Abstract
Background
Reduced estimated glomerular filtration rate (eGFR) and elevated urinary albumin‐to‐creatinine ratio (ACR) individually increase risk of cardiovascular disease (CVD). We hypothesized that these associations are stronger among people with abnormal (both low and high) hemoglobin levels.
Methods and Results
Using 5801 participants with available hemoglobin measures of the ARIC (Atherosclerosis Risk in Community) study in 1996–1998, we explored the cross‐sectional association of eGFR and ACR with hemoglobin levels and their longitudinal associations with CVD (heart failure, coronary heart disease, and stroke) risk through 2013. At baseline, 8.8% had anemia (<13 g/dL in men and <12 g/dL in women) and 7.2% had high hemoglobin (≥16 g/dL in men and ≥15 g/dL in women). The adjusted prevalence ratio of anemia was 2.12 (95% confidence interval, 1.59–2.82) for eGFR 30 to 59 compared with ≥90 mL/min per 1.73 m2 and 1.45 (1.07–1.95) for ACR ≥30 compared with <10 mg/g. ACR ≥30 mg/g was also associated with high hemoglobin (prevalence ratio, 1.57 [1.12–2.19] compared with <10 mg/g). During follow‐up, there were 1069 incident CVDs among 5098 CVD‐free participants at baseline. In multivariable Cox models, lower eGFR, higher ACR, and anemia were each independently associated with CVD risk, with the association of low eGFR being slightly stronger in anemia (P‐for‐interaction, 0.072). There was no hemoglobin‐ACR interaction; however, when CVD subtypes were analyzed separately, risk of coronary heart disease and stroke associated with high ACR was slightly stronger in high hemoglobin (P‐for‐interaction, 0.074).
Conclusions
Kidney function, albuminuria, and anemia were correlated and independently associated with CVD risk. Correlation and potential interaction for atherosclerotic CVD between albuminuria and high hemoglobin deserve further investigation.
Keywords: anemia, anemia and chronic kidney disease, cardiovascular disease, chronic kidney disease
Subject Categories: Chronic Ischemic Heart Disease, Heart Failure, Ischemic Stroke
Clinical Perspective
What Is New?
In a community cohort of the ARIC (Atherosclerosis Risk in Communities) study, lower kidney function and higher albuminuria were each associated with higher prevalence of anemia; and higher albuminuria was also associated with higher prevalence of high hemoglobin.
In prospective analysis, the association of low kidney function with risk of cardiovascular disease tended to be stronger among people with anemia.
When cardiovascular subtypes were separately analyzed, the association of albuminuria with risk of coronary heart disease and stroke tended to be stronger among people with high hemoglobin.
What Are the Clinical Implications?
Potential synergistic effect of low kidney function and anemia on cardiovascular risk should warrant clinical attention.
The cross‐sectional association between albuminuria and high hemoglobin and their potential interaction on risk of coronary heart disease and stroke in prospective analysis deserve further investigation.
Cardiovascular disease (CVD) is an important complication of chronic kidney disease (CKD). Underlying mechanisms are multifactorial, but cardiac overload, vascular calcification, and arterial stiffness in reduced glomerular filtration rate (GFR) can cause ventricular‐arterial coupling mismatch.1, 2 In addition, albuminuria, the other important marker for defining and staging CKD,3 reflects endothelial function4 and low‐grade inflammation5 and is strongly associated with CVD risk.6
Anemia may also play a role in the relationship between CKD and CVD. Anemia is highly prevalent among people with reduced kidney function attributed to decreased synthesis of erythropoietin,7 iron deficiency,8 and chronic inflammation,9 and it contributes to increased cardiac workload10 and left ventricular hypertrophy.11 Previous studies showed that the association of reduced kidney function was stronger in anemia with the risk of coronary heart disease12 and stroke.13
On the other hand, the association between albuminuria and hemoglobin has been understudied, and the few existing studies have shown conflicting results. A few cross‐sectional studies of diabetic population showed positive association between albuminuria and anemia,14, 15 whereas a study of individuals living in high‐altitude showed an association between proteinuria and high hemoglobin.16 No studies have explored a potential interaction between albuminuria and hemoglobin in terms of future risk of CVD.
In the present study, we sought to investigate the cross‐sectional association of estimated GFR (eGFR) and albuminuria with hemoglobin levels and, subsequently, assess their potential interactions for CVD risk in a biethnic community‐based cohort, the ARIC (Atherosclerosis Risk in Communities) study. We hypothesized that the association of GFR and albuminuria with risk of CVD is stronger among people with deranged hemoglobin levels.
