Abstract
Background
Black race has been shown to be a risk factor for amputation in peripheral artery disease (PAD); however, race has been argued to be a marker for socioeconomic status (SES) rather than true disparity. The aim of this study is to study the impact of race and SES on amputation risk in PAD patients.
Methods and Results
Patients with incident PAD in the national Veterans Affairs Corporate Data Warehouse were identified from 2003 to 2014 (N=155 647). The exposures were race and SES (measured by median income in residential ZIP codes). The outcome was incident major amputation. Black veterans were significantly more likely to live in low‐SES neighborhoods and to present with advanced PAD. Black patients had a higher amputation risk in each SES stratum compared with white patients. In Cox models (adjusting for covariates), black race was associated with a 37% higher amputation risk compared with white race (hazard ratio: 1.37; 95% confidence interval, 1.30–1.45), whereas low SES was independently predictive of increased risk of amputation (hazard ratio: 1.12; 95% confidence interval, 1.06–1.17) and showed no evidence of interaction with race. In predicted amputation risk analysis, black race and low SES continued to be significant risk factors for amputation regardless of PAD presentation.
Conclusions
Black race significantly increases the risk of amputation within the same SES stratum compared with white race and has an independent effect on limb loss after controlling for comorbidities, severity of PAD at presentation, and use of medications.
Keywords: amputations, disparities, race, socioeconomic position
Subject Categories: Peripheral Vascular Disease, Quality and Outcomes
Clinical Perspective
What Is New?
Black race and low socioeconomic status (SES) are independently predictive of major amputations in patients with peripheral artery disease.
In the presence of diabetes mellitus and chronic kidney disease or end‐stage renal disease, the predicted major amputation risk of black patients with claudication and low SES approaches the risk of white patients with critical limb ischemia living in high‐SES neighborhoods.
What Are the Clinical Implications?
Black race should be considered not as a surrogate for access disparity and low SES in patients with peripheral artery disease but rather as an independent risk factor for major amputations.
Early diagnosis and medical management of peripheral artery disease and associated risk factors should be considered for black patients with claudication, especially with low SES, given their higher predicted risk of major amputations.
Peripheral artery disease (PAD) is a global pandemic affecting 8 million to 10 million adults in the United States and >200 million people worldwide.1 Black patients have a 2‐ to 3‐fold higher prevalence of PAD than white patients.2 A number of studies have shown black race to be a significant risk factor for amputation in PAD.3, 4, 5, 6 Advanced clinical stage, presence of comorbid diabetes mellitus (DM) and chronic kidney disease (CKD), and higher anatomic complexity of disease not amenable to revascularization have been put forth as reasons for poor limb salvage in black patients.7 Poorer patency of lower extremity bypasses and endovascular interventions in black patients suggest biological pathways affecting higher risk of amputation.4, 8, 9 Racial differences in endothelial oxidative stress and inflammation may be mechanisms for poorer outcomes in PAD patients.10
Some authors have argued that race could be a marker for socioeconomic status (SES) and thus for healthcare access, as the reason for racial disparity in vascular outcomes.11, 12, 13 Lack of access to care and regional clustering have been shown to affect black patients disproportionately.3, 7 Low SES has been clearly linked with higher prevalence of coronary artery disease (CAD), DM, hypertension, smoking, and physical inactivity as well as poor CAD outcomes such as myocardial infarction and cardiovascular death.14, 15 Low income has been shown to be associated with prevalence of PAD, but no research has clearly addressed the impact of SES on PAD outcomes or the interaction of race and SES on the risk of amputation for PAD patients.
The aim of this study is to examine the impact of racial disparity (black versus white) and low SES on limb loss in patients with PAD. We also aim to gain better understanding of the interaction of race and SES in the presence of high‐risk comorbidities (DM and CKD) and of the severity of PAD at presentation on the risk of amputation in veterans across the United States.
Methods
This study was approved by the Emory University institutional review board and the Atlanta VA (US Department of Veterans Affairs) Medical Center research and development committee. Informed consent was waived for a retrospective cohort study design with no human subject contact and minimal privacy risks. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure given the identifiable nature of the data sources.
Sample and Database
We reviewed the national Veterans Health Administration Corporate Data Warehouse to identify patients with incident symptomatic PAD (N=155 647) from 2003 to 2014, using a validated algorithm for the International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis code for PAD plus any 1 of 3 criteria: (1) 2 ankle‐brachial indexes in 14 months, (2) 2 visits to a vascular surgeon or clinic in 14 months, or (3) any PAD procedure code.16 A patient entered the PAD cohort on the earliest date of a PAD diagnosis code during the study time frame; this is referred to as the PAD diagnosis date. We excluded anyone with a PAD diagnosis code in the previous 3 years (2000–2002) to capture incident cases.
