Coronary chronic total occlusions (CTOs) are defined as 100% occlusions with TIMI (Thrombolysis in Myocardial Infarction) 0 flow with at least a 3‐month duration.1 Treatment options for patients with coronary CTOs include lifestyle changes and medications (as is appropriate for all patients with coronary artery disease) and coronary revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). In the previous version of the appropriateness use criteria for coronary revascularization,2 revascularization recommendations were different for patients with and without a coronary CTO, but this is no longer the case in the current (2016 and 2017) versions.3, 4
The goal of this review is to summarize the available evidence on the clinical benefits, likelihood of success, risk for complications, and crossing strategies for CTO PCI and provide practical clinical recommendations.
Clinical Benefits
Randomized Trials
The potential benefits of CTO PCI have been and continue to be controversial given the scarcity of randomized controlled trials (Table 1).5, 6, 7, 8, 9, 10
Table 1.
Overview of Randomized Controlled Trials and Large Observational Studies That Compared CTO PCI With MT in Patients With Coronary CTOs
| Study (Author) Name | No. of Patients Enrolled | No. of Centers | Study Period | Study Design | Compared Study Groups | Primary End Point | Overall Success Rate, % | Follow‐Up Period | MACE Rate, % |
|---|---|---|---|---|---|---|---|---|---|
| Randomized trials | |||||||||
| EXPLORE (Henriques et al)5 | 304 | 14 | 2007–2015 | Randomized, prospective | Early CTO PCI after STEMI vs MT after STEMI | LVEF and LVEDV | 73 | 4 mo | 5.4 vs 2.6 |
| DECISION‐CTO (Park)6 | 834 | 19 | 2010–2016 | Randomized, prospective | CTO PCI vs OMT (non‐CTO PCI performed in both study groups) | Death, MI, stroke, TVR | 91.1 | 3 y | 19.0 vs. 21.4 |
| EURO‐CTO (Werner)7 | 407 | 26 | 2012–2015 | Randomized, prospective | CTO PCI vs OMT (non‐CTO PCI not performed) | Health status | 86.3 | 12 mo | 6.7 vs 5.2 |
| Observational studies | |||||||||
| IRCTO (Tomasello et al)9 | 1777 | 12 | 2008–2009 | Observational, prospective | PCI vs OMT vs CABG | MACCE, cardiac death | 75.4 | 1 y | 2.6 vs 8.2 vs 6.9 |
| Jang et al10 | 738 | 1 | 2003–2012 | Observational, retrospective | OMT+PCI/CABG vs OMT (all Rentrop 3 collateral filling grade.) | MACE, cardiac death | 80.1 | 42 mo | 3.4 vs 9.7 |
CABG indicates coronary artery bypass grafting; CTO, chronic total occlusion; DECISION‐CTO, Drug‐Eluting Stent Implantation Versus Optimal Medical Treatment in Patients with Chronic Total Occlusion; EURO‐CTO, Randomized Multicenter Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions; EXPLORE, Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST‐Elevation Myocardial Infarction; IRCTO, Italian Registry of Chronic Total Occlusions; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular event; MACE, major adverse cardiac event; MI, myocardial infarction; MT, medical therapy; OMT, optimal medical treatment; OPEN‐CTO, Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and TVR, target vessel revascularization.
Only one randomized controlled trial comparing CTO PCI with medical therapy alone has been published to date, the EXPLORE (Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST‐Elevation Myocardial Infarction) trial.5 The EXPLORE trial randomized 304 patients who underwent primary PCI for ST‐segment–elevation myocardial infarction (MI) and had a coexisting non–infarct‐related artery CTO to CTO PCI versus medical therapy alone. CTO PCI success was 73%. Cardiac magnetic resonance imaging performed after 4 months showed similar left ventricular ejection fraction and left ventricular end‐diastolic volume in the 2 study groups.5 Despite its limitations (enrollment of patients regardless of symptoms and regardless of viability and ischemia of the myocardium supplied by the CTO; potential selection bias given slow enrollment over 7 years at 14 sites; low CTO PCI success rate; and use of a surrogate rather than a clinical primary end point), the EXPLORE trial findings do not support routine PCI of nonculprit CTOs for improving the ejection fraction of patients with recent ST‐segment‐–elevation acute MI.
Two other randomized controlled trials were presented in 2017, but neither has been published as of November 2017. The DECISION‐CTO (Drug‐Eluting Stent Implantation Versus Optimal Medical Treatment in Patients with Chronic Total Occlusion; Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT01078051) trial was presented at the 2017 American College of Cardiology meeting.6 The EuroCTO (Randomized Multicenter Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions; Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT01760083) was presented at the 2017 EuroPCR meeting.7 Both trials were stopped before completion of the planned enrollment and were, hence, underpowered.
DECISION‐CTO trial planned to enroll 1284 patients but was stopped early because of slow enrollment, after randomizing 834 patients with coronary CTOs to CTO PCI or optimal medical therapy alone (OMT). CTO PCI was performed with a high success rate (91%). Concurrent nonocclusive lesions were revascularized in many patients in both groups (77% and 79% for the OMT and CTO PCI groups, respectively). Nearly 20% of the OMT group crossed over to CTO PCI. At 3 years, the primary end point of death, MI, stroke, or repeated revascularization occurred in 19% of the OMT versus 21.4% of the CTO PCI group, suggesting noninferiority of OMT. Measures of quality of life (QoL; Seattle Angina Questionnaire) were similar between study groups. DECISION‐CTO trial has important design and execution limitations, hindering interpretation of its results and limiting their applicability to daily clinical practice. These limitations include the following: (1) high prevalence of non‐CTO lesions that were treated after enrollment in both study groups without knowledge of the presence of ischemia or symptoms after non‐CTO lesion revascularization; (2) high rates of crossover from OMT to CTO PCI; (3) mild baseline symptoms; (4) suboptimal primary end point, because the main benefit of CTO PCI is expected to be symptom improvement and not improvement in mortality or MI; (5) inappropriate design (noninferiority, although CTO PCI would need to be superior to replace the less invasive OMT); and (6) low power.
In contrast to DECISION‐CTO trial, QoL was the primary efficacy end point in EuroCTO (Seattle Angina Questionnaire components at 12 months). Patients with non‐CTO lesions could not be enrolled until after such lesions were successfully recanalized. The initial plan was to enroll 1200 patients but because of slow enrollment, the study ended after randomizing 407 patients 2:1 to CTO PCI and OMT or OMT alone. Procedural success was 86.3%, and 7.3% of the OMT‐only group crossed over to CTO PCI. Likely because of small sample size, the study showed statistically significant improvement with CTO PCI in only 1 of the 5 components of the Seattle Angina Questionnaire (ie, angina frequency; P=0.009).
Although randomized controlled clinical trials are the gold standard for determining the efficacy and safety of an intervention, only 15% of the American College of Cardiology/American Heart Association guideline recommendations are based on level A evidence.11 Therefore, in most cases, clinical decision making relies on less robust clinical evidence, such as retrospective and observational studies.
Observational Studies: Revascularization Versus No Revascularization of CTOs
Several observational studies have compared the outcomes of CTO PCI with no revascularization; the largest ones are summarized in Table 1.
Tomasello et al9 examined the long‐term outcomes of 1777 CTO patients from the Italian CTO Registry, according to treatment strategy: PCI, 43.7%; medical therapy, 46.5%; or surgery, 9.8%. At 1‐year follow‐up, the incidence of cardiac death (1.4% versus 4.7% versus 6.3%; P<0.001) and major adverse cardiac events (MACEs) (2.6% versus 8.2% and 6.9%; P<0.001) were significantly lower in the PCI group. After propensity matching (n=619), medical therapy was associated with the higher MACE rate (7.6% versus 1.7%; P<0.001), cardiac death (4.4% versus 1.5%; P=0.002), acute MI (2.9% versus 1.1%), and rehospitalization (4.4% versus 2.3%; P=0.04), compared with CTO PCI.
Well‐developed collateral circulation to the CTO target vessel distal to the occlusion has been used as an argument against CTO revascularization, despite studies demonstrating ischemia in nearly all such cases.12, 13 Jang et al10 examined the long‐term outcomes of different treatment strategies among 738 patients with at least one CTO lesion and well‐developed collateral channels. During a median follow‐up of 42 months, patients who underwent coronary revascularization (with PCI or CABG; n=502) had a lower incidence of cardiac death (hazard ratio [HR], 0.29; 95% confidence interval [CI], 0.15–0.58; P<0.01) and MACE (HR, 0.32; 95% CI, 0.21–0.49; P<0.01), even after propensity matching (cardiac death: HR, 0.27; 95% CI, 0.09–0.80; P=0.02; and MACE: HR, 0.44; 95% CI, 0.23–0.82; P=0.01).10
In summary, large observational studies suggest benefit with CTO recanalization versus medical therapy; however, they are subject to selection and ascertainment bias.
Observational Studies: Impact of CTO PCI on Depression, Exercise Capacity, and Ventricular Arrhythmias
Several small observational studies have explored the potential effect of CTO PCI on various surrogate end points, such as depression, exercise capacity, and the risk for ventricular arrhythmias.
