
Simple obesity
Obesity causes progressive cardiometabolic dysfunction (Lumeng et al., 2007; Mauvais-Jarvis et al., 2013; Tchkonia et al., 2013). Expanded AT depots release more FFA, which increase insulin secretion, decrease insulin sensitivity and increase hepatic production of very low-density lipoproteins. In addition, hypertrophic adipocytes attract macrophages into the AT, which induces a sterile inflammation-like state characterized by secretion of numerous proinflammatory cytokines and adipokines. Finally, AT vasculature often fails to expand sufficiently in obesity, leading to local hypoxia that exacerbates the inflammatory state (Pasarica et al., 2009; Sun et al., 2013).
These processes are influenced importantly by ovarian hormones. For example, in rats, ovariectomy increases immune-cell infiltration into the AT and increases insulin resistance even if adiposity is controlled (Rogers et al., 2009; Vieira Potter et al., 2012). Increases in circulating adipokines and immune factors in postmenopausal women suggest similar effects contribute to increased risk of cardiometabolic disease in these women (Pfeilschifter et al., 2002; Polotsky and Polotsky, 2010). Conversely, in younger postmenopausal women, HT reduces or delays these pathological processes (Santen et al., 2010; Manson et al., 2013; Manson and Kaunitz, 2016). Thus, studies of the roles of estrogens are an emerging theme in obesity-related cardiometabolic disease (DeClercq et al., 2008; Monteiro et al., 2014; Blenck et al., 2016).
An additional potential mechanism through which estrogens improve AT function is related to the ‘browning’ of white adipocytes (Palmer and Clegg, 2015), i.e. increased expression of uncoupling protein-1 (UCP-1), which generates heat without synthesizing ATP, thus increasing EE, and reduces cardiometabolic risk (Rosen and Spiegelman, 2014). Estrogens may induce browning in two ways. First, estrogens act in the heart to increase the secretion of cardiac natriuretic peptides (Jankowski et al., 2001; Wang et al., 2002), which in turn act in the AT to increase browning (Collins, 2014). Second, estrogens may increase hypothalamic expression of brain-derived neurotrophic factor, which increases sympathetic outflow to the AT and increases browning (Cao et al., 2011; Palmer and Clegg, 2015).
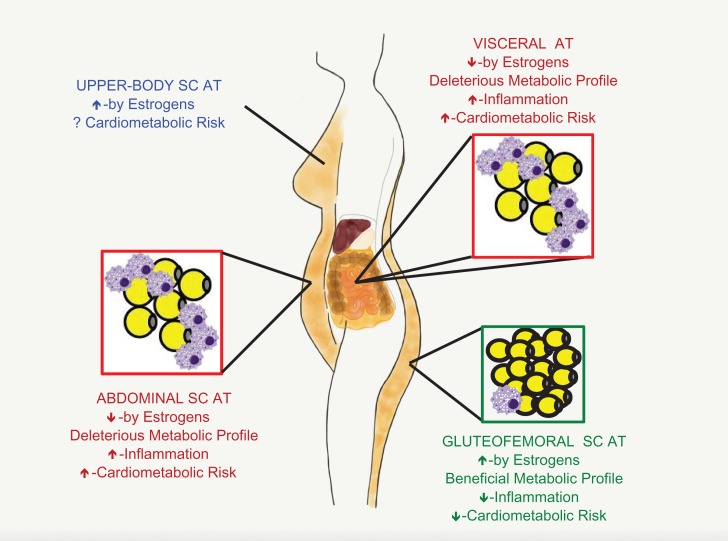
Different AT depots are differentially predisposed to obesity-related pathophysiology (Lee et al., 2013; Tchkonia et al., 2013; Fried et al., 2015; Karpe and Pinnick, 2015) (Fig. 2). Centrally located AT, especially visceral AT, brings the greatest health risk (Wang et al., 2005; Després and Lemieux, 2006; Pischon et al., 2008). A variety of data indicate that this is related to the direct delivery of FFA and proinflammatory immune mediators to the liver (Bergman et al., 2006; Rytka et al., 2011; Item and Konrad, 2012). Indeed, omental AT resection added further metabolic benefits to Roux-en-Y gastric bypass surgery (Dillard et al., 2013). Mesenteric AT appears to be even more toxic, as in comparison to subcutaneous or omental AT, it is more densely innervated by sympathetic efferents, expresses more glucocorticoid receptors and is more prone to macrophage migration (Tchkonia et al., 2013). Abdominal subcutaneous AT also has adverse metabolic characteristics (Tchkonia et al., 2013), especially deep abdominal subcutaneous AT (Smith et al., 2001; Koster et al., 2010).
Figure 2.
Female-typical adiposity and associated cardiometabolic risk. Following puberty, girls and women (F) tend to deposit excess triacylglycerol as gluteofemoral SC AT, whereas males (M) tend to deposit it as abdominal SC AT; males also have more visceral AT, i.e. AT whose vasculature enters the portal vein and liver. Gluteofemoral AT is cardiometabolically benign (green) because it traps triacylglycerol, so contributes little to plasma lipid levels, and because it is resistant to inflammation. In contrast, abdominal SC AT and visceral AT are cardiometabolically toxic (red) because they have higher relative rates of lipolysis, which increases plasma lipids, and because they are prone to inflammation, which leads to increased levels of circulating proinflammatory molecules. The relative amounts of upper-body SC AT are variable, and its cardiometabolic consequences are poorly understood (blue). For further explanation, see text. SC AT, subcutaneous adipose tissue.
In contrast to all other AT depots, superficial gluteofemoral subcutaneous AT actually reduces cardiometabolic-disease risk (Snijder et al., 2004; Yusuf et al., 2005; Koster et al., 2010; Lee et al., 2013). Indeed, women with marked gluteofemoral obesity often remain metabolically healthy (Karpe and Pinnick, 2015). Many factors appear to contribute to the development of larger gluteofemoral subcutaneous AT depots in women and to this depot's protective nature (Santosa et al., 2008; Lee et al., 2013; Tchkonia et al., 2013; Karpe and Pinnick, 2015). Gluteofemoral subcutaneous AT releases more of the insulin-sensitizing adipokine palmitoleate than abdominal subcutaneous AT (Pinnick et al., 2012). Adipocytes in gluteofemoral subcutaneous AT also have greater lipoprotein-lipase (LPL) activity in women than men, indicating that they more effectively clear triacylglyceride after meals (Votruba and Jensen, 2007). Moreover, uptake of plasma FFA, lipogenesis and triacylglycerol re-esterification is greater in women's gluteofemoral subcutaneous AT than in their abdominal subcutaneous AT, whereas the opposite is true in men (Koutsari et al., 2011; Søndergaard et al., 2012). Together, these characteristics of women's gluteofemoral subcutaneous AT contribute to the greater size of this depot, the greater stability of stored triacylglycerol, and the lower plasma FFA levels.
Another factor relevant to sex differences in AT function is the greater proliferative potential of preadipocytes in subcutaneous versus visceral AT. For example, Tchoukalova et al. (2010) overfed healthy-weight men and women (mean BMI, 22.1 kg/m2) for 8 weeks, leading to a gain of 3.8 kg fat mass and an increase in mean BMI to 23.6 kg/m2. Strikingly, women displayed greater AT hyperplasia, so that their average adipocyte size decreased with weight gain. This was most evident in femoral fat samples and was associated with relatively greater expansion of gluteofemoral than central AT. In addition, women with larger baseline abdominal subcutaneous adipocytes also displayed marked hyperplasia in that depot. This suggests that as AT expands in obesity, the larger number of gluteofemoral adipocytes in women permits them to store more triacylglycerol before reaching dysfunctional degrees of hypertrophy (Tchkonia et al., 2013). Unfortunately, the specific effects of ovarian hormones on AT cell biology have not yet been intensively researched. This is a major challenge facing women's health research.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, chronic anovulation and polycystic ovaries (Dumesic et al., 2015; McCartney and Marshall, 2016). One of the primary pathological changes thought to lead to PCOS is increased ovarian androgen secretion. Indeed, increased androgen production occurs even in theca cells cultured from women with PCOS (Nelson et al., 1999). This pathology may be caused in some women by polymorphisms of DENND1A, which encodes a protein affecting the placement of cell-surface receptors (McAllister et al., 2014).
Although the prevalence of PCOS is similar in healthy-weight, overweight and obese women (Yildiz et al., 2008), about 40–50% of PCOS patients are obese (Carmina et al., 2009; Teede et al., 2010). Some studies suggest that PCOS is associated with greater abdominal obesity (Carmina et al., 2007), but this is controversial (Barber et al., 2008). Although obesity pathophysiology related to PCOS is poorly understood, several results support key roles of increased estrogen and androgen secretion. Androgens can lead to dyslipidemia indirectly by exacerbating insulin resistance, leading to altered lipid metabolism and body composition, and directly, through effects in the AT (Diamanti-Kandarakis, 2007). Whether AT dysfunction is primary or secondary to hyperandrogenism or other abnormalities in PCOS, however, remains unknown (Villa and Pratley, 2011). Women with PCOS have lower LPL expression in subcutaneous AT than healthy women (Mannerås-Holm et al., 2014). Both androgens and estrogens inhibit AT LPL activity (Pedersen et al., 2004; Blouin et al., 2010), and even after controlling for BMI, expression of LPL in the subcutaneous AT correlated negatively with plasma estradiol (Mannerås-Holm et al., 2014). Excessive visceral-fat distribution (Diamanti-Kandarakis, 2007; Escobar-Morreale and San Millán, 2007) and disturbed adipokine release seem to influence PCOS development (Torres-Leal et al., 2010; Villa and Pratley, 2011). For example, in women with PCOS, leptin expression was reduced in the subcutaneous AT, adiponectin expression was reduced in both subcutaneous and omental AT and adiponectin receptor-2 expression was reduced in subcutaneous AT (Carmina et al., 2008; Mannerås-Holm et al., 2014). These and other adipokines may modulate the hypothalamic-pituitary-gonadal axis through receptors in pituitary FSH, LH and TSH cells (Sone et al., 2001; Taheri et al., 2002; Psilopanagioti et al., 2009) so as to increase ovarian hormone secretion in patients with PCOS (Olszanecka-Glinianowicz et al., 2011, 2013). In addition, some adipokines may directly influence ovarian steroidogenesis (Tersigni et al., 2011). Consistent with a link between decreased adipokines and increased ovarian hormone secretion, plasma estradiol levels were found to be negatively correlated with adiponectin receptor-1 in the subcutaneous AT of women with PCOS independent of BMI; testosterone levels, however, were not significantly correlated with adiponectin receptor-1 (Mannerås-Holm et al., 2014). Interestingly, although AT inflammation was not different in women with PCOS and BMI-matched control women (Lindholm et al., 2011), adiponectin release in response to the proinflammatory cytokine tumor necrosis factor-α was decreased in adipocytes obtained from overweight or obese women with PCOS (Chazenbalk et al., 2010).
Eating
General aspects of the control of eating
The intake of metabolic energy occurs mainly in the form of meals (including snacks). Meals and the surrounding affective and cognitive processes are the products of a widely distributed information-processing network in the brain, described below, that produces conscious and unconscious responses that underlie planning to obtain food, motivational urges to eat, eating per se, the pleasurable, or hedonic, aspects of eating, satiation and the effects of nutrient repletion on learning.