Study Exposures and Outcome
The following exposures were of interest. Race was abstracted from the national VA Corporate Data Warehouse and from VA Medical SAS administrative databases and was split into 3 categories: white, black, and other. Other race composed only 1.3% of the cohort; therefore, this group was excluded from further analyses. SES was operationalized as median household income of the patient's most recent residential ZIP code tabulation area (ZCTA). We used data from the American Community Survey, accessed through American Fact Finder. Specifically, we used 5‐year 2010–2014 estimates from the “Selected Economic Characteristics” table, stratified by ZCTA.17 We linked the income data to the ZCTAs we had for our patients from the VA data. We investigated crude associations between our SES measures and amputation risk and elected to dichotomize this variable (≈$40 000 per year), representing meaningful changes in risk. For sensitivity analyses, we used 2 other measures of SES: (1) neighborhood poverty, as defined by the percentage of individuals living below the poverty line in residential ZCTAs (dichotomized as ≤20% and >20%), and (2) area deprivation index (ADI). The ADI uses 17 US Census poverty, education, housing, and employment indicators to characterize neighborhood socioeconomic disadvantage and has been correlated with a number of health outcomes including mortality and rehospitalizations.18, 19 We used the methodology described by Kind et al by estimating ADI at a 5‐digit ZCTA level (Table S1) and then divided ADI into 4 “national ADI” categories: ADI‐0 (least deprived) composed 85% of the population, whereas ADI‐1, ‐2, and ‐3 were equally sized 5% groupings, with deprived neighborhoods in ascending order (ADI‐3 indicated most deprived).
The outcome of interest was incident major amputation (below‐ and above‐knee amputations) after PAD diagnosis during follow‐up. Specific amputation codes are defined in Table S2. The follow‐up continued through amputation occurrence, death (treated as a competing risk), or December 31, 2015 (at which the subject was censored). Patients with prior amputations were included in the analysis, but incident amputation was defined as the first major amputation after PAD diagnosis.
Covariates
A comprehensive list of patient demographics (age at PAD diagnosis, sex), body mass index (underweight, normal weight, overweight, or obese), smoking (current, former, never smoker, or unknown, classified using a validated method for text‐based health factors17), PAD severity (claudication, critical limb ischemia [CLI], or unspecified, using ICD‐9 codes and additional tissue loss codes for CLI20; Table S2), patient comorbidities (DM, hypertension, CAD, chronic obstructive pulmonary disease, congestive heart failure, atrial fibrillation, carotid disease, depression, CKD or end‐stage renal disease [ESRD]), medication use (antiplatelets, statins, cilostazol, and antiglycemics), serum creatinine, and urban/rural status of residence. All covariates were measured as closely as possible to the PAD diagnosis date with a 6‐month limit. Data were abstracted from the national VA Corporate Data Warehouse and VA Medical SAS administrative databases using administrative codes (Table S2) and pharmacy data tables.
Statistical Analyses
Demographic and clinical variables were assessed for the entire cohort and stratified by race and SES. Continuous variables were expressed as mean±SD or as median (interquartile range [IQR]) if they were not normally distributed. These were compared using independent‐sample t tests or the Wilcoxon rank‐sum test. Discrete variables were compared using χ2 tests for proportions. Proportions of missing data were also calculated, and participants with and without missing data were compared (Table S3). Complete case analysis was used (except for smoking, for which missing category was included), given the large sample size and comparable exposures of race and SES in the participants included in the Cox model and those missing other covariates. The Cox models included higher proportions of patients with current smoking and prevalence of comorbidities, likely biasing our Cox model estimates toward the null. A large proportion of patients with PAD did not have granular information on PAD severity (claudication versus CLI), which is a known drawback of the ICD‐9 coding system; therefore, these patients were handled as their ICD‐9 code category of unspecified symptoms.
Cumulative incidence function curves of amputation‐free survival, which account for death as a competing risk, were plotted by race and SES for up to 10 years of follow‐up.21 We then calculated crude 1‐, 3‐, 5‐, and 10‐year survival estimates from these curves.
Cause‐specific Cox proportional hazards regression models were then constructed to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for amputation. We began with a crude model containing only race (model 1), then adjusted sequentially for age and sex (model 2) and DM, CKD/ESRD, and PAD severity (model 3) as well‐known risk factors for amputation in PAD patients. We then added SES, as defined by median household income by ZCTA (model 4), to the previous model to examine the change in HRs for race on amputation risk and to assess the impact of SES. Model 5 was then constructed by the addition of all other covariates in Table 1 to the Cox model to obtain a fully adjusted model for amputation risk. We considered separate Cox models with alternative measures of SES (neighborhood poverty and ADI). We also tested interaction terms for race and SES, but all interaction terms were nonsignificant, so we report only the results of models without interaction. All variables were found to meet the proportional hazards assumption via log−log survival curves for amputation and mortality. Wald confidence limits were constructed for all HRs, and an additional Wald test was run to compare the within‐group comparisons. Furthermore, we constructed predicted risk curves at 3 years (1‐amputation–specific survival) for each race–SES stratum of patients with claudication and CLI and stratified these curves by DM and CKD/ESRD, as these are major predictors of amputation risk in this population.
Table 1.