Bruckel et al reported a high prevalence of depression among patients with CTOs with significant reduction after successful CTO PCI (40.0% versus 11.1%; P=0.01).14 Patients who were depressed derived the most benefit from CTO PCI.14
Rossello et al demonstrated increased 6‐minute walking distance (417±126 m versus 463±103 m; P=0.002) and decreased angina frequency after successful CTO PCI, especially in patients with a large baseline ischemic burden.15 Abdullah et al demonstrated increased peak oxygen uptake during cardiopulmonary exercise testing (7.7±4.3 to 19.1±4.0 mL/kg per minute; P=0.02) and decreased plasma B‐type natriuretic peptide levels (143±138 to 102±123 pg/mL; P=0.01) in 28 patients 5 months after successful CTO PCI.16 Mashayekhi et al showed improved exercise capacity (peak oxygen consumption and anaerobic threshold increased by 12% and 28%, respectively; P=0.001 for both), decrease in mean Canadian Cardiology Society angina score (1.88±0.12 to 1.14±0.08; P<0.0001), and increase of left ventricular ejection fraction (by 6.79%; 95% CI, 2.18%–11.40%; P=0.007) in 50 patients at 7 months after CTO PCI.17
Di Marco et al found that 56% of 84 patients with a prior MI who were referred for ventricular tachycardia ablation had coexisting infarct‐related CTO. These patient had larger scar tissue area (34 versus 19 cm2; P=0.001) and a higher incidence of readmission because of recurrent ventricular tachycardia during a median follow‐up of 19 months (47% versus 16%; P=0.003).18 The VACTO (Ventricular Arrhythmias and Chronic Total Coronary Occlusion) study explored the impact of CTOs on the outcomes of 162 patients with ischemic cardiomyopathy who received an implantable cardioverter‐defibrillator (ICD). At least one CTO was present in 44% of the patients and was independently associated with appropriate ICD therapy (adjusted HR, 3.5; 95% CI, 1.5–8.3; P=0.003) and mortality (adjusted HR, 5.6; 95% CI, 1.4–21; P=0.02) during a median follow‐up of 257 days.19 In another study of 425 patients who had a prior ventricular arrhythmia and underwent ICD implantation, the incidence of appropriate ICD therapy was significantly higher in patients with CTOs (51.7% versus 36.3%; P=0.0001).20 Raja et al21 compared long‐term mortality and the incidence of ventricular arrhythmias in patients with ischemic cardiomyopathy and ICD (n=307) divided into 3 groups: no CTOs (n=94), nonrevascularized CTOs (n=114), and revascularized CTOs (n=99). During a median follow‐up of 4.1 years, the 3 groups had similar mortality (P=0.274) and incidence of ventricular arrhythmias (P=0.306).21
Ischemia testing can facilitate the planning of CTO PCI. Secemsky et al compared the concordance between target vessel selection and ischemic burden (assessed by stress imaging) in patients with CTOs (N=532) who underwent CTO PCI (n=100 [18.8%]) or non‐CTO PCI (n=432 [81.2%]).22 The concordance between target vessel and areas of ischemia was significantly higher in patients who underwent CTO PCI versus those who underwent non‐CTO PCI (90.7% versus 67.9%; P<0.0001).
Observational Studies: Successful Versus Failed Procedures
Several studies have compared successful with failed CTO PCI: a meta‐analysis of 25 studies compared successful (71%) with failed (29%) CTO PCIs in 28 486 patients. During a mean follow‐up of 3.11 years, compared with failed procedures, successful CTO PCI was associated with lower mortality (odds ratio, 0.52), less residual angina (odds ratio, 0.38), lower risk for stroke (odds ratio, 0.72), and less need for subsequent CABG (odds ratio, 0.18).23
The OPEN‐CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures) used the Seattle Angina Questionnaire, Rose Dyspnea Scale, and Patient Health Questionnaire in 1000 consecutive patients undergoing CTO PCI using the hybrid approach at 12 experienced US centers. At 1‐month follow‐up, the Seattle Angina Questionnaire QoL score improved (from 49.4±0.9 to 75.0±0.7; P<0.01), with a simultaneous decrease in symptoms of dyspnea (Rose Dyspnea Scale score decreased from 2.0±0.1 to 1.1±0.1; P<0.01) and depression (Patient Health Questionnaire score decreased from 6.2±0.2 to 3.5±0.1; P<0.01). The most prominent difference in SAQ (Seattle Angina Questionnaire) scores was detected in the QoL domain, with a 10.8 (95% CI, 6.3–15.3) point greater improvement observed in patients with successful versus failed procedures (P<0.001).8
Comparison of successful versus failed CTO PCIs has important shortcomings, because it is not a randomized comparison and it is likely that patients in whom CTO PCI fails have more complex angiographic characteristics and more comorbidities that can adversely affect subsequent outcomes.
Success of CTO PCI
A meta‐analysis of 65 studies published between 2000 and 2011 reported 77% angiographic success and 3.1% risk for MACE.24 In recent years, several large multicenter registries have reported higher success (≈85%–90%) with acceptable complication rates in CTO PCI at various experienced centers and with operators from all around the world, although less optimal results have been reported from all‐comer PCI registries (Table 2).8, 25, 26, 27, 28, 29, 30, 31, 32
Table 2.
Procedural Outcomes of Multicenter CTO and General PCI Registries in Recent Years
| Authors | Study Period | No. of Centers | No. of Cases | Technical Success, % | Procedural Success, % | Overall MACE, % | Death, % | Acute MI, % | Stroke, % | TVR, % | Pericardial Tamponade, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dedicated CTO PCI registries | |||||||||||
| Christopoulos et al25 | 2012–2015 | 11 | 1036 | 91 | 90 | 1.7 | 0.3 | 0.7 | 0.1 | 0.2 | 0.5 |
| Habara et al26 | 2012–2013 | 56 | 3229 | ··· | 88 | 0.5 | 0.2 | 0.1 | 0.1 | 0.0 | 0.3 |
| Wilson et al27 | 2012–2014 | 7 | 1156 | 90 | ··· | 1.6 | 0 | 0.8 | 0.4 | 0.0 | 0.7 |
| Maeremans et al28 | 2014–2015 | 17 | 1253 | 89 | 86 | 2.6 | 0.2 | 0.2 | 2.2 | 0.1 | 1.3 |
| Sapontis et al8 | 2013–2017 | 12 | 1000 | 86 | 85 | 7.0 | 0.9 | 2.6 | 0.0 | 0.1 | ···a |
| CTO PCI analyses from all‐comer PCI registries | |||||||||||
| Brilakis et al30 | 2009–2013 | ··· | 22365 | ··· | 59 | 1.6 | 0.4 | 1.9 | 0.1 | 0.4 | 0.1 |
| Hannan et al29 | 2009–2012 | 61 | 4030 | ··· | 61.3 | 1.1 | ··· | ··· | ··· | ··· | ··· |
| Ramunddal et al32 | 2005–2012 | ··· | 6442 | ··· | 54.2 | ··· | ··· | ··· | ··· | ··· | ··· |
| Tsai et al31 | 2007–2013 | 79 | 2394 | 79.8 | 79.7 | 4.3 | 0.0 | 0.13 | 0.0 | 0.0 | 0.0 |
CTO indicates chronic total occlusion; MACE, major adverse cardiovascular event; MI, myocardial infarction; PCI, percutaneous coronary intervention; and TVR, target vessel revascularization.
The incidence of tamponade was not reported.
Success of CTO PCI at Experienced Centers
The PROGRESS‐CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) reported 91% technical success among 1036 consecutive CTO PCIs performed at 11 US centers.25
The Japanese Retrograde Summit registry reported 88% success and a remarkably low 0.53% in‐hospital complication rate among 3229 CTO PCIs performed at 56 centers in Japan. Higher‐volume centers had slightly higher success rates but similar complication rates.26
The UK Hybrid CTO Registry reported a 90% final technical success rate and a 1.6% 30‐day MACE rate among 1156 patients. Compared with low‐complexity lesions (J‐CTO score, 0–1), more complex lesions (J‐CTO score, ≥2) were more likely to require dissection reentry techniques (56% versus 15%) and use of multiple approaches. The technical success rate of the first attempted CTO PCI was 79%, but taking into account repeated procedures, the final success rate increased to 90%.27
The RECHARGE (Registry of Crossboss and Hybrid Procedures in France, the Netherlands, Belgium, and United Kingdom) reported 89% and 86% technical and procedural success rates, respectively, with a 2.6% incidence of in‐hospital major complications among 1253 CTO PCIs performed at 17 European centers by 22 experienced high‐volume operators.28
The OPEN‐CTO Registry reported 86% technical success and 7% incidence of in‐hospital MACEs in 1000 consecutive CTO PCIs.33 The major complication rates were higher than in other studies, mainly related to higher rates of perforation and periprocedural MI (0.9% mortality, 2.6% MI, 0% stroke, 0.7% emergency surgery, and 4.8% clinical perforation).8
Success of CTO PCI in All‐Comer Registries
The outcomes of CTO PCI in all‐comer PCI registries have not been as good as those achieved at experienced centers.
In an analysis from the National Cardiovascular Data Registry in the United States, CTO PCI represented 3.8% of all PCIs for stable coronary artery disease (22 365 of 594 510) performed between 2009 and 2013. CTO PCI was associated with lower success (59% versus 96%; P<0.001) and higher complication (1.6% versus 0.8%; P<0.001) rates, compared with treatment of nonocclusive lesions. Higher CTO PCI volume was associated with higher success (53% for operators performing <5 CTO PCIs/year versus 75% for operators performing >10 CTO PCIs/year; P<0.001) and lower MACE (1.7% versus 1.4%, respectively; P=0.05) rates.30
Hannan et al29 examined 4030 patients undergoing CTO PCI (of 156 043 PCIs) at 61 centers participating in the New York State PCI Registry. CTO PCI was performed infrequently (3.1%), with low technical success (61.3%) and acceptable complication rates (1.07%). On multivariable analysis, incomplete revascularization (with ≥1 existing lesion) in the setting of successful CTO PCI was not associated with higher mortality during 2.5 years of follow‐up (HR, 1.11; 95% CI, 0.74–1.68; P=0.6090). Patients in whom CTO intervention failed, however, had a higher mortality (HR, 1.63; 95% CI, 1.28–2.08; P<0.0001), regardless of whether other lesions were treated or not.29
In the SCAAR (Swedish Coronary Angiography and Angioplasty Registry), the prevalence of CTO among patients with at least one 50% luminal coronary stenosis was 16.1% (14 441 of 89 872 patients). Approximately half of all patients who had a CTO (6442 of 14 441) underwent CTO PCI, with a 54.2% success rate. Successful CTO PCI was associated with lower long‐term mortality (HR, 0.85; 95% CI, 0.73–0.98; P<0.034) compared with failed procedures.32
Tsai et al31 examined 111 273 patients who underwent coronary angiography at 79 Veterans Affairs centers and were included in the Veterans Affairs Clinical Assessment Reporting and Tracking program. At least one CTO was found in 26.4% of all patients (n=29 399), of whom 8.1% underwent CTO PCI. CTO PCI procedural success rate was 79.7%, and MACE rate was 4.3%. During 2 years of follow‐up, successful CTO PCI was associated with lower mortality (HR, 0.67; 95% CI, 0.47–0.95; P=0.02) and need for CABG (HR, 0.14; 95% CI, 0.08–0.24; P<0.01) compared with failed CTO PCI.31
CTO PCI Scores
Several scoring systems have been developed to determine the likelihood of CTO PCI technical success and the potential difficulty of the procedure (measured as time required to cross the occlusion), such as the CL Clinical and lesion‐related) score,34 Ellis score,35 Japan‐chronic total occlusion score (J‐CTO),36 Ostial, Rentrop grade, Age score (ORA),37 PROGRESS‐CTO score,38 and RECHARGE score39 (Table 3). These scores combine various clinical and angiographic characteristics and can provide a quantitative assessment of the likelihood of success and the difficulty of the case, as long as they are applied in similar settings as the ones that they were developed on. CTO PCI scores may also be useful for scheduling procedures (eg, by avoiding performing multiple high‐complexity lesions on the same day).41
Table 3.