The primary internal stimuli controlling eating include the subjective value assigned to food and neural and endocrine feedbacks from the gastrointestinal (GI) tract, from AT, and from metabolic processes. ‘Food value’ in this context refers to reinforcement, i.e. the ability of food stimuli to support learning, to generate approach behavior and to elicit emotional responses, including flavor hedonics (Schultz, 2015). Beyond these, there are several secondary or modulatory internal factors, ranging from circadian rhythm to psychological factors and motivators such as stress, emotional state, cognitive control, etc. As reviewed below, reproductive hormones importantly modulate these physiological controls of eating (Asarian and Geary, 2013; Mauvais-Jarvis et al., 2013; López and Tena-Sempere, 2015). External stimuli, in particular stimuli with learned cognitive and affective meanings, also play important modulatory roles in eating (French et al., 2012; Higgs et al., 2012). There is a dearth of research on the influence of reproductive hormones on these controls of eating. This is unfortunate because, for example, estrogens and their lack after menopause can influence affective (Joffe et al., 2011; Schmidt et al., 2015) and cognitive (Hara et al., 2015) functions in women.
Roles of ovarian hormones
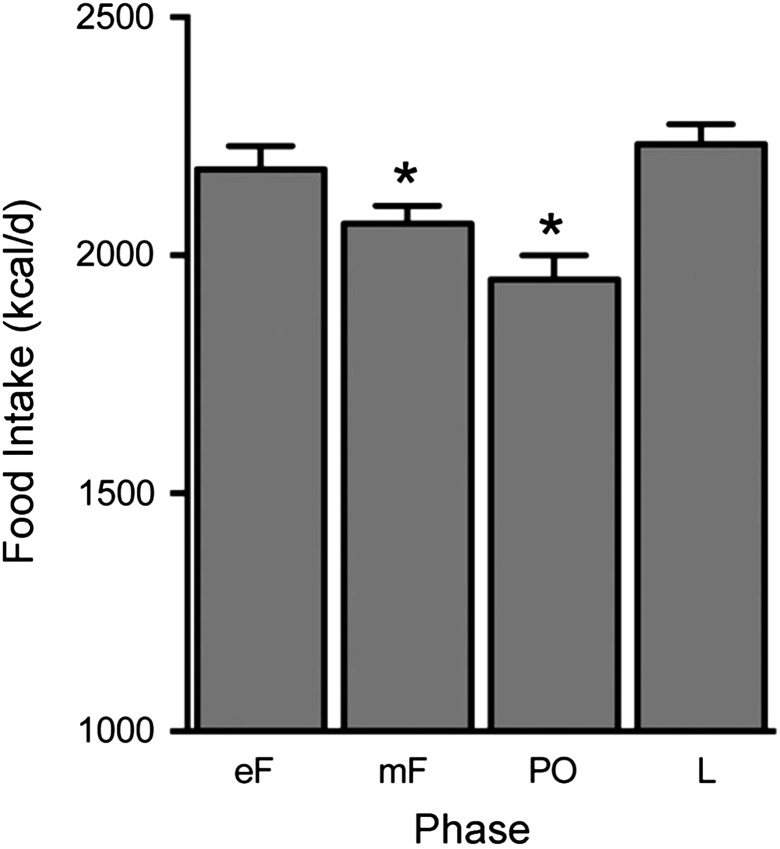
The best-established effect of ovarian hormones on eating is the progressive decrease in eating during the follicular phase of the menstrual cycle. In healthy-weight cycling women, food intake decreases ~200–300 kcal/d from the luteal maximum to the peri-ovulatory minimum (Fig. 3), an amount relevant to body-weight regulation (Hall et al., 2011; Asarian and Geary, 2013). Available data indicate that eating does not decrease during anovulatory cycles (Barr et al., 1995; Rock et al., 1996), and that the decrease in ovulatory cycles is due to decreased meal size rather than decreased meal frequency (Pohle-Krauza et al., 2008; Brennan et al., 2009) and is related to a decrease in the intake of sweet foods (Bowen and Grunberg, 1990; Fong and Kretsch, 1993). Old-world monkeys and apes (Parvorder Catarrhini), which have ovarian cycles similar to those of women (Zeleznik and Pohl, 2006), as well as mice, rats and many other species, display comparable cyclic decreases in meal size and food intake. Loss of estrogens after ovariectomy increases meal size, food intake and body weight in old-world monkeys, rats and mice (Bellino and Wise, 2003; Sullivan et al., 2005; Asarian and Geary, 2013), suggesting that increased eating contributes to the increase in AT after menopause in women, but whether this is true is unknown.
Figure 3.
Daily food intake during the ovarian cycle in women. Note the progressive decrease in energy intake during the follicular phase, the nadir around ovulation and the high level during most of the luteal phase. Data are amounts eaten per day (kcal, means and standard errors of the mean) calculated from three studies in which food intake was measured by weighing and the cycle phase was monitored with urinary luteinizing hormone tests and reports of menses in a total of 34 women. In each study, data were averaged across the early-follicular (eF; 4 d), midfollicular (mF; ~9 d), peri-ovulatory (PO; 4 d) and luteal (L; ~11 d) phases. *Significantly different from luteal phase. Original data are from Fong and Kretsch (1993), Gong et al. (1989) and Lyons et al. (1989), and the figure is reprinted from Asarian and Geary (2013); used with permission.
Experiments in rats and mice indicate that estrogens signaling via Esr1 mediate the cyclic change in eating in those species. This cyclic effect appears to correspond to the decrease in eating during the human follicular and peri-ovulatory phases (Asarian and Geary, 2013). Rats and mice do not have luteal phases, however, and the control of eating in this phase of the menstrual cycle is less well understood. Although it is reasonable to hypothesize that progestins oppose the inhibitory effects of estrogens during the luteal phase, data fail to support this. Physiological doses of estradiol were shown to inhibit eating in ovariectomizued rhesus macaques, and this was not affected by progesterone treatment (Czaja, 1978). Similarly, estrogen together with escalating progestin doses failed to affect eating in women (Eck et al., 1997), although the experiment was limited because the participants were cycling, so that the treatment effects may have been obscured by the effects of endogenous hormones, and because diet records, rather than measure of actual eating, were used. Yet in addition, depot medroxyprogesterone failed to affect food intake in an adequately powered, prospective, placebo-controlled study in cycling women (Pelkman et al., 2001).
Binge eating, referring to eating an abnormally large amount of food on a single occasion with a feeling of loss of control overeating, is a dysregulated form of eating especially prevalent in girls and women (Reichborn-Kjennerud et al., 2003; Hilbert et al., 2012). Binge eating is prodromal to bulimia nervosa, binge-eating disorder and obesity. It is also highly heritable (Davis, 2015). Female rats are much more prone than male rats to develop binge-like eating (Klump et al., 2013). Related to binge eating is emotional eating, i.e. eating in response to negative emotions, and this is also more prevalent in girls and women.
Binge eating develops most often during puberty, in association with higher estrogen levels (Klump et al., 2010). In addition, binge and emotional eating vary through the menstrual cycle, with lower rates in the late-luteal through the peri-ovulatory phases and higher rates during the mid-luteal phase (Culbert et al., 2016). A time-series analysis of binge tendencies and ovarian hormone levels through the cycle suggested that estrogens inhibit emotional and binge eating and progestins oppose this effect of estrogens, i.e. indirectly stimulate emotional and binge eating (Klump et al., 2014). This is the strongest indication that progestins are clinically important in dysregulated eating in human females. Estradiol, but not progesterone, was shown to reduce amounts eaten during binge-like episodes in rats (Yu et al., 2011), but whether this is a useful model of binge size or frequency in women is not known.
Peripheral mechanisms
Taste
Although the perception of sweet and creaminess differs in men and women (Bartoshuk et al., 1994; Hayes and Duffy, 2008) and women's intake of sweet food increases during the luteal phase (Bowen and Grunberg, 1990) and during pregnancy (Belzer et al., 2010), the roles of ovarian hormones in these phenomena have not been carefully investigated (Asarian and Geary, 2013).
GI signals
A variety of GI neural and endocrine signals are candidate meal-control mechanisms (Camilleri, 2015; Steinert et al., 2016). Unfortunately, research on their operation in women remains sparse (Asarian and Geary, 2013). Ghrelin, a hormone secreted by the stomach, stimulates meal initiation and increases meal size. In rats, there was an estradiol-dependent increase in ghrelin's eating-stimulatory effect during the first 2 days of the 4-day ovarian cycle (ovulation occurs on the last night), and ghrelin levels increased after ovariectomy in parallel to the increase in eating (Clegg et al., 2007). The small-intestinal hormone CCK reduces meal size by hastening meal termination, i.e. by producing satiation. CCK is the only endocrine meal-control signal that can be considered to have a proven normal or ‘physiological’ role in humans. That is, intravenous infusion of CCK in amounts that mimic prandial changes in plasma levels is sufficient to reduce meal size; CCK-receptor antagonists reduce the satiating potency of intraduodenal fat infusions and CCK-receptor antagonists administered alone increase meal size (Steinert et al., 2016). In rats, estrogens act via Esr1 in the caudal medial NTS (cmNTS) to increase CCK's satiating action (see below). Unfortunately, whether estrogens affect CCK satiation in women is unknown. The intestinal hormones GLP-1 and peptide YY(3-36) are also candidate eating-inhibitory signals, and estradiol increases the GLP-1 satiating action in ovariectomized rats (Asarian and Geary, 2013).
Leptin
Endocrine signals related to AT mass, notably leptin and insulin, contribute to the control of eating (Levin et al., 2011; Le Foll et al., 2014). Leptin is secreted by white adipocytes, and basal (fasting) plasma leptin levels are highly correlated with AT mass. Interestingly, for a given level of fat mass, leptin levels are higher in women than men, and this difference decreases after menopause. Estrogen levels and differences in intra-abdominal and subcutaneous AT distribution contribute to these effects (Rosenbaum and Leibel, 1999; Rosenbaum et al., 2001). Originally hypothesized to be the crucial link between body adiposity and the controls of eating and EE, leptin is now considered to defend only against reductions in body weight, not against weight increases (Ravussin et al., 2014). Whether estrogens interact with leptin to control eating are controversial. In ovariectomized rats, estradiol increased the eating-inhibitory potency of acute leptin injections (Clegg et al., 2003), but not that of chronic leptin treatment (Chen and Heiman, 2001). Furthermore, food intake was not correlated to plasma leptin levels in cycling women (Paolisso et al., 1999). None of these studies, however, was done in the weight-reduced state, when leptin's contribution to eating is greatest.
The brain mechanisms mediating potential interactive effects of leptin and estrogens on eating are also uncertain. In mice and rats, Esr1 is expressed in ~10% of neurons that express the signaling leptin receptor (Leprb) in the arcuate nucleus of the hypothalamus (ARC), the cmNTS and several other areas thought to mediate the control of eating (Asarian, unpublished data; Kim et al., 2016). Esr1/Leprb co-expression is much higher, ∼80%, in the preoptic area (Kim et al., 2016), one of the brain sites containing the GnRH neurons that control FSH and LH secretion (Herbison, 2006). Kim et al. (2016) reported that although food intake was increased in transgenic mice lacking Esr1 in Leprb neurons globally, estradiol treatment did not activate Leprb neurons in the ARC of ovariectomized mice, suggesting that estrogens and leptin do not affect ARC Esr1/Leprb neurons to inhibit eating. In contrast, Asarian (unpublished data) found that knockdown of Leprb neurons in the mNTS with RNA interference increased eating and completely prevented estradiol from inhibiting food intake in ovariectomized rats, suggesting that an Esr1/Leprb interaction in the mNTS is necessary for the normal control of eating.
Insulin
Basal plasma levels of insulin also correlate with body fat mass, and insulin may act in the brain to control eating and EE. Estrogens appear to regulate the effects of insulin on eating. Central administration of insulin was found to inhibit eating more in ovariectomized than in intact rats, and this was reversed by central estradiol administration (Clegg et al., 2006). These effects do not occur in peripubertal rats (Keen-Rhinehart et al., 2009), further suggesting an activational role of estradiol. Interestingly, female, but not male, transgenic mice with brain-specific null mutations of the insulin receptor are hyperphagic, which in females was related to profound reductions of LH and signs of defective ovarian follicle maturation, indicating a role for neural insulin receptors in the normal function of the hypothalamic-pituitary-gonadal axis (Brüning et al., 2000).
