Abstract
Background
Excessive daytime sleepiness (EDS), a common symptom among patients with sleep‐disordered breathing, is closely associated with the development of cardiovascular diseases, but its long‐term prognostic value is not completely understood. The aim of this study was to investigate whether EDS would be an independent prognostic factor after myocardial infarction.
Methods and Results
We prospectively recruited 112 post–myocardial infarction patients. The Epworth Sleepiness Scale was completed before polysomnography, and EDS was defined as a score ≥11. After exclusion of 8 patients who accepted treatment with continuous positive airway pressure, 104 patients were followed up for 48 months. The primary composite end point was major adverse cardiac events. Patients with EDS had higher rates of major adverse cardiac events (48.4% versus 27.4%, χ2=5.27, P=0.022) and reinfarction (29.0% versus 5.5%, χ2=13.51, P=0.0002) compared with those without EDS. In the Cox proportional hazards model, patients with EDS had 2.15 times (95% confidence interval, 1.08–4.18; P=0.030) higher crude risk of major adverse cardiac events, with prognostic significance persisting after adjusting for age, diabetes mellitus, depression, left ventricular ejection fraction, apnea–hypopnea index, and nocturnal nadir oxygen saturation (hazard ratio: 2.13, 95% confidence interval, 1.04–4.26, P=0.039). Furthermore, among participants with moderate to severe sleep‐disordered breathing, the presence of EDS was associated with higher risk of major adverse cardiac events than those without EDS, after adjusting for age and nadir oxygen saturation (hazard ratio: 3.17, 95% confidence interval, 1.22–7.76, P=0.019).
Conclusions
EDS may be an independent prognostic factor of adverse outcome in post–myocardial infarction patients with moderate to severe sleep‐disordered breathing. Evaluation of EDS may shed new light on risk stratification and identify treatment responders for this patient population.
Keywords: excessive daytime sleepiness, major adverse cardiac event, myocardial infarction, sleep disordered breathing
Clinical Perspective
What Is New?
Patients with excessive daytime sleepiness (EDS) had significantly higher rates of major adverse cardiac events and reinfarction compared with those without EDS.
EDS in patients with moderate to severe sleep‐disordered breathing independently predicts major adverse cardiac events in a multivariate Cox proportional hazards model.
In patients with moderate to severe sleep‐disordered breathing and without EDS, the probability of major adverse cardiac events at 36 months is similar to the 36‐month outcome in those without sleep‐disordered breathing.
What Are the Clinical Implications?
Because of the strong prognostic implications of EDS in patients with moderate to severe sleep‐disordered breathing, we suggest routine evaluation of patients with myocardial infarction to identify those who are at risk and who may need intervention such as continuous positive airway pressure.
Larger clinical trials are warranted to evaluate strategies to alleviate EDS and the effect on long‐term prognosis.
Introduction
Epidemiological studies have shown that sleep‐disordered breathing (SDB) is associated with increased risk for cardiovascular disease (CVD) and poor prognosis.1, 2 Excessive daytime sleepiness (EDS) is a common symptom in patients with SDB and has been associated with an increased risk of motor vehicle accidents, occupational injuries, and the development of cerebrovascular diseases and CVD.3, 4 Moreover, longitudinal studies have shown that EDS is an independent risk factor for adverse outcomes in patients with coronary artery disease5 and predicts cardiovascular mortality.6, 7 Although continuous positive airway pressure (CPAP) is recommended for patients with moderate to severe SDB,8 evidence for its use in SDB patients with CVD but without EDS is questionable.9, 10 Recently, SAVE (Sleep Apnea Cardiovascular Endpoints Study) showed no reduction in cardiovascular events after CPAP treatment of predominantly nonsleepy patients with moderate to severe obstructive sleep apnea (OSA) and established CVD11; however, disease severity and poor CPAP adherence may have affected the results of this trial. More studies are required to evaluate the prognostic value of EDS in patients with specific CVD conditions. We previously reported that EDS is present in almost a third of patients after acute myocardial infarction (MI).12 Consequently, we sought to investigate whether subjective EDS would predict adverse outcomes for patients after MI, independent of traditional risk factors.