Materials and Methods
Study Population
Availability of data and material detailed policies for accessing ARIC data can be found at https://www2.cscc.unc.edu/aric/. It is also possible to obtain the study's data from the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/). The ARIC study is a prospective cohort of middle‐aged US adults designed to investigate risk factors for CVD. In brief, 15 792 participants aged between 45 and 64 years were enrolled from 4 US communities (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD) between 1987 and 1989. Subsequent visits were conducted every ≈3 years between 1990 and 1992 (visit 2), 1993 and 1995 (visit 3), and 1996 and 1998 (visit 4). More details of the ARIC study have been described previously.17 Visit 4 was set as baseline for the current study because the 3 primary exposures of eGFR, urinary albumin‐to‐creatinine ratio (ACR), and hemoglobin were simultaneously assessed for the first time at this visit. The study population was restricted to 6036 participants from the 2 field centers (Forsyth County, NC; Washington County, MD) who had hemoglobin levels measured voluntarily. We excluded those with (1) missing eGFR or ACR (n=160), (2) missing hemoglobin (n=33), (3) ethnicities other than white or black (n=22); and (4) eGFR <30 mL/min/1.73 m2 or end‐stage renal disease at baseline (n=20), leaving 5801 individuals included in the analysis. The study was conducted complying with the Declaration of Helsinki. Written informed consent was obtained from all participants, and the institutional review board at each study site approved the study (#H.34.99.07.02.A1 at Johns Hopkins University).
Exposures
GFR was estimated by the Chronic Kidney Disease Epidemiology Collaboration equation using serum creatinine and cystatin C.18 Serum creatinine was measured using a modified kinetic Jaffé method, calibrated to the Cleveland Clinic laboratory measurements,19 and then standardized to the isotope‐dilution mass spectrometry traceable method.20 Serum cystatin C was measured using an enhanced immunonephelometric assay (Siemens Healthcare Diagnostics, Tarrytown, NY). Urine albumin and creatinine were measured by nephelometry and the Jaffé method. For ACR, the detectable threshold was 2 mg/L for urine albumin and 1 mg/dL for urine creatinine. For 2386 participants with urine albumin values below the threshold, we imputed a value of 1 mg/L (no participants had urine creatinine below the detectable thresholds). Almost all participants with undetectable urine albumin (n=2373; 99.5%) were categorized as ACR <10 mg, followed by ACR 10 to 29 mg (n=12), and ACR >30 mg/g (n=1). eGFR and ACR were classified into 3 categories of eGFR ≥90, 60 to 89, and 30 to 59 mL/min/1.73 m2 and ACR <10, 10 to 29, and ≥30 mg/g, according to the clinical guidelines and literature.3, 21, 22
Hemoglobin was measured using automated hematology analyzers: Coulter S+IV (calibration S‐Cal; Beckman Coulter, Fullerton, CA) and Technicon H‐6000 (calibration Fisher; Technicon Corporation, Tarrytown, NY). Using the World Health Organization anemia definition,23 hemoglobin level was classified into 4 categories with different cutoffs for men and women as follows; high hemoglobin (≥16 g/dL in men and ≥15 g/dL in women), normal hemoglobin (≥13 and <16 g/dL in men and ≥12 and <15 g/dL in women), mild anemia (≥12 and <13 g/L in men and ≥11 and <12 g/dL in women), and moderate/severe anemia (<12 g/dL in men and <11 g/dL in women).
Outcomes
The primary outcome of interest was incident CVD, defined as incident heart failure (HF), coronary heart disease (CHD), and stroke. Secondarily, we analyzed HF and atherosclerotic CVD (ASCVD; including CHD and stroke) separately. The ARIC study has conducted active surveillance of hospital discharge records and linkage to death certificates to capture all hospitalizations and deaths with the International Disease Classification of Diseases, Ninth or Tenth Revision (ICD‐9 or ‐10) codes. Cases of HF were defined as hospitalizations and deaths with ICD‐9 or ‐10 codes indicating a diagnosis of HF (428 or I‐50, respectively). Incident ASCVD has been centrally adjudicated by a panel of physicians since the beginning of the ARIC study. Participants were followed until an event of interest and were censored by death, loss to follow‐up, or the end of study follow‐up on December 31, 2013.
Covariates
Covariate data were derived from visit 4 except years of education, which was assessed at visit 1. Age, sex, race, smoking status, alcohol consumption, and years of education were based on self‐reported questionnaires. Body mass index was calculated by dividing weight in kilograms by height in meters squared. Systolic and diastolic blood pressure were taken twice by trained technicians using a random‐zero sphygmomanometer in a sitting position after 5 minutes of rest and were averaged. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or taking antihypertensive drug. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, a casual glucose ≥200 mg/dL, a self‐reported physician diagnosis of diabetes mellitus, or antidiabetic medication use. Prevalent chronic obstructive pulmonary disease was defined as a history of hospitalization with ICD‐9 codes indicating chronic obstructive pulmonary disease (490, 491, 492, 494, and 496) between visit 1 and visit 4. Prevalent HF, CHD, and stroke were based on a self‐questionnaire at visit 1, and incident cases recorded between visit 1 and visit 4.