Intra-nasal insulin delivery has been used to selectively stimulate brain insulin receptors in humans. Similar to the rat studies, intra-nasal insulin before meals decreased eating more in men than in reproductive-age women (Benedict et al., 2008). In apparent contrast to the findings of Clegg et al. (2006) in rats described above, premeal intra-nasal insulin failed to inhibit eating in postmenopausal women (Krug et al., 2010). In contrast, intra-nasal insulin given after meals reduced subsequent intake of preferred snack foods in young women taking high-estrogen contraceptives, suggesting that insulin increases postprandial satiety in women (Hallschmid et al., 2012). These data suggest that central insulin-based pharmacotherapy might be an effective obesity treatment in women.
Central mechanisms
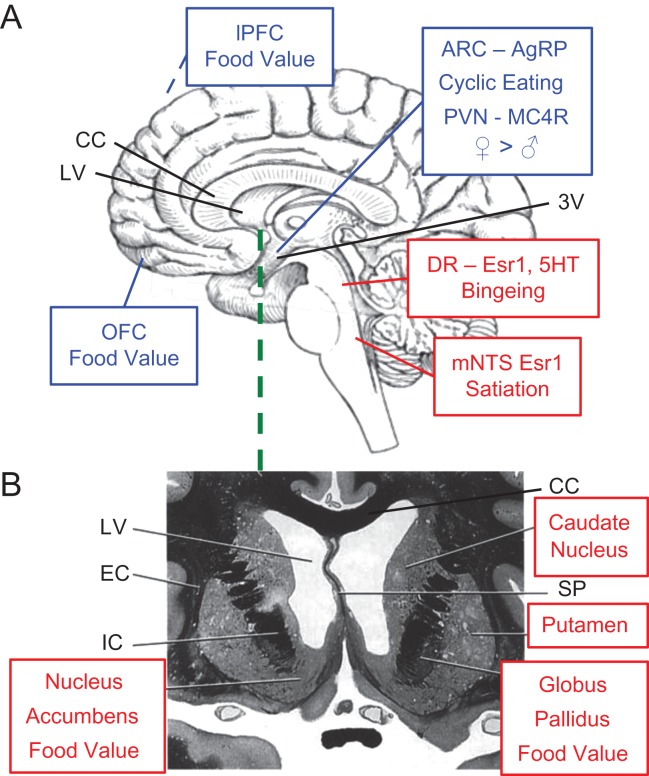
A widely distributed central neural network controls eating (Berthoud, 2002; Shin et al., 2009; Grill and Hayes, 2012; Castro and Berridge, 2014; Schultz, 2015; Val-Laillet et al., 2015; Farr et al., 2016; Geary and Moran, 2016). Local circuits in the caudal brainstem integrate a variety of sensory inputs, including gustatory, vagal afferent, spinal-visceral afferent and many GI-hormone signals, and also produce the consummatory behaviors of eating. Animal studies indicate that the hypothalamus plays the leading role in homeostatic eating, i.e. eating stimulated by nutrient depletion. Additional cerebral regions involved in eating include primary and secondary gustatory regions (insula and orbitofrontal cortex, OFC) as well as regions involved in memory (hippocampus) and cognitive control (dorsolateral prefrontal cortex, inferior frontal cortex and cingulate cortex). Neuropharmacological and molecular-genetic studies in animals and fMRI studies in humans indicate that the striatum (which consists of the nucleus accumbens, caudate nucleus and putamen), the OFC, the amygdala and the midbrain dopamine neurons perform neural computation of the subjective value of food stimuli (‘value’ was defined above). Estrogens and other gonadal steroids diffuse freely through the blood–brain barrier (Pardridge et al., 1980), act on cognate receptors in several of these areas and through them affect eating-related neural information processing in these areas and in areas linked to them via neural projections (López and Tena-Sempere, 2015) (Fig. 4).
Figure 4.
Some brain areas where estrogens affect the control of eating, based on animal (A) and human (B) research. Shown in red are brain areas where estrogens act directly via estrogen receptor 1 (Esr1) to affect eating; in blue are areas where estrogen may affect neural processing directly or indirectly through actions in other brain areas. (A) A schematic mid-sagittal section of the human brain highlighting areas in which estrogens affect eating. In the cmNTS, estrogen actions on Esr1 reduce meal size, in part by increasing the satiating potency of CCK; in the dorsal raphe nucleus (DR), estrogens bind to Esr1 to change serotonergic (5HT) neurotransmission so as to reduce binge-like eating. In the ARC (just lateral to the third ventricle, 3V) estrogens affect the activity of neurons expressing AgRP to reduce food intake during the early phase of the ovarian cycle. In the paraventricular nucleus of the hypothalamus (just lateral to the 3V), estrogens affect the activity of neurons expressing MC4R to reduce food intake in females (♀) more than in males (♂). Numerous fMRI studies implicate the OFC and lPFC (out of sight on the lateral surface of the cerebral hemisphere) in the neural computation of the value of food stimuli, which, as explained in the text, includes reinforcement, approach generation and affect. Ovarian hormones influence these processes. For example, visual food stimuli elicited larger responses in the lPFC in sated than in hungry women when tested during the late follicular phase, but not when tested during the early-follicular phase. (B) A schematic frontal section of the ventromedial area of the frontal lobes at the level shown by the green line in A. fMRI studies implicate the dorsal striatum (which consists of the head of the caudate and the putamen), the nucleus accumbens and the globus pallidus in the neural computation of food value. Again, ovarian hormones influence these processes; for example, pictures of high energy-foods elicited larger responses in women tested during the peri-ovulatory phase than during the luteal phase. See text for details. Abbreviations: LV, lateral ventricle; CC, corpus callosum; EC, extreme capsule; IC, internal capsule; SP, septum pellucidum; ARC, arcuate nucleus of the hypothalamus; CCK, cholecystokinin; lPFC, lateral prefrontal cortex; fMRI, functional MRI. Brain schematics are from http://www.clker.com/clipart-90953.html; the frontal lobe section was originally published in Haines (2012); used with permission.
Studies in rats indicate that estrogen-Esr1 signaling in neurons in the cmNTS in the caudal brainstem controls the estrogenic inhibition of normal eating (Asarian and Geary, 2013). That is, knockdown of cmNTS Esr1 with RNA interference increases eating and body weight and completely blocks the usual eating-inhibitory effect of estradiol in ovariectomized rats. No other brain site has been similarly linked to the estrogenic control of eating (e.g. ARC data were discussed in the leptin sections) (Asarian and Geary, 2013). The network effects of cmNTS estrogen stimulation, of course, may involve the hypothalamus and other forebrain sites. For example, expression of the neurotransmitter AgRP in ARC neurons changes across the ovarian cycle in mice, and transgenic lesions of AgRP neurons prevent the cyclic decrease in eating; these neurons do not express Esr1, however, indicating that these effects are functionally downstream of estrogens’ neural actions (Olofsson et al., 2009).
AgRP neurons appear to be selectively involved in the aversive, homeostatic hunger produced by prolonged food deprivation, in contrast to the incentive-state like hunger that is more operative in spontaneous eating (Betley et al., 2015). AgRP neurons secrete the neurotransmitters NPY and glutamate as well as AgRP, and these molecules appear to make temporally distinct contributions to hunger (Krashes et al., 2013). Unfortunately, the roles of ovarian hormones in these effects have not yet been tested. It is important to note in this regard that specific aspects of eating are likely to involve Esr in different sites. For example, binge-like eating in mice apparently depends on estrogen-Esr1 signaling in serotoninergic neurons in the dorsal raphe nuclei (Cao et al., 2014). This is especially interesting because women with bulimia nervosa have reduced serotonin-transporter binding in the dorsal raphe both during active bulimia nervosa and after recovery (Pichika et al., 2012).
Pro-opiomelanocortin neurons, which release the neurotransmitters α-MSH and β-endorphin, also may be involved in the estrogenic inhibition of eating. Brain α-MSH neurons signal mainly via MC3R in the ARC and via MC4R in the paraventricular nucleus of the hypothalamus (Biebermann et al., 2012), and loss-of-function polymorphisms of human MC4R lead to voracious appetite and obesity (Dempfle et al., 2004; Stutzmann et al., 2008; Valette et al., 2013). MC4R defects have a prevalence of ∼2–5% in obese Europeans, making it one of the commonest of all genetic diseases (O'Rahilly and Farooqi, 2006; Tao, 2010). Importantly, weight gain is about twice as much in women as in men, ∼8–9 kg/m2 versus ∼4–5 kg/m2. Mice with null mutations of this gene (Mc4r−/− mice) show a similar syndrome, again with a marked sex difference that begins around puberty, suggestive of activational effects of reproductive hormones (Huszar et al., 1997). In contrast, obesity was already present in pre-pubertal boys and girls with either of two MC4R polymorphisms (Vogel et al., 2011). Separate analyses of just one of these, SNP rs17782313, however, unveiled a pronounced sex difference. In women bearing this SNP, but not in men, there were increases in the eating traits disinhibition and emotional eating as well as differences in gray-matter volume in eating-related areas including the right amygdala, right hippocampus and medial OFC (Horstmann et al., 2013). The effects of estrogens on pro-opiomelanocortin (POMC) neurons may be mediated by both Esr1 (Xu et al., 2011) and the recently discovered Gq-coupled membrane estrogen-receptor (Smith et al., 2013). Whether estrogens act directly or indirectly on POMC neurons and the sites involved, however, are unknown.
Human functional neuroimaging studies converge with animal studies in identifying subcortical and cortical brain structures that are involved in the control of eating. Typically, these studies localize regions that respond differently to food and non-food stimuli, to hunger and satiety, and in obese and normal-weight women (Morris and Dolan, 2001; Wang et al., 2004; Small and Prescott, 2005; Stoeckel et al., 2008; Schur et al., 2009; Salem and Dhillo, 2015). Both animal and human receptor expression studies suggest that reproductive hormones may affect the function of these regions. For example, in humans, ESR1 are abundant in the hypothalamus, amygdala, hippocampus, nucleus accumbens and several cortical regions (Hara et al., 2015). Importantly, this includes membrane estrogen receptors, which mediate rapid effects on neural activity, as well as classical nuclear estrogen receptors (Almey et al., 2015). Furthermore, estrogens are required to maintain dopamine innervation of the striatum, and estrogen and/or progesterone replacement can rescue ovariectomy-induced reductions in caudate dopamine (Kritzer et al., 2003). Progesterone receptors are also expressed in food-related regions beyond the hypothalamus, including the caudate (Zhu et al., 2003), amygdala, hippocampus and frontal cortex (Brinton et al., 2008). Although the exact role of hormones in many of these regions is not entirely clear, the widespread distribution of their receptors in areas mediating hedonics, motivation and cognition reinforces the notion of their involvement in non-reproductive neural functions, particularly in the control of normal and dysregulated eating.
There are sex-related structural differences in brain areas involved in processing food-related information (Ruigrok et al., 2014); for example, women compared with men express relative increases in regional gray-matter volume or density in frontal cortex, dorsal striatum and right insula, but relative reductions in ventral striatum, amygdala, hippocampus and left insula. Sex modulates the functional activation of these regions in a corresponding way. Obese women show different neural responses to pictures of high energy-food than lean women or obese men. For example, dorsal-striatal activity to high energy-food pictures is enhanced in obese women (Rothemund et al., 2007; Geliebter et al., 2013). Rodent research implicates the dorsal striatum in the development of inflexible, habitual control of eating (Furlong et al., 2014). Accordingly, eating habits may be particularly inflexible in obese women.