Demographic and Baseline Information for the PAD Cohort Stratified by Race and SES
| All | Racea | SESb | |||
|---|---|---|---|---|---|
| Black | White | Income ≤$40 000 | Income >$40 000 | ||
| Patients, n | 155 647 | 23 204 | 118 762 | 46 893 | 105 117 |
| Race, % | |||||
| White | 82.6 | ··· | ··· | 72.4 | 87.2 |
| Black | 16.1 | ··· | ··· | 26.4 | 11.5 |
| Other | 1.3 | ··· | ··· | 1.2 | 1.3 |
| Median household income of residential ZCTA, % | |||||
| ≤$40 000 | 30.9 | 50.7 | 27.1 | ··· | ··· |
| >$40 000 | 69.2 | 49.3 | 72.9 | ··· | ··· |
| Individuals below poverty line in residential ZCTA, % | |||||
| ≤20.0% | 66.5 | 41.2 | 71.6 | 18.3 | 88.1 |
| >20.0% | 33.5 | 58.8 | 28.4 | 81.7 | 11.9 |
| ADI, mean (SD) | 103.1 (12.3) | 105.4 (11.2) | 102.8 (12.3) | 111.2 (4.6) | 99.5 (12.9) |
| Urban vs rural ZCTA, % | |||||
| Urban (population >50 000) | 67.3 | 87.1 | 63.2 | 60.3 | 70.4 |
| Urban cluster (population ≤50 000) | 20.1 | 8.8 | 22.4 | 23.8 | 18.5 |
| Rural | 12.6 | 4.1 | 14.5 | 15.8 | 11.1 |
| Age, y, mean (SD) | 66.7 (9.9) | 64.8 (10.3) | 66.9 (9.7) | 66.0 (9.8) | 66.9 (9.8) |
| Male sex (%) | 97.9 | 97.4 | 98.1 | 97.9 | 97.9 |
| Smoking | |||||
| Current | 51.4 | 56.5 | 51.0 | 54.3 | 50.1 |
| Former | 19.0 | 15.4 | 19.7 | 17.3 | 19.8 |
| Never | 6.5 | 6.7 | 6.1 | 6.4 | 6.5 |
| Missing | 23.2 | 21.4 | 23.2 | 22.1 | 23.6 |
| BMI categories, kg/m2 | |||||
| Underweight (<18.5) | 2.3 | 3.4 | 2.1 | 2.8 | 2.0 |
| Normal (18.5–25) | 26.3 | 30.5 | 25.2 | 28.5 | 25.3 |
| Overweight (25–30) | 36.0 | 34.2 | 36.4 | 25.6 | 36.2 |
| Obese (>30.0) | 35.4 | 31.9 | 36.4 | 33.1 | 36.5 |
| Comorbidities (reference: no) | |||||
| DM | 45.5 | 51.6 | 44.1 | 45.9 | 45.3 |
| Hypertension | 84.2 | 90.1 | 83.1 | 85.4 | 83.7 |
| CAD | 46.3 | 37.6 | 48.1 | 45.2 | 46.7 |
| CHF | 16.4 | 18.9 | 15.9 | 16.6 | 16.2 |
| COPD | 8.5 | 7.1 | 8.9 | 8.5 | 8.5 |
| AF | 12.0 | 7.9 | 12.9 | 10.9 | 12.5 |
| Carotid disease | 63.8 | 59.7 | 64.7 | 63.2 | 64.1 |
| Depression | 16.0 | 15.6 | 16.3 | 16.2 | 15.8 |
| CKD or ESRD | 7.4 | 15.6 | 5.6 | 8.1 | 7.0 |
| Taking any medications (%) | |||||
| Statins | 72.1 | 66.5 | 73.4 | 70.7 | 72.8 |
| Antiplatelets | 79.4 | 75.9 | 80.6 | 79.4 | 79.5 |
| Antiglycemics | 39.7 | 44.4 | 38.6 | 40.3 | 39.4 |
| Cilostazol | 8.0 | 8.0 | 8.0 | 8.3 | 7.9 |
| PAD severity per ICD‐9 codes (%) | |||||
| Unspecified | 60.1 | 56.1 | 60.8 | 59 | 60.6 |
| Claudication | 19 | 17.7 | 19.3 | 18.9 | 19.1 |
| CLI (rest pain/ulcer) | 20.9 | 26.3 | 19.9 | 22.1 | 20.3 |
| Laboratory | |||||
| Creatinine, mean (IQR) | 1.1 (0.9–1.4) | 1.2 (1.0–1.7) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) | 1.1 (0.9–1.4) |
| Outcomes | |||||
| Mortality, % | 40.7 | 38.3 | 40.2 | 41.9 | 39.7 |
| Amputation, % | 6.1 | 10.7 | 5.3 | 7.2 | 5.6 |
Antiplatelets include prasugrel, ticagrelor, dipyridamole, clopidogrel, or aspirin. ADI indicates area deprivation index; AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESRD, end‐stage renal disease; ICD‐9, International Classification of Diseases, Ninth Revision; IQR, interquartile range; PAD, peripheral artery disease; SES, socioeconomic status; ZCTA, ZIP code tabulation area.
