Abstract
Background
Epidemiologic evidence has emerged to reveal an association of albuminuria and low estimated glomerular filtration rate (eGFR) with dementia, but the findings are inconsistent. In addition, there are limited studies addressing the association between albuminuria and Alzheimer disease (AD).
Methods and Results
A total of 1562 community‐dwelling Japanese subjects aged ≥60 years without dementia were followed up for 10 years. The outcomes were incidence of all‐cause dementia and its subtypes, namely, AD and vascular dementia (VaD). The hazard ratios for the outcomes were estimated according to urine albumin–creatinine ratio (UACR) and eGFR levels using a Cox proportional hazards model. During the follow‐up, 358 subjects developed all‐cause dementia (238 AD and 93 VaD). Higher UACR level was significantly associated with greater multivariable‐adjusted risks of all‐cause dementia (hazard ratios [95% confidence intervals]: 1.00 [reference], 1.12 [0.78–1.60], 1.65 [1.18–2.30], and 1.56 [1.11–2.19] for UACR of ≤6.9, 7.0–12.7, 12.8–29.9, and ≥30.0 mg/g, respectively), AD (1.00 [reference], 1.20 [0.77–1.86], 1.75 [1.16–2.64], and 1.58 [1.03–2.41], respectively), and VaD (1.00 [reference], 1.03 [0.46–2.29], 1.94 [0.96–3.95], and 2.19 [1.09–4.38], respectively). On the other hand, lower eGFR level was marginally associated with greater risk of VaD, but not AD. Subjects with UACR ≥12.8 mg/g and eGFR of <60 mL/min per 1.73 m2 had 3.3‐fold greater risk of VaD than those with UACR <12.8 mg/g and eGFR of ≥60 mL/min per 1.73 m2.
Conclusions
Albuminuria is a significant risk factor for the development of both AD and VaD in community‐dwelling Japanese elderly. Moreover, albuminuria and low eGFR are mutually associated with a greater risk of VaD.
Keywords: albuminuria, Alzheimer disease, estimated glomerular filtration rate, prospective cohort study, vascular dementia
Subject Categories: Epidemiology
Clinical Perspective
What Is New?
This community‐based prospective cohort study revealed a significant association between albuminuria and risk for the development of all‐cause dementia, Alzheimer disease, and vascular dementia.
The combined influence of albuminuria and low estimated glomerular filtration rate conferred increased risk for the development of vascular dementia, but not Alzheimer disease.
What Are the Clinical Implications?
The findings of this study indicate that subjects with albuminuria should be considered a high‐risk population for Alzheimer disease as well as vascular dementia, and those with concurrent low estimated glomerular filtration rate should be considered a more severe high‐risk population for vascular dementia.
Further studies are warranted to determine whether albuminuria and low estimated glomerular filtration rate are risk factors for dementia independent of each other.
Introduction
Dementia is a worldwide priority both in terms of public health and social care because of the rapidly increasing burdens it places on communities. Approximately 46.8 million people worldwide have dementia, and there are 9.9 million new cases per year.1 Moreover, the number of people living with dementia will nearly double every 20 years.1 Although the causes of dementia, especially Alzheimer disease (AD), are gradually being revealed, they are still incompletely understood. Therefore, the early identification of possible risk factors for dementia and the establishment of preventive and treatment strategies for dementia have become increasingly important.2
Chronic kidney disease, which is characterized by albuminuria or proteinuria, and estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2, is also increasingly recognized as a substantial public health problem. Since the prevalence of chronic kidney disease has been increasing in the elderly,3 we must determine its influence on the risk of dementia. To date, several prospective epidemiologic studies have explored the association of albuminuria and low eGFR with cognitive decline4, 5, 6, 7 and dementia.8, 9 Most of these studies assessed cognitive decline or dementia by using only a simple cognitive score (eg, the Mini Mental State Examination), but few studies examined the development of dementia and its subtypes, especially AD and vascular dementia (VaD), on the basis of detailed neurologic and morphologic examination. In addition, there have been few studies investigating the mutual association between albuminuria and low eGFR on the development of dementia.
The Hisayama Study is a population‐based cohort study conducted to explore the risk factors for cardiovascular disease and dementia in a general Japanese population.10, 11 The most important feature of the study is that the dementia subtypes were diagnosed by detailed neurologic and morphologic examination, including neuroimaging and autopsy. The purpose of the present study was to assess the influence of albuminuria and low eGFR on the development of dementia and its subtypes, which were determined on the basis of precise information, in an elderly population enrolled in the Hisayama Study.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
A population‐based prospective study of cerebro‐ and cardiovascular diseases was begun in 1961 in the town of Hisayama, which is located in a suburb of the Fukuoka metropolitan area on Kyushu Island, Japan. The population of the town in 2016 was ≈8500. Full community surveys of the residents have been repeated since 1961.10 Comprehensive surveys of cognitive impairment in the elderly of this town have been conducted since 1985.11 In 2002 and 2003, 1760 residents aged 60 and older (participation rate 83.4%) underwent a screening examination for the present study.12 After excluding 122 subjects who already had dementia at baseline, 31 for whom no urine sample was obtained, 1 for whom no blood sample was obtained, and 44 for whom no educational information was obtained, the remaining 1562 subjects (672 men, 890 women) were enrolled in this study.