Statistical Analysis
In cross‐sectional analyses, baseline characteristics were compared across the categories of hemoglobin using ANOVA and chi‐square tests. Prevalence ratios of high hemoglobin or anemia associated with categories of eGFR and ACR were analyzed in separate Poisson regression models. Models were adjusted for age, sex, race, body mass index, smoking status, alcohol consumption, education level, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, past HF, past CHD, past stroke, and ACR for the analysis of eGFR and eGFR for the analysis of ACR. P values for linear trends among categorical variables were assessed by assigning the mean value for each category and modeling as a continuous variable.
In longitudinal analyses, individuals with past history of CVD at baseline were excluded to assess the incident cases. Crude incidence rates (IRs) and 95% confidence intervals (CIs) were estimated using Poisson regression models. Adjusted hazard ratios were estimated using Cox proportional hazards models with the same covariates for the cross‐sectional analysis described above except past CVD. Proportional hazards assumptions were verified using a Schoenfeld residual method.24 Interactions were assessed using the log‐likelihood tests comparing models with and without multiplicative interaction terms (ie, hemoglobin status×eGFR or ACR category). To obtain reliable estimates, mild and moderate/severe anemia were grouped together in the longitudinal analysis.
We conducted subgroup analyses by demographic factors of age (<65 versus ≥65 years) and sex (male versus female). Also, to validate some unique findings for the relationship between high hemoglobin and albuminuria in the primary analysis of ARIC (detailed in Results), we repeated the analysis using data from the National Health and Nutrition Examination Surveys (NHANES). Briefly, we analyzed 13 884 people aged ≥18 years in NHANES 1999–2004 who were assessed for hemoglobin, eGFR, and ACR and followed for up to 12.7 years (median, 9.5) through 2011 for mortality. Cardiovascular mortality was defined as deaths attributed to heart disease or cerebrovascular disease. The detail study designs and methods of the NHANES have been described elsewhere.25 A 2‐sided P value <0.05 was considered statistically significant. All statistical analyses were performed using STATA software (version 13; StataCorp LP, College Station, TX).
Results
Baseline Characteristics
Mean age of the study population was 63.3 (SD, 5.7) years, 54.3% were female, and 4.7% were black. Table 1 shows baseline characteristics stratified by hemoglobin status. At baseline, 4872 (84.0%) had normal hemoglobin and 509 (8.8%) had anemia, including 403 (7.0%) with mild anemia and 106 (1.8%) with moderate/severe anemia. There were 420 (7.2%) individuals with high hemoglobin. Individuals with anemia were more likely to be older, female, and black compared with those in the normal group. Individuals with high hemoglobin were more likely to be male, white, and current smokers compared with those with normal hemoglobin. Prevalence of diabetes mellitus and CVD was higher in both anemia and high hemoglobin compared with normal hemoglobin.
Table 1.
Baseline Characteristics by Hemoglobin Status
| Characteristics | Total (n=5801) | High Hemoglobina (n=420) | Normal Hemoglobina (n=4872) | Mild Anemiaa (n=403) | Moderate/Severe Anemiaa (n=106) | P Value |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 63.3 (5.7) | 62.9 (5.7) | 63.2 (5.6) | 64.0 (6.0) | 66.0 (5.6) | <0.001 |
| Female, N (%) | 3152 (54.3) | 118 (28.1) | 2697 (55.4) | 278 (69.0) | 59 (55.7) | <0.001 |
| Black race, N (%) | 273 (4.7) | 6 (1.4) | 206 (4.2) | 45 (11.2) | 16 (15.1) | <0.001 |
| Body mass index mean (SD), kg/m2 | 28.3 (5.4) | 28.9 (5.0) | 28.3 (5.4) | 27.6 (6.2) | 27.2 (5.0) | <0.001 |
| Blood pressure mean (SD), mm Hg | ||||||
| Systolic | 126.1 (18.7) | 126.9 (17.8) | 126.0 (18.5) | 126.0 (21.0) | 125.7 (19.2) | 0.806 |
| Diastolic | 68.2 (10.2) | 71.0 (10.1) | 68.3 (10.0) | 65.9 (11.1) | 62.3 (12.5) | <0.001 |
| Current/former smoker, N (%) | 3386 (58.4) | 311 (74.0) | 2809 (57.7) | 201 (49.9) | 65 (61.3) | <0.001 |
| Current/former alcohol consumer, N (%) | 4455 (76.8) | 351 (83.6) | 3730 (76.6) | 293 (72.7) | 81 (76.4) | 0.002 |
| 12 y or more of education, N (%) | 4665 (80.4) | 335 (79.8) | 3927 (80.6) | 324 (80.4) | 79 (74.5) | 0.470 |
| Medical history, N (%) | ||||||
| Hypertension | 2010 (34.6) | 144 (34.3) | 1675 (34.4) | 146 (36.2) | 45 (42.5) | 0.337 |
| Diabetes mellitus | 899 (15.5) | 84 (20.0) | 738 (15.1) | 59 (14.6) | 18 (17.0) | 0.057 |
| COPD | 202 (3.5) | 21 (5.0) | 158 (3.2) | 11 (2.7) | 12 (11.3) | <0.001 |
| Past heart failure | 136 (2.3) | 18 (4.3) | 93 (1.9) | 18 (4.5) | 7 (6.6) | 0.002 |
| Past coronary heart disease | 559 (9.6) | 47 (11.2) | 455 (9.3) | 43 (10.7) | 14 (13.2) | 0.282 |
| Past stroke | 113 (1.9) | 12 (2.9) | 86 (1.8) | 10 (2.5) | 5 (4.7) | 0.057 |
COPD indicates chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Anemia was defined as Hb <13 g/dL for men and Hb <12 g/dL for women. High hemoglobin was defined as Hb ≥16 g/dL for men and Hb ≥15 g/dL in women.