P for comparisons across race categories <0.0001 for all variables except total cholesterol, depression, and cilostazol (P<0.05).
P for comparisons across 2‐level income categories <0.0001 for all variables except DM, carotid disease, cilostazol, and antiglycemics (P<0.05) and CHD, COPD, depression, antiplatelets, and sex (P>0.05).
The statistical analysis was done using SAS version 9.4 (SAS Institute). Two‐sided P<0.05 was considered statistically significant.
Results
Our cohort consisted of 155 647 veterans with clinical PAD with a median follow‐up of 5.9 years. The majority of the cohort was male (97.9%), with a mean age of 66.7±9.8 years. There were 9517 major amputations and 63 287 deaths identified during follow‐up. The cohort was 82.6% white and 16.1% black. SES variable distributions and other demographics are listed in Table 1. Black veterans were significantly more likely than white veterans to live in ZCTAs with lower SES, represented by lower median household income (≤$40 000: 50.7% versus 27.1%), higher neighborhood poverty (>20% individuals below the federal poverty level: 58.8% versus 28.4%), and higher mean ADI (105.4 versus 102.8). More black patients presented with CLI compared with white patients (26.3% versus 19.9%; P<0.0001) and had higher prevalence of DM (51.6% versus 44.1%; P<0.0001) and CKD/ESRD (15.5% versus 5.6%; P<0.0001). White PAD patients had higher prevalence of CAD (48.1% versus 37.6%; P<0.0001) and atrial fibrillation (12.9% versus 7.9%; P<0.0001). Black patients were less likely to be on a statin (66.5% versus 73.4%; P<0.0001) or antiplatelet medication (75.9% versus 80.6%; P<0.0001) but more likely to be on DM medication (44.4% versus 38.6%; P<0.0001) compared with white patients. However, after PAD diagnosis, black patients had more ankle‐brachial index measurements (median: 1.7 [IQR: 0.8–3.3] versus 1.6 [IQR: 0.8–3.2]; Kruskal–Wallis test, P<0.001) and vascular procedures (median: 0.8 [IQR: 0.5–1.8] versus 0.75 [IQR: 0.4–1.5]; Kruskal–Wallis test, P<0.001) than white patients during follow‐up.
Unadjusted Associations of Race and SES With Amputation and Mortality
The cumulative incidence function curves (Figure 1) plot amputation‐free survival over time for patients of different race and SES categories. They depict statistically significant differences in amputation‐free survival across race–SES categories, with black patients at particularly high risk and white patients at the lowest risk. White patients in the high‐SES category (median ZCTA income >$40 000) had the lowest amputation risk. Within races, differences in SES categories were statistically significant only for white patients. Amputation risks at 1, 3, 5, and 10 years were highest for black patients in the low‐SES group (4.4%, 7.2%, 9.4%, and 13.0%, respectively) and lowest for whites in the high‐SES group (2.1%, 3.3%, 4.3%, and 6.0%, respectively), with the risk in the highest group being roughly double that of the lowest group at each time point (Table 2).
Figure 1.
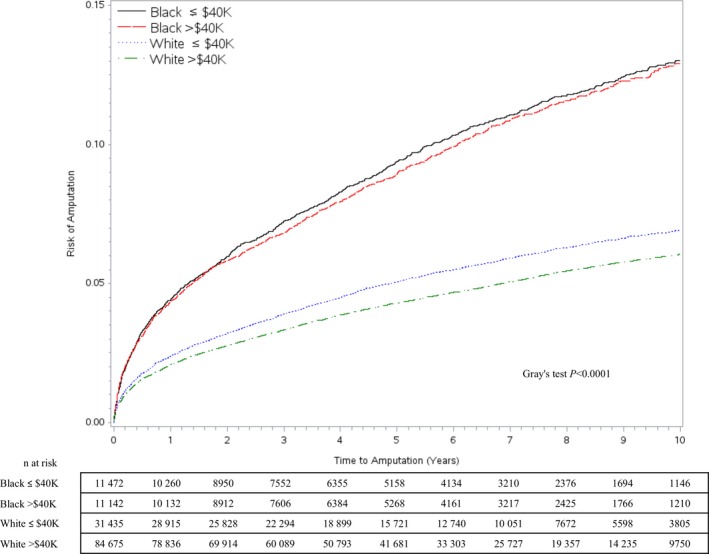
Cumulative incidence function curves of amputations in patients with incident peripheral artery disease from 2003 to 2014 and data on race and socioeconomic status.
Table 2.