Changes in neural responses to food pictures through the menstrual cycle suggest a role of ovarian hormones in processing food-related information. Accordingly, the reduced food intake in the peri-ovulatory phase of the menstrual cycle is reflected in changes in the responses of eating-related regions. Specifically, the ventral striatum shows enhanced activity to high versus low energy-food pictures during the peri-ovulatory compared with the luteal phase (Frank et al., 2010), in line with the notion of enhanced dopaminergic reward activity during the peri-ovulatory phase (Dreher et al., 2007; Frank et al., 2010). In a separate group of subjects, these neural effects were mirrored by reduced appeal of high energy-food pictures during the peri-ovulatory phase compared with the luteal phase, with unchanged appeal of low energy-food pictures. Thus, the meal size-limiting effects of estrogens during the peri-ovulatory phase are mediated by increased ventral striatal responses to high-calorie food, reflecting increased reward sensitivity induced by higher levels of estrogens. Indeed, separate research showed a positive correlation between estradiol levels and amphetamine-induced euphoria in women during the follicular, but not the luteal, phase, suggesting that estrogens make women more sensitive to (striatal) dopamine (Justice and de Wit, 1999). These findings converge with reports of reduced reward sensitivity and dopamine release in obesity and therefore conceptualize increased food intake as an attempt to compensate for reduced reward value of food (for review, see Val-Laillet et al., 2015).
Moreover, neural responses to food pictures reflect interactions between prandial state and menstrual-cycle phase. Specifically, responses to food pictures in the lateral prefrontal cortex (lPFC) are enhanced in the fed compared with the hungry state during the late-follicular (high-estrogen), but not the early-follicular (low-estrogen), phase (Alonso-Alonso et al., 2011). Thus, estrogens may act in the prefrontal cortex to facilitate cognitive control over appetite in the fed state, i.e. reduce the tendency to ‘eat in the absence of hunger,’ a trait associated with increased obesity risk (French et al., 2012). In line with this notion, the lPFC is less activated after meals in obese women than in healthy-weight or formerly obese women (Le et al., 2007). Furthermore, stronger lateral prefrontal responses to food pictures predict reduced subsequent energy intake (Cornier et al., 2010), and these responses are smaller in obese compared with healthy-weight individuals (Batterink et al., 2010).
Although converging evidence from human neuroimaging studies identifies regions involved in mediating the influence of ovarian hormones on processing food-related stimuli, there is a dearth of studies that directly investigate the effects of sex and ovarian hormones on precisely defined components of neural processing related to eating. Addressing this gap would involve using real food rather than pictures and using paradigms established in the fields of reinforcement learning, affective neuroscience and decision neuroscience. For example, it would be interesting to directly test the effects of estrogens on the neural mechanisms underlying Pavlovian and goal-directed aspects of eating. One hypothesis would be that estrogens act in the striatum to make eating behavior flexible and goal-directed.
Energy expenditure
General aspects of human EE
EE is energy lost in work or heat (urinary nitrogen and glucose losses can also contribute in pathological situations). Total EE includes REE, the postprandial increase in EE due to digestive and metabolic handling of ingesta (known as diet-induced thermogenesis (DIT) or the thermic effect of food), thermoregulatory responses, weight-regulatory EE responses and physical-activity EE. REE is also known as the resting or basal metabolic rate and is sometimes subdivided into sleeping and awake, resting components. In moderately active individuals in a thermoneutral environment, REE usually accounts for ~60–70% of total EE (Levine, 2004), DIT for ~10–15% (D'Alessio et al., 1988; Reed and Hill, 1996) and physical activity for the rest. Physical-activity EE can be further subdivided into exercise or sports activities (including walking for exercise) and non-exercise-activity thermogenesis, which is the energetic cost of occupational physical activity and incidental daily activities, such as sitting, talking, standing, fidgeting, etc.
REE is strongly correlated with FMM (r2 ~0.6–0.7), with no sex difference (Johnstone et al., 2005; Blundell et al., 2010). Individuals with lower REE/FFM values are at increased risk for weight gain (Ravussin et al., 1988), suggesting that genetic differences in the cellular components of REE may contribute to individual differences in obesity risk (Konarzewski and Książek, 2013). About half of REE is due to membrane processes, mainly Na+/K+-ATPase and UCP-mediated mitochondrial-proton leak (Hulbert and Else, 2005). A decline in UCP activity may explain the decrease in REE with aging (St-Onge and Gallagher, 2010; Saito, 2013) which, as reviewed below, complicates studies of menopausal effects on REE.
The much lower requirement for physical-activity EE in developed societies is thought to contribute to the obesity epidemic and to disease risk (Woolf et al., 2008). Physical activity not only directly increases EE, but also increases REE (Gilliat-Wimberly et al., 2001; Van Pelt et al., 2001). Physical-activity EE can be expressed as metabolic-energy equivalents (MET), the ratio of the rate of EE during the activity and a standard rate, which was originally based on EE while sitting quietly (ASHRAE standard 55, 2016). Only a few physical laborers or athletes regularly exceed a daily average level of 2 MET (Levine, 2004). The recommended minimal level of physical activity for good health in the USA is 500 MET-min/wk (ODPHP, 2016). As moderate-intensity activity, such as walking at ~5 km/h, expends ~3.3 MET and vigorous-intensity activity, such as jogging at ~8 km/h, expends ~6 MET, this recommendation can be met by walking for ~150 min/wk or jogging for ~75 min/wk. Epidemiological data indicate that any level of physical activity reduces adult all-cause mortality risk and that exceeding the MET recommendation 2-fold or more reduces it by ~30–40% (Arem et al., 2015b). Only 40% of US Americans, however, meet this recommendation.
Increased physical-activity EE improves cardiovascular fitness and reduces all-cause mortality independent of BMI (Barry et al., 2014). Furthermore, higher levels of physical-activity EE predict success in maintaining diet-induced weight loss (Phelan et al., 2006). Thus, women should be encouraged to increase physical-activity EE, especially after menopause and in societies in which they tend to be less active (Ford et al., 1991; Livingstone et al., 2001; Sadarangani et al., 2014). The physiological mechanisms mediating these mortality effects are not known. Unfortunately, the substantial error in available methods for measuring habitual physical-activity EE (Arem et al., 2015a; Lim et al., 2015) impedes research progress in this area.
Finally, alterations in total EE contribute to body-weight regulation (Leibel et al., 1995; Hall et al., 2011; Dhurandhar et al., 2015). If body weight decreases, total EE decreases markedly more than predicted by the lower body mass, thus strongly resisting further weight loss. This adaptive reduction in EE is one reason that weight loss is so difficult. For example, a reduction in habitual intake by 200 kcal/d will lead to 19 kg weight loss in 3 y, versus a predicted weight loss of 78 kg if there were no adaptation in EE (Hall et al., 2011). The underlying mechanisms are not fully understood. Changes in the amount or efficiency of physical-activity EE (Leibel et al., 1995; Levine et al., 1999; Levine, 2004) and in UCP-1 expression in brown AT (Fromme and Klingenspor, 2011; Morrison et al., 2014) appear to contribute.
Roles of ovarian hormones
Resting energy expenditure
REE per kg body mass is lower in women due to their higher percent AT, but REE per kg FFM does not appear to differ between the sexes (Buchholz et al., 2001; Johnstone et al., 2005). This deserves more research, however, with more accurate measurement methods; in the studies cited, residual variability was ~25% of REE, i.e. ~300–400 kcal/d (Wang et al., 2011). Interestingly, women's AT is more metabolically active and ~10 fold more variable than men's (Buchholz et al., 2001). This is due in part to a 5-fold higher expression of UCP-1 in women's brown adipocytes (Nookaew et al., 2013).
REE varies over the menstrual cycle, from a minimum in the early-follicular phase to a maximum ~50–100 kcal/d more in the luteal phase (Day et al., 2005). Administration of a GnRH antagonist during the mid-luteal phase reduced REE to the early-follicular level, indicating a role of ovarian hormones (Day et al., 2005). Progestins seem not to be involved because high-progestin contraceptives have little effect on REE (Pelkman et al., 2001). Estrogens may be the cause because transdermal estrogen treatment increases REE in premenopausal women in whom endogenous ovarian hormones are suppressed by GnRH antagonism for either 6 days or 5 months (Day et al., 2005; Melanson et al., 2015; Van Pelt et al., 2015).
The effect of menopause on REE has not received much investigation. In one 4-year longitudinal study beginning with regularly cycling women at least 43 years of age, sleeping REE decreased by 7.9% in the women who became postmenopausal during the study compared with 5.3% in those who remained premenopausal, but the difference was not statistically significant (Lovejoy et al., 2008). In a 2-week study of postmenopausal women, estrogen treatment did not affect REE (Bessesen et al., 2015). Three-month treatment with a selective estrogen-receptor agonist also failed to increase REE in ovariectomized old-world monkeys (Sullivan et al., 2012). In contrast, estrogens clearly increase REE in laboratory rodents (Rogers et al., 2009), and REE is decreased in Esr1 knockout mice of both sexes, although not in Esr2 knockouts (reviewed in Mauvais-Jarvis, 2011; Van Pelt et al., 2015).
Dietary-induced thermogenesis
Studies of reproductive hormonal effects on DIT are contradictory. DIT has been found to increase, decrease or not to change in the luteal phase of the menstrual cycle compared with the follicular phase (Piers et al., 1995; Melanson et al., 1996; Tai et al., 1997; Li et al., 1999). Estrogen treatment did not affect DIT in premenopausal women in whom endogenous ovarian hormones were suppressed by GnRH antagonism (Melanson et al., 1996). We know of no studies of DIT across menopause.
Physical-activity EE
Estrogens increase physical activity in rats, mice and monkeys, but whether this is so in humans is unknown (Asarian and Geary, 2013). One study found that healthy-weight and overweight women walked ~1600 more steps/day (~100–200 kcal/d) during the early-follicular phase compared with the mid-luteal phase (Day et al., 2005). It is interesting to note that physical-activity EE decreased during the luteal phase, whereas REE increased. Estrogen treatment, however, did not affect physical-activity EE in premenopausal women in whom endogenous ovarian hormones were acutely suppressed by GnRH antagonists (Melanson et al., 1996). Around the age of menopause, physical-activity EE tends to decrease substantially, but similarly in women who become postmenopausal and those who do not (30% and 39%, respectively) (Lovejoy et al., 2008). Even if menopause is not the cause, the magnitude of the average decrease in physical-activity EE in aging women is clearly clinically relevant (Woolf et al., 2008).
Surprisingly, most studies indicate that acute physical activity results in negative energy balance, i.e. energy intake either does not change or is reduced following increased physical activity (King et al., 2011; Deighton et al., 2013; Howe et al., 2014; Blundell et al., 2015). This was recently confirmed in a study in which participants expended ∼1.3 MET for two consecutive days with no change in food intake (Douglas et al., 2015). Obviously, at some point regular moderate or high intensity physical activity EE must lead to increase energy intake to avoid weight loss, but how this occurs is also unknown (Stensel, 2010).
Central mechanisms
REE
Genetic and physiological studies in mice and rats indicate that estrogens acting via Esr1 in the ventromedial nucleus of the hypothalamus increase EE by increasing hypothalamic sympathetic outflow, resulting in increased brown-AT UCP-1 activity (Musatov et al., 2007; Xu et al., 2011; Martínez de Morentin et al., 2014). Because the animals were maintained below thermoneutrality in these studies, however, it is difficult to distinguish REE from thermoregulatory EE. Pronounced species differences also complicate EE research. For example, glucocorticoids decrease brown-AT UCP-1 activity in mice, but stimulate it in humans (Ramage et al., 2016).