Cross‐Sectional Associations of eGFR and ACR With Hemoglobin Status
Figure shows the distribution of hemoglobin status across eGFR and ACR categories. Prevalence of anemia was higher with lower eGFR and higher ACR. When stratified by eGFR categories, anemia was present in 8.2% of participants with eGFR ≥90 mL/min/1.73 m2, 7.8% with eGFR 60 to 89 mL/min/1.73 m2, and 18.8% with eGFR 30 to 59 mL/min/1.73 m2. When stratified by ACR categories, prevalence of anemia was 8.1% in participants with ACR <10 mg/g, 10.7% with ACR 10 to 29 mg/g, and 13.3% with ACR ≥30 mg/g. Generally, similar patterns were observed for mild and moderate/severe anemia (Figure).
Figure 1.
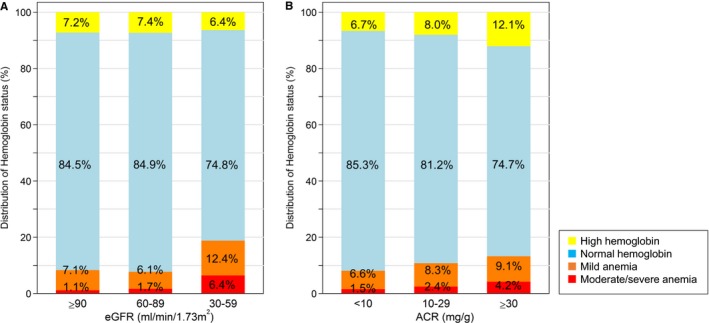
Proportion of high hemoglobin, normal hemoglobin, mild anemia, and moderate/severe anemia across (A) eGFR and (B) ACR categories: Percentage in the bar indicates the proportion of individuals with each hemoglobin category within eGFR and ACR category. High hemoglobin (Hb) was Hb ≥16 g/dL in men and Hb ≥15 g/dL in women, normal hemoglobin was Hb 13 to 16 in men and 12 to 15 g/dL in women, mild anemia was Hb 12 to 13 g/L in men and 11 to 12 g/dL in women, and moderate/severe anemia was <12 g/dL in men and <11 g/dL in women. ACR indicates albumin‐to‐creatinine ratio; eGFR, estimated glomerular filtration rate.
Regarding prevalence of high hemoglobin, there was no association with eGFR categories (7.2% of participants with eGFR 90 mL/min/1.73 m2, 7.4% with eGFR 30–89 mL/min/1.73 m2, and 6.4% with eGFR 30–59 mL/min/1.73 m2). In contrast, prevalence of high hemoglobin was incrementally higher among increasing categories of ACR (6.7% in ACR <10 mg/g, 8.0% in ACR 10–29 mg/g, and 12.1% in ACR ≥30 mg/g).
The association of eGFR and ACR with hemoglobin status remained consistent after adjusting for potential confounders (Table 2). The adjusted prevalence of anemia was significantly higher in participants with an eGFR of 30 to 59 mL/min/1.73 m2 (prevalence ratio, 2.12 [95% CI, 1.59–2.82]; P‐for‐trend, <0.001) compared with an eGFR ≥90 mL/min/1.73 m2. When ACR was examined, the adjusted prevalence of anemia was higher in participants with an ACR ≥30 mg/g (prevalence ratio, 1.45 [95% CI, 1.07–1.95]; P‐for‐trend, 0.022) compared with an ACR <10 mg/g. Regarding prevalence of high hemoglobin, there was no association with eGFR categories, whereas prevalence was significantly higher in participants with an ACR ≥30 mg/g (prevalence ratio, 1.57 [95% CI, 1.12–2.19]; P‐for‐trend, 0.009) compared with an ACR <10 mg/g (Table 2).
Table 2.