Crude Amputation Risk Estimates From Cumulative Incidence Function Curves at 1, 3, 5, and 10 Years
| Race×SES (Income) | Major Amputation Risk, % (95% CI) | |||
|---|---|---|---|---|
| 1 y | 3 y | 5 y | 10 y | |
| Black | ||||
| ≤$40 000 | 4.4 (4.1–4.8) | 7.2 (6.8–7.7) | 9.4 (8.8–9.9) | 13.0 (12.3–13.8) |
| >$40 000 | 4.3 (4.0–4.7) | 6.8 (6.4–7.3) | 8.9 (8.4–9.5) | 12.9 (12.2–13.7) |
| White | ||||
| ≤$40 000 | 2.4 (2.2–2.6) | 3.9 (3.7–4.1) | 5.0 (4.8–5.3) | 6.9 (6.6–7.2) |
| >$40 000 | 2.1 (2.0–2.2) | 3.3 (3.2–3.4) | 4.3 (4.2–4.4) | 6.0 (5.9–6.2) |
CI indicates confidence interval; SES, socioeconomic status.
Adjusted Associations of Race and SES With Amputation and Mortality
In the crude model (model 1), black patients had roughly twice the amputation risk of white patients (HR: 2.08; 95% CI, 1.98–2.19; Table 3). Adjusting for age and sex (model 2) only slightly attenuated the HR for black patients (HR: 2.02; 95% CI, 1.93–2.12). Adjusting for DM, CKD, and PAD severity in model 3 reduced the HR for black patients (HR: 1.56) versus white patients (Table 3), suggesting substantial confounding of the impact of race on amputation risk by these traditional risk factors. In model 3, with the addition of PAD severity, the HR for PAD with unspecified severity (versus claudication) was 0.88, suggesting a predominance of mild claudication, asymptomatic PAD, or atypical forms of PAD in the unspecified cohort, whereas CLI (resting pain, tissue loss) had an HR of 7.8 for risk of amputations (versus claudication).
Table 3.
Cause‐Specific Cox Proportional Hazards Models for Impact of Race and SES on Amputations
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5a |
|---|---|---|---|---|---|
| Unadjusted | Race, Age, and Sex | Race, Age, Sex, DM, Kidney Disease, and PAD Severity | Race, Age, Sex, DM, Kidney Disease, PAD Severity, and SES | Fully Adjusted Model | |
| Race | |||||
| White | Ref. | Ref. | Ref. | Ref. | Ref. |
| Black | 2.08 (1.98–2.19) | 2.02 (1.93–2.12) | 1.56 (1.49–1.64) | 1.53 (1.45–1.60) | 1.37 (1.30–1.45) |
| Ageb | ··· | 0.99 (0.98–0.99) | 0.99 (0.99–0.99) | 0.99 (0.98–0.99) | 0.99–0.98–0.99) |
| Sex | |||||
| Male | ··· | Ref. | Ref. | Ref. | Ref. |
| Female | ··· | 0.39 (0.31–0.49) | 0.46 (0.37–0.57) | 0.46 (0.36–0.57) | 0.46 (0.37–0.57) |
| DM | ··· | ··· | 1.71 (1.63–1.79) | 1.72 (1.64–1.80) | 1.64 (1.49–1.82) |
| CKD or ESRD | ··· | ··· | 2.02 (1.89–2.15) | 2.03 (1.90–2.16) | 1.59 (1.46–1.73) |
| PAD severity | |||||
| Claudication | ··· | ··· | Ref. | Ref. | Ref. |
| Unspecifiedc | ··· | ··· | 0.88 (0.81–0.96 | 0.88 (0.81–0.96) | 0.88 (0.80–0.96) |
| CLI | ··· | ··· | 7.76 (7.72–8.41) | 7.74 (7.14–8.39) | 6.43 (5.92–6.98) |
| SES: median income (household) of residential ZCTA | |||||
| >$40 001 | ··· | ··· | ··· | Ref. | Ref. |
| ≤$40 000 | ··· | ··· | ··· | 1.12 (1.07–1.17) | 1.12 (1.06–1.17) |
Data are shown as hazard ratio (95% confidence interval). CKD indicates chronic kidney disease; CLI, critical limb ischemia; DM, diabetes mellitus; ESRD, end‐stage renal disease; PAD, peripheral artery disease; Ref., referent; SES, socioeconomic status; ZCTA, ZIP code tabulation area.
Fully adjusted model also adjusted for comorbidities including hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, atrial fibrillation, carotid disease, depression, body mass index, urban/rural population mix, smoking, creatinine level, and medication use (statins, antiplatelet agents, cilostazol, and antiglycemics [insulin/oral]).
One‐year increase.
Per International Classification of Diseases, Ninth Revision codes.