Physical-activity EE
Female rats and mice are much more spontaneously active than their male conspecifics. Estrogens appear to act in at least three brain areas to stimulate physical activity in mice and rats. First, Fahrbach et al. (1985) found that estradiol injections into the medial preoptic area increase physical activity in rats. Second, Musatov et al. (2007) found that knockdown of Esr1 in the VMH reduces physical activity in female rats. Using complementary site-specific knockout and pharmacogenetic stimulation of Esr1, Correa et al. (2015) demonstrated that VMH Esr1 neurons stimulate physical activity in female, but not in male, mice and are a distinct subpopulation not involved in controlling eating, brown-AT activation or fertility, as indicated by the maturation of corpora lutea. Finally, using site-specific Esr1 knockout or knockdown, Xu et al. (2015) found that Esr1 neurons in the medial nucleus of the amygdala also control physical activity, but do so similarly in male and female mice.
Physical activity-induced increases in EE affect activity of reward regions of the brain in humans. For example, an escalating 6-month daily treadmill regimen reduced insula responses to highly valued food pictures (vs non-food) in overweight and obese subjects in the fasted state, and this reduction in insula activation correlated with reduction in fat mass and body weight (Cornier et al., 2012; McFadden et al., 2013). Moreover, after an acute 60-minute exercise, normal-weight subjects showed increased dorsolateral prefrontal activity and reduced insula, putamen, orbitofrontal and hippocampal activity to pictures of high-calorie food (vs non-food) (Evero et al., 2012; Crabtree et al., 2014). These data suggest that exercise downregulates the food-related responses of cortical and subcortical reward regions and upregulates the responses of prefrontal control regions. How ovarian hormones influence the relation between exercise and central food processing, however, remains largely unknown.
Discussion
Obesity results from tonically greater energy intake than EE. This simple truth belies the facts that the controls of eating and EE, the development of obesity, and the cardiometabolic consequences of the amount and distribution of AT are each multifactorial processes involving genetic, epigenetic, metabolic, endocrine, psychological, social and cultural factors, most of which are poorly understood. Because obesity remediation is a worldwide health challenge and because current strategies for weight reduction, other than bariatric surgery, have limited efficacy, there is an urgent need for research aimed at developing, first, further preventive and therapeutic strategies to achieve and maintain healthy levels of adiposity, and second, to minimize cardiometabolic and other risks of increased adiposity. Physiological sex differences are important variables in these processes. This should be clear from the marked sex differences in obesity prevalence, especially prevalence of severe obesity (BMI ≥35 kg/m2).
We find that sex-specific physiological mechanisms, especially estrogen-mediated effects, play significant roles in the development of obesity in women as well as in its pathophysiological consequences. Table II summarizes well-established and probable female-specific obesity-related mechanisms, based on a wide variety of human and animal research. Estrogens act in the brain, in the AT and in other peripheral sites to affect: (i) the proximal causes of obesity, i.e. relative increases in eating and decreases in EE, (ii) the amount and regional distribution of AT and (iii) AT depot-specific pathophysiological changes that lead to morbidity. As a result, estrogens contribute both to male–female sex differences in obesity and obesity co-morbidities and to changes in obesity and obesity co-morbidities throughout women's lives (Fig. 5). Therefore, understanding the full impact of AT on women's health will require consideration of hormonal status on each of these processes. Currently astonishingly few data are available to address the normal physiology of these processes, much less the effects of surgical and hormonal treatments on female overweight and obesity or the pathophysiology of endocrine disorders with obesity as a frequent co-morbidity.
Table II.
Sex differences and effects of estrogens on adiposity, eating and EE in women.
| Adiposity |
| ♦ Beginning at puberty, positive energy balance leads to female-typical AT distribution, i.e. greater accretion of gluteofemoral subcutaneous AT and less accretion of visceral AT. |
| ◊ Estrogens and adipocyte genes synergize to produce female-typical AT distribution. |
| ◊ Loss of estrogens after menopause increases total adiposity and decreases lean body mass, and estrogenic HRT prevents this. |
| ◊ Loss of estrogens after menopause partially reverses female-typical regional AT distribution, and estrogenic HRT prevents this. |
| Eating |
| ♦ Increasing estrogen secretion through the follicular phase progressively reduces daily food intake. |
| ○ Estrogens act on Esr1-expressing neurons in the cmNTS to increase CCK-mediated satiation; several other meal-control mechanisms may participate. |
| ◊ Sex differences in gustatory sensory function affect eating. |
| ♦ Genetic defects in brain α-MSH–MC4R signaling lead to a more marked overeating and obesity syndrome in females than males. |
| ◊ Neuroimaging studies indicate that estrogens increase the activity of striatal dopaminergic neurons that processing of flavor hedonics. |
| ◊ Neuroimaging studies indicate that estrogens increase lPFC processing of cognitive controls inhibiting eating. |
| EE |
| ◊ Estrogens increase REE during the luteal phase. |
| ○ Estrogens act in several brain sites to increase physical-activity EE in rats and mice. |
Note: ♦ indicates well-established effects in humans with proven or likely clinical relevance, as reviewed in text; ○ indicates effects apparent effects in humans with as yet uncertain clinical relevance; ◊ indicates effects established in animal research. Abbreviations: α-MSH, α-melanocyte-stimulating hormone; MC4R, melanocortin 4 receptor; lPFC, lateral prefrontal cortex; REE, resting energy expenditure; CCK, cholecystokinin; cmNTS, caudal medial nucleus of the solitary tract.
Figure 5.
Summary of the principal effects of estrogens, represented by relative estradiol concentrations, on eating, EE and AT through women's life cycles. Note: Estradiol concentrations and durations of epochs are not to scale. See text for details. EE, energy expenditure; AT, adipose tissue.
Many of the effects described in Table II may appear to be small. For example, changes in food intake and EE through the menstrual cycle are on average only a few hundred kcal/d. It should be realized, however, that regular small changes in energy balance can lead to substantial differences in body weight and, therefore, be of considerable physiological relevance. The best available estimates indicate that an average increase of only 240 kcal/d in energy intake over EE is sufficient to produce the mean increase in average body weight that underlies the obesity epidemic in the USA (Hall et al., 2011). Thus, the phenomena reviewed here are medically relevant and worthy of increased basic and clinical research.
It is also clear that obesity-related physiological characteristics vary widely across individual women. For example, as reviewed above, although most obese women display the female-typical pattern of relatively more gluteofemoral AT than central AT, some women have male-typical, central AT distribution (Karpe and Pinnick, 2015). The effects of menstrual cycle on flavor preferences also display great inter-subject variability (Bartoshuk et al., 1994, 2006). Such variability may arise for many reasons, ranging from psychological, social and cultural influences on one side to genetic and epigenetic effects on the other side (Shepard et al., 2009). Inter-subject variability together with the modest effect sizes mentioned above is a great challenge for research and an impediment to translation of research data into clinical practice. Research can meet this challenge in a variety of ways. Sample sizes should be based on power calculations taking effect size and variability into account. In addition, special care is required in sex research to consider subject characteristics, including age, race, alcohol use, stress levels or ‘allostatic load’, psychological traits such as tendencies for emotional or binge eating, etc., that can alter the sex-specific influences on AT physiology, eating and EE. In such cases, appropriate statistical tools are helpful; for example, the multiple-regression analyses described above that parse the separate effects of aging and menopause on AT. Finally, researchers should avail themselves of the many methodological improvements that can increase the efficiency and meaning of pre-clinical and clinical sex-related research (Rubinow and Schmidt, 2002; Keitt et al., 2003; Becker et al., 2005; Greenspan et al., 2007; McCarthy et al., 2012).
The homogeneity of study groups with respect to reproductive physiology status itself is another important issue that is often overlooked, in part because findings in one domain fail to reach researchers in other domains. This is one of many reasons that inter-disciplinary investigator groups should be facilitated. Thus, postmenopausal study participants should be characterized, menstrual cycles should be evaluated more accurately in both obese and non-obese women, and effects of the type and duration of HT, including the interval between menopause and beginning HT, should be assessed. In women with surgical menopause, the indication for ovariectomy and whether hysterectomy was also done should be considered. In addition, as it is likely that indications for gynecological surgery differ in obese and non-obese women, due, for example to the higher prevalence of endometrial cancer and disturbed bleeding patterns in obese women (Madsen et al., 2013; Crujeiras and Casanueva, 2015), associations between surgical menopause and adiposity and AT distribution need to be explored. A related issue is that reproductive physiology, in particular estrogens, also may influence experimental outcomes via ‘off-target’ physiological functions. For example, pharmacological data in women may be influenced by differences in the bioactivity of many drugs due to sex differences in cytochrome p450 enzyme activity, effects of estrogens on hepatic drug metabolism and effects of estrogens on plasma levels of sex-hormone binding globulin and other globulins (Soldin and Mattison, 2009; Spoletini et al., 2012).
We acknowledge weaknesses of the review. We focused on the classical AT depots, although excess adiposity is also associated with increased hepatic, intramuscular and cardiovascular fat, which are independently associated with cardiometabolic risk and appear to be affected by hormonal status in women (Oosthuyse and Bosch, 2012; El Khoudary et al., 2015; Marino and Jornayvaz, 2015). We focused mainly on pre-clinical whole-body mechanistic studies, at the expense of detailed reviews of cell- and tissue-level studies, genome-wide gene association studies and epidemiological studies, despite the many plausible mechanistic hypotheses that these suggest. We did not consider pregnancy, although postpartum weight gain is an important obesity risk (Gore et al., 2003). We did not consider tissue-specific synthesis of estrogens in the brain or AT or production of neurally active estrogen metabolites or other neurosteroids (Azcoitia et al., 2011; Do Rego et al., 2012; Porcu et al., 2016).
In conclusion, current human and animal research supports significant roles of ovarian hormones, especially estrogens, in the regulation of female AT. These involve influences on eating, EE, AT amount, regional AT distribution and the physiological function of adipocytes in the various AT depots. In the coming years, improvements in research design and analyses and the development of additional powerful methodologies should lead to important progress across the range of diverse influences of female physiology on adiposity. Continued animal research should better characterize, for example, the site and nature of estrogen receptors in the brain that affect eating and EE. Animal research should also lead the way to identifying the downstream molecular mechanisms responding to the activation of estrogen response elements in the DNA, which contribute the hormone's effects on gluteofemoral AT and on most, if not all, of its neural effects. A pressing issue in this connection is the better understanding of the interaction of estrogenic and genetic controls leading to the protective cardiometabolic effects of gluteofemoral AT. Similarly, improvements in the design of fMRI studies should help specify the neural information-processing mechanisms underlying sex-related hedonic and cognitive controls of eating. Progress in these and other research directions described here should lead to better understanding of female obesity, including clinically challenging syndromes such as PCOS, and, ultimately, to better strategies to maintain and improve women's health in the face of the obesity epidemic.
Authors’ roles
All authors contributed to the identification and critical evaluation of the relevant literature and to drafting the manuscript, and all authors approved the final version of the manuscript. B.L. conceived of the theme, prepared the proposal, and contributed clinical and scientific expertise as a gynecological endocrinologist. N.G. contributed expertise in adipose-tissue physiology, exercise physiology and the neuroendocrine regulation of eating and body weight. P.N.T. contributed expertise in functional neuroimaging and cognitive neuroscience. L.A. prepared the proposal and contributed expertise in adipose-tissue physiology and the neuroendocrine regulation of eating and body weight.
Funding
NIH grant DK 092608 (to L.A.); Swiss NSF grants PP00P1_150739 and 00014_165884 (to P.N.T.) and a private foundation (Philhuman Stiftung, Lichtenstein) (to B.L.).