Scoring Systems for Predicting the Success and Efficiency of CTO PCI
| Score Variables | J‐CTO Score36 | CL Score34 | PROGRESS‐CTO Score38 | ORA Score37 | RECHARGE Score39 | Ellis Score35 |
|---|---|---|---|---|---|---|
| No. of cases | 494 | 1657 | 781 | 1073 | 1253 | 456 |
| End point | Guidewire crossing <30 min | Technical success | Technical success | Technical success | Technical success | Technical success |
| Age, y | − | − | − | + (≥75) | + (>65) | − |
| Prior CABG | − | + | − | − | + | − |
| Prior failure | + | − | − | − | − | − |
| Proximal cap | + (Blunt) | + (Blunt) | + (Ambiguous) | + (Ostial) | + | + (Ambiguous, ostial) |
| Tortuosity | + (>45° in lesion) | − | + (Moderatea proximal) | − | + | + |
| Calcification | + | + (Severe) | − | − | + | + |
| Lesion length | + (≥20 mm) | + (≥20 mm) | − | − | + | + |
| Target vessel | − | + (Non‐LAD) | + (LCX) | − | − | + (Poor distal target) |
| Collateral quality | − | − | + (Interventional) | + (Rentrop <2) | − | +b |
| Other | − | Prior myocardial infarction | − | − | BMI >30 kg/m2, nonproximal location | Operator experience |
+ Indicates present; −, absent; BMI, body mass index; CABG, coronary artery bypass grafting; CL, ; CTO, chronic total occlusion; J‐CTO, Japan Chronic Total Occlusion score; LAD, left anterior descending artery; LCX, circumflex artery; ORA, ostial location, Rentrop grade <2, age ≥75 years; PCI, percutaneous coronary intervention; PROGRESS‐CTO, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; and RECHARGE, Registry of Crossboss and Hybrid Procedures in France, the Netherlands, Belgium and United Kingdom.
Moderate tortuosity was defined as 2 bends >70° or 1 bend >90° proximal to the lesion.
Applying specific collateral classification scoring (range, 0–2) combining Werner collateral classification,40 tortuosity, and collateral type (septal, epicardial, or other).
Bridging the Gap
In summary, the CTO PCI success rates in all‐comer registries (54%–80%) are significantly lower than those achieved at experienced centers (85%–90%). Bridging the gap will likely require development of novel equipment and techniques as well as development of comprehensive, high‐volume,42 CTO PCI programs43 and continued education through live case demonstrations,44 CTO PCI workshops, and proctorships.45 CTO PCI is one of the key components of the growing complex, high‐risk, PCI programs.46, 47
Complications of CTO PCI
Because of higher lesion and technical complexity, CTO PCI carries a higher risk than non‐CTO PCI. In the National Cardiovascular Data Registry, CTO PCI had a 2‐fold higher periprocedural complication rate than non‐CTO PCI (1.6% versus 0.8%; P<0.0001).30 Among experienced centers and operators, the contemporary risk of CTO PCI is ≈3% (Tables 1 and 2). A scoring system was recently developed for determining the risk of CTO PCI, the PROGRESS‐CTO complication score, which uses 3 variables: age >65 years, lesion length ≥23 mm, and use of the retrograde approach.48
The estimated procedural risk should be taken into account when decisions are made about proceeding with CTO PCI. Moreover, operators and catheterization laboratories performing CTO PCI should be vigilant and prepared to treat any complications that may arise.
Perforation
Perforation is one of the most feared complications of CTO PCI. Coronary perforations are infrequent (0.33% of all cases) but are associated with poor short‐ and long‐term outcomes. Kinnaird et al analyzed 527 121 interventions of the British Cardiovascular Intervention Society Database, showing that female sex, older age, rotational atherectomy, and CTO PCI were independent predictors of perforation.49 The incidence of coronary perforations among 26 807 CTO interventions from the same registry was 1.40%. Patients with coronary perforations had a higher incidence of in‐hospital and 12‐month death, MI, and bleeding requiring transfusion.50 In another multicenter registry, the incidence of perforation among 2097 CTO PCIs was 4.1%, but only 0.6% required pericardiocentesis.51
Coronary perforation in patients who underwent prior CABG had been considered less perilous previously, because pericardial adhesions form after sternotomy, potentially preventing tamponade. Recent reports, however, suggest that perforation in patients who underwent prior CABG may result in loculated hematomas, potentially causing localized tamponade and cardiogenic shock. In such cases, computed tomography–guided drainage or surgical intervention may be required to drain the effusion.52, 53, 54 Intramural bleeding should be suspected if the clinical picture is suggestive of pericardial tamponade, but no pericardial effusion can be detected by echocardiography.55
Radiation
CTO PCI often requires long procedure and fluoroscopy time and may expose both the patient and the operator to high radiation doses that can cause skin injury and increase the subsequent risk for malignancy. Acute dermatitis can develop at the exposed skin area and may progress to chronic skin ulcer if left undiscovered or untreated, occasionally requiring surgical intervention and skin transplantation.56 Wei et al examined 2124 patients who underwent 2579 PCIs (238 of which were CTO PCIs) and found that a chronic skin ulcer developed in 0.34% (n=9 patients, in 5 of whom the ulcer developed after CTO PCI) and required surgical treatment in 8 patients.57 The threshold for stopping the procedure because of high radiation exposure is 7 to 10 Gy air kerma dose, and postprocedural monitoring and referral for dermatologist follow‐up are recommended for air kerma doses >5 Gy.
In part because of an increase in popularity of CTO PCI and increasing emphasis on procedural safety, there have been significant reductions in patient radiation dose over time, by using low fluoroscopy rates (6–7.5 frames per second), newer x‐ray systems that administer a lower radiation dose,58 and better radiation safety techniques.59 Werner et al60 examined 984 CTO PCIs performed in 863 patients between 2010 and 2015. During that period, fluoroscopy settings were changed (15 to 7.5 and then to 6 pulses/second), with a cine frame rate of 15 to 7.5/second. Despite an increase in lesion complexity and similar fluoroscopy times, dose area product significantly decreased during the study period (initially by 20%, followed by an additional 7% reduction).60 Additional shielding (eg, using disposable sterile radiation shields) could further reduce operator radiation dose.61
CTO PCI Techniques
The most challenging portion of most CTO PCIs is crossing the occlusion with a guidewire. There are 3 main crossing techniques: antegrade wire escalation (AWE), antegrade dissection/reentry, and the retrograde approach.
Antegrade Wire Escalation
AWE (ie, sequential use of various guidewires in the antegrade direction, from the proximal to the distal part of the vessel) is the most commonly used initial and final CTO crossing technique (66%–78%),25, 26, 27, 28 especially for less complex occlusions.62
Antegrade Dissection and Reentry
Antegrade dissection and reentry (ADR) refers to use of the subintimal (or subadventitial) space for crossing the occlusion, followed by reentry into the distal true lumen using guidewires or dedicated systems, such as the Stingray balloon and guidewire (Boston Scientific, Natick, MA).63, 64, 65, 66, 67 The early version of these techniques created extensive areas of dissection and was associated with suboptimal short‐ and long‐term outcomes68; hence, limited dissection techniques (minimizing the length of dissection and consequent side branch loss) are preferred.64
Danek et al69 reported use of ADR in 459 of 1313 CTO PCIs (34.9%) performed at 11 US centers between 2012 and 2105. ADR was more commonly performed using dedicated devices (CrossBoss, 53.7%; Stingray, 54.8%) and was associated with comparable technical success rate as AWE (92.7% versus 94.2%; P=0.43) and similar complication rates (2.1% versus 0.6%; P=0.12).69
As part of RECHARGE, Maeremans et al70 analyzed the outcomes and safety of the antegrade dissection reentry in 292 patients. ADR techniques overall were used for complex lesions (J‐CTO score, 2.7±1.1), with 78% per‐lesion success rate and 3.1% complication rate.70
Azzalini et al compared the long‐term outcomes of CTO PCIs using device‐based antegrade dissection reentry strategies (CrossBoss/Stingray [Boston Scientific]) with wire‐based dissection reentry techniques (subintimal tracking and reentry and limited antegrade subintimal tracking) in a multicenter registry of 223 cases.71 When comparing device‐based reentry with subintimal tracking and reenty (STAR) and limited antegrade subintimal tracking, the device‐based reentry strategies (ie, CrossBoss/Stingray) had significantly lower incidence of MACE (4.3% versus 15.4% and 17.5%; P=0.02) and target vessel revascularization (3.1% versus 7.7% and 15.5%; P=0.02).