Methods
In consideration of the privacy of patients, the data, the analytic methods, and the study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We conducted a prospective study of post‐MI patients that included 112 patients who completed overnight attended polysomnography. This study was conducted between April 2004 and March 2010. Although consecutive patients were eligible, recruitment was based on exclusion criteria, availability of research personnel, and patients consenting to participate. The diagnosis of MI was made by the attending physician according to guidelines.13 Patients were excluded if they were previously given a diagnosis of SDB and treated with CPAP. Patients taking sedatives were also excluded from participation in the study because of influences on sleep pattern. This study was approved by the Mayo Clinic institutional review board, and all patients completed written informed consent. Note that outcomes data related to OSA from a large population of these participants were published previously.2
Questionnaires
Patients were asked to complete a paper version of the Epworth Sleepiness Scale (ESS) before polysomnography.14 The ESS is a self‐completed instrument consisting of 8 questions assessing the propensity to fall asleep while engaged in activities of daily living, with each item rated from 0 (not at all likely to fall asleep) to 3 (very likely to fall asleep). The total score ranges from 0 to 24, and subjectively quantified EDS was defined as a score ≥11.14 Scoring of the questionnaire was done while blinded to the results of the polysomnography and follow‐up.
Sleep Monitoring
All participants underwent fully attended overnight polysomnography within a median of 7 days after MI to identify the presence of SDB.2, 12, 15 Polysomnography was recorded on an E‐Series Comprehensive Networked‐Linked Amplifier System for Sleep/EEG (Compumedics) or Siesta 802 wireless amplifier/recorder for ambulatory polysomnography and long‐term electroencephalography (Compumedics). Airflow was monitored by nasal pressure transducer (integrated nasal pressure transducer with Pro‐Tech Pro‐Flow nasal cannulas) and oronasal thermocouple (Pro‐Tech 1‐channel oronasal thermal airflow sensor). During all polysomnography studies, the electroencephalogram, electro‐oculogram, and submental electromyogram were recorded with surface electrodes, according to American Academy of Sleep Medicine standards.16 Polysomnograms were scored by an experienced registered polysomnography technologist. Apneas were defined as ≥90% decrease of airflow for at least 10 seconds (as viewed on the thermal airflow channel), and hypopneas were defined as ≥30% decline in airflow for at least 10 seconds (as viewed on the nasal pressure channel) accompanied by oxygen desaturation ≥4%. Severity of disordered breathing was determined by calculating the apnea–hypopnea index (AHI; number of events per hour), and patients with AHI ≥5 were considered to have SDB, whereas those with AHI ≥15 were regarded as having moderate to severe SDB. Patients were considered to have central sleep apnea if the total AHI ≥15, with >50% of the breathing events of central origin.17
Patient Management and Follow‐up
Patients with an AHI ≥5 were advised to seek formal clinical assessment to confirm the diagnostic research findings and to initiate intervention at the treating physician's discretion. Treatment strategies for MI were at the discretion of the attending physician at the Mayo Clinic. Follow‐up contact was made by mail or telephone every 6 months to ascertain vital status and incidence of the outcome variables. The primary composite end point was major adverse cardiac events (MACE), including death (either cardiovascular or all‐cause mortality), readmission for recurrent nonfatal MI, hospitalized unstable angina regardless of revascularization, hospitalized heart failure, stroke, and significant arrhythmic events.18 Reinfarction was diagnosed according to patients' symptoms, elevated cardiac enzymes, ECG, and available angiographic data, as described previously.19
Statistical Analyses
Continuous variables were described as medians and 25th and 75th percentiles, whereas dichotomous variables were described as frequency and percentage. Wilcoxon tests were used for comparison of continuous variables, whereas Pearson χ2 tests were used for dichotomous variables. To minimize potential confounding of CPAP therapy, 8 post‐MI CPAP users (of whom 25% had EDS), after polysomnography testing, were not included in the analysis. Kaplan–Meier cumulative survival curves were constructed, and rates of MACE and reinfarction were compared by log‐rank test. Cox proportional hazards analysis was used to compute hazard ratios (HRs) for EDS in the prediction of MACE and reinfarction during follow‐up. Diabetes mellitus, left ventricular ejection fraction, and nocturnal nadir oxygen saturation (minSaO2), which have been reported to be strong prognostic factors in this patient group,2 were incorporated, together with age, AHI, and depression for adjustment in the statistical models of MACE. Only age and minSaO2 (ie, strongest prognostic factor2) were adjusted for in several multivariate models given the relatively small number of outcome events (if ≤20 events) to avoid overfitting of the Cox model. The appropriateness of the proportional hazards assumption was examined using graphical methods and tested with the Schoenfeld residuals test using Stata 12 (StataCorp). Analyses were performed using JMP version 10 (SAS Institute), and a P value <0.05 was considered statistically significant.