Conflict of interest
None of the authors has any conflict of interest.
References
- Abdulnour J, Doucet E, Brochu M, Lavoie J-M, Strychar I, Rabasa-Lhoret R, Prud'homme D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause 2012;19:760–767. [DOI] [PubMed] [Google Scholar]
- Aguiar AL, Couto-Silva AC, Vicente EJ, Freitas IC, Cruz T, Adan L. Weight evolution in girls treated for idiopathic central precocious puberty with GnRH analogues. J Pediatr Endocrinol Metab 2006;19:1327–1334. [DOI] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav 2015;74:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Alonso MM, Ziemke FF, Magkos FF, Barrios FAF, Brinkoetter MM, Boyd II, Rifkin-Graboi AA, Yannakoulia MM, Rojas RR, Pascual-Leone AA et al. . Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr 2011;94:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arem H, Keadle SK, Matthews CE. Invited commentary: meta-physical activity and the search for the truth. Am J Epidemiol 2015. a;181:656–658. [DOI] [PubMed] [Google Scholar]
- Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO et al. . Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015. b;175:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H et al. . Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011;478:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 2013;305:R1215–R1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHRAE: American Society of Heating, Refrigerating and Air-Conditioning Engineers https://www.ashrae.org/resources--publications/bookstore/standard-55 (March 2016, date last accessed).
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience 2011;191:139–147. [DOI] [PubMed] [Google Scholar]
- Barber TM, Golding SJ, Alvey C, Wass JAH, Karpe F, Franks S, McCarthy MI. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:999–1004. [DOI] [PubMed] [Google Scholar]
- Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr 1995;61:39–43. [DOI] [PubMed] [Google Scholar]
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis 2014;56:382–390. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci 2006;361:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav 1994;56:1165–1171. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 2010;52:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M et al. . Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005;146:1650–1673. [DOI] [PubMed] [Google Scholar]
- Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod 2003;68:10–18. [DOI] [PubMed] [Google Scholar]
- Belzer LM, Smulian JC, Lu S-E, Tepper BJ. Food cravings and intake of sweet foods in healthy pregnancy and mild gestational diabetes mellitus. A prospective study. Appetite 2010;55:609–615. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008;93:1339–1344. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14:16S–19S. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 2002;26:393–428. [DOI] [PubMed] [Google Scholar]
- Bessesen DH, Cox-York KA, Hernandez TL, Erickson CB, Wang H, Jackman MR, Van Pelt RE. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: impact of menopause and estradiol. Obesity (Silver Spring) 2015;23:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 2015;521:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebermann H, Kühnen P, Kleinau G, Krude H. The neuroendocrine circuitry controlled by POMC, MSH, and AGRP. Handb Exp Pharmacol 2012;209:47–75. [DOI] [PubMed] [Google Scholar]
- Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res 2016;118:1294–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, Mailloux J, Luu-The V, Tchernof A. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–188. [DOI] [PubMed] [Google Scholar]
- Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H et al. . Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010;11:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell JE, Gibbons C, Caudwell P, Finlayson G, Hopkins M. Appetite control and energy balance: impact of exercise. Obes Rev 2015;16:67–76. [DOI] [PubMed] [Google Scholar]
- Boucher JG, Husain M, Rowan-Carroll A, Williams A, Yauk CL, Atlas E. Identification of mechanisms of action of bisphenol a-induced human preadipocyte differentiation by transcriptional profiling. Obesity (Silver Spring) 2014;22:2333–2343. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Grunberg NE. Variations in food preference and consumption across the menstrual cycle. Physiol Behav 1990;47:287–291. [DOI] [PubMed] [Google Scholar]
- Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol 2009;297:G602–G610. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ et al. . Progesterone receptors: form and function in brain. Front Neuroendocrinol 2008;29:313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125. [DOI] [PubMed] [Google Scholar]
- Buchholz AC, Rafii M, Pencharz PB. Is resting metabolic rate different between men and women. Br J Nutr 2001;86:641–646. [DOI] [PubMed] [Google Scholar]
- Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015;148:1219–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011;14:324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Xu P, Oyola MG, Xia Y, Yan X, Saito K, Zou F, Wang C, Yang Y, Hinton A et al. . Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest 2014;124:4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 2007;92:2500–2505. [DOI] [PubMed] [Google Scholar]
- Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril 2009;91:1332–1335. [DOI] [PubMed] [Google Scholar]
- Carmina E, Chu MC, Moran C, Tortoriello D, Vardhana P, Tena G, Preciado R, Lobo R. Subcutaneous and omental fat expression of adiponectin and leptin in women with polycystic ovary syndrome. Fertil Steril 2008;89:642–648. [DOI] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Advances in the neurobiological bases for food ‘liking’ versus ‘wanting’. Physiol Behav 2014;136:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G, Trivax BS, Yildiz BO, Bertolotto C, Mathur R, Heneidi S, Azziz R. Regulation of adiponectin secretion by adipocytes in the polycystic ovary syndrome: role of tumor necrosis factor-{alpha}. J Clin Endocrinol Metab 2010;95:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 2012;8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab 2001;280:E315–E322. [DOI] [PubMed] [Google Scholar]
- Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes (Lond) 2005;29:1252–1258. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006;55:978–987. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007;56:1051–1058. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Riedy CA, Smith KAB, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 2003;52:682–687. [DOI] [PubMed] [Google Scholar]
- Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat Rev Endocrinol 2014;10:157–163. [DOI] [PubMed] [Google Scholar]
- Cornier M-A, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav 2012;105:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier M-A, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav 2010;99:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 2015;10:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images of food. Am J Clin Nutr 2014;99:258–267. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011;12:722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crujeiras AB, Casanueva FF. Obesity and the reproductive system disorders: epigenetics as a potential bridge. Hum Reprod Update 2015;21:249–261. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, Klump KL. Hormonal factors and disturbances in eating disorders. Curr Psychiatry Rep 2016;18:65. [DOI] [PubMed] [Google Scholar]
- Czaja JA. Ovarian influences on primate food intake: assessment of progesterone actions. Physiol Behav 1978;21:923–928. [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, Owen LR, Bushman MC, Boden G, Owen OE. Thermic effect of food in lean and obese men. J Clin Invest 1988;81:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal'Ava N, Bahamondes L, Bahamondes MV, Bottura BF, Monteiro I. Body weight and body composition of depot medroxyprogesterone acetate users. Contraception 2014;90:182–187. [DOI] [PubMed] [Google Scholar]
- Dalgaard K, Landgraf K, Heyne S, Lempradl A, Longinotto J, Gossens K, Ruf M, Orthofer M, Strogantsev R, Selvaraj M et al. . Trim28 haploinsufficiency triggers Bi-stable epigenetic obesity. Cell 2016;164:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. The epidemiology and genetics of binge eating disorder (BED). CNS Spectr 2015;20:522–529. [DOI] [PubMed] [Google Scholar]
- Davis KE, D Neinast M, Sun K, M Skiles W, D Bills J, A Zehr J, Zeve D, D Hahner L, W Cox D, M Gent L et al. . The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2013;2:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and {beta}-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab 2005;90:3312–3317. [DOI] [PubMed] [Google Scholar]
- DeClercq V, Taylor C, Zahradka P. Adipose tissue: the link between obesity and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets 2008;8:228–237. [DOI] [PubMed] [Google Scholar]
- Deighton K, Barry R, Connon CE, Stensel DJ. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur J Appl Physiol 2013;113:1147–1156. [DOI] [PubMed] [Google Scholar]
- Dempfle A, Hinney A, Heinzel-Gutenbrunner M, Raab M, Geller F, Gudermann T, Schäfer H, Hebebrand J. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet 2004;41:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887. [DOI] [PubMed] [Google Scholar]
- Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes (Lond) 2015;39:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E. Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes (Lond) 2007;31:S8–S13. discussion S31–S32. [DOI] [PubMed] [Google Scholar]
- Dillard TH, Purnell JQ, Smith MD, Raum W, Hong D, Laut J, Patterson EJ. Omentectomy added to Roux-en-Y gastric bypass surgery: a randomized, controlled trial. Surg Obes Relat Dis 2013;9:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Vaudry D, Luu-The V, Tonon M-C, Tsutsui K, Pelletier G, Vaudry H. Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Front Endocrinol (Lausanne) 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JA, King JA, McFarlane E, Baker L, Bradley C, Crouch N, Hill D, Stensel DJ. Appetite, appetite hormone and energy intake responses to two consecutive days of aerobic exercise in healthy young men. Appetite 2015;92:57–65. [DOI] [PubMed] [Google Scholar]
- Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 2007;104:2465–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 2015;36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck LH, Bennett AG, Egan BM, Ray JW, Mitchell CO, Smith MA, Klesges RC. Differences in macronutrient selections in users and nonusers of an oral contraceptive. Am J Clin Nutr 1997;65:419–424. [DOI] [PubMed] [Google Scholar]
- El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell LH, Matthews KA. Cardiovascular fat, menopause, and sex hormones in women: the SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab 2015;100:3304–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 2007;18:266–272. [DOI] [PubMed] [Google Scholar]
- Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol 2012;112:1612–1619. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav 1985;35:985–992. [DOI] [PubMed] [Google Scholar]
- Farr OM, Li C-SR, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metab Clin Exp 2016;65:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, Gunatilake R, Kalaitzoglou E. Air displacement plethysmography: cradle to grave. Nutr Clin Pract 2015;30:219–226. [DOI] [PubMed] [Google Scholar]
- Fong AK, Kretsch MJ. Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle. Am J Clin Nutr 1993;57:43–46. [DOI] [PubMed] [Google Scholar]
- Ford ES, Merritt RK, Heath GW, Powell KE, Washburn RA, Kriska A, Haile G. Physical activity behaviors in lower and higher socioeconomic status populations. Am J Epidemiol 1991;133:1246–1256. [DOI] [PubMed] [Google Scholar]
- Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010;1363:81–92. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Ploutz-Snyder L, Kanaley JA. Longitudinal changes in abdominal fat distribution with menopause. Metab Clin Exp 2009;58:311–315. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010;17:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 2012;59:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SK, Lee M-J, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl F, Riedl D, Fessler S, Wildt L, Walter M, Richter R, Schüßler G, Böttcher B. Impact of endometriosis on quality of life, anxiety, and depression: an Austrian perspective. Arch Gynecol Obstet 2015;292:1393–1399. [DOI] [PubMed] [Google Scholar]
- Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 2011;300:R1–R8. [DOI] [PubMed] [Google Scholar]
- Fu J, Hofker M, Wijmenga C. Apple or pear: size and shape matter. Cell Metab 2015;21:507–508. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Jayaweera HK, Balleine BW, Corbit LH. Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. J Neurosci 2014;34:5012–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsson BG, Johansson JM, Lönn M, Jernås M, Olbers T, Peltonen M, Larsson I, Lönn L, Sjöström L, Carlsson B et al. . High expression of complement components in omental adipose tissue in obese men. Obes Res 2003;11:699–708. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups. Am J Epidemiol 1996;143:228–239. [DOI] [PubMed] [Google Scholar]
- Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev 2014;1:CD003987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Benussi C, De Simone L, Genazzani AR. Climacteric modifications in body weight and fat tissue distribution. Climacteric 1999;2:37–44. [DOI] [PubMed] [Google Scholar]
- Geary N, Moran TH. Appetite In: Sadock BJ, Sadock VA, Ruiz P (eds).. Comprehensive Textbook of Psychiatry, 10th edn Philadelphia, PA: Lippincott Williams & Wilkens, 2016. (in press). [Google Scholar]
- Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res 2013;243:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA 2006;103:6676–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliat-Wimberly M, Manore MM, Woolf K, Swan PD, Carroll SS. Effects of habitual physical activity on the resting metabolic rates and body compositions of women aged 35 to 50 years. J Am Diet Assoc 2001;101:1181–1188. [DOI] [PubMed] [Google Scholar]
- Głab E, Barg E, Wikiera B, Grabowski M, Noczyńska A. Influence of GnRH analog therapy on body mass in central precocious puberty. Pediatr Endocrinol Diabetes Metab 2009;15:7–11. [PubMed] [Google Scholar]
- Goh LGH, Dhaliwal SS, Welborn TA, Lee AH, Della PR. Anthropometric measurements of general and central obesity and the prediction of cardiovascular disease risk in women: a cross-sectional study. BMJ Open 2014;4:e004138–e004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr 1989;49:252–258. [DOI] [PubMed] [Google Scholar]
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med 2003;26:149–159. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA et al. . Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132:S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 2012;16:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DE. Fundamental Neuroscience for Basic and Clinical Applications, 3rd edn Philadelphia, PA: Churchill Livingstone/Elsevier, 2012. [Google Scholar]
- Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 2012;61:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev 2015;95:785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav 2008;95:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison A. Physiology of the gonadotropin-releasing hormone neuronal network In: Knobil and Neill's Physiology of Reproduction; Vol 1 Amsterdam, The Netherlands: Elsevier, 2006;1415–1482. [Google Scholar]
- Higgs S, Robinson E, Lee M. Learning and memory processes and their role in eating: implications for limiting food intake in overeaters. Curr Obes Rep 2012;1:91–98. [Google Scholar]
- Hilbert A, de Zwaan M, Braehler E. How frequent are eating disturbances in the population? Norms of the eating disorder examination-questionnaire. PLoS One 2012;7:e29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SC, Wu S, Chan SG, Sham A. Menopausal transition and changes of body composition: a prospective study in Chinese perimenopausal women. Int J Obes (Lond) 2010;34:1265–1274. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N et al. . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann A, Kovacs P, Kabisch S, Boettcher Y, Schloegl H, Tönjes A, Stumvoll M, Pleger B, Villringer A. Common genetic variation near MC4R has a sex-specific impact on human brain structure and eating behavior. PLoS One 2013;8:e74362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SM, Hand TM, Manore MM. Exercise-trained men and women: role of exercise and diet on appetite and energy intake. Nutrients 2014;6:4935–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL. Membranes and the setting of energy demand. J Exp Biol 2005;208:1593–1599. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD et al. . Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997;88:131–141. [DOI] [PubMed] [Google Scholar]
- Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev 2012;13:30–39. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Natl Acad Sci USA 2001;98:11765–11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Petrillo LF, Koukopoulos A, Viguera AC, Hirschberg A, Nonacs R, Somley B, Pasciullo E, White DP, Hall JE et al. . Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab 2011;96:E1044–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005;82:941–948. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. [DOI] [PubMed] [Google Scholar]
- Kamel EG, McNeill G, Van Wijk MC. Usefulness of anthropometry and DXA in predicting intra-abdominal fat in obese men and women. Obes Res 2000;8:36–42. [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA et al. . Abdominal fat distribution in pre- and postmenopausal women: the impact of physical activity, age, and menopausal status. Metab Clin Exp 2001;50:976–982. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Fried SK, Xie H, Lee M-J, Divoux A, Rosencrantz MA, Chang RJ, Smith SR. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab 2013;98:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol 2015;11:90–100. [DOI] [PubMed] [Google Scholar]
- Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Desai M, Ross MG. Central insulin sensitivity in male and female juvenile rats. Horm Behav 2009;56:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitt SK, Wagner CR, Tong C, Marts SA. Understanding the biology of sex and gender differences: using subgroup analysis and statistical design to detect sex differences in clinical trials. Med Gen Med 2003;5:39. [PubMed] [Google Scholar]
- Kiernan M, Winkleby MA. Identifying patients for weight-loss treatment: an empirical evaluation of the NHLBI obesity education initiative expert panel treatment recommendations. Arch Intern Med 2000;160:2169–2176. [DOI] [PubMed] [Google Scholar]
- Kim JS, Rizwan MZ, Clegg DJ, Anderson GM. Leptin signaling is not required for anorexigenic estradiol effects in female mice. Endocrinology 2016;157:1991–2001. [DOI] [PubMed] [Google Scholar]
- King JA, Wasse LK, Ewens J, Crystallis K, Emmanuel J, Batterham RL, Stensel DJ. Differential acylated ghrelin, peptide YY3-36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J Clin Endocrinol Metab 2011;96:1114–1121. [DOI] [PubMed] [Google Scholar]
- Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight Management and Obesity Prevention, NAASO, The Obesity Society, American Society for Nutrition, et al . Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 2007;85:1197–1202. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med 2010;40:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine S, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. Int J Eat Disord 2013;46:729–736. [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S, Keel PK. Ovarian hormone influences on dysregulated eating: a comparison of associations in women with versus without binge episodes. Clin Psychol Sci 2014;2:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Lee HS, Lim JS, Kim SM, Hwang JS. Changes in bone mineral density and body composition in children with central precocious puberty and early puberty before and after one year of treatment with GnRH agonist. Horm Res Paediatr 2011;75:174–179. [DOI] [PubMed] [Google Scholar]
- Konarzewski M, Książek A. Determinants of intra-specific variation in basal metabolic rate. J Comp Physiol B Biochem Syst Environ Physiol 2013;183:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Marcus CL, Kim C, Gallagher PR, Schwab R, Bradford RM, Zemel BS. Anthropometric predictors of visceral adiposity in normal-weight and obese adolescents. Pediatr Diabetes 2013;14:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA et al. . Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 2011;60:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 2013;18:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Bethea CL. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience 2003;122:757–772. [DOI] [PubMed] [Google Scholar]
- Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab 2010;95:E468–E472. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome. Diabetes Care 2006;29:679–684. [DOI] [PubMed] [Google Scholar]
- Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun 2014;5:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DSN, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr 2007;86:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 2014;63:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê K-A, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, Beale E, Xie C, Greenberg AS, Allayee H et al. . Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway. Diabetes 2011;60:2802–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Mattson MP. The neuropathology of obesity: insights from human disease. Acta Neuropathol 2014;127:3–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré F, Godin G, Guilbert E, Laperrière L, Dodin S. Determinants of the intention to adopt hormone replacement therapy among premenopausal women. Maturitas 2000;34:211–218. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–628. [DOI] [PubMed] [Google Scholar]
- Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how. Endocrinology 2011;152:2552–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab 2004;286:E675–E685. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999;283:212–214. [DOI] [PubMed] [Google Scholar]
- Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 1992;55:950–954. [DOI] [PubMed] [Google Scholar]
- Li ET, Tsang LB, Lui SS. Resting metabolic rate and thermic effects of a sucrose-sweetened soft drink during the menstrual cycle in young Chinese women. Can J Physiol Pharmacol 1999;77:544–550. [PubMed] [Google Scholar]
- Lim S, Wyker B, Bartley K, Eisenhower D. Measurement error of self-reported physical activity levels in New York City: assessment and correction. Am J Epidemiol 2015;181:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm A, Blomquist C, Bixo M, Dahlbom I, Hansson T, Sundström Poromaa I, Burén J. No difference in markers of adipose tissue inflammation between overweight women with polycystic ovary syndrome and weight-matched controls. Hum Reprod 2011;26:1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MB, Robson PJ, McCarthy S, Kiely M, Harrington K, Browne P, Galvin M, Wareham NJ, Rennie KL. Physical activity patterns in a nationally representative sample of adults in Ireland. Public Health Nutr 2001;4:1107–1116. [DOI] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J et al. . Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LM, Edelman A, Chen M, Otterness C, Trussell J, Helmerhorst FM. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev 2013;7:CD008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab 2015;26:411–421. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007;56:16–23. [DOI] [PubMed] [Google Scholar]
- Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr 1989;49:1164–1168. [DOI] [PubMed] [Google Scholar]
- Madsen AM, El-Nashar SA, Hopkins MR, Khan Z, Famuyide AO. Endometrial ablation for the treatment of heavy menstrual bleeding in obese women. Int J Gynaecol Obstet 2013;121:20–23. [DOI] [PubMed] [Google Scholar]
- Mannerås-Holm L, Benrick A, Stener-Victorin E. Gene expression in subcutaneous adipose tissue differs in women with polycystic ovary syndrome and controls matched pair-wise for age, body weight, and body mass index. Adipocyte 2014;3:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ et al. . Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Kaunitz AM. Menopause management--getting clinical care back on track. N Engl J Med 2016;374:803–806. [DOI] [PubMed] [Google Scholar]
- Marino L, Jornayvaz FR. Endocrine causes of nonalcoholic fatty liver disease. World J Gastroenterol 2015;21:11053–11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Morentin PB, González-García I, Martins L, Lage R, Fernández-Mallo D, Martínez-Sánchez N, Ruíz-Pino F, Liu J, Morgan DA, Pinilla L et al. . Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 2014;20:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Abrams B, Crawford S, Miles T, Neer R, Powell LH, Wesley D. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord 2001;25:863–873. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 2011;22:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci 2014;111:E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci 2012;32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney CR, Marshall JC. Clinical practice. Polycystic ovary syndrome. N Engl J Med 2016;375:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Cornier M-A, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport 2013;24:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson EL, Gavin KM, Shea KL, Wolfe P, Wierman ME, Schwartz RS, Kohrt WM. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol 2015;119:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melanson KJ, Saltzman E, Russell R, Roberts SB. Postabsorptive and postprandial energy expenditure and substrate oxidation do not change during the menstrual cycle in young women. J Nutr 1996;126:2531–2538. [DOI] [PubMed] [Google Scholar]
- Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm 2014;2014:615917–615920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JR, Stout JR, Walter AA, Smith AE, Stock MS, Herda TJ, Sherk VD, Young KC, Lockwood CM, Kendall KL et al. . Mechanical scale and load cell underwater weighing: a comparison of simultaneous measurements and the reliability of methods. J Strength Cond Res 2011;25:652–661. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 2001;21:5304–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014;19:741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 2007;104:2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 1999;13:946–957. [DOI] [PubMed] [Google Scholar]
- Nookaew I, Svensson P-A, Jacobson P, Jernås M, Taube M, Larsson I, Andersson-Assarsson JC, Sjöström L, Froguel P, Walley A et al. . Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab 2013;98:E370–E378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RJ, Flight IH, Rees MC. Oestrogen and progestogen hormone replacement therapy for peri-menopausal and post-menopausal women: weight and body fat distribution. Cochrane Database Syst Rev 2000;2:CD001018. [DOI] [PubMed] [Google Scholar]
- O'Rahilly S, Farooqi IS. Genetics of obesity. Philos Trans R Soc Lond B Biol Sci 2006;361:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode JJ, Pivarnik JM, Reeves MJ, Knous JL. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc 2007;39:403–409. [DOI] [PubMed] [Google Scholar]
- ODPHP (Office of Disease Prevention and Health Promotion, US Dept Health and Human Services) Physical Activity Guidelines, Appendix 1, 2016. https://health.gov/paguidelines/.