Adjustedb Prevalence Ratios of Anemia and High Hemoglobin
| Prevalence of Anemiaa | Prevalence of High Hemoglobina | |||
|---|---|---|---|---|
| Adjusted Prevalence Ratio (95% CI) | P Trend | Adjusted Prevalence Ratio (95% CI) | P Trend | |
| eGFR, mL/min per 1.73 m2 | ||||
| ≥90 | 1 [Reference] | <0.001 | 1 [Reference] | 0.601 |
| 60 to 89 | 0.95 (0.78–1.17) | 1.02 (0.82–1.26) | ||
| 30 to 59 | 2.12 (1.59–2.82) | 0.84 (0.55–1.29) | ||
| ACR, mg/g | ||||
| <10 | 1 [Reference] | 0.022 | 1 [Reference] | 0.009 |
| 10 to 29 | 1.21 (0.94–1.56) | 1.12 (0.82–1.53) | ||
| ≥30 | 1.45 (1.07–1.95) | 1.57 (1.12–2.19) | ||
ACR indicates albumin‐to‐creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
Anemia was defined as Hb <13 g/dL for men and Hb <12 g/dL for women. High hemoglobin was defined as Hb ≥16 g/dL for men and Hb ≥15 g/dL in women.
The model was adjusted for age, race, body mass index, smoking status, alcohol consumption, education level, hypertension, diabetes mellitus, past coronary disease, past stroke, chronic obstructive pulmonary disease, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR.
Longitudinal Associations of eGFR, ACR, and Hemoglobin Status With CVD Risk
During a median follow‐up of 15.6 years, there were 1069 cases of incident CVD among 5098 participants who were free of past history of CVD at baseline (crude IR, 15.4 per 1000 person‐years [95% CI, 14.5–16.4]). Table 3 shows the crude IRs across hemoglobin status and eGFR/ACR categories (as noted above, mild and moderate/severe anemia were grouped together). The IR per 1000 person‐years was higher in participants with an eGFR of 30 to 59 compared with ≥90 mL/min/1.73 m2 (IR, 34.0 [95% CI, 28.7–40.3] versus 11.0 [9.8–12.3]) and in participants with an ACR ≥30 compared with <10 mg/g (IR, 42.1 [95% CI, 35.6–49.7] versus 13.0 [12.1–14.0]). For hemoglobin status, the IR was higher with both high hemoglobin (IR, 18.3 [95% CI, 14.8–22.6]) and anemia (IR, 20.3 [95% CI, 16.8–24.4]) compared with normal hemoglobin (IR, 14.8 [95% CI, 13.8–15.8]). Both lower eGFR and higher ACR were associated with increased risk of CVD within each category of hemoglobin status, with the exception of eGFR in the category of high hemoglobin. However, there were few participants and events with eGFR 30 to 59 mL/min/1.73 m2 and high hemoglobin (3 events among 18 participants).
Table 3.
Crude Incidence Rates of Cardiovascular Disease Per 1000 Person‐Years
| High Hemoglobinb | Normal Hemoglobinb | Anemiab | Overall | |
|---|---|---|---|---|
| Events/No. of Subjects | Events/No. of Subjects | Events/No. of Subjects | Events/No. of Subjects | |
| Incidence Ratea (95% CI) | Incidence Ratea (95% CI) | Incidence Ratea (95% CI) | Incidence Ratea (95% CI) | |
| eGFR | ||||
| ≥90 | 27/143 | 263/1687 | 22/166 | 312/1996 |
| 14.2 (9.7–20.7) | 10.9 (9.6–12.3) | 9.4 (6.2–14.2) | 11.0 (9.8–12.3) | |
| 60 to 89 | 54/194 | 517/2349 | 52/205 | 623/2748 |
| 21.9 (16.8–28.6) | 16.2 (14.9–17.7) | 20.8 (15.8–27.3) | 16.9 (15.6–18.3) | |
| 30 to 59 | 3/18 | 95/275 | 36/61 | 134/354 |
| 13.3 (4.3–41.4) | 30.3 (24.8–37.0) | 62.0 (44.7–86.0) | 34.0 (28.7–40.3) | |
| ACR | ||||
| <10 | 48/276 | 643/3596 | 73/337 | 764/4209 |
| 12.6 (9.5–16.7) | 12.7 (11.8–13.7) | 16.6 (13.2–20.9) | 13.0 (12.1–14.0) | |
| 10 to 29 | 20/48 | 125/476 | 21/54 | 166/578 |
| 38.8 (25.0–60.2) | 20.8 (17.4–24.7) | 33.4 (21.8–51.2) | 23.2 (19.9–27.0) | |
| ≥30 | 16/31 | 107/239 | 16/41 | 139/311 |
| 59.3 (36.3–96.8) | 40.7 (33.7–49.2) | 39.2 (24.0–64.1) | 42.1 (35.6–49.7) | |
| Overall | 84/355 | 875/4311 | 110/432 | |
| 18.3 (14.8–22.6) | 14.8 (13.8–15.8) | 20.3 (16.8–24.4) | ||
ACR indicates albumin‐to‐creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
Incidence rates were presented in number of cases per 1000 person‐years.
High hemoglobin (Hb ≥16 g/dL in men and Hb ≥15 g/dL in women), normal hemoglobin (Hb 13–16 in men and 12–15 g/dL in women), and anemia (Hb <13 g/L in men and <12 g/dL in women).