Results
Patient Characteristics
The final study sample consisted of 104 participants (82 male) with a median age of 62 years. SDB (AHI ≥5) was present in 76 patients analyzed (73.1%), of whom 6 (7.9%) had predominant central sleep apnea, and 46 (44.2%) had moderate to severe SDB (AHI ≥15). EDS (ESS score ≥11) was present in 31 participants (29.8%). The groups had similar prevalence of comorbidities, except that patients with EDS had slightly higher fasting triglyceride levels in comparison to patients without EDS (Table 1). Patients diagnosed with SDB had ESS scores similar to those of patients without SDB (median score 9 versus 8, P=0.149). The prevalence of EDS was not significantly different between patients with and without moderate to severe SDB (23.9% versus 34.5%, P=0.242).
Table 1.
Characteristics of Post‐MI Patients Classified by Presence or Absence of EDS (ESS ≥11)
| Variable | Non‐EDS (n=73) | EDS (n=31) | P Value |
|---|---|---|---|
| Age, y | 62 (54–72) | 62 (49–71) | 0.447 |
| Male, n (%) | 56 (76.7) | 26 (83.9) | 0.414 |
| Body mass index, kg/m2 | 28 (26–31) | 29 (26–33) | 0.336 |
| Total cholesterol, mmol/L | 4.32 (3.59–5.15) | 4.58 (3.72–5.58) | 0.293 |
| Triglyceride, mmol/L | 1.26 (0.69–1.86) | 1.59 (1.13–2.33) | 0.036 |
| Low‐density cholesterol, mmol/L | 2.59 (1.94–3.39) | 2.72 (2.02–3.26) | 0.821 |
| High‐density cholesterol, mmol/L | 1.06 (0.91–1.19) | 0.97 (0.86–1.16) | 0.370 |
| Fasting glucose, mmol/L | 6.11 (5.55–6.99) | 6.33 (5.77–7.27) | 0.179 |
| Systolic blood pressure, mm Hg | 118 (107–134) | 115 (101–126) | 0.322 |
| Diastolic blood pressure, mm Hg | 67 (60–74) | 66 (60–77) | 0.768 |
| Left ventricular ejection fraction, % | 53 (43–60) | 53 (40–61) | 0.915 |
| ST‐segment–elevation myocardial infarction, n (%) | 54 (74.0) | 22 (71.0) | 0.752 |
| Hypertension, n (%) | 43 (58.9) | 16 (51.6) | 0.492 |
| Diabetes mellitus, n (%) | 14 (19.2) | 6 (19.4) | 0.983 |
| Previous MI, n (%) | 13 (17.8) | 5 (16.1) | 0.836 |
| Major depressive disorder, n (%) | 12 (16.4) | 8 (25.8) | 0.268 |
| Current smoker, n (%) | 19 (26.0) | 9 (29.0) | 0.752 |
| Aspirin, n (%) | 68 (93.2) | 30 (96.8) | 0.469 |
| Adenosine diphosphate receptor inhibitor, n (%) | 60 (82.2) | 26 (83.9) | 0.836 |
| Beta‐blockade, n (%) | 71 (97.3) | 31 (100) | 0.352 |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, n (%) | 60 (82.2) | 23 (74.2) | 0.353 |
| Statins, n (%) | 68 (93.2) | 31 (100) | 0.135 |
| AHI, events/h | 13 (5–22) | 10 (6–25) | 0.899 |
| MinSaO2, % | 86 (83–90) | 85 (81–87) | 0.433 |
| Nocturnal saturation <90%, % | 99 (91–100) | 99 (97–100) | 0.847 |
| ESS score | 7 (5–9) | 13 (12–14) | <0.001 |
| Moderate to severe SDB, n (%) | 35 (48.0) | 11 (35.5) | 0.242 |
AHI indicates apnea–hypopnea index; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; MI, myocardial infarction; MinSaO2, nocturnal nadir oxygen saturation; SDB, sleep disordered breathing.