After adjusting for SES in model 4, the adjusted amputation HR for black patients (HR: 1.53; 95% CI, 1.45–1.60) did not change substantially. Low SES in this model was associated with a 12% greater risk of amputation versus high SES (HR: 1.12; 95% CI, 1.07–1.17). The HR for SES did not change with additional adjustments in model 5, although the HR for black versus white patients was further attenuated (HR: 1.37; 95% CI, 1.30–1.45) with the addition of other covariates. The additional covariates with the largest impact on amputation risk were female sex (HR: 0.5), congestive heart failure (HR: 1.34), current smoking (HR: 1.26), use of insulin (HR: 1.5), and body mass index (HR: underweight, 1.7; overweight, 0.7; and obese, 0.6; Table S4, model 1). Prevalent use of medications including statins (HR: 0.8), antiplatelet medications (HR: 0.9), and cilostazol (HR: 0.8) was protective for amputation risk. When we omitted medication use from the adjusted Cox model, we found that the race–amputation risk association was stronger (HR: 1.41 versus 1.37 in model with medication use), whereas the SES–amputation risk HRs remained the same (1.12), suggesting that medication use was modifying or attenuating the association between race and amputation risk (Table S4, model 2). On replacing the CKD/ESRD variable (as defined by ICD‐9 diagnoses codes) by CKD stages (as defined by calculation of estimated glomerular filtration rate), we find a similar association of race and SES with amputation risk (Table S4, model 3). However, most of the elevated risk associated with CKD is confined to CKD stage 4 (HR: 1.35; 95% CI, 1.2–1.5) and CKD stage5/ESRD (HR: 1.6; 95% CI, 1.5–1.8). Neighborhood poverty (HR: 1.16) and ADI (HR: 1.12 for ADI‐2) had a similar association with increased amputation risk when used as a measure of SES in the adjusted Cox models (Table 4) instead of median income by ZCTA. Black race remained an independent predictor of worse amputation risk in each model (HR: 1.35, Model 1 neighborhood poverty; and 1.40, Model 2 ADI, respectively).
Table 4.
Cause‐Specific Cox Proportional Hazards Models for Impact of Race and SES on Amputations in PAD: Sensitivity Analysis Using Alternative Measures of SES
| Variable | Model 1a | Model 2a |
|---|---|---|
| SES Measure: Neighborhood Poverty | SES Measure: ADI | |
| Race | ||
| White | Ref. | Ref. |
| Black | 1.35 (1.28–1.43) | 1.40 (1.33–1.47) |
| Individuals below poverty line in residential ZCTA (%) | ||
| ≤20% | Ref. | ··· |
| >20% | 1.16 (1.11–1.22) | ··· |
| ADI categories | ||
| ADI‐0 (least disadvantaged) | ··· | Ref |
| ADI‐1 | ··· | 0.99 (0.89–1.09) |
| ADI‐2 | ··· | 1.12 (1.02–1.23) |
| ADI‐3 (most disadvantaged) | ··· | 1.05 (0.96–1.16) |
Data are shown as hazard ratio (95% confidence interval). ADI indicates area deprivation index; PAD indicates peripheral artery disease; Ref., referent; SES, socioeconomic status; ZCTA, ZIP code tabulation area.
Fully adjusted models also adjusted for age, sex, comorbidities (diabetes mellitus, chronic kidney disease/end‐stage renal disease, hypertension, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation, carotid disease), depression, smoking, creatinine level, PAD severity, diagnosis year, urban/rural ZIP code area, body mass index.
Predicted Amputation Risk by Race, SES, DM, Kidney Disease, and PAD Severity
We then looked at predicted amputation risk at three years stratified by presentation of claudication (Figure 2) and CLI (Figure 3), based on the fully adjusted Cox model. The predicted risk was further assessed by subgroups for race, SES, and presence of comorbid DM or CKD/ESRD. As expected, our analysis showed worse amputation risk for patients with CLI across the board (7–26%) versus patients with claudication (1–5%). In both claudication and CLI patients, those with both DM and CKD/ESRD experienced the worst amputation risk regardless of SES category, followed by patients with one or the other at roughly the same risk. Patients with neither DM nor CKD had the best amputation‐free survival in all race–SES categories. Among patients with claudication (Figure 2), black patients had higher amputation risks than white patients (with those of other races falling in the middle) in both low‐ and high‐SES categories. For all races, higher SES was associated with fewer amputations (Figure 2). Similar patterns were observed among CLI patients (Figure 3). Interestingly, black patients with claudication, low SES, and presence of DM and CKD/ESRD had a predicted 3‐year amputation risk of 5%, which is comparable to white CLI patients with no DM or kidney disease (7%). In models with interaction terms between race and SES, those terms were not significant, and only noninteraction models are presented.
Figure 2.
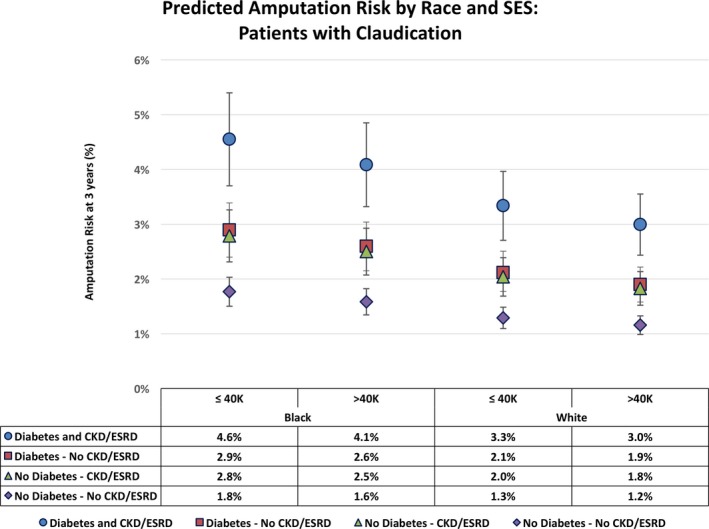
Three‐year predicted risk of amputation in each race–socioeconomic status (SES) stratum of patients with claudication, stratified by diabetes mellitus and chronic kidney disease/end stage renal disease (CKD/ESRD) status.