Hasegawa et al72 compared the midterm outcomes of intimal with subintimal tracking by using both antegrade and retrograde crossing. Subintimal tracking occurred less frequently in the antegrade group (11.6% versus 30.9%; P<0.01), requiring target vessel revascularization more frequently in the retrograde group (7.1% versus 16.0%; P=0.03) but not in the antegrade group (2.8% versus 3.6%; P=0.99).72
Song et al73 compared intraplaque with subintimal wire crossing using intravascular ultrasonographic assessment for acute vessel damage and complications. Subintimal tracking was more common with dissection reentry strategies than with wire escalation (86.7% versus 27.9%) in more complex lesions (J‐CTO score: 2.5±1.1 versus 1.6±1.1; P<0.001). Subintimal crossing was associated with a higher composite of in‐hospital all‐cause death, periprocedural MI, and target lesion revascularization (7.9% versus 1.9%; P=0.04), driven by periprocedural MI (7.0% versus 1.9%; P=0.1). Subintimal tracking was associated with a higher rate of intravascular ultrasound–detected vascular injury (89.5% versus 52.4%; P<0.001), angiographic dye staining/extravasation (14.0% versus 3.8%; P=0.01), and branch occlusion (48.2% versus 16.2%; P<0.001).73
In summary, limited ADR techniques are a key component of contemporary CTO PCI, especially for crossing complex CTOs, and are associated with favorable short‐ and long‐term outcomes.
Retrograde Approach
The retrograde approach involves advancement of a guidewire through a collateral vessel or bypass grafts into the distal true lumen, followed by CTO crossing against the former direction of blood flow. Similar to ADR, the retrograde approach is an essential tool for achieving high CTO PCI success rates,74, 75, 76 especially in more complex cases and when antegrade crossing strategies are not feasible or fail to achieve crossing.25, 27 However, the retrograde approach carries higher risk for procedure‐related complications. Results of the largest retrograde CTO PCI registries (>300 cases) published to date are summarized in Table 4.74, 75, 76, 77, 78 Careful selection of collateral channels is essential for optimizing the success and safety of the retrograde approach. Various pathways (septal and epicardial collaterals, saphenous vein grafts, and arterial grafts) can be used for retrograde crossing.
Table 4.
Largest Published Registries of Retrograde CTO PCI
| Authors | No. of Retrograde Cases | Study Period | Initial Retrograde Approach, % | Initial Retrograde Technical Success, % | Use of Reverse CART, % | Overall Technical Success, % | Overall MACE Rate, % |
|---|---|---|---|---|---|---|---|
| Yamane et al74 | 378 | 2009 | 75 | 70.4 | 42.5 | 83.6 | 0.5 |
| Tsuchikane et al75 | 801 | 2009–2010 | 67 | 71.2 | 55.2 | 84.8 | 1.6 |
| Karmpaliotis et al76 | 462 | 2006–2011 | 46 | 83.4 | 47.2 | 81.4 | 2.6 |
| Galassi et al77 | 1582 | 2008–2012 | 76 | 83.2 | 16.0 | 75.3 | 0.8 |
| Karmpaliotis et al78 | 539 | 2012–2015 | 46 | 82.1 | 62.2 | 84.8 | 4.3 |
CART indicates controlled antegrade and retrograde subintimal tracking; CTO, chronic total occlusion; MACE, major adverse cardiac event; and PCI, percutaneous coronary intervention.
The European CTO Club reported 75.3% technical and 71.2% clinical success among 1582 retrograde CTO PCIs, with significant improvement over time (73.5%, 65.8%, 73.0%, 74.7%, and 79.2%, between 2008 and 2012). The overall procedural complication rate was 6.8%, whereas the in‐hospital major adverse cardiac and cerebrovascular event (defined as any cardiac death, stroke, and repeated revascularization during the hospital stay) rate was 0.8%. During a mean follow‐up of 24.7 months, all‐cause mortality rate was 3.9%, cardiac mortality rate was 1.9%, and overall major adverse cardiac and cerebrovascular event rate was 13.6%.77
Karmpaliotis et al78 analyzed 539 retrograde interventions among 1301 CTO PCIs performed at 11 US centers between 2012 and 2015. Retrograde CTO PCI, as compared with antegrade‐only cases, was associated with higher clinical (prior CABG, 48% versus 24% [P<0.001]; prior PCI, 70.4% versus 60.8% [P<0.001]; prior CTO PCI failure, 20.7% versus 15.4% [P=0.017]) and lesion (J‐CTO score, 3.1±1.0 versus 2.1±1.2; P<0.001) complexity, lower technical success (85% versus 94%; P<0.001), and higher in‐hospital MACE rate (4.3% versus 1.1%; P<0.001).78
Okamura et al79 analyzed the retrograde procedure‐related complications of the Retrograde Summit Registry in Japan. Retrograde success was 71.9%, and the complication rate was 11.3%. The most common complication that occurred during retrograde cases was collateral channel injury (9.5%), yet additional treatment was required in only 2.1% of all cases. Use of septal collaterals was associated with longer procedure (197.0±84.6 versus 184.6±81.4 minutes; P=0.063) and fluoroscopy (97.2±51.9 versus 87.1±41.4 minutes; P=0.007) times but had a lower rate of non–Q‐wave MI (0.1% versus 1.1%; P=0.021) and channel injuries (1.1% versus 3.8%; P=0.005) compared with use of epicardial collaterals.79
Saphenous vein grafts80 and septal collaterals are the preferred pathways for retrograde crossing, because they are easier to cross and carry lower risk for complications.74, 76, 79 Dautov et al assessed the safety and effectivity of the septal surfing technique in 240 retrograde PCIs, showing successful septal crossing in 81% of the cases.81 Mashayekhi et al82 compared the outcomes of retrograde CTO PCI via ipsilateral (n=44 [28%]) with contralateral (n=114 [72%]) collateral connections. The overall retrograde success was similar in the ipsilateral and contralateral group (80% versus 82%; P>0.05), with low crossover rates (ipsilateral to contralateral, 11%; contralateral to ipsilateral, 4%) and a similar major complications rate (5% versus 7%; P=1.00).82 However, epicardial collateral perforation can rapidly cause tamponade and may require urgent sealing from both directions,83 usually with coils, fat, or thrombin.84 Use of epicardial collaterals is usually reserved for highly experienced operators and centers (Figure 1).85
Figure 1.
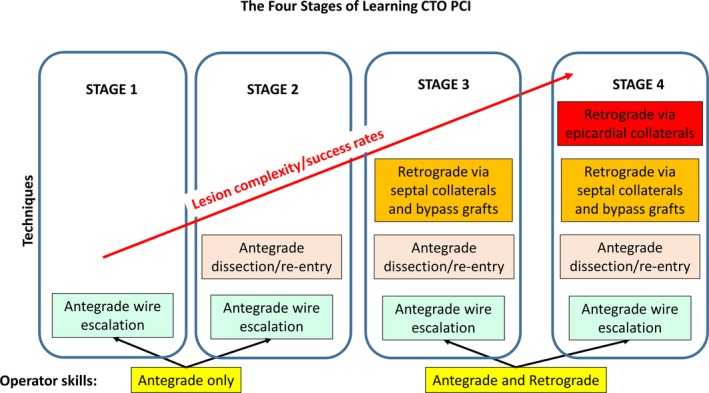
The 4 stages of learning chronic total occlusion (CTO) percutaneous coronary intervention (PCI). Reprinted from Azzalini and Brilakis85 with permission. Copyright 2017, John Wiley and Sons.
In summary, retrograde is an important CTO PCI technique but should be used with caution, because it carries increased risk for complications, especially through epicardial collaterals and in patients who underwent prior CABG.
Selection of Crossing Technique
Determining the optimal initial and subsequent crossing strategy depends on the angiographic characteristics of the lesion and should be selected after detailed review of the angiogram. The hybrid approach (Figure 2) starts with dual angiography and focuses on assessment of 4 anatomic features (proximal cap ambiguity, distal target vessel, interventional collateral, and lesion length), recommending early change if the initially selected strategy is not successful.33
Figure 2.
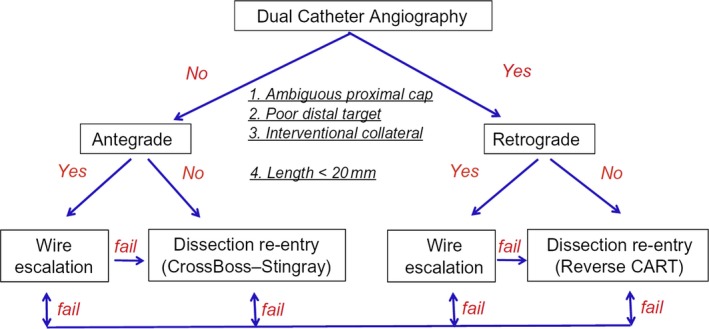
The hybrid algorithm for crossing coronary chronic total occlusions (CTOs). CART, controlled antegrade and retrograde tracking and dissection. Reprinted from Brilakis1 with permission. Copyright 2017, Elsevier.
Another algorithm is the Asia Pacific algorithm (http://apcto.club/apcto-algorithm), which is also based on the CTO anatomic characteristics and gives preference to parallel wiring and intravascular ultrasound–guided wiring86 over antegrade dissection and reentry, reflecting the local expertise in the countries where it was developed.40, 87
Remaining Controversies
Randomized Controlled Trials
As described earlier, only 3 randomized controlled clinical trials comparing CTO PCI with medical therapy have been performed to date, the EXPLORE, DECISION‐CTO, and EuroCTO trials. Of the 3 trials, 2 (the DECISION‐CTO and EuroCTO trials) were stopped prematurely before completion of planned enrollment and were, thus, underpowered. Moreover, DECISION‐CTO trial randomized several patients with multivessel coronary artery disease before revascularization of the non‐CTO lesions, limiting assessment of the impact of CTO PCI. Given the potential placebo effect of CTO PCI on QoL, there is a need for a well‐designed and adequately powered sham‐controlled, randomized clinical trial to definitively answer the question of the impact of CTO PCI on patient symptoms.