Follow‐Up Survey
The subjects were followed prospectively for 10 years, from when they underwent a screening examination to November 2012 (median 10.2 years; interquartile range 7.7–10.3 years), during which time health examinations were performed every year. Details of the methods for screening potential dementia events have been reported elsewhere.13, 14 The postal service or telephone was used to collect the health information of subjects who did not have examinations or who had moved out of town. To gather information about new events, including stroke, cognitive impairment, and dementia, we established a daily monitoring system among the study team and local physicians or members of the town's Health and Welfare Office. We also acquired new events of stroke and dementia missed by the monitoring system at regular annual health examinations. Follow‐up screening surveys of cognitive function, including neuropsychological tests, the Hasegawa Dementia Scale‐Revised,15 or the Mini Mental State Examination,16 were conducted in 2005 and 2012 to detect dementia cases precisely. When a subject was suspected to have new neurologic symptoms, including cognitive impairment, the study team, which consisted of stroke physicians and psychiatrists, carefully evaluated the subject by conducting comprehensive investigations including interviews of the family or attending physician, physical and neurologic examinations, and a review of the clinical records. When a subject died, we reviewed all available clinical information, interviewed the attending physician and the family of the deceased subject, and tried to obtain permission for an autopsy from the family. During follow‐up, 326 subjects died; of those, 215 subjects underwent a brain examination at autopsy. No subjects were lost to follow‐up.
Diagnosis of Dementia
The guidelines of the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition, were used to define the diagnosis of dementia.17 Subjects diagnosed with AD met the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association,18 and subjects diagnosed with VaD met the criteria of the National Institute of Neurological Disorders and Stroke‐Association Internationale pour la Recherche et l'Enseignement en Neurosciences.19 Clinical information, including neuroimaging, was used to diagnose possible and probable dementia subtypes. Definite dementia subtypes were also determined on the basis of clinical and neuropathologic information in subjects with dementia who underwent autopsy. The diagnostic procedure for autopsy cases has been previously reported.20 For each autopsy case, we evaluated 15 areas of the brain: namely, the middle frontal gyrus, precentral gyrus, temporal lobe, parietal lobe, occipital lobe, basal ganglia, thalamus, hippocampus, amygdala, orbital gyri, temporal pole, cerebellar hemisphere, midbrain, pons, and medulla oblongata. Each of the specimens was cut into 8‐ to 15‐mm sections except for the brainstem, which was cut into 5‐mm sections, and the microinfarcts and bleeding were carefully evaluated in each section. A neuropathologic diagnosis of AD was made following the National Institute on Aging―Reagan Institute criteria21; the frequencies of neuritic plaques and neurofibrillary tangles were evaluated using the Consortium to Establish a Registry for Alzheimer's Disease criteria22 and Braak stage.23 Definite VaD cases were confirmed with causative stroke or cerebrovascular change and no neuropathologic evidence of other forms of dementia. Expert stroke physicians and psychiatrists adjudicated every case of dementia.
During the 10 years of follow‐up, 358 subjects (132 men, 226 women) developed dementia. 319 subjects (89.1%) were evaluated using brain imaging (129 [36.0%] brain magnetic resonance image alone, 68 [19.0%] brain computed tomography alone, and 122 [34.1%] both brain magnetic resonance image and computed tomography), 87 (24.3%) underwent autopsy, and both were performed in 77 (21.5%). Therefore, 329 subjects (91.9%) had some kind of morphologic examination. Of subjects with dementia, 21 AD cases and 22 VaD cases had other coexisting subtypes of dementia, 16 of which were a mixed type of AD and VaD. These cases were counted as events in the analyses for each subtype. Finally, 238 subjects experienced AD, 93 subjects experienced VaD, and 43 subjects experienced other subtypes of dementia. Other subtypes of dementia included 43 cases of the following subtypes: 8 cases of dementia with Lewy bodies, 10 cases of senile dementia of the neurofibrillary tangle type, 4 cases of trauma‐induced dementia, 2 cases of alcohol‐induced dementia, 2 cases of progressive supranuclear palsy, 1 case of frontotemporal lobar degeneration, 1 case of acute subdural hematoma, 1 case of metastatic brain tumor, 1 case of hypoxic ischemic encephalopathy, 1 case of motor neuron disease, and 12 cases of unknown causes.
Albuminuria and Kidney Function
Fresh voided urine samples were collected at the health examination, and urine creatinine and albumin were measured using the turbidimetric immunoassay method. Urine albumin‐creatinine ratio (UACR) (mg/g) was calculated by dividing urinary albumin values by the urinary creatinine concentrations. UACR was categorized as normoalbuminuria (UACR <30 mg/g) and albuminuria (UACR ≥30 mg/g) using the cut‐off point from the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.24 UACR in the normoalbuminuria range was further divided into the following tertile categories: low‐normal (≤6.9 mg/g), medium‐normal (7.0–12.7 mg/g), and high‐normal (12.8–29.9 mg/g). For the sensitivity analysis using age‐ and sex‐specific UACR level, the UACR level was recategorized by using sex‐specific criteria of albuminuria (men: ≥20.0 mg/g; women: ≥30.0 mg/g)25 and the tertile categories of normoalbuminuria level according to age (10‐year interval) and sex.