EDS Predicts MACE and Reinfarction
Thirty‐five patients (33.7%) experienced at least 1 MACE during the 4‐year follow‐up. The frequencies of MACE and the MACE components are shown in Table 2. Ten patients had ≥2 types of MACE; 15 patients had MACE ≥2 times. Reinfarction was the most common event (15 events) followed by angina (14 events), death (10 events), heart failure (9 events), significant arrhythmias (9 events), and stroke (3 events). Five patients did not complete the 48‐month investigation and reported no MACE during the mean follow‐up time of 34.8 months. Patients with EDS had significantly higher rates of MACE (48.4% versus 27.4%, χ2=5.27, P=0.022) and reinfarction (29.0% versus 5.5%, χ2=13.51, P=0.0002) compared with those without EDS (Figure 1). As shown in Table 3, patients with EDS had more than twice the risk of MACE 4 years after MI than those without EDS (HR: 2.15; 95% confidence interval [CI], 1.08–4.18; P=0.03). The prognostic value of EDS persisted after adjusting for age, diabetes mellitus, depression, left ventricular ejection fraction, AHI, and minSaO2 (HR: 2.13; 95% CI, 1.04–4.26; P=0.039). In addition, patients with EDS had a higher risk of reinfarction compared with those without EDS (HR: 6.76; 95% CI, 2.19–25.03; P<0.001), and that risk persisted after adjustment for age and minSaO2 (HR: 6.62; 95% CI, 2.12–24.8; P=0.001). Only 1 of 8 long‐term compliant CPAP users (1 had EDS and 7 had moderate to severe SDB) had MACE without death during the 48‐month follow‐up.
Table 2.
Frequency of MACE and MACE Components
| Overall Population (N=104) | Patients With EDS (n=31) | Patients Without EDS (n=73) | ||||
|---|---|---|---|---|---|---|
| Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | |
| MACE | 35 (33.7) | 60 | 15 (48.4) | 29 | 20 (27.4) | 31 |
| Death | 10 (9.6) | 10 | 4 (12.9) | 4 | 6 (8.2) | 6 |
| Hospitalization | ||||||
| Reinfarction | 13 (12.5) | 15 | 9 (29.0) | 11 | 4 (5.5) | 4 |
| Angina | 9 (8.7) | 14 | 2 (6.5) | 4 | 7 (9.6) | 10 |
| Heart failure | 9 (8.7) | 9 | 2 (6.5) | 2 | 7 (9.6) | 7 |
| Significant arrhythmias | 6 (5.8) | 9 | 4 (12.9) | 7 | 2 (2.7) | 2 |
| Stroke | 3 (2.9) | 3 | 1 (3.2) | 1 | 2 (2.7) | 2 |
EDS indicates excessive daytime sleepiness; MACE, major adverse cardiac events.
Figure 1.
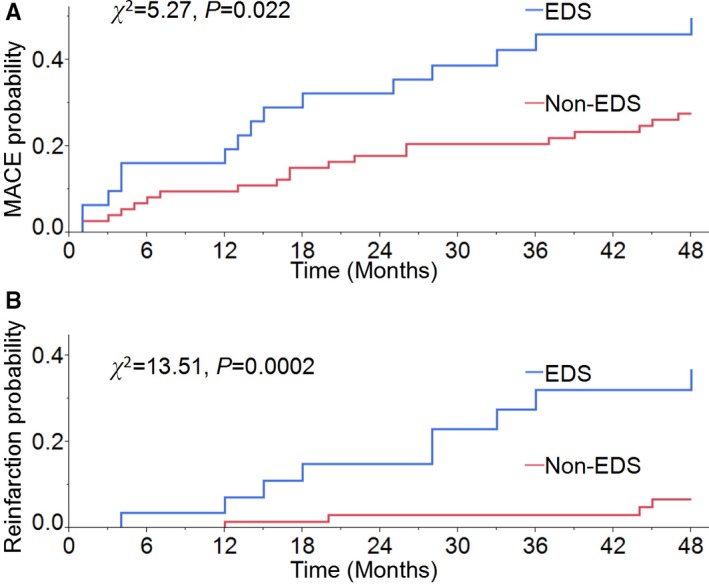
Kaplan–Meier curves show post–myocardial infarction patients with EDS had higher rates of MACE (A) and reinfarction (B) than those without EDS. EDS indicates excessive daytime sleepiness; MACE, major adverse cardiac events.
Table 3.