- Ohlstein JF, Strong AL, McLachlan JA, Gimble JM, Burow ME, Bunnell BA. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J Mol Endocrinol 2014;53:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci 2009;106:15932–15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanecka-Glinianowicz M, Kuglin D, Dąbkowska-Huć A, Skałba P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2011;154:51–56. [DOI] [PubMed] [Google Scholar]
- Olszanecka-Glinianowicz M, Madej P, Nylec M, Owczarek A, Szanecki W, Skałba P, Chudek J. Circulating apelin level in relation to nutritional status in polycystic ovary syndrome and its association with metabolic and hormonal disturbances. Clin Endocrinol (Oxf) 2013;79:238–242. [DOI] [PubMed] [Google Scholar]
- Oosthuyse T, Bosch AN. Oestrogen's regulation of fat metabolism during exercise and gender specific effects. Curr Opin Pharmacol 2012;12:363–371. [DOI] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panotopoulos G, Ruiz JC, Raison J, Guy-Grand B, Basdevant A. Menopause, fat and lean distribution in obese women. Maturitas 1996;25:11–19. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Rizzo MR, Mazziotti G, Rotondi M, Tagliamonte MR, Varricchio G, Carella C, Varricchio M. Lack of association between changes in plasma leptin concentration and in food intake during the menstrual cycle. Eur J Clin Invest 1999;29:490–495. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Mietus LJ, Frumar AM, Davidson BJ, Judd HL. Effects of human serum on transport of testosterone and estradiol into rat brain. Am J Physiol 1980;239:E103–E108. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab 2004;89:1869–1878. [DOI] [PubMed] [Google Scholar]
- Pelkman CL, Chow M, Heinbach RA, Rolls BJ. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr 2001;73:19–26. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23:90–119. [DOI] [PubMed] [Google Scholar]
- Phelan S, Wyatt HR, Hill JO, Wing RR. Are the eating and exercise habits of successful weight losers changing. Obesity (Silver Spring) 2006;14:710–716. [DOI] [PubMed] [Google Scholar]
- Phillips GB, Jing T, Heymsfield SB. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metab Clin Exp 2008;57:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichika R, Buchsbaum MS, Bailer U, Hoh C, Decastro A, Buchsbaum BR, Kaye W. Serotonin transporter binding after recovery from bulimia nervosa. Int J Eat Disord 2012;45:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers LS, Diggavi SN, Rijskamp J, van Raaij JM, Shetty PS, Hautvast JG. Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr 1995;61:296–302. [DOI] [PubMed] [Google Scholar]
- Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes 2012;61:1399–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KGM, Tjønneland A et al. . General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120. [DOI] [PubMed] [Google Scholar]
- Pohle-Krauza RJ, Carey KH, Pelkman CL. Dietary restraint and menstrual cycle phase modulated L-phenylalanine-induced satiety. Physiol Behav 2008;93:851–861. [DOI] [PubMed] [Google Scholar]
- Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Semin Reprod Med 2010;28:426–434. [DOI] [PubMed] [Google Scholar]
- Porcu P, Barron AM, Frye CA, Walf AA, Yang S-Y, He X-Y, Morrow AL, Panzica GC, Melcangi RC. Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol 2016;28:12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994;73:460–468. [DOI] [PubMed] [Google Scholar]
- Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology 2009;89:38–47. [DOI] [PubMed] [Google Scholar]
- Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, van Beek EJR, Morton NM, Walker BR, Stimson RH. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 2016;24:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318:467–472. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Leibel RL, Ferrante AW. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab 2014;20:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr 1996;63:164–169. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Røysamb E, Maes H, Tambs K, Harris JR. Gender differences in binge-eating: a population-based twin study. Acta Psychiatr Scand 2003;108:196–202. [DOI] [PubMed] [Google Scholar]
- Rock CL, Gorenflo DW, Drewnowski A, Demitrack MA. Nutritional characteristics, eating pathology, and hormonal status in young women. Am J Clin Nutr 1996;64:566–571. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009;150:2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014;156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Clinical review 107: role of gonadal steroids in the sexual dimorphisms in body composition and circulating concentrations of leptin. J Clin Endocrinol Metab 1999;84:1784–1789. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Pietrobelli A, Vasselli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentrations is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord 2001;25:1365–1371. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–421. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Gonadal steroids, brain, and behavior: role of context. Dialogues Clin Neurosci 2002;4:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 2014;39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 2011;60:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani KP, Hamer M, Mindell JS, Coombs NA, Stamatakis E. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes Care 2014;37:1016–1023. [DOI] [PubMed] [Google Scholar]
- Saito M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab J 2013;37:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem V, Dhillo WS. Imaging in endocrinology: the use of functional MRI to study the endocrinology of appetite. Eur J Endocrinol 2015;173:R59–R68. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M et al. . Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab 2010;95:s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa S, Hensrud DD, Votruba SB, Jensen MD. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am J Clin Nutr 2008;88:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne) 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry 2015;72:714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev 2015;95:853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Gantz M, Velasquez G, Punyanitya M, Heymsfield SB. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity (Silver Spring) 2012;20:2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Silva AM, Chen J, Gallagher D, Sardinha LB, Allison DB, Heymsfield SB. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study. Nutr Metab (Lond) 2009;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 2003;11:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard KN, Michopoulos V, Toufexis DJ, Wilson ME. Genetic, epigenetic and environmental impact on sex differences in social behavior. Physiol Behav 2009;97:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Berthoud H-R. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav 2009;97:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE et al. . New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver HJ, Welch EB, Avison MJ, Niswender KD. Imaging body composition in obesity and weight loss: challenges and opportunities. Diabetes Metab Syndr Obes 2010;3:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res 2005;166:345–357. [DOI] [PubMed] [Google Scholar]
- Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab 2013;305:E632–E640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GI, Reeds DN, Okunade AL, Patterson BW, Mittendorfer B. Systemic delivery of estradiol, but not testosterone or progesterone, alters very low density lipoprotein-triglyceride kinetics in postmenopausal women. J Clin Endocrinol Metab 2014;99:E1306–E1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Lovejoy JC, Greenway F, Ryan D, de Jonge L, la Bretonne de J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab Clin Exp 2001;50:425–435. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord 2004;28:402–409. [DOI] [PubMed] [Google Scholar]
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 2009;48:143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søndergaard E, Gormsen LC, Nellemann B, Jensen MD, Nielsen S. Body composition determines direct FFA storage pattern in overweight women. Am J Physiol Endocrinol Metab 2012;302:E1599–E1604. [DOI] [PubMed] [Google Scholar]
- Sone M, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res 2001;305:351–356. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Crutchfield M, Jannausch ML, Russell-Aulet M. Longitudinal changes in body composition in women approaching the midlife. Ann Hum Biol 1996;23:253–265. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Vitale C, Malorni W, Rosano GMC. Sex differences in drug effects: interaction with sex hormones in adult life. Handb Exp Pharmacol 2012;214:91–105. [DOI] [PubMed] [Google Scholar]
- St-Onge M-P, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation. Nutrition 2010;26:152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert R, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Nori Geary N. Ghrelin, CCK, GLP-1 and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity and after RYGB. Physiol Rev 2016. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 2010;57:36–42. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–647. [DOI] [PubMed] [Google Scholar]
- Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O'Rahilly S et al. . Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 2008;57:2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 2005;13:2072–2080. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Shearin J, Koegler FH, Cameron JL. Selective estrogen receptor modulator promotes weight loss in ovariectomized female rhesus monkeys (Macaca mulatta) by decreasing food intake and increasing activity. Am J Physiol Endocrinol Metab 2012;302:E759–E767. [DOI] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 2013;18:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metab Clin Exp 1995;44:369–373. [DOI] [PubMed] [Google Scholar]
- Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem Biophys Res Commun 2002;291:1208–1212. [DOI] [PubMed] [Google Scholar]
- Tai MM, Castillo TP, Pi-Sunyer FX. Thermic effect of food during each phase of the menstrual cycle. Am J Clin Nutr 1997;66:1110–1115. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 2012;81:715–736. [DOI] [PubMed] [Google Scholar]
- Tao Y-X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 2010;31:506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 2010;18:1410–1416. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013;17:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci 2010;107:18226–18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersigni C, Di Nicuolo F, D'Ippolito S, Veglia M, Castellucci M, Di Simone N. Adipokines: new emerging roles in fertility and reproduction. Obstetr Gynecol Surv 2011;66:47–63. [DOI] [PubMed] [Google Scholar]
- Tobias JH, Steer CD, Vilarino-Güell C, Brown MA. Effect of an estrogen receptor-alpha intron 4 polymorphism on fat mass in 11-year-old children. J Clin Endocrinol Metab 2007;92:2286–2291. [DOI] [PubMed] [Google Scholar]
- Tom SE, Cooper R, Patel KV, Guralnik JM. Menopausal characteristics and physical functioning in older adulthood in the National Health and Nutrition Examination Survey III. Menopause 2012;19:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Leal FL, Fonseca-Alaniz MH, Rogero MM, Tirapegui J. The role of inflamed adipose tissue in the insulin resistance. Cell Biochem Funct 2010;28:623–631. [DOI] [PubMed] [Google Scholar]
- Trémollieres FA, Pouilles JM, Ribot CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol 1996;175:1594–1600. [DOI] [PubMed] [Google Scholar]
- van der Sluis IM, Boot AM, Krenning EP, Drop SLS, de Muinck Keizer-Schrama SMPF. Longitudinal follow-up of bone density and body composition in children with precocious or early puberty before, during and after cessation of GnRH agonist therapy. J Clin Endocrinol Metab 2002;87:506–512. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Dinneno FA, Seals DR, Jones PP. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab 2001;281:E633–E639. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Gavin KM, Kohrt WM. Regulation of body composition and bioenergetics by estrogens. Endocrinol Metab Clin North Am 2015;44:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vague J. La Differenciation Sexuelle Facteur Determinant Des Formes De Lobesite. Presse Med 1947;55:339–340. [PubMed] [Google Scholar]
- Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, Alonso-Alonso M, Audette M, Malbert CH, Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin 2015;8:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valette M, Bellisle F, Carette C, Poitou C, Dubern B, Paradis G, Hercberg S, Muzard L, Clément K, Czernichow S. Eating behaviour in obese patients with melanocortin-4 receptor mutations: a literature review. Int J Obes (Lond) 2013;37:1027–1035. [DOI] [PubMed] [Google Scholar]
- Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 2012;153:4266–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep 2011;11:179–184. [DOI] [PubMed] [Google Scholar]
- Vogel CIG, Boes T, Reinehr T, Roth CL, Scherag S, Scherag A, Hebebrand J, Hinney A. Common variants near MC4R: exploring gender effects in overweight and obese children and adolescents participating in a lifestyle intervention. Obes Facts 2011;4:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 2012;354:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba SB, Jensen MD. Sex differences in abdominal, gluteal, and thigh LPL activity. Am J Physiol Endocrinol Metab 2007;292:E1823–E1828. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A et al. . Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 2004;21:1790–1797. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PWF, Sutherland P, Omland T, Vasan RS. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–258. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005;81:555–563. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Heller M, Later W, Heymsfield SB, Müller MJ. Evaluation of specific metabolic rates of major organs and tissues: comparison between men and women. Am J Hum Biol 2011;23:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters B, Lass N, Reinehr T. Treatment with gonadotropin-releasing hormone analogues: different impact on body weight in normal-weight and overweight children. Horm Res Paediatr 2012;78:304–311. [DOI] [PubMed] [Google Scholar]
- Woolf K, Reese CE, Mason MP, Beaird LC, Tudor-Locke C, Vaughan LA. Physical activity is associated with risk factors for chronic disease across adult women's life cycle. J Am Diet Assoc 2008;108:948–959. [DOI] [PubMed] [Google Scholar]
- Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO, Zou F et al. . Estrogen receptor-α in medial amygdala neurons regulates body weight. J Clin Invest 2015;125:2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD et al. . Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Geary N, Corwin RL. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol Behav 2011;104:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L et al. . Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteal function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates In: Niell JD (ed).. Knobil and Neill's Physiology of Reproduction, 3rd edn Amsterdam: Elsevier/Academic, 2006, 2449–2510. [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 2003;100:2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]