Serum creatinine (SCr) concentrations were measured using the enzymatic method. eGFR was calculated using the following Chronic Kidney Disease Epidemiology Collaboration equation with the Japanese coefficient26 of 0.813, where SCr is serum creatinine, κ is 0.7 for women, and 0.9 for men, α is −0.329 for women and −0.411 for men, min (SCr/κ, 1) indicates the minimum of SCr/κ or 1, and max (SCr/κ, 1) indicates the maximum of SCr/κ or 1:
Low eGFR was defined as eGFR <60 mL/min per 1.73 m2 according to the same KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.24
Risk Factors
At the baseline, a self‐administered questionnaire covering information on educational status, smoking habits, alcohol intake, regular exercise, and antihypertensive and antidiabetic treatments was completed for each subject and checked by trained interviewers. History of stroke was defined as a preexisting sudden onset of nonconvulsive and focal neurologic deficit persisting for >24 hours on the basis of all available clinical data. Educational level was categorized as 0 to 6, 7 to 9, 10 to 12, and ≥13 years of formal education. Smoking habits and alcohol intake were categorized as being either current habitual or not. Subjects engaging in sports or other forms of exercise ≥3 times per week during their leisure time were defined as having regular exercise. Blood samples were collected after an overnight fast. Diabetes mellitus was defined as a fasting plasma glucose level of ≥7.0 mmol/L, 2‐hour 75 g oral glucose postloaded or casual glucose levels of ≥11.1 mmol/L, and/or current use of oral glucose‐lowering agents or insulin. Total cholesterol was measured enzymatically.
Blood pressure was measured 3 times with patients in the sitting position using an automated sphygmomanometer after at least 5 minutes of rest. The mean of the 3 measurements was used for the present analysis. Hypertension was defined as blood pressure ≥140/90 mm Hg and/or current use of antihypertensive agents. Body height and weight were measured in light clothing without shoes, and body mass index (kg/m2) was calculated.
Statistical Analysis
The linear trends in the means and frequencies of risk factors across UACR and eGFR levels were tested using a linear regression analysis and a logistic regression analysis, respectively. The linear trend tests were examined as an ordinal variable representing the 4 categories of UACR and the 2 categories of eGFR. UACR was logarithmically transformed, because its distribution was skewed. Kaplan‐Meier curves were used to estimate the cumulative incidence of all‐cause dementia and to compare data between UACR and eGFR levels. A Cox proportional hazards model was used to estimate the hazard ratio (HR) with 95% confidence interval (CI) for the development of all‐cause dementia and its subtypes (ie, AD, VaD, and their composite) according to the UACR and eGFR levels. We evaluated 3 different models: (1) model 1, adjusted for age and sex; (2) model 2, adjusted for model 1 plus educational level, history of stroke, systolic blood pressure, the use of antihypertensive agents, diabetes mellitus, total cholesterol, body mass index, smoking habits, alcohol intake, and regular exercise; and (3) model 3, adjusted for model 2 plus log UACR or eGFR. The sensitivity analyses were also performed by using the method of Fine and Gray27 in order to take into account the competing risk of death. We also assessed the association between UACR level and the risk of VaD with and without prior stroke. All data analyses were done with SAS version 9.4 (SAS Institute, Cary, NC) for statistical computing. A 2‐tailed value of P<0.05 was considered statistically significant in all analyses.
Ethical Consideration
This study was conducted with the approval of the Kyushu University Institutional Board for Clinical Research. Written informed consent was obtained from all subjects.
Results
Baseline characteristics of the study subjects according to the UACR level are listed in Table 1. The mean values of age, systolic blood pressure, diastolic blood pressure, body mass index, and the frequencies of women, antihypertensive agents, diabetes mellitus, and history of stroke increased with higher UACR level, while subjects with higher UACR level had lower eGFR and were less likely to have higher formal education, smoking habits, and alcohol intake. Baseline characteristics according to the eGFR level are listed in Table S1. Lower eGFR level was associated with higher mean values of age, systolic blood pressure, and UACR, and higher frequencies of antihypertensive agents and history of stroke, while the frequency of women decreased at lower eGFR level.
Table 1.