Use of Dissection/Reentry Strategies
There is ongoing controversy on the use and timing of dissection/reentry (both antegrade and retrograde) techniques in contemporary CTO PCI. As described in the Antegrade Dissection and Reentry section, extensive dissection/reentry has been associated with a high risk for periprocedural complications (eg, periprocedural MI) and high rates of restenosis, likely because of side branch loss, and is only used as a bailout.
The CrossBoss First trial compared upfront use of the CrossBoss catheter with AWE for CTO crossing.88 It demonstrated similar crossing time, success and complication rates, and costs, suggesting that both strategies are acceptable as an initial approach. Crossing time was shorter with upfront use of the CrossBoss catheter in in‐stent restenotic lesions. Antegrade dissection/reentry was the final successful strategy in 22% of the AWE group, demonstrating the importance of these techniques at various stages of contemporary CTO PCI. Additional studies comparing various crossing strategies are important for further optimizing the initial and subsequent strategy selection for CTO PCI.
Radial Versus Femoral Approach in CTO PCI
Access site selection is important in CTO PCI for providing appropriate support and allowing enough space for multiple devices during various techniques. Eight French guiding catheters, via transfemoral access, are often recommended, because they provide strong support, but may carry a higher risk for vascular complications.89 Access with ultrasound and fluoroscopic guidance could reduce the risk for arterial access complications.90
Murakami et al showed that transradial CTO PCI can be effective in appropriately selected cases91; however, bifemoral access was used for more complex cases. Tanaka et al92 compared transradial (n=280) with transfemoral (n=305) CTO PCI, reporting similar technical success in the 2 groups (74.6% versus 72.5%; P=0.51). However, complex (J‐CTO score, ≥3) cases performed using transradial access had significantly lower technical success rates than those done via transfemoral access (35.7% versus 58.2%; P=0.004).92 Alaswad et al demonstrated that CTO PCI using at least one transradial access was feasible, with high success rates, but at the cost of longer fluoroscopy and mean procedure time.93 The 8F sheathless guide catheters94 or 7F slender sheaths (Terumo, Somerset, NJ) are increasingly being used for radial CTO PCI and may facilitate wider adoption of the radial approach for CTO (and other complex) PCIs.
Conclusions
All decisions in medicine should be based on the risk/benefit ratio (Figure 3). The main and best documented to date benefit of CTO PCI is symptom improvement (ie, improvement in angina or angina equivalents). Consequently, for truly asymptomatic patients, there should be a high threshold for doing CTO PCI for other indications, such as ischemia reduction or improvement in ejection fraction. Deriving benefit requires successful recanalization; hence, estimating the likelihood of success (85%–90% at experienced centers) is critical for accurate estimation of the potential benefit. The risk of major procedural complications is ≈3% and depends on patient age, lesion complexity, and crossing techniques used. Use of scoring systems can be useful for providing the patient and operator with an objective assessment of the likelihood of success and procedural risk, provided that the scores are used by operators and patients similar to those from whom the scores were derived. Successful CTO PCI in appropriately selected patients can provide significant clinical benefits and improve their QoL.
Figure 3.
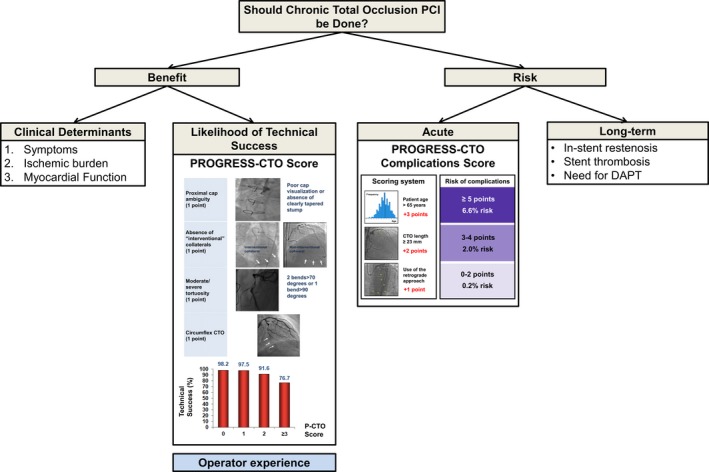
Algorithm for potential risk/benefit assessment in chronic total occlusion percutaneous coronary intervention. DAPT indicates dual antiplatelet therapy; PCI, percutaneous coronary intervention; PROGRESS‐CTO, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention. Reprinted from Brilakis1 with permission. Copyright 2017, Elsevier.
Disclosures
Brilakis reports consulting/speaker honoraria from Abbott Vascular, ACIST, Amgen, Asahi, CSI, Elsevier, GE Healthcare, Medicure, and Nitiloop; he has received research support from Boston Scientific and Osprey; he is on the Board of Directors for Cardiovascular Innovations Foundation and the Board of Trustees for the Society of Cardiovascular Angiography and Interventions. Tajti has no disclosures to report.
(J Am Heart Assoc. 2018;7:e006732 DOI: 10.1161/JAHA.117.006732.)29330258
References
- 1. Brilakis E. Manual of Coronary Chronic Total Occlusion Interventions: A Step‐by‐Step Approach. 2nd ed Cambridge, MA: Elsevier; 2017. [Google Scholar]
- 2. Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology: endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Circulation. 2009;119:1330–1352. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 appropriate use criteria for coronary revascularization in patients with acute coronary syndromes: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:570–591. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 5. Henriques JP, Hoebers LP, Ramunddal T, Laanmets P, Eriksen E, Bax M, Ioanes D, Suttorp MJ, Strauss BH, Barbato E, Nijveldt R, van Rossum AC, Marques KM, Elias J, van Dongen IM, Claessen BE, Tijssen JG, van der Schaaf RJ; EXPLORE Trial Investigators . Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68:1622–1632. [DOI] [PubMed] [Google Scholar]
- 6. Park S. Drug‐eluting stent implantation versus optimal medical treatment in patients with chronic total occlusion (DECISION‐CTO). American College of Cardiology's 66th Annual Scientific Session & Expo; Washington, DC; 2017. [Google Scholar]
- 7. Werner G. A randomized multicentre trial to evaluate the utilization of revascularization or optimal medical therapy for the treatment of chronic total coronary occlusions (EuroCTO). EuroPCR; Paris, France; 2017. [Google Scholar]
- 8. Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, McCabe JM, Karmpaliotis D, Moses J, Nicholson WJ, Pershad A, Wyman RM, Spaedy A, Cook S, Doshi P, Federici R, Thompson CR, Marso SP, Nugent K, Gosch K, Spertus JA, Grantham JA. Early procedural and health status outcomes after chronic total occlusion angioplasty: a report from the OPEN‐CTO Registry (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures). JACC Cardiovasc Interv. 2017;10:1523–1534. [DOI] [PubMed] [Google Scholar]
- 9. Tomasello SD, Boukhris M, Giubilato S, Marza F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, Desideri A, Rubartelli P, Cioppa A, Baralis G, Galassi AR. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36:3189–3198. [DOI] [PubMed] [Google Scholar]
- 10. Jang WJ, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Kim WS, Lee YT, Gwon HC. Long‐term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well‐developed collateral circulation. JACC Cardiovasc Interv. 2015;8:271–279. [DOI] [PubMed] [Google Scholar]
- 11. Han H, Chao H, Guerra A, Sosa A, Christopoulos G, Christakopoulos GE, Rangan BV, Maragkoudakis S, Jneid H, Banerjee S, Brilakis ES. Evolution of the American College of Cardiology/American Heart Association Clinical Guidelines. J Am Coll Cardiol. 2015;65:2726–2734. [DOI] [PubMed] [Google Scholar]
- 12. Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. 2014;83:9–16. [DOI] [PubMed] [Google Scholar]
- 13. Werner GS, Surber R, Ferrari M, Fritzenwanger M, Figulla HR. The functional reserve of collaterals supplying long‐term chronic total coronary occlusions in patients without prior myocardial infarction. Eur Heart J. 2006;27:2406–2412. [DOI] [PubMed] [Google Scholar]
- 14. Bruckel JT, Jaffer FA, O'Brien C, Stone L, Pomerantsev E, Yeh RW. Angina severity, depression, and response to percutaneous revascularization in patients with chronic total occlusion of coronary arteries. J Invasive Cardiol. 2016;28:44–51. [PubMed] [Google Scholar]
- 15. Rossello X, Pujadas S, Serra A, Bajo E, Carreras F, Barros A, Cinca J, Pons‐Llado G, Vaquerizo B. Assessment of inducible myocardial ischemia, quality of life, and functional status after successful percutaneous revascularization in patients with chronic total coronary occlusion. Am J Cardiol. 2016;117:720–726. [DOI] [PubMed] [Google Scholar]
- 16. Abdullah SM, Hastings JL, Amsavelu S, Garcia‐Morales F, Hendrix F, Karatasakis A, Danek BA, Karacsonyi J, Rangan BV, Roesle M, Khalili H, Banerjee S, Brilakis ES. Percutaneous coronary intervention of coronary chronic total occlusions improves peak oxygen uptake during cardiopulmonary exercise testing. J Invasive Cardiol. 2017;29:83–91. [PubMed] [Google Scholar]