In multivariable Cox analyses, results were largely consistent, with a higher adjusted risk of CVD with lower eGFR, higher ACR, and anemia, but not with high hemoglobin (Table 4). The association of incident CVD with low eGFR was stronger among participants with anemia compared with normal or high hemoglobin, with a borderline significance (P‐for‐interaction, 0.072; Table 4). On the other hand, there was no interaction between ACR and anemia (P‐for‐interaction, 0.262), suggesting any multiplicative associations with CVD risk (Table 4). There was also no interaction between eGFR and ACR (P‐for‐interaction, P=0.653).
Table 4.
Adjustedc Hazard Ratios of Cardiovascular Disease Across Hemoglobin and eGFR/ACR Categories
| Hemoglobin Categories Adjusted Hazard Ratio (95% CI) | All | |||
|---|---|---|---|---|
| High Hemoglobin | Normal Hemoglobin | Anemia | ||
| eGFRa, mL/min per 1.73 m2 | ||||
| ≥90 | 1.13 (0.74–1.72) | 1 [Reference] | 1.03 (0.66–1.62) | 1 [Reference] |
| 60 to 89 | 1.30 (0.96–1.78) | 1.14 (0.97–1.34) | 1.55 (1.14–2.10) | 1.16 (1.00–1.35) |
| 30 to 59 | 0.61 (0.19–1.92) | 1.55 (1.20–2.00) | 2.80 (1.93–4.06) | 1.62 (1.29–2.03) |
| ACRb, mg/g | ||||
| <10 | 0.95 (0.70–1.28) | 1 [Reference] | 1.38 (1.07–1.76) | 1 [Reference] |
| 10 to 29 | 2.05 (1.26–3.34) | 1.40 (1.15–1.71) | 2.66 (1.71–4.13) | 1.51 (1.27–1.80) |
| ≥30 | 2.43 (1.39–4.24) | 2.13 (1.71–2.66) | 2.07 (1.23–3.50) | 2.06 (1.69–2.52) |
| All | 1.06 (0.84–1.35) | 1 [Reference] | 1.38 (1.12–1.70) | |
ACR indicates albumin‐to‐creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate.
P‐for‐interaction (eGFR): 0.072.
P‐for‐interaction (ACR): 0.262.
The model was adjusted for age, race, body mass index, smoking status, alcohol consumption, education level, hypertension, diabetes mellitus, past coronary disease, past stroke, chronic obstructive pulmonary disease, and the ACR categories for the analysis of eGFR and the eGFR categories for the analysis of ACR.
The results were consistent when separating mild and moderate/severe anemia, with the risk of incident CVD being higher in moderate/severe anemia compared with mild anemia (Table S1). The associations were largely consistent in both older and younger participants (<65 versus ≥65 years) and men and women, without significant 3‐way interaction for the hemoglobin‐eGFR interaction or the hemoglobin‐ACR interaction (3‐way P‐for‐interaction, all >0.1).
Generally, consistent results were observed when assessing HF (921 cases in Table S2) and ASCVD (517 cases in Table S3) separately, although a borderline significant interaction was observed between hemoglobin status and ACR for ASCVD, where the association of high ACR with risk of ASCVD tended to be stronger among those with high hemoglobin compared with normal hemoglobin or anemia (P‐for‐interaction, 0.074; Table S3).
Analysis in the NHANES
To replicate the findings of the relationship between higher ACR and high hemoglobin in ARIC, we repeated the analysis in the NHANES (Table S4). Consistent with the cross‐sectional association in the primary analysis, prevalence of high hemoglobin was significantly higher in higher ACR ≥30 mg/g compared with <10 mg/g (prevalence ratio, 1.81 [95% CI, 1.37–2.38]; Table S5). For longitudinal analysis, 497 deaths attributed to CVD were recorded. Although the P value for interaction was not significant (P‐for‐interaction, 0.14), the risk gradient associated with high ACR appeared to be most evident within the category of high hemoglobin (>10‐fold risk gradient in high hemoglobin [hazard ratio, 4.97 in ACR ≥30 versus 0.34 in ACR <10 mg/g] versus ≈2.5‐fold risk gradient in anemia [hazard ratio, 5.07 in ACR ≥30 versus 2.14 in ACR <10 mg/g]; Table S6).
Discussion
In this community‐based cohort, both lower eGFR and higher ACR were independently associated with a higher prevalence of anemia. Of note, higher ACR was also significantly associated with a higher prevalence of high hemoglobin. In longitudinal analyses, lower eGFR, higher ACR, and anemia were independently associated with risk of CVD, with a slightly stronger association of lower eGFR in anemia. These findings were generally consistent in subgroup analyses by age and sex, and in separate analyses for risk of HF and ASCVD. Of interest, the association of high ACR with risk of ASCVD was slightly stronger in participants who had concomitant high hemoglobin. Similar cross‐sectional and longitudinal associations were observed in the NHANES.