Figure 3.
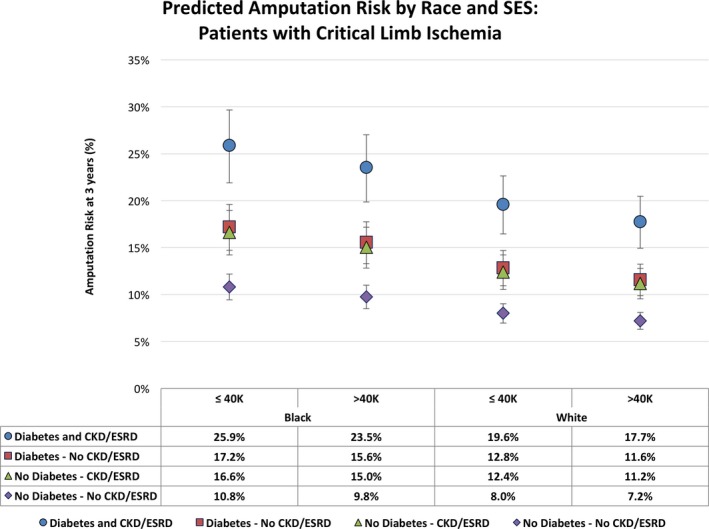
Three‐year predicted risk of amputation in each race–socioeconomic status (SES) stratum of patients with critical limb ischemia (CLI), stratified by diabetes mellitus and chronic kidney disease/end stage renal disease (CKD/ESRD) status.
Discussion
Our study is one of the largest population‐based studies showing the independent impact of race and SES on lifetime risk of amputations in incident PAD patients. We confirmed the negative impact of well‐known risk factors such as DM, kidney disease, and CLI at presentation on limb loss for PAD patients and showed how black race is disproportionately affected even after accounting for these comorbidities and advanced presentation. Low SES increases the risk of amputation for each race and risk subgroup. Traditionally, claudication has been regarded as an early sign of PAD and of having a low risk of amputation; however, we showed that black patients with claudication, DM, and kidney disease have a high risk of amputation comparable to that of white healthier CLI patients. This finding suggests a role for early diagnosis and aggressive management of PAD in black patients.
Our findings are consistent with other studies showing that racial disparity and low SES may contribute to the increased amputation rate of PAD patients.7, 22, 23 The biological mechanisms postulated for this disparity include a proinflammatory state that tends to affect black patients10, 24 and people with low SES25 disproportionately, even after adjustment for difference in cardiovascular risk. Black race, DM, and kidney disease have all been associated with arterial stiffness, small‐vessel disease, and infrapopliteal occlusive disease.26, 27 These differences in comorbidities and inflammation may alter the natural progression of PAD and the success of medical or surgical interventions, supporting the independent effects seen in our study for black patients and those in poor socioeconomic strata. A recent analysis of lower extremity bypasses in the National Surgical Quality Improvement Project database found that black patients had a higher incidence of femoral–tibial or popliteal–tibial bypasses compared with white PAD patients.28 Involvement of distal tibial vessels in PAD may preclude successful attempts at revascularization and increase the need for primary amputation.7, 22, 23 Despite revascularization, black race has been shown to affect patency of open and endovascular procedures.4, 8, 9, 28 The predilection for more aggressive atherosclerotic disease burden is further supported by our findings of the higher predicted 3‐year amputation risk (5%) seen in black patients with DM and CKD/ESRD living in low‐SES neighborhoods despite presenting early with claudication. In contrast, white patients with CLI and without the other risk factors have only a 7% predicted risk of limb loss over the same time.
Most studies, including our analysis, find that black patients present late in the course of their disease and more frequently with gangrene compared with white patients.7, 22, 23, 29 Recent publications have attempted to evaluate whether the well‐described occurrence of primary amputation rather than attempt at revascularization for black patients is due to provider bias or lack of access to quality care compared with clinical presentation. We saw a higher number of post diagnosis ankle‐brachial indexes and revascularizations at follow‐up for black patients, suggesting a lack of bias in the VA system. Conversely, Regenbogen et al found that black Medicare beneficiaries undergoing lower extremity amputations were less likely to be treated by vascular specialists and less likely to be treated at high‐volume hospitals. Racial gaps persisted even among high‐performing physicians, suggesting differences in provider clinical decision‐making and management.29 Holman et al also found that black Medicare beneficiaries were less likely to undergo limb‐salvage procedures than their white counterparts leading up to amputation and that differences in rates of care were due to differences in how black and white patients were treated within hospital referral regions and not to regional differences in where they received care.23 Durazzo et al found worsening of the racial gap in hospitals with the greatest capacity to perform revascularizations. Contrary to our findings, they saw increased disparity in limb loss for black patients living in wealthier areas even though there was an overall increase in the likelihood of receiving revascularization for all patients residing in wealthier ZIP codes.7 Provider bias, influenced by race, in the management of cardiovascular disease has been described in the literature.30, 31 We found that black patients had lower rates of prescriptions for statins and antiplatelet agents in our cohort compared with white patients. Low SES was associated with lower use of statins but not of antiplatelet agents, which are largely available as generic products, suggesting a role for medication affordability and copayments. Nevertheless, our findings in adjusted models of independent association of black race and low SES with increased amputations accounted for the medical management of PAD.