Baseline Characteristics of Subjects According to UACR Level, the Hisayama Study, 2002
| Variable | UACR, mg/g | P for Trend | |||
|---|---|---|---|---|---|
| ≤6.9 (n=386) | 7.0 to 12.7 (n=372) | 12.8 to 29.9 (n=391) | ≥30.0 (n=413) | ||
| Age, y | 69 (7) | 70 (7) | 72 (8) | 73 (8) | <0.001 |
| Women, % | 43.8 | 60.0 | 68.0 | 56.2 | <0.001 |
| Formal education | 0.02a | ||||
| 0–6 y, % | 8.3 | 6.2 | 8.4 | 9.7 | |
| 7–9 y, % | 38.3 | 41.4 | 47.6 | 49.4 | |
| 10–12 y, % | 42.0 | 41.9 | 34.5 | 32.7 | |
| ≥13 y, % | 11.4 | 10.5 | 9.5 | 8.2 | |
| Systolic blood pressure, mm Hg | 129 (20) | 133 (19) | 138 (18) | 148 (21) | <0.001 |
| Diastolic blood pressure, mm Hg | 76 (10) | 78 (11) | 79 (11) | 83 (12) | <0.001 |
| Antihypertensive agent, % | 22.5 | 27.4 | 39.6 | 51.6 | <0.001 |
| Hypertension, % | 39.9 | 51.1 | 63.9 | 78.5 | <0.001 |
| Diabetes mellitus, % | 15.0 | 18.8 | 21.7 | 32.2 | <0.001 |
| Total cholesterol, mmol/L | 5.16 (0.88) | 5.35 (0.86) | 5.21 (0.94) | 5.24 (0.89) | 0.66 |
| Body mass index, kg/m² | 22.6 (2.9) | 23.1 (3.0) | 23.1 (3.4) | 23.4 (3.4) | <0.001 |
| Obesity, % | 21.5 | 25.0 | 27.9 | 32.7 | <0.001 |
| eGFR, mL/min per 1.73 m² | 74 (9) | 74 (9) | 72 (10) | 68 (16) | <0.001 |
| History of stroke, % | 3.1 | 3.8 | 4.4 | 8.2 | 0.001 |
| Smoking habits, % | 19.2 | 13.7 | 12.3 | 14.0 | 0.04 |
| Alcohol intake, % | 43.5 | 37.6 | 28.6 | 35.1 | 0.002 |
| Regular exercise, % | 11.1 | 15.1 | 15.9 | 10.2 | 0.74 |
Values are shown as means (SDs) or frequencies. eGFR indicates estimated glomerular filtration rate; UACR, urine albumin‐creatinine ratio.
P value for χ2 test.
Figure 1 and Figure S1 show the cumulative incidence of all‐cause dementia according to UACR and eGFR levels, respectively. The cumulative incidence of all‐cause dementia significantly increased with higher UACR level (P for trend <0.001) and lower eGFR level (P<0.001). Similar findings were observed for the cumulative incidence of AD and/or VaD according to UACR and eGFR levels (Figures S2 and S3).
Figure 1.
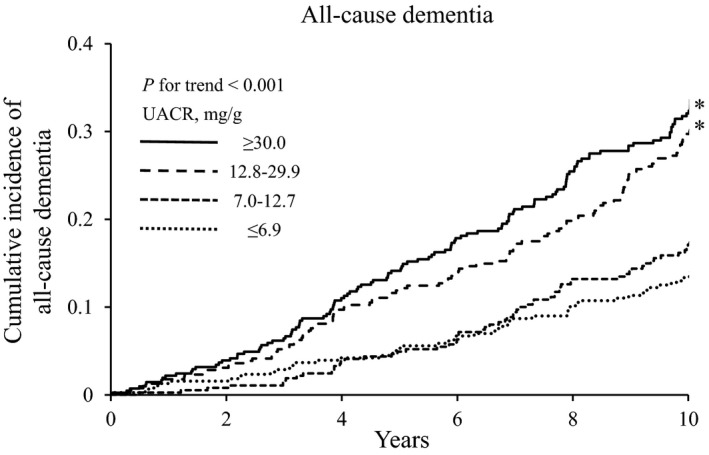
Cumulative incidence of all‐cause dementia according to urine albumin‐creatinine ratio level. *P<0.05 vs urine albumin‐creatinine ratio ≤6.9 mg/g. UACR indicates urine albumin‐creatinine ratio.
The age‐ and sex‐adjusted HR for the development of all‐cause dementia increased significantly with higher UACR level (P for trend <0.001, Table 2). This association was not altered substantially after adjustment for potential confounding factors: namely, age, sex, educational level, history of stroke, systolic blood pressure, the use of antihypertensive agents, diabetes mellitus, total cholesterol, body mass index, smoking habits, alcohol intake, and regular exercise (P for trend=0.001). In addition, further adjustment for eGFR also did not change the association (P for trend=0.002). Compared with subjects with UACR of ≤6.9 mg/g, the multivariable‐adjusted HR for the development of all‐cause dementia was 1.12 (95% CI, 0.78–1.60), 1.65 (1.18–2.30), and 1.56 (1.11–2.19) in those with UACR of 7.0 to 12.7 mg/g, 12.8 to 29.9 mg/g, and ≥30.0 mg/g, respectively. Similar linear association was observed for the development of AD (HR 1.20 [95% CI, 0.77–1.86] for UACR 7.0–12.7 mg/g, 1.75 [1.16–2.64] for UACR 12.8–29.9 mg/g, and 1.58 [1.03–2.41] for UACR ≥30 mg/g) and VaD (1.03 [0.46–2.29], 1.94 [0.96–3.95], and 2.19 [1.09–4.38], respectively). The multivariable risk for the development of AD and/or VaD also increased significantly with higher UACR level. The sensitivity analysis after excluding AD cases who were diagnosed using computed tomography or only clinical information (n=46) also showed a significant association between UACR level and the multivariable HR for AD (Table S2).
Table 2.
Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to UACR Level
| UACR, mg/g | Person‐Years at Risk, n | No. of Events, n | Model 1 HR (95% CI) | P for Trend | Model 2 HR (95% CI) | P for Trend | Model 3 | P for Trend |
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | ||||||||
| All‐cause dementia | ||||||||
| ≤6.9 | 3498 | 55 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | 0.001 | 1.00 (Reference) | 0.002 |
| 7.0–12.7 | 3429 | 65 | 1.10 (0.76–1.57) | 1.11 (0.77–1.60) | 1.12 (0.78–1.60) | |||
| 12.8–29.9 | 3279 | 109 | 1.66 (1.19–2.31)† | 1.64 (1.17–2.30)† | 1.65 (1.18–2.30)† | |||
| ≥30.0 | 3293 | 129 | 1.66 (1.20–2.29)† | 1.61 (1.15–2.26)† | 1.56 (1.11–2.19)* | |||
| Alzheimer disease | ||||||||
| ≤6.9 | 3498 | 35 | 1.00 (Reference) | 0.02 | 1.00 (Reference) | 0.01 | 1.00 (Reference) | 0.01 |
| 7.0–12.7 | 3429 | 47 | 1.19 (0.77–1.85) | 1.19 (0.77–1.86) | 1.20 (0.77–1.86) | |||
| 12.8–29.9 | 3279 | 76 | 1.68 (1.12–2.52)* | 1.75 (1.15–2.64)† | 1.75 (1.16–2.64)† | |||
| ≥30.0 | 3293 | 80 | 1.51 (1.01–2.27)* | 1.60 (1.05–2.45)* | 1.58 (1.03–2.41)* | |||
| Vascular dementia | ||||||||
| ≤6.9 | 3498 | 12 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | 0.004 | 1.00 (Reference) | 0.008 |
| 7.0–12.7 | 3429 | 12 | 0.99 (0.44–2.20) | 1.02 (0.46–2.27) | 1.03 (0.46–2.29) | |||
| 12.8–29.9 | 3279 | 26 | 2.08 (1.04–4.17)* | 1.94 (0.95–3.93) | 1.94 (0.96–3.95) | |||
| ≥30.0 | 3293 | 43 | 2.88 (1.50–5.54)† | 2.32 (1.16–4.62)* | 2.19 (1.09–4.38)* | |||
| Alzheimer disease and/or vascular dementia | ||||||||
| ≤6.9 | 3498 | 46 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | <0.001 | 1.00 (Reference) | 0.002 |
| 7.0–12.7 | 3429 | 58 | 1.16 (0.79–1.71) | 1.18 (0.80–1.74) | 1.18 (0.80–1.74) | |||
| 12.8–29.9 | 3279 | 97 | 1.75 (1.22–2.50)† | 1.75 (1.22–2.52)† | 1.75 (1.22–2.52)† | |||
| ≥30.0 | 3293 | 114 | 1.74 (1.22–2.46)† | 1.72 (1.19–2.48)† | 1.66 (1.15–2.39)† | |||
Model 1: adjusted for age and sex. Model 2: adjusted for Model 1 plus educational level, history of stroke, systolic blood pressure, the use of antihypertensive agents, diabetes mellitus, total cholesterol, body mass index, smoking habits, alcohol intake, and regular exercise. Model 3: adjusted for Model 2 plus eGFR. CI indicates confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; UACR, urine albumin‐creatinine ratio.
*P<0.05, † P<0.01 vs reference.
Lower eGFR level was marginally associated with the development of all‐cause dementia after adjustment for age and sex (HR 1.25 [95% CI, 0.96–1.63]) and potential confounding factors (1.12 [0.85–1.48]) (Table S3). With regard to subtypes of dementia, lower eGFR level was a significant risk factor for the development of VaD after adjustment for potential confounding factors (1.67 [1.02–2.74]), but this association did not reach the level of statistical significance after additional adjustment for log UACR (HR 1.48 [95% CI, 0.90–2.44]). There was no evidence of a significant association between eGFR level and the development of not only AD but also AD and/or VaD.
Sensitivity analyses using Fine and Gray models, which took into account the competing risk of death, did not significantly change the association of albuminuria and low eGFR with dementia (Tables S4 and S5). In addition, we addressed the association between age‐ and sex‐specific UACR level and the dementia risk (Table S6), and found a significant linear association of age‐ and sex‐specific UACR level with the risk of all‐cause dementia and its subtypes. To address the influence of prior stroke events on the association between albuminuria and VaD, we also estimated the association of UACR level with the risk of VaD with and without prior stroke events, separately. As a result, higher UACR level was significantly associated with a higher risk for the development of VaD with prior stroke events, but not for VaD without it (Table S7).
As shown in Figure 2, we assessed the combined influence of albuminuria and kidney function on the development of dementia subtypes. With regard to AD, UACR of ≥12.8 mg/g was associated with a significantly higher multivariable‐adjusted risk of AD than UACR of <12.8 mg/g among subjects with eGFR of ≥60 mL/min per 1.73 m2, but not among subjects with eGFR of <60 mL/min per 1.73 m2. Nonetheless, there was no evidence of an interaction between the UACR level and eGFR level on AD (P for interaction >0.12). With regard to VaD, higher UACR level and lower eGFR level were mutually associated with greater risk of VaD after adjustment for confounding factors. There was no evidence of an interaction between UACR level and eGFR level on VaD (P for interaction >0.68).
Figure 2.