Cox Proportional Hazards Analysis of EDS (ESS ≥11) for MACE in Post‐MI Patients
| HR (95% CI) | P Value | |
|---|---|---|
| All post‐MI patients (n=104) | ||
| Unadjusted | 2.15 (1.08–4.18) | 0.030 |
| Adjusted for age | 2.15 (1.08–4.19) | 0.029 |
| Adjusted for age, diabetes mellitus, and LVEF | 2.15 (1.08–4.22) | 0.031 |
| Adjusted for age, diabetes mellitus, LVEF, depression, AHI, and minSaO2 | 2.13 (1.04–4.26) | 0.039 |
| Subgroup patients with moderate to severe SDB (n=46) | ||
| Unadjusted | 2.80 (1.10–6.75) | 0.032 |
| Adjusted for age and minSaO2 | 3.17 (1.22–7.76) | 0.019 |
AHI indicates apnea–hypopnea index; CI, confidence interval; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MI, myocardial infarction; minSaO2, nocturnal nadir oxygen saturation; SDB, sleep‐disordered breathing.
Prognostic Value of EDS in Patients With Moderate to Severe SDB
MACE rates were 17.9% in patients with no SDB (AHI<5), 30% with mild SDB (AHI 5 to <15), 37.1% with moderate to severe SDB (AHI ≥15) without EDS, and 72.7% with both moderate to severe SDB and EDS (χ2=12.68, P=0.005; Figure 2). In patients with moderate to severe SDB, MACE risk differed significantly in those with versus without EDS (HR: 2.80; 95% CI, 1.10–6.75; P=0.032), with prognostic significance persisting after adjusting for age and minSaO2 (HR: 3.17; 95% CI, 1.22–7.76; P=0.019). Patients with moderate to severe SDB without EDS (n=35) had no increase in MACE compared with patients without SDB (n=28) in an unadjusted model (HR: 2.23; 95% CI, 0.84–6.96; P=0.109).
Figure 2.
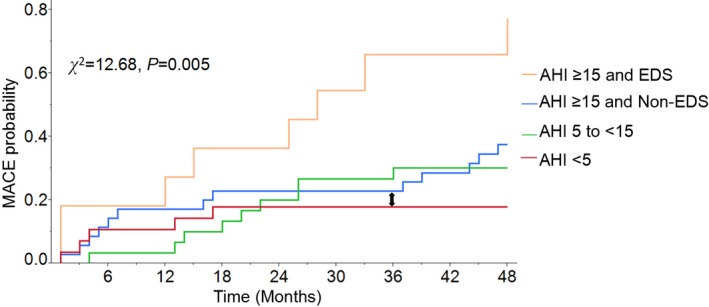
MACE estimates for post–myocardial infarction patients with and without SDB and EDS. Patients with both EDS and moderate to severe SDB (AHI ≥15) had the highest risk of MACE. Note that in those patients with moderate to severe SDB (AHI ≥15) and without EDS (blue line), the probability of MACE at 36 months (indicated by arrow) is very similar to the 36‐month outcome in those without SDB (AHI <5; red line). AHI indicates apnea–hypopnea index; EDS, excessive daytime sleepiness; MACE, major adverse cardiac events; SDB, sleep‐disordered breathing.
Discussion
These data are the first to suggest that subjective EDS is independently associated with an increased risk of MACE after MI. The prognostic value is most significant in patients with moderate to severe SDB. As an easily recognized complaint, EDS can be readily incorporated into routine evaluations for patients receiving secondary prevention therapy after MI.