Although several studies have reported high prevalence of anemia among people with reduced kidney function,26 most investigated clinical populations with advanced kidney failure,27 end‐stage renal disease,28 or diabetic nephropathy.14, 15 In addition, very few studies explored the association between albuminuria and the prevalence of anemia in the general population.29 In our study, the relationship between albuminuria and anemia was independent of kidney function. Although underlying mechanisms are not clear, albuminuria is linked to proximal tubule injury.30 Thus, function of interstitial fibroblasts producing erythropoietin may be impaired in this process. In addition, albuminuria is associated with low‐grade inflammation,31 a condition tightly linked to anemia.9
Derangements in kidney function and hemoglobin levels could both lead to limited cardiac oxygen supply and increase in cardiac workload. Thus, risk of CVD could be more evident when reduced kidney function and anemia concomitantly exist. In the present study, we noted that the association of low eGFR with CVD risk was slightly stronger in participants with anemia, which was generally consistent with previous studies investigating CHD17 and stroke18 outcomes.
Interestingly, the presence of albuminuria was significantly associated with high hemoglobin, which is in line with a study reporting a positive correlation between polycythemia and proteinuria among individuals living in a high altitude.16 High hemoglobin levels could increase blood viscosity,32 and this increased viscosity could elevate systemic blood pressure and glomerular perfusion pressure,33 which can lead to albuminuria.34 Nonetheless, further investigations are warranted regarding the pathophysiological link between albuminuria and high hemoglobin.
The positive association between albuminuria and high hemoglobin may provide important clinical implications given that we observed potential interactions for risk of some CVD outcomes. Previous studies report that individuals with high hemoglobin have an increased susceptibility to thrombogenesis, including an increased risk of CHD35 and thrombotic events.36 Given that endothelial injury plays a major role in the development of albuminuria,5 it is plausible that thrombogenesis attributed to high hemoglobin could be accelerated by the presence of endothelial dysfunction. Nonetheless, the potential interaction between high ACR and high hemoglobin for poor prognosis may deserve future investigations.
Several limitations should be acknowledged. First, our outcome ascertainment for HF relied on ICD codes, which might lead to misclassification. However, an abstract adjudication for acute decompensated HF showed the positive predictive value of hospitalizations with a primary ICD‐9 code 428 “heart failure” to be greater than 90%.37 Second, some cross‐categories of CKD measures and hemoglobin status had a relatively small number of events, which might reduce statistical power. Third, we could not exclude the possibility of residual confounding. For example, we did not have information on cause of kidney disease. Fourth, our study population in the ARIC study predominantly consisted of white adults, and thus generalizability of our findings to other races is not certain. However, similar results were observed in the NHANES, which consisted of representative population in the United States including blacks and Hispanics.
In conclusion, as anticipated, both lower eGFR and higher ACR were independently associated with high prevalence of anemia and higher CVD risk. Of note, ACR ≥30 mg/g was also associated with high prevalence of high hemoglobin. Anemia was also independently associated with risk of CVD, and we observed a slightly stronger association of low eGFR with CVD in anemia. In addition, the association of high ACR with risk of ASCVD was slightly stronger in high hemoglobin. These correlations and potential interaction between albuminuria and high hemoglobin would deserve further investigations.
Sources of Funding
Ishigami was supported by National Heart, Lung, and Blood Institute grant T32HL007024. The Atherosclerosis Risk in Communities Study is performed as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
Dr. Matsushita received research funding and personal fee out of the work from Kyowa Hakko Kirin and personal fee out of the work from Akebia Therapeutics.
Supporting information
Table S1. Adjusted Hazard Ratio of Cardiovascular Disease Across Hemoglobin and eGFR/ACR Category Separating Mild and Moderate/Severe Anemia
Table S2. Adjusted Hazard Ratio of Heart Failure
Table S3. Adjusted Hazard Ratio of Atherosclerotic Cardiovascular Disease
Table S4. Baseline Characteristics in NHANES 1999–2004
Table S5. Prevalence Ratio of Anemia and High Hemoglobin in the NHNAES 1999–2004
Table S6. Adjusted Hazard Ratio of Cardiovascular Mortality in NHANES 1999–2004 Through 2011
Acknowledgments
We thank the staff and participants of the Atherosclerosis Risk in Communities Study for their important contributions. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
(J Am Heart Assoc. 2018;7:e007209 DOI: 10.1161/JAHA.117.007209.)29330257
This study was presented in abstract form at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health Scientific Sessions; March 1–4, 2016, in Phoenix, AZ.