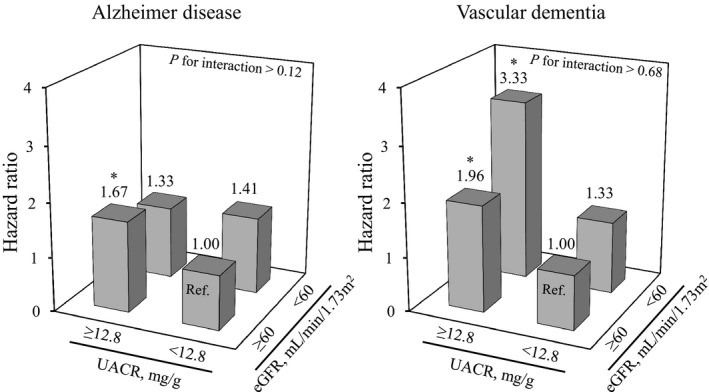
Combined influence of albuminuria and kidney function on the development of dementia subtypes. *P<0.05 vs reference. Adjusted for age, sex, educational level, history of stroke, systolic blood pressure, the use of antihypertensive agents, diabetes mellitus, total cholesterol, BMI, smoking habits, alcohol intake, and regular exercise. BMI indicates body mass index; UACR, urine albumin‐creatinine ratio.
Discussion
The present results clearly showed that albuminuria was significantly associated with higher risk for the development of all‐cause dementia, AD, and VaD independent of other potential risk factors for dementia. On the other hand, subjects with a lower eGFR level tended to have a higher risk of incident VaD. In addition, albuminuria and low eGFR mutually increased the risk of VaD, but not AD. To the best of our knowledge, this is the first study to prospectively demonstrate a clear association between albuminuria and the future risk of AD.
Several prospective epidemiologic studies have investigated the association between albuminuria and cognitive decline5, 6, 7 or the development of dementia.9 The Reasons for Geographic and Racial Disparities in Stroke Study reported that higher UACR level was associated with the development of cognitive impairment (defined as a 6‐Item Screener score ≤4) in 19 399 US individuals.7 The Rancho Bernardo Study found that only men with UACR of ≥30.0 mg/g had a significantly higher risk of cognitive decline determined by Mini Mental State Examination and category fluency scores during a 6.6‐year study period among 759 community‐based US individuals.5 The Three‐City Study demonstrated that proteinuria (defined as UACR >30.0 mg/g or proteinuria >300 mg/g) was associated with an increased risk of VaD, but not AD.9 However, Wang et al reported that albuminuria (defined as UACR ≥17 mg/g for men and ≥26 mg/g for women) was not associated with cognitive decline (defined as a Mini Mental State Examination score decrease of ≥2) during a 4‐year follow‐up.6 The possible reasons for the discrepancy include differences in the sample size and follow‐up period, since most of these studies had smaller sample size and shorter follow‐up period than the present study. Further studies with a large sample size and long follow‐up will be needed to explore the association between albuminuria and dementia.
Albuminuria is well recognized as a good biomarker for endothelial dysfunction and atherosclerotic vascular disease, including stroke.28, 29 As we therefore expected, the present study showed that higher UACR level was significantly associated with a higher risk for the development of VaD, especially VaD with prior stroke events. In addition, the present study revealed a significant association between the UACR level and the risk of AD. The exact mechanisms underlying this association remain unclear, but we speculate that increased permeability of various plasma proteins and impaired amyloid β clearance at the blood–brain barrier via endothelial dysfunction may be involved in the association.30, 31 Further prospective studies will be needed to clarify this issue.
The present study demonstrated that subjects with lower eGFR level were likely to have a higher risk for the development of only VaD, but this association was not statistically significant. Several longitudinal studies in community‐based settings demonstrated conflicting results in terms of the association of low eGFR with cognitive decline4, 5 or dementia.8, 9 The Northern Manhattan Study showed that subjects with eGFR <60 mL/min per 1.73 m2 had greater cognitive decline than those with eGFR ≥90 mL/min per 1.73 m2.4 The Cardiovascular Health Cognition Study demonstrated that moderate renal insufficiency (defined as Cr ≥1.5 mg/dL for men and ≥1.3 mg/dL for women) was associated with an increased risk of VaD, but not AD.8 In addition, the Three‐City Study showed that subjects with eGFR 45 to 59 mL/min per 1.73 m2 had significantly higher risk for the development of VaD, but not AD, than those with eGFR ≥60 mL/min per 1.73 m2.9 However, the Rancho Bernardo Study reported that there was no clear association between moderately to severely decreased kidney function (defined as eGFR <60 mL/min per 1.73 m2) and any domains of cognitive decline.5 Differences in the covariates included in the model may explain the conflicting findings of these previous studies. That is, the Northern Manhattan Study, the Cardiovascular Health Cognition Study, and the Three‐City Study did not adjust for albuminuria and showed a significant association of lower kidney function with cognitive decline and the development of VaD. Similarly, in the present study, lower eGFR level was a significant risk factor for the development of VaD unless we adjusted for albuminuria. Therefore, it would be necessary to consider the influence of albuminuria when assessing the association between low eGFR and dementia.