- 17. Mashayekhi K, Neuser H, Kraus A, Zimmer M, Dalibor J, Akin I, Werner G, Aurel T, Neumann FJ, Behnes M. Successful percutaneous coronary intervention improves cardiopulmonary exercise capacity in patients with chronic total occlusions. J Am Coll Cardiol. 2017;69:1095–1096. [DOI] [PubMed] [Google Scholar]
- 18. Di Marco A, Paglino G, Oloriz T, Maccabelli G, Baratto F, Vergara P, Bisceglia C, Anguera I, Sala S, Sora N, Dallaglio P, Marzi A, Trevisi N, Mazzone P, Della Bella P. Impact of a chronic total occlusion in an infarct‐related artery on the long‐term outcome of ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2015;26:532–539. [DOI] [PubMed] [Google Scholar]
- 19. Nombela‐Franco L, Mitroi CD, Fernandez‐Lozano I, Garcia‐Touchard A, Toquero J, Castro‐Urda V, Fernandez‐Diaz JA, Perez‐Pereira E, Beltran‐Correas P, Segovia J, Werner GS, Javier G, Luis AP. Ventricular arrhythmias among implantable cardioverter‐defibrillator recipients for primary prevention: impact of chronic total coronary occlusion (VACTO Primary Study). Circ Arrhythm Electrophysiol. 2012;5:147–154. [DOI] [PubMed] [Google Scholar]
- 20. Nombela‐Franco L, Iannaccone M, Anguera I, Amat‐Santos IJ, Sanchez‐Garcia M, Bautista D, Calvelo MN, Di Marco A, Moretti C, Pozzi R, Scaglione M, Canadas V, Sandin‐Fuentes M, Arenal A, Bagur R, Perez‐Castellano N, Fernandez‐Perez C, Gaita F, Macaya C, Escaned J, Fernandez‐Lozano I. Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter‐defibrillator recipients (VACTO secondary study): insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interv. 2017;10:879–888. [DOI] [PubMed] [Google Scholar]
- 21. Raja V, Wiegn P, Obel O, Christakopoulos G, Christopoulos G, Rangan BV, Roesle M, Abdullah SM, Luna M, Addo T, Ayers C, Garcia S, de Lemos JA, Banerjee S, Brilakis ES. Impact of chronic total occlusions and coronary revascularization on all‐cause mortality and the incidence of ventricular arrhythmias in patients with ischemic cardiomyopathy. Am J Cardiol. 2015;116:1358–1362. [DOI] [PubMed] [Google Scholar]
- 22. Secemsky EA, Gallagher R, Harkness J, Pomerantsev E, Gewirtz H, Jaffer FA, Yeh RW. Target vessel revascularization and territory of myocardial ischemia in patients with chronic total occlusions. J Am Coll Cardiol. 2017;70:1196–1197. [DOI] [PubMed] [Google Scholar]
- 23. Christakopoulos GE, Christopoulos G, Carlino M, Jeroudi OM, Roesle M, Rangan BV, Abdullah S, Grodin J, Kumbhani DJ, Vo M, Luna M, Alaswad K, Karmpaliotis D, Rinfret S, Garcia S, Banerjee S, Brilakis ES. Meta‐analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115:1367–1375. [DOI] [PubMed] [Google Scholar]
- 24. Patel VG, Brayton KM, Tamayo A, Mogabgab O, Michael TT, Lo N, Alomar M, Shorrock D, Cipher D, Abdullah S, Banerjee S, Brilakis ES. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta‐analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6:128–136. [DOI] [PubMed] [Google Scholar]
- 25. Christopoulos G, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Wyman RM, Lombardi WL, Menon RV, Grantham JA, Kandzari DE, Lembo N, Moses JW, Kirtane AJ, Parikh M, Green P, Finn M, Garcia S, Doing A, Patel M, Bahadorani J, Tarar MN, Christakopoulos GE, Thompson CA, Banerjee S, Brilakis ES. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int J Cardiol. 2015;198:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Habara M, Tsuchikane E, Muramatsu T, Kashima Y, Okamura A, Mutoh M, Yamane M, Oida A, Oikawa Y, Hasegawa K; Retrograde Summit Investigators . Comparison of percutaneous coronary intervention for chronic total occlusion outcome according to operator experience from the Japanese retrograde summit registry. Catheter Cardiovasc Interv. 2016;87:1027–1035. [DOI] [PubMed] [Google Scholar]
- 27. Wilson WM, Walsh SJ, Yan AT, Hanratty CG, Bagnall AJ, Egred M, Smith E, Oldroyd KG, McEntegart M, Irving J, Strange J, Douglas H, Spratt JC. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102:1486–1493. [DOI] [PubMed] [Google Scholar]
- 28. Maeremans J, Walsh S, Knaapen P, Spratt JC, Avran A, Hanratty CG, Faurie B, Agostoni P, Bressollette E, Kayaert P, Bagnall AJ, Egred M, Smith D, Chase A, McEntegart MB, Smith WH, Harcombe A, Kelly P, Irving J, Smith EJ, Strange JW, Dens J. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE Registry. J Am Coll Cardiol. 2016;68:1958–1970. [DOI] [PubMed] [Google Scholar]
- 29. Hannan EL, Zhong Y, Jacobs AK, Stamato NJ, Berger PB, Walford G, Sharma S, Venditti FJ, King SB III. Patients with chronic total occlusions undergoing percutaneous coronary interventions: characteristics, success, and outcomes. Circ Cardiovasc Interv. 2016;9:e003586. [DOI] [PubMed] [Google Scholar]
- 30. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. [DOI] [PubMed] [Google Scholar]
- 31. Tsai TT, Stanislawski MA, Shunk KA, Armstrong EJ, Grunwald GK, Schob AH, Valle JA, Alfonso CE, Nallamothu BK, Ho PM, Rumsfeld JS, Brilakis ES. Contemporary incidence, management, and long‐term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: insights from the VA CART program. JACC Cardiovasc Interv. 2017;10:866–875. [DOI] [PubMed] [Google Scholar]
- 32. Ramunddal T, Hoebers LP, Henriques JP, Dworeck C, Angeras O, Odenstedt J, Ioanes D, Olivecrona G, Harnek J, Jensen U, Aasa M, Jussila R, James S, Lagerqvist B, Matejka G, Albertsson P, Omerovic E. Chronic total occlusions in Sweden: a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS One. 2014;9:e103850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brilakis ES, Grantham JA, Rinfret S, Wyman RM, Burke MN, Karmpaliotis D, Lembo N, Pershad A, Kandzari DE, Buller CE, Demartini T, Lombardi WL, Thompson CA. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2012;5:367–379. [DOI] [PubMed] [Google Scholar]
- 34. Alessandrino G, Chevalier B, Lefevre T, Sanguineti F, Garot P, Unterseeh T, Hovasse T, Morice MC, Louvard Y. A clinical and angiographic scoring system to predict the probability of successful first‐attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8:1540–1548. [DOI] [PubMed] [Google Scholar]
- 35. Ellis SG, Burke MN, Murad MB, Graham JJ, Badawi R, Toma C, Meltser H, Nair R, Buller C, Whitlow PL; CAPS Group . Predictors of successful hybrid‐approach chronic total coronary artery occlusion stenting: an improved model with novel correlates. JACC Cardiovasc Interv. 2017;10:1089–1098. [DOI] [PubMed] [Google Scholar]
- 36. Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J‐CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–221. [DOI] [PubMed] [Google Scholar]
- 37. Galassi AR, Boukhris M, Azzarelli S, Castaing M, Marza F, Tomasello SD. Percutaneous coronary revascularization for chronic total occlusions: a novel predictive score of technical failure using advanced technologies. JACC Cardiovasc Interv. 2016;9:911–922. [DOI] [PubMed] [Google Scholar]
- 38. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, Alaswad K, Lombardi W, Grantham JA, Moses J, Christakopoulos G, Tarar MN, Rangan BV, Lembo N, Garcia S, Cipher D, Thompson CA, Banerjee S, Brilakis ES. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv. 2016;9:1–9. [DOI] [PubMed] [Google Scholar]
- 39. Maeremans J, Spratt JC, Knaapen P, Walsh S, Agostoni P, Wilson W, Avran A, Faurie B, Bressollette E, Kayaert P, Bagnall AJ, Smith D, McEntegart MB, Smith WHT, Kelly P, Irving J, Smith EJ, Strange JW, Dens J. Towards a contemporary, comprehensive scoring system for determining technical outcomes of hybrid percutaneous chronic total occlusion treatment: the RECHARGE score. Catheter Cardiovasc Interv. 2017;00:00–00. May 4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40. Werner GS, Ferrari M, Heinke S, Kuethe F, Surber R, Richartz BM, Figulla HR. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107:1972–1977. [DOI] [PubMed] [Google Scholar]
- 41. Karatasakis A, Danek BA, Karmpaliotis D, Alaswad K, Jaffer FA, Yeh RW, Patel M, Bahadorani JN, Lombardi WL, Wyman RM, Grantham JA, Kandzari DE, Lembo NJ, Doing AH, Toma C, Moses JW, Kirtane AJ, Parikh MA, Ali ZA, Garcia S, Kalsaria P, Karacsonyi J, Alame AJ, Thompson CA, Banerjee S, Brilakis ES. Comparison of various scores for predicting success of chronic total occlusion percutaneous coronary intervention. Int J Cardiol. 2016;224:50–56. [DOI] [PubMed] [Google Scholar]
- 42. Michael TT, Karmpaliotis D, Brilakis ES, Alomar M, Abdullah SM, Kirkland BL, Mishoe KL, Lembo N, Kalynych A, Carlson H, Banerjee S, Luna M, Lombardi W, Kandzari DE. Temporal trends of fluoroscopy time and contrast utilization in coronary chronic total occlusion revascularization: insights from a multicenter United States registry. Catheter Cardiovasc Interv. 2015;85:393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brilakis ES, Vo MN. How to develop a successful chronic total occlusion percutaneous coronary intervention program. Cardiovasc Revasc Med. 2016;17:3–4. [DOI] [PubMed] [Google Scholar]