References
- 1. Hung SC, Kuo KL, Peng CH, Wu CH, Lien YC, Wang YC, Tarng DC. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014;85:703–709. [DOI] [PubMed] [Google Scholar]
- 2. Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23:586–593. [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 4. Satchell S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol. 2013;9:717–725. [DOI] [PubMed] [Google Scholar]
- 5. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106–2111. [DOI] [PubMed] [Google Scholar]
- 6. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J; CKD Prognosis Consortium . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta‐analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fehr T, Ammann P, Garzoni D, Korte W, Fierz W, Rickli H, Wuthrich RP. Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 2004;66:1206–1211. [DOI] [PubMed] [Google Scholar]
- 8. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL‐6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. [DOI] [PubMed] [Google Scholar]
- 10. Anand IS, Chandrashekhar Y, Ferrari R, Poole‐Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stritzke J, Mayer B, Lieb W, Luchner A, Doring A, Hense HW, Schunkert H. Haematocrit levels and left ventricular geometry: results of the MONICA Augsburg Echocardiographic Substudy. J Hypertens. 2007;25:1301–1309. [DOI] [PubMed] [Google Scholar]
- 12. Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community‐based Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919–2925. [DOI] [PubMed] [Google Scholar]
- 13. Abramson JL, Jurkovitz CT, Vaccarino V, Weintraub WS, McClellan W. Chronic kidney disease, anemia, and incident stroke in a middle‐aged, community‐based population: the ARIC study. Kidney Int. 2003;64:610–615. [DOI] [PubMed] [Google Scholar]
- 14. Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes: a cross‐sectional survey. Diabetes Care. 2003;26:1164–1169. [DOI] [PubMed] [Google Scholar]
- 15. Adetunji OR, Mani H, Olujohungbe A, Abraham KA, Gill GV. ‘Microalbuminuric anaemia’—the relationship between haemoglobin levels and albuminuria in diabetes. Diabetes Res Clin Pract. 2009;85:179–182. [DOI] [PubMed] [Google Scholar]
- 16. Jefferson JA, Escudero E, Hurtado ME, Kelly JP, Swenson ER, Wener MH, Burnier M, Maillard M, Schreiner GF, Schoene RB, Hurtado A, Johnson RJ. Hyperuricemia, hypertension, and proteinuria associated with high‐altitude polycythemia. Am J Kidney Dis. 2002;39:1135–1142. [DOI] [PubMed] [Google Scholar]
- 17. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 21. Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. [DOI] [PubMed] [Google Scholar]
- 22. Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 24. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 25. CDC . National Center for Health Statistics. Available at: http://www.Cdc.Gov/nchs/index.Htm. Accessed September 13, 2016.
- 26. Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162:1401–1408. [DOI] [PubMed] [Google Scholar]
- 27. Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38:803–812. [DOI] [PubMed] [Google Scholar]
- 28. Obrador GT, Roberts T, St Peter WL, Frazier E, Pereira BJ, Collins AJ. Trends in anemia at initiation of dialysis in the United States. Kidney Int. 2001;60:1875–1884. [DOI] [PubMed] [Google Scholar]
- 29. Inker LA, Coresh J, Levey AS, Tonelli M, Muntner P. Estimated GFR, albuminuria, and complications of chronic kidney disease. J Am Soc Nephrol. 2011;22:2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waikar SS, Sabbisetti V, Arnlov J, Carlsson AC, Coresh J, Feldman HI, Foster MC, Fufaa GD, Helmersson‐Karlqvist J, Hsu CY, Kimmel PL, Larsson A, Liu Y, Lind L, Liu KD, Mifflin TE, Nelson RG, Riserus U, Vasan RS, Xie D, Zhang X, Bonventre JV; for the Chronic Kidney Disease Biomarkers Consortium Investigators . Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule‐1 in five cohort studies. Nephrol Dial Transplant. 2016;31:1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS; CRIC Study Investigators . Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wade JP, Pearson TC, Russell RW, Wetherley‐Mein G. Cerebral blood flow and blood viscosity in patients with polycythaemia secondary to hypoxic lung disease. Br Med J (Clin Res Ed). 1981;283:689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson LO. Increased blood viscosity as the pathogenic agent in minimal change nephrosis: a new hypothesis. Med Hypotheses. 1981;7:1421–1429. [DOI] [PubMed] [Google Scholar]
- 34. Jae SY, Kurl S, Laukkanen JA, Heffernan KS, Choo J, Choi YH, Park JB. Higher blood hematocrit predicts hypertension in men. J Hypertens. 2014;32:245–250. [DOI] [PubMed] [Google Scholar]
- 35. Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease—the Framingham study: a 34‐year follow‐up. Am Heart J. 1994;127:674–682. [DOI] [PubMed] [Google Scholar]
- 36. Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Adjusted Hazard Ratio of Cardiovascular Disease Across Hemoglobin and eGFR/ACR Category Separating Mild and Moderate/Severe Anemia
Table S2. Adjusted Hazard Ratio of Heart Failure
Table S3. Adjusted Hazard Ratio of Atherosclerotic Cardiovascular Disease
Table S4. Baseline Characteristics in NHANES 1999–2004
Table S5. Prevalence Ratio of Anemia and High Hemoglobin in the NHNAES 1999–2004
Table S6. Adjusted Hazard Ratio of Cardiovascular Mortality in NHANES 1999–2004 Through 2011