The present study found that albuminuria and low eGFR were mutually associated with a greater risk of incident VaD. Several epidemiologic studies have demonstrated that both albuminuria and low eGFR had influence on the risk of clinical and subclinical cerebrovascular disease.32, 33, 34 Increased permeability of the blood–brain barrier, which is induced by endothelial dysfunction, causes white‐matter hyperintensities.35 Low eGFR is associated with the prevalence of silent brain infarcts and microbleeding.36 These cerebrovascular diseases lead to ischemic death of nerve cells, and are likely to lead to VaD. Further studies are warranted to confirm whether albuminuria and low eGFR are risk factors for dementia independent of each other or not.
Some limitations of the present study should be noted. First, UACR and eGFR were based on only 1 measurement at baseline. During follow‐up, UACR and eGFR in subjects with chronic kidney disease may have been changed by modifications in lifestyle or medication. This limitation would have introduced misclassification of UACR and eGFR levels, which would be expected to bias results toward the null, reducing the observed association found in the current study. Second, we could not completely rule out the potential effects of selection bias in our exclusion of 352 residents who did not participate in the baseline examination, since in general these residents were likely to be less healthy than those who attended the health examination. However, we believe that such a selection bias may have had little influence on our findings, because the participation rate at the baseline examination was very high (83.4%). Third, we could not completely exclude the potential influence of residual confounders on the association of albuminuria and low eGFR with dementia risk. Finally, among the 1562 subjects in this study, only 215 (13.8%) subjects were available for a detailed brain examination at autopsy. Moreover, 46 of the 238 AD cases (19.3%) were diagnosed using computed tomography or only clinical information. Thus, we cannot deny the possibility that VaD cases were mixed in with the AD cases, because lesions of small vessel disease (eg, microinfarcts, leukoaraiosis, and microbleedings) might be missed, even using brain magnetic resonance image examination. These limitations could have caused some misclassification of the dementia subtypes.
Conclusion
Albuminuria was a significant risk factor for the development of all‐cause dementia, AD, and VaD in a general population of community‐dwelling Japanese elderly. In addition, subjects with both albuminuria and low eGFR had increased risk for VaD but not AD. These results suggest that subjects with albuminuria should be considered a high‐risk population for AD as well as VaD, and those with concurrent low eGFR should be considered a more severe high‐risk population for VaD. These populations should be recommended for careful observation for the potential development of dementia in clinical practice.
Sources of Funding
This study was supported in part by Grants‐in‐Aid for Scientific Research (A) (JP16H02644 and JP16H02692) and (B) (JP16H05850, JP16H05557, and JP17H04126) and (C) (JP15K09267, JP15K08738, JP15K09835, JP16K09244, JP17K09114, JP17K09113, and JP17K01853) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (H25‐Junkankitou [Seishuu]‐Sitei‐022, H29‐Junkankitou‐Ippan‐003, and H27‐Shokuhin‐[Sitei]‐017); and by the Japan Agency for Medical Research and Development (JP17dk0207025, JP17ek0210082, JP17gm0610007, JP17ek0210083, JP17km0405202, JP17ek0210080).
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Subjects According to eGFR Level, the Hisayama Study, 2002
Table S2. Multivariable‐Adjusted Hazard Ratios for the Development of Alzheimer Disease According to Urine Albumin‐Creatinine Ratio Level When Excluding 46 Cases Diagnosed as Alzheimer Disease Using CT or Only Clinical Information
Table S3. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to eGFR Level
Table S4. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to Urine Albumin‐Creatinine Ratio Level, Taking the Competing Risk of Death into Account
Table S5. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to eGFR Level, Taking the Competing Risk of Death into Account
Table S6. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to Age‐ and Sex‐Specific Level of Urine Albumin‐Creatinine Ratio
Table S7. Age‐ and Sex‐Adjusted and Multivariable‐Adjusted Hazard Ratios or the Development of Vascular Dementia With and Without Prior Stroke According to Urine Albumin‐Creatinine Ratio Level
Figure S1. Cumulative incidence of all‐cause dementia according to eGFR level.
Figure S2. Cumulative incidence of Alzheimer disease and/or vascular dementia according to urine albumin‐creatinine ratio level.
Figure S3. Cumulative incidence of Alzheimer disease and/or vascular dementia according to eGFR level.
Acknowledgments
The authors thank the residents of the town of Hisayama for their participation in the survey and the staff of the Division of Health and Welfare of Hisayama for their cooperation. We are also very grateful to Yoshinao Oda, Toru Iwaki, and their colleagues in the Department of Anatomic Pathology and Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, who provided insight and expertise for the autopsy findings that greatly assisted the research.
(J Am Heart Assoc. 2018;7:e006693 DOI: 10.1161/JAHA.117.006693.)29353232
References
- 1. World Alzheimer report 2015: the global impact of dementia. An analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International. Available at: https://www.alz.co.uk/research/worldalzheimerreport2015.pdf. Accessed March 16, 2017.