The prognostic value of EDS in post‐MI patients is a novel finding and is consistent with previous studies in non‐MI patients,5, 6, 7 although other investigations did not report significant correlation of EDS with CVD prevalence in a cross‐sectional study20 or incidence of new‐onset CVD in longitudinal observation.21 A number of factors have been hypothesized as accounting for the unfavorable cardiovascular profile in sleepy individuals. First, EDS is closely associated with traditional cardiovascular risk factors, such as obesity, diabetes mellitus, uncontrolled hypertension, and physical inactivity.22, 23, 24, 25 It is conceivable that mechanisms implicated in CVD risk (eg, inflammation26) may also underlie EDS. Second, aside from SDB, insufficient sleep and other sleep disorders such as insomnia, which may undermine patients' sleep quality and cause EDS, have also been associated with poor cardiovascular outcomes.27 Third, although the basic mechanistic correlation between EDS and cardiovascular disorders remains unclear, assessment of inflammation, sympathetic overdrive, oxidative stress, and endocrine dysfunction, as found in sleep‐deprived participants, may provide insights into the mechanisms linking EDS to cardiovascular outcomes in post‐MI patients.28, 29, 30
Interestingly, although EDS occurs frequently in post‐MI patients, its presence is unrelated to a diagnosis of SDB. The relationship between SDB and EDS is also inconsistent in patients with atrial fibrillation31 and with heart failure.32 The reason for the absence of EDS in some post‐MI patients with SDB is not completely understood. It has been suggested that in patients with coexisting heart failure and OSA,33 the lack of EDS can be partially explained by a state of physiological hyperarousal provoked by elevated sympathetic activation during wakefulness. This hypothesis, however, cannot be extrapolated to post‐MI patients because if increased sympathetic activity accounted for lack of subjective EDS, we would expect to observe a worse prognosis than those with EDS, who presumably have lower sympathetic drive. Therefore, the mechanisms of the absence of EDS in patients with CVD need to be further investigated.
Combating hypersomnolence in post‐MI patients may be challenging. Because EDS is a frequent symptom of several CVD conditions,12, 33 chronic disease management rather than stimulant therapy may reasonably be prioritized to alleviate EDS and to improve cardiac outcomes as well. Meanwhile, because several preventable risk factors for coronary artery disease, such as inactivity, obesity, and a high‐carbohydrate diet,22, 24, 34 are closely related to EDS, lifestyle adjustment may be recommended. As one of the most important symptoms of moderate to severe SDB, the presence of EDS is an indicator for CPAP therapy.8 The recently published SAVE study excluded moderate to severe OSA patients with either severe hypoxemia or an ESS score >15 (mean ESS of 7.3 for participants receiving CPAP) and found that the use of CPAP therapy had no significant effect on the prevention of recurrent serious cardiovascular events.11 This result is in line with previous studies9, 10 that showed no favorable outcomes in nonsleepy SDB patients treated with CPAP. Importantly in our study—even in a high‐risk group such as post‐MI patients—in the absence of EDS, even moderate to severe SDB did not result in any increase in MACE. Note that in those patients with moderate to severe SDB and without EDS (Figure 2), the MACE probability at 36 months is very similar to the 36‐month outcome of those without SDB; therefore, it is conceivable that the absence of benefit of treatment in SAVE may in fact reflect the absence of increased cardiovascular risk in nonsleepy OSA patients with established CVD. Consequently, randomized controlled trials of the effects of OSA treatment of cardiovascular outcomes, which are limited to only nonsleepy patients, may not demonstrate any reduction in cardiovascular events, given that nonsleepy OSA patients may not be at higher risk than similar patients without OSA. Furthermore, adherence to CPAP therapy in SAVE was relatively low (3.3 h/night), which may have been associated with the lack of EDS. It is likely that poor adherence to CPAP therapy may have accounted for the negative result. In fact, in prior studies, patients using CPAP for >4 h/night exhibited better outcomes than those who had poor CPAP compliance.9, 10 Consequently, EDS can be used as an indicator of which patients could be the best candidates for CPAP treatment, since adherence to therapy may be better in symptomatic patients. However, a recent report suggests that other variables such as AHI and nocturnal desaturation may also be important for influencing adherence to CPAP.35 This personalized strategy for treatment selection may identify responders at greatest risk of adverse events and thus most likely to benefit with improved outcomes.
Strengths and Limitations
Strengths of this study include the prospective design and a blind evaluation of EDS, polysomnography, and follow‐up. Weaknesses include the modest sample size and thus limitations related to adjustment for more variables during prognostic modeling. The low percentages of female and young participants may also limit the generalizability of our findings. Meanwhile, ≈75% of the sample had SDB, and of those participants, only 8 effectively used CPAP. Those 8 patients were excluded from the analysis. We cannot rule out potential effects of accepting (or not accepting) therapy recommendations on outcomes. It is conceivable that not accepting therapy recommendations may itself actually be the core risk factor, rather than the sleepiness (or SDB) itself, in that the noncompliance itself is a risk factor.36 Last, patients' habitual sleep durations were not assessed and the degree of EDS at follow‐up may have differed from the levels reported at baseline. Incorporating a repeat questionnaire of EDS into follow‐up may be pragmatic and meaningful for the evaluation of high‐risk patients.