- 44. Shimura T, Yamamoto M, Tsuchikane E, Teramoto T, Kimura M, Satou H, Matsuo H, Kawase Y, Suzuki Y, Kano S, Habara M, Nasu K, Kinoshita Y, Terashima M, Matsubara T, Suzuki T. Safety of live case demonstrations in patients undergoing percutaneous coronary intervention for chronic total occlusion. Am J Cardiol. 2016;118:967–973. [DOI] [PubMed] [Google Scholar]
- 45. Sharma V, Jadhav ST, Harcombe AA, Kelly PA, Mozid A, Bagnall A, Richardson J, Egred M, McEntegart M, Shaukat A, Oldroyd K, Vishwanathan G, Rana O, Talwar S, McPherson M, Strange JW, Hanratty CG, Walsh SJ, Spratt JC, Smith WH. Impact of proctoring on success rates for percutaneous revascularisation of coronary chronic total occlusions. Open Heart. 2015;2:e000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kirtane AJ, Doshi D, Leon MB, Lasala JM, Ohman EM, O'Neill WW, Shroff A, Cohen MG, Palacios IF, Beohar N, Uriel N, Kapur NK, Karmpaliotis D, Lombardi W, Dangas GD, Parikh MA, Stone GW, Moses JW. Treatment of higher‐risk patients with an indication for revascularization: evolution within the field of contemporary percutaneous coronary intervention. Circulation. 2016;134:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalra A, Bhatt DL, Pinto DS, Kirtane AJ, Kapadia SR, Makkar RR, Rihal CS, Kleiman NS, Cutlip DE. Accreditation and funding for a 24‐month advanced interventional cardiology fellowship program: a call‐to‐action for optimal training of the next generation of interventionalists. Catheter Cardiovasc Interv. 2016;88:1010–1015. [DOI] [PubMed] [Google Scholar]
- 48. Danek BA, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Patel MP, Mahmud E, Lombardi WL, Wyman MR, Grantham JA, Doing A, Kandzari DE, Lembo NJ, Garcia S, Toma C, Moses JW, Kirtane AJ, Parikh MA, Ali ZA, Karacsonyi J, Rangan BV, Thompson CA, Banerjee S, Brilakis ES. Development and validation of a scoring system for predicting periprocedural complications during percutaneous coronary interventions of chronic total occlusions: the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO) complications score. J Am Heart Assoc. 2016;5:e004272 DOI: 10.1161/JAHA.116.004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kinnaird T, Kwok CS, Kontopantelis E, Ossei‐Gerning N, Ludman P, deBelder M, Anderson R, Mamas MA; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Incidence, determinants, and outcomes of coronary perforation during percutaneous coronary intervention in the United Kingdom between 2006 and 2013: an analysis of 527 121 cases from the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv. 2016;9:e003449. [DOI] [PubMed] [Google Scholar]
- 50. Kinnaird T, Anderson R, Ossei‐Gerning N, Cockburn J, Sirker A, Ludman P, deBelder M, Walsh S, Smith E, Hanratty C, Spratt J, Strange J, Hildick‐Smith D, Mamas MA; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Legacy effect of coronary perforation complicating percutaneous coronary intervention for chronic total occlusive disease: an analysis of 26 807 cases from the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv. 2017;10:e004642. [DOI] [PubMed] [Google Scholar]
- 51. Danek BA, Karatasakis A, Tajti P, Sandoval Y, Karmpaliotis D, Alaswad K, Jaffer F, Yeh RW, Kandzari DE, Lembo NJ, Patel MP, Mahmud E, Choi JW, Doing AH, Lombardi WL, Wyman RM, Toma C, Garcia S, Moses JW, Kirtane AJ, Hatem R, Ali ZA, Parikh M, Karacsonyi J, Rangan BV, Khalili H, Burke MN, Banerjee S, Brilakis ES. Incidence, treatment, and outcomes of coronary perforation during chronic total occlusion percutaneous coronary intervention. Am J Cardiol. 2017;120:1285–1292. [DOI] [PubMed] [Google Scholar]
- 52. Wilson WM, Spratt JC, Lombardi WL. Cardiovascular collapse post chronic total occlusion percutaneous coronary intervention due to a compressive left atrial hematoma managed with percutaneous drainage. Catheter Cardiovasc Interv. 2015;86:407–411. [DOI] [PubMed] [Google Scholar]
- 53. Adusumalli S, Morris M, Pershad A. Pseudo‐pericardial tamponade from right ventricular hematoma after chronic total occlusion percutaneous coronary intervention of the right coronary artery: successfully managed percutaneously with computerized tomographic guided drainage. Catheter Cardiovasc Interv. 2016;88:86–88. [DOI] [PubMed] [Google Scholar]
- 54. Karatasakis A, Akhtar YN, Brilakis ES. Distal coronary perforation in patients with prior coronary artery bypass graft surgery: the importance of early treatment. Cardiovasc Revasc Med. 2016;17:412–417. [DOI] [PubMed] [Google Scholar]
- 55. Franks RJ, de Souza A, Di Mario C. Left atrial intramural hematoma after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2015;86:E150–E152. [DOI] [PubMed] [Google Scholar]
- 56. Lee BL, Ma H, Perng CK, Wang TH, Liao WC, Yeh FL, Shih YC. Clinical manifestation, diagnosis, and surgical treatment of chronic radiation ulcers related to percutaneous coronary intervention. Ann Plast Surg. 2016;76(suppl 1):S68–S73. [DOI] [PubMed] [Google Scholar]
- 57. Wei KC, Yang KC, Mar GY, Chen LW, Wu CS, Lai CC, Wang WH, Lai PC. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross‐sectional study. Medicine (Baltimore). 2015;94:e2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christopoulos G, Christakopoulos GE, Rangan BV, Layne R, Grabarkewitz R, Haagen D, Latif F, Abu‐Fadel M, Banerjee S, Brilakis ES. Comparison of radiation dose between different fluoroscopy systems in the modern catheterization laboratory: results from bench testing using an anthropomorphic phantom. Catheter Cardiovasc Interv. 2015;86:927–932. [DOI] [PubMed] [Google Scholar]
- 59. Christopoulos G, Makke L, Christakopoulos G, Kotsia A, Rangan BV, Roesle M, Haagen D, Kumbhani DJ, Chambers CE, Kapadia S, Mahmud E, Banerjee S, Brilakis ES. Optimizing radiation safety in the cardiac catheterization laboratory: a practical approach. Catheter Cardiovasc Interv. 2016;87:291–301. [DOI] [PubMed] [Google Scholar]
- 60. Werner GS, Glaser P, Coenen A, Moehlis H, Tischer KH, Koch M, Klingenbeck R. Reduction of radiation exposure during complex interventions for chronic total coronary occlusions: implementing low dose radiation protocols without affecting procedural success rates. Catheter Cardiovasc Interv. 2017;89:1005–1012. [DOI] [PubMed] [Google Scholar]
- 61. Shorrock D, Christopoulos G, Wosik J, Kotsia A, Rangan B, Abdullah S, Cipher D, Banerjee S, Brilakis ES. Impact of a disposable sterile radiation shield on operator radiation exposure during percutaneous coronary intervention of chronic total occlusions. J Invasive Cardiol. 2015;27:313–316. [PubMed] [Google Scholar]
- 62. Nombela‐Franco L, Urena M, Jerez‐Valero M, Nguyen CM, Ribeiro HB, Bataille Y, Rodes‐Cabau J, Rinfret S. Validation of the J‐chronic total occlusion score for chronic total occlusion percutaneous coronary intervention in an independent contemporary cohort. Circ Cardiovasc Interv. 2013;6:635–643. [DOI] [PubMed] [Google Scholar]
- 63. Valenti R, Vergara R, Migliorini A, Parodi G, Carrabba N, Cerisano G, Dovellini EV, Antoniucci D. Predictors of reocclusion after successful drug‐eluting stent‐supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61:545–550. [DOI] [PubMed] [Google Scholar]
- 64. Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. 2012;5:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Godino C, Viani GM, Spagnolo P, Pavon AG, Colombo A, Carlino M. Management of large coronary dissection after STAR. Cardiovasc Revasc Med. 2014;15:58–60. [DOI] [PubMed] [Google Scholar]
- 66. Carlino M, Figini F, Ruparelia N, Uretsky BF, Godino C, Latib A, Bertoldi L, Brilakis E, Karmpaliotis D, Antoniucci D, Margonato A, Colombo A. Predictors of restenosis following contemporary subintimal tracking and reentry technique: the importance of final TIMI flow grade. Catheter Cardiovasc Interv. 2016;87:884–892. [DOI] [PubMed] [Google Scholar]
- 67. Mogabgab O, Patel VG, Michael TT, Fuh E, Alomar M, Rangan BV, Abdullah SM, Banerjee S, Brilakis ES. Long‐term outcomes with use of the CrossBoss and stingray coronary CTO crossing and re‐entry devices. J Invasive Cardiol. 2013;25:579–585. [PubMed] [Google Scholar]
- 68. Colombo A, Mikhail GW, Michev I, Iakovou I, Airoldi F, Chieffo A, Rogacka R, Carlino M, Montorfano M, Sangiorgi GM, Corvaja N, Stankovic G. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc Interv. 2005;64:407–411; discussion 412. [DOI] [PubMed] [Google Scholar]