- 2. Fratiglioni L, Qiu C. Prevention of cognitive decline in ageing: dementia as the target, delayed onset as the goal. Lancet Neurol. 2011;10:778–779. [DOI] [PubMed] [Google Scholar]
- 3. Fung E, Kurella Tamura M. Epidemiology and public health concerns of CKD in older adults. Adv Chronic Kidney Dis. 2015;23:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MS, Sacco RL, Wright CB. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20:2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jassal SK, Kritz‐Silverstein D, Barrett‐Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo Study. Am J Epidemiol. 2010;171:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang F, Zhang L, Liu L, Wang H. Level of kidney function correlates with cognitive decline. Am J Nephrol. 2010;32:117–121. [DOI] [PubMed] [Google Scholar]
- 7. Kurella Tamura M, Muntner P, Wadley V, Cushman M, Zakai NA, Bradbury BD, Kissela B, Unverzagt F, Howard G, Warnock D, McClellan W. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011;58:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seliger SL, Siscovick DS, Stehman‐Breen CO, Gillen DL, Fitzpatrick A, Bleyer A, Kuller LH. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. [DOI] [PubMed] [Google Scholar]
- 9. Helmer C, Stengel B, Metzger M, Froissart M, Massy ZA, Tzourio C, Berr C, Dartigues JF. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77:2043–2051. [DOI] [PubMed] [Google Scholar]
- 10. Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y. Secular trends in cardiovascular disease and its risk factors in Japanese: half‐century data from the Hisayama Study (1961–2009). Circulation. 2013;128:1198–1205. [DOI] [PubMed] [Google Scholar]
- 11. Ohara T, Hata J, Yoshida D, Mukai N, Nagata M, Iwaki T, Kitazono T, Kanba S, Kiyohara Y, Ninomiya T. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88:1925–1932. [DOI] [PubMed] [Google Scholar]
- 12. Takae K, Nagata M, Hata J, Mukai N, Hirakawa Y, Yoshida D, Kishimoto H, Tsuruya K, Kitazono T, Kiyohara Y, Ninomiya T. Serum uric acid as a risk factor for chronic kidney disease in a Japanese community: the Hisayama Study. Circ J. 2016;80:1857–1862. [DOI] [PubMed] [Google Scholar]
- 13. Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, Kanba S, Kiyohara Y. Glucose tolerance status and risk of dementia in the community: the Hisayama Study. Neurology. 2011;77:1126–1134. [DOI] [PubMed] [Google Scholar]
- 14. Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late‐life blood pressure and dementia in Japanese elderly: the Hisayama Study. Hypertension. 2011;58:22–28. [DOI] [PubMed] [Google Scholar]
- 15. Katoh S, Simogaki H, Onodera A, Ueda H, Oikawa K, Ikeda K, Kosaka A, Imai Y, Hasegawa K. Development of the revised version of Hasegawa's Dementia Scale (HDS‐R) [in Japanese]. Jpn J Geriatr Psychiatry. 1991;2:1339–1347. [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 18. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 19. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 20. Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T. Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama Study. Brain Pathol. 2008;18:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The National Institute on Aging and Reagan Institute. Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease . Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 22. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 23. Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 24. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter. 2013;3(suppl):1–150. [Google Scholar]
- 25. de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: public health perspectives. J Am Soc Nephrol. 2006;17:2120–2126. [DOI] [PubMed] [Google Scholar]
- 26. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD‐EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. [DOI] [PubMed] [Google Scholar]
- 27. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, Jensen T, Kofoed‐Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32:219–226. [DOI] [PubMed] [Google Scholar]
- 29. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators . Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 30. Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. [DOI] [PubMed] [Google Scholar]
- 31. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta‐analysis. Nephrol Dial Transplant. 2015;30:1162–1169. [DOI] [PubMed] [Google Scholar]
- 33. Toyoda K, Ninomiya T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. 2014;13:823–833. [DOI] [PubMed] [Google Scholar]
- 34. Wada M, Nagasawa H, Iseki C, Takahashi Y, Sato H, Arawaka S, Kawanami T, Kurita K, Daimon M, Kato T. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross‐sectional study in community‐based Japanese elderly. J Neurol Sci. 2008;272:36–42. [DOI] [PubMed] [Google Scholar]
- 35. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Y, Lv P, Jin H, Cui W, Niu C, Zhao M, Fan C, Teng Y, Pan B, Peng Q, Luo J, Zheng L, Huang Y. Association between low estimated glomerular filtration rate and risk of cerebral small‐vessel diseases: a meta‐analysis. J Stroke Cerebrovasc Dis. 2016;25:710–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Subjects According to eGFR Level, the Hisayama Study, 2002
Table S2. Multivariable‐Adjusted Hazard Ratios for the Development of Alzheimer Disease According to Urine Albumin‐Creatinine Ratio Level When Excluding 46 Cases Diagnosed as Alzheimer Disease Using CT or Only Clinical Information
Table S3. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to eGFR Level
Table S4. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to Urine Albumin‐Creatinine Ratio Level, Taking the Competing Risk of Death into Account
Table S5. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to eGFR Level, Taking the Competing Risk of Death into Account
Table S6. Multivariable‐Adjusted Hazard Ratios for the Development of All‐Cause Dementia and Its Subtypes According to Age‐ and Sex‐Specific Level of Urine Albumin‐Creatinine Ratio
Table S7. Age‐ and Sex‐Adjusted and Multivariable‐Adjusted Hazard Ratios or the Development of Vascular Dementia With and Without Prior Stroke According to Urine Albumin‐Creatinine Ratio Level
Figure S1. Cumulative incidence of all‐cause dementia according to eGFR level.
Figure S2. Cumulative incidence of Alzheimer disease and/or vascular dementia according to urine albumin‐creatinine ratio level.
Figure S3. Cumulative incidence of Alzheimer disease and/or vascular dementia according to eGFR level.