Conclusions
EDS occurs frequently in post‐MI patients regardless of SDB. Its presence is predictive of poor prognosis after MI, especially in patients with moderate to severe SDB, although its pathophysiological mechanisms and interventional strategies are still to be determined. The high prevalence and prognostic value of EDS in patients with CVD warrants further investigation because they may identify those at highest risk of mortality and in need of intervention to improve outcomes.
Sources of Funding
This study was supported by funding from National Institutes of Health grants HL114676, HL65176, HL114024, and UL1TR002377. Dr Xie is supported by Wu Yingkai Foundation for Medical Research and Development, Beijing, China (no. 201402). Dr Kuniyoshi was supported by American Heart Association grant 06‐15709Z. Dr Somers is supported by the NIH (grant HL65176). Dr Covassin is supported by American Heart Association grant 16SDG27250156. Dr Chahal is supported by American Heart Association grant 17POST33400211. This study was also supported in part by a gift to the Mayo Foundation by the Philips Respironics Foundation.
Disclosures
Dr Somers has served as a consultant for ResMed, Philips, GlaxoSmithKline, Respicardia, Ronda Grey, Biosense Webster, Dane Garvin, Bayer, and U‐Health. He has spoken at meetings sponsored by Philips, and ResMed. He works with Mayo Health Solutions and their industry partners on intellectual property related to sleep and to obesity. Dr Kuniyoshi became a full‐time employee of Philips Respironics after the collection of the baseline data provided in this article. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Acknowledgments
We would like to thank Debra L. Pfeifer and Ann B. Peterson for their superb secretarial and administrative assistance and Diane E. Davison, RN, MA, for her expertise in coordinating the studies.
(J Am Heart Assoc. 2018;7:e007221 DOI: 10.1161/JAHA.117.007221.)29352093
References
- 1. Hla KM, Young T, Hagen EW, Stein JH, Finn LA, Nieto FJ, Peppard PE. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Wang S, Chahal CA, Wei Y, Somers VK. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5:e003162 DOI: 10.1161/JAHA.115.003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boden‐Albala B, Roberts ET, Bazil C, Moon Y, Elkind MS, Rundek T, Paik MC, Sacco RL. Daytime sleepiness and risk of stroke and vascular disease findings from the Northern Manhattan Study (NOMAS). Circ Cardiovasc Qual Outcomes. 2012;5:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Endeshaw Y, Rice TB, Schwartz AV, Stone KL, Manini TM, Satterfield S, Cummings S, Harris T, Pahor M; Health ABC Study . Snoring, daytime sleepiness, and incident cardiovascular disease in the health, aging, and body composition study. Sleep. 2013;36:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee CH, Ng WY, Hau W, Ho HH, Tai BC, Chan MY, Richards AM, Tan HC. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9:1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Empana JP, Dauvilliers Y, Dartigues JF, Ritchie K, Gariepy J, Jouven X, Tzourio C, Amouyel P, Besset A, Ducimetiere P. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community‐dwelling elderly: the Three City Study. Stroke. 2009;40:1219–1224. [DOI] [PubMed] [Google Scholar]
- 7. Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–123. [DOI] [PubMed] [Google Scholar]
- 8. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long‐term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 9. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613–620. [DOI] [PubMed] [Google Scholar]
- 10. Barbé F, Durán‐Cantolla J, Sánchez‐de‐la‐Torre M, Martínez‐Alonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia‐Rio F, Diaz de Atauri J, Terán J, Mayos M, de la Peña M, Monasterio C, del Campo F, Montserrat JM; Spanish Sleep And Breathing Network . Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. [DOI] [PubMed] [Google Scholar]
- 11. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi‐Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