- 69. Danek BA, Karatasakis A, Karmpaliotis D, Alaswad K, Yeh RW, Jaffer FA, Patel M, Bahadorani J, Lombardi WL, Wyman MR, Grantham JA, Doing A, Moses JW, Kirtane A, Parikh M, Ali ZA, Kalra S, Kandzari DE, Lembo N, Garcia S, Rangan BV, Thompson CA, Banerjee S, Brilakis ES. Use of antegrade dissection re‐entry in coronary chronic total occlusion percutaneous coronary intervention in a contemporary multicenter registry. Int J Cardiol. 2016;214:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maeremans J, Dens J, Spratt JC, Bagnall AJ, Stuijfzand W, Nap A, Agostoni P, Wilson W, Hanratty CG, Wilson S, Faurie B, Avran A, Bressollette E, Egred M, Knaapen P, Walsh S; RECHARGE Investigators . Antegrade dissection and reentry as part of the hybrid chronic total occlusion revascularization strategy: a subanalysis of the RECHARGE Registry (Registry of CrossBoss and Hybrid Procedures in France, the Netherlands, Belgium and United Kingdom). Circ Cardiovasc Interv. 2017;10:e004791. [DOI] [PubMed] [Google Scholar]
- 71. Azzalini L, Dautov R, Brilakis ES, et al. Procedural and longer‐term outcomes of wire‐ versus device‐based antegrade dissection and re‐entry techniques for the percutaneous revascularization of coronary chronic total occlusions. Int J Cardiol. 2017;231:78–83. [DOI] [PubMed] [Google Scholar]
- 72. Hasegawa K, Tsuchikane E, Okamura A, Fujita T, Yamane M, Oikawa Y, Suzuki Y, Igarashi Y, Kyo E, Muramatsu T. Incidence and impact on midterm outcome of intimal versus subintimal tracking with both antegrade and retrograde approaches in patients with successful recanalisation of chronic total occlusions: J‐PROCTOR 2 study. EuroIntervention. 2017;12:e1868–e1873. [DOI] [PubMed] [Google Scholar]
- 73. Song L, Maehara A, Finn MT, Kalra S, Moses JW, Parikh MA, Kirtane AJ, Collins MB, Nazif TM, Fall KN, Hatem R, Liao M, Kim T, Green P, Ali ZA, Batres C, Leon MB, Mintz GS, Karmpaliotis D. Intravascular ultrasound analysis of intraplaque versus subintimal tracking in percutaneous intervention for coronary chronic total occlusions and association with procedural outcomes. JACC Cardiovasc Interv. 2017;10:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamane M, Muto M, Matsubara T, Nakamura S, Muramatsu T, Oida A, Igarashi Y, Nozaki Y, Kijima M, Tuschikane E. Contemporary retrograde approach for the recanalisation of coronary chronic total occlusion: on behalf of the Japanese Retrograde Summit Group. EuroIntervention. 2013;9:102–109. [DOI] [PubMed] [Google Scholar]
- 75. Tsuchikane E, Yamane M, Mutoh M, Matsubara T, Fujita T, Nakamura S, Muramatsu T, Okamura A, Igarashi Y, Oida A. Japanese multicenter registry evaluating the retrograde approach for chronic coronary total occlusion. Catheter Cardiovasc Interv. 2013;82:E654–E661. [DOI] [PubMed] [Google Scholar]
- 76. Karmpaliotis D, Michael TT, Brilakis ES, Papayannis AC, Tran DL, Kirkland BL, Lembo N, Kalynych A, Carlson H, Banerjee S, Lombardi W, Kandzari DE. Retrograde coronary chronic total occlusion revascularization: procedural and in‐hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5:1273–1279. [DOI] [PubMed] [Google Scholar]
- 77. Galassi AR, Sianos G, Werner GS, Escaned J, Tomasello SD, Boukhris M, Castaing M, Buttner JH, Bufe A, Kalnins A, Spratt JC, Garbo R, Hildick‐Smith D, Elhadad S, Gagnor A, Lauer B, Bryniarski L, Christiansen EH, Thuesen L, Meyer‐Gessner M, Goktekin O, Carlino M, Louvard Y, Lefevre T, Lismanis A, Gelev VL, Serra A, Marza F, Di Mario C, Reifart N; Euro CTO Club . Retrograde recanalization of chronic total occlusions in Europe: procedural, in‐hospital, and long‐term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol. 2015;65:2388–2400. [DOI] [PubMed] [Google Scholar]
- 78. Karmpaliotis D, Karatasakis A, Alaswad K, Jaffer FA, Yeh RW, Wyman RM, Lombardi WL, Grantham JA, Kandzari DE, Lembo NJ, Doing A, Patel M, Bahadorani JN, Moses JW, Kirtane AJ, Parikh M, Ali ZA, Kalra S, Nguyen‐Trong P‐KJ, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, Thompson CA, Banerjee S, Brilakis ES. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv. 2016;9:e003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Okamura A, Yamane M, Muto M, Matsubara T, Igarashi Y, Nakamura S, Muramatsu T, Fujita T, Oida A, Tsuchikane E. Complications during retrograde approach for chronic coronary total occlusion: sub‐analysis of Japanese multicenter registry. Catheter Cardiovasc Interv. 2016;88:7–14. [DOI] [PubMed] [Google Scholar]
- 80. Dautov R, Manh Nguyen C, Altisent O, Gibrat C, Rinfret S. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv. 2016;9:e003515. [DOI] [PubMed] [Google Scholar]
- 81. Dautov R, Urena M, Nguyen CM, Gibrat C, Rinfret S. Safety and effectiveness of the surfing technique to cross septal collateral channels during retrograde chronic total occlusion percutaneous coronary intervention. EuroIntervention. 2017;12:e1859–e1867. [DOI] [PubMed] [Google Scholar]
- 82. Mashayekhi K, Behnes M, Valuckiene Z, Bryniarski L, Akin I, Neuser H, Neumann FJ, Reifart N. Comparison of the ipsi‐lateral versus contra‐lateral retrograde approach of percutaneous coronary interventions in chronic total occlusions. Catheter Cardiovasc Interv. 2017;89:649–655. [DOI] [PubMed] [Google Scholar]
- 83. Boukhris M, Tomasello SD, Azzarelli S, Elhadj ZI, Marza F, Galassi AR. Coronary perforation with tamponade successfully managed by retrograde and antegrade coil embolization. J Saudi Heart Assoc. 2015;27:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kotsia AP, Brilakis ES, Karmpaliotis D. Thrombin injection for sealing epicardial collateral perforation during chronic total occlusion percutaneous coronary interventions. J Invasive Cardiol. 2014;26:E124–E126. [PubMed] [Google Scholar]
- 85. Azzalini L, Brilakis ES. Ipsilateral vs. contralateral vs. no collateral (antegrade only) chronic total occlusion percutaneous coronary interventions: what is the right choice for your practice? Catheter Cardiovasc Interv. 2017;89:656–657. [DOI] [PubMed] [Google Scholar]
- 86. Galassi AR, Sumitsuji S, Boukhris M, Brilakis ES, Di Mario C, Garbo R, Spratt JC, Christiansen EH, Gagnor A, Avran A, Sianos G, Werner GS. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: An overview. JACC Cardiovasc Interv. 2016;9:1979–1991. [DOI] [PubMed] [Google Scholar]
- 87. Karacsonyi J, Alaswad K, Jaffer FA, Yeh RW, Patel M, Bahadorani J, Karatasakis A, Danek BA, Doing A, Grantham JA, Karmpaliotis D, Moses JW, Kirtane A, Parikh M, Ali Z, Lombardi WL, Kandzari DE, Lembo N, Garcia S, Wyman MR, Alame A, Nguyen‐Trong PK, Resendes E, Kalsaria P, Rangan BV, Ungi I, Thompson CA, Banerjee S, Brilakis ES. Use of intravascular imaging during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Am Heart Assoc. 2016;5:e003890 DOI: 10.1161/JAHA.116.003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Karacsonyi J, Tajti P, Rangan BV et al. Randomized Comparison of a CrossBoss First vs. Standard Wire Escalation Strategy for Crossing Coronary Chronic Total Occlusions: the “CrossBoss First” trial. JACC: Cardiovascular Interventions 2017. [DOI] [PubMed]
- 89. Kinnaird T, Anderson R, Ossei‐Gerning N, Gallagher S, Large A, Strange J, Ludman P, de Belder M, Nolan J, Hildick‐Smith D, Mamas M. Vascular access site and outcomes among 26,807 chronic total coronary occlusion angioplasty cases from the British Cardiovascular Interventions Society National Database. JACC Cardiovasc Interv. 2017;10:635–644. [DOI] [PubMed] [Google Scholar]
- 90. Fairley SL, Lucking AJ, McEntegart M, Shaukat A, Smith D, Chase A, Hanratty CG, Spratt JC, Walsh SJ. Routine use of fluoroscopic‐guided femoral arterial puncture to minimise vascular complication rates in CTO intervention: multi‐centre UK experience. Heart Lung Circ. 2016;25:1203–1209. [DOI] [PubMed] [Google Scholar]
- 91. Murakami T, Masuda N, Torii S, Ijichi T, Natsumeda M, Fujii T, Nakano M, Ohno Y, Nakazawa G, Shinozaki N, Tammam KO, Matsukage T, Ogata N, Yoshimachi F, Ikari Y. The efficacy and feasibility of chronic total occlusion by transradial intervention: a Japanese single‐center retrospective study. J Invasive Cardiol. 2015;27:E177–E181. [PubMed] [Google Scholar]
- 92. Tanaka Y, Moriyama N, Ochiai T, Takada T, Tobita K, Shishido K, Sugitatsu K, Yamanaka F, Mizuno S, Murakami M, Matsumi J, Takahashi S, Akasaka T, Saito S. Transradial coronary interventions for complex chronic total occlusions. JACC Cardiovasc Interv. 2017;10:235–243. [DOI] [PubMed] [Google Scholar]
- 93. Alaswad K, Menon RV, Christopoulos G, Lombardi WL, Karmpaliotis D, Grantham JA, Marso SP, Wyman MR, Pokala NR, Patel SM, Kotsia AP, Rangan BV, Lembo N, Kandzari D, Lee J, Kalynych A, Carlson H, Garcia SA, Thompson CA, Banerjee S, Brilakis ES. Transradial approach for coronary chronic total occlusion interventions: insights from a contemporary multicenter registry. Catheter Cardiovasc Interv. 2015;85:1123–1129. [DOI] [PubMed] [Google Scholar]
- 94. Dautov R, Ribeiro HB, Abdul‐Jawad Altisent O, Nombela‐Franco L, Gibrat C, Nguyen CM, Rinfret S. Effectiveness and safety of the transradial 8Fr sheathless approach for revascularization of chronic total occlusions. Am J Cardiol. 2016;118:785–789. [DOI] [PubMed] [Google Scholar]