- 12. Sert Kuniyoshi FH, Zellmer MR, Calvin AD, Lopez‐Jimenez F, Albuquerque FN, van der Walt C, Trombetta IC, Caples SM, Shamsuzzaman AS, Bukartyk J, Konecny T, Gami AS, Kara T, Somers VK. Diagnostic accuracy of the Berlin Questionnaire in detecting sleep‐disordered breathing in patients with a recent myocardial infarction. Chest. 2011;140:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 14. Johns MW. A new method for measuring daytime sleepiness—the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 15. Sert Kuniyoshi FH, Singh P, Gami AS, Garcia‐Touchard A, van der Walt C, Pusalavidyasagar S, Wright RS, Vasquez EC, Lopez‐Jimenez F, Somers VK. Patients with obstructive sleep apnea exhibit impaired endothelial function after myocardial infarction. Chest. 2011;140:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Academy of Sleep Medicine Task Force . Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:662–689. [PubMed] [Google Scholar]
- 17. Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS; CANPAP Investigators . Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. [DOI] [PubMed] [Google Scholar]
- 18. Ng LL, Sandhu JK, Narayan H, Quinn PA, Squire IB, Davies JE, Bergmann A, Maisel A, Jones DJ. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2014;63:280–289. [DOI] [PubMed] [Google Scholar]
- 19. Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA; OASIS‐6 Trial Group . Effects of fondaparinux on mortality and reinfarction in patients with acute ST‐segment elevation myocardial infarction: the OASIS‐6 randomized trial. JAMA. 2006;295:1519–1530. [DOI] [PubMed] [Google Scholar]
- 20. Campos‐Rodríguez F, Reina‐González A, Reyes‐Núñez N, Beiztegui‐Sillero A, Almeida‐González C, Peña‐Griñán N. Clinical and cardiovascular characteristics of patients with obstructive sleep apnoeas without excessive daytime sleepiness. Arch Bronconeumol. 2010;46:594–599. [DOI] [PubMed] [Google Scholar]
- 21. Gangwisch JE, Rexrode K, Forman JP, Mukamal K, Malaspina D, Feskanich D. Daytime sleepiness and risk of coronary heart disease and stroke: results from the Nurses' Health Study II. Sleep Med. 2014;15:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bixler E, Vgontzas A, Lin H‐M, Calhoun S, Vela‐Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. [DOI] [PubMed] [Google Scholar]
- 23. Calhoun SL, Vgontzas AN, Fernandez‐Mendoza J, Mayes SD, Tsaoussoglou M, Basta M, Bixler EO. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep. Sleep. 2011;34:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basta M, Lin H‐M, Pejovic S, Sarrigiannidis A, Bixler EO, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 25. Mbatchou Ngahane BH, Nganda MM, Dzudie A, Luma H, Kamdem F, Ngote HR, Monkam Y, Kuaban C. Prevalence and determinants of excessive daytime sleepiness in hypertensive patients: a cross‐sectional study in Douala. Cameroon. BMJ open. 2015;5:e008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. [DOI] [PubMed] [Google Scholar]
- 27. Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. JAMA Intern Med. 2003;163:205–209. [DOI] [PubMed] [Google Scholar]
- 28. Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin‐2 levels in humans: clinical implications. J Clin Endocrinol Meta. 1999;84:1979–1985. [DOI] [PubMed] [Google Scholar]
- 29. Mullington JM, Haack M, Toth M, Serrador JM, Meierewert HK. Cardiovascular, inflammatory and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spiegel K, Leproult R, Van CE. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 31. Albuquerque FN, Calvin AD, Sert Kuniyoshi FH, Konecny T, Lopez‐Jimenez F, Pressman GS, Kara T, Friedman P, Ammash N, Somers VK, Caples SM. Sleep‐disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arzt M, Young T, Finn L, Skatrud JB, Ryan CM, Newton GE, Mak S, Parker JD, Floras JS, Bradley TD. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. [DOI] [PubMed] [Google Scholar]
- 33. Taranto Montemurro L, Floras JS, Millar PJ, Kasai T, Gabriel JM, Spaak J, Coelho FMS, Bradley TD. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142:1222–1228. [DOI] [PubMed] [Google Scholar]
- 34. Lowden A, Holmbäck U, Åkerstedt T, Forslund J, Lennernäs M, Forslund A. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol Psychol. 2004;65:251–263. [DOI] [PubMed] [Google Scholar]
- 35. Camposrodriguez F, Martinezalonso M, Sanchezdelatorre M, Barbe F. Long‐term adherence to continuous positive airway pressure therapy in non‐sleepy sleep apnea patients. Sleep Med. 2016;17:1–6. [DOI] [PubMed] [Google Scholar]
- 36. Truong KK, Ross M, Massoudi N, Hashemzadeh M, Jafari B. CPAP non‐compliance increases risk for 30‐day readmission in obstructive sleep apnea patients. Am J Respir Crit Care Med. 2016;193:A3109. (Abstract). [Google Scholar]