Studies have also shown that black patients tend to reside in residential ZIP codes with low income.7, 32 Although insurance status has been shown to increase the likelihood of amputation in PAD in the United States,22, 33 socioeconomic deprivation is associated with lower extremity amputations in a nationalized health system like the United Kingdom.34 Studies from the VA system have shown better health outcomes for minorities with CAD, heart failure, and hospitalizations.35, 36, 37 Our study shows that efforts may be needed in the VA system to decrease the disparity for black and low‐SES patients regarding PAD amputations. A striking finding from our analysis is the high predicted risk of ≈5% for 3‐year amputation risk in black patients with DM and kidney disease despite early presentation as patients with claudication. This emphasizes the need for strategies to identify PAD early in especially vulnerable populations and to target aggressive risk factor control and optimal medical management. Supervised exercise therapy, which is now covered by Medicare for first‐line treatment of claudication, may help prevent progression of PAD to CLI and amputation. Contemporary studies have shown a decline in amputation rates for Medicare beneficiaries with PAD in general and across racial subgroups, although disparities still exist.3, 38 Multidisciplinary preventative measures such as diabetic control and wound care, along with patient and community engagement, seem to decrease racial disparity in limb loss among diabetic patients and may hold promise in PAD care.12, 38, 39 Increased awareness and education of providers in reducing implicit bias in the treatment of PAD is also needed.40 Future research directions include implementation and dissemination of quality‐driven metrics and evaluation of longitudinal management programs of PAD patients to improve limb salvage whether using medical or surgical treatment options.
Our study has several limitations. First, the observational nature of this study using administrative data and clinical care records makes it susceptible to bias due to data collection and missingness, although we have described in detail our analytical methods for covariate identification and handling of missing data. Complete case analysis may introduce bias due to unmeasured differences in missing data; however, we performed a detailed comparison of our missing and included cohorts in Table S3. Our main source of missingness was the exposure of interest (ie, race and SES), thus we chose complete case analysis over imputation techniques. Another limitation is a high number of patients with unspecified disease severity (59%); this may reflect weaknesses of the ICD‐9 coding scheme in chronic disease41 or underrecognition of disease severity as a prognostic factor in PAD.42, 43 However, the amputation risk estimates for the unspecified category are <1 (HR: 0.88), suggesting mostly less severe disease, including asymptomatic PAD or minor symptoms. For the purposes of adjusted or predicted amputation risk, we used model estimates that were clearly classified as claudication or CLI to obtain accurate risk prediction for racial subgroups and to show disparity in amputation risk. The occurrence of amputation was captured by a combination of procedure codes and diagnostic codes. Those happening outside the VA would be captured as diagnoses codes if they were seen by a VA provider following that surgery or if the VA paid for that care. We could miss the occurrence of amputations in a small percentage of patients who sought all care outside the VA system and did not use VA coverage (fee basis). We made comprehensive efforts to show the etiological nature of the racial and SES disparity using multiple models with additional covariates based on known risk factors for PAD amputations, but the analysis may be susceptible to residual confounding. The analysis focuses on risk factors at baseline, and we did not account for time‐varying covariates in terms of progression of DM or CKD and suspect that it may further highlight the disparity faced by black patients in terms of biological risk. Our study is based on Veterans Health Administration data, and it is overwhelmingly composed of male patients. Results may differ in a non‐VA population. Given the nature of administrative data, we cannot determine the role of patient preference and choices in the outcome of amputation, and its relationship to race or SES is unknown.
In conclusion, our study is a large population‐based study using national veteran health administrative data and shows the importance of black race and low SES in independently affecting amputation risk of incident PAD patients, despite controlling for multiple risk factors and medical management of PAD. Furthermore, we showed the variation in predicted risk of amputation for claudication and CLI patients by race, low SES, and presence of DM and kidney disease. Black patients seem to be continually disadvantaged in terms of high occurrence of comorbid conditions, residence in low‐SES areas, and increased risk of limb loss despite early presentation as patients with claudication compared with white patients, even after adjusting for multiple risk factors.
Sources of Funding
This work was supported by the following grants: Arya: American Heart Association Mentored Clinical and Population Research Award (15MCPRP25580005); National Institutes of Health–National Institute of Aging (NIH‐NIA), 1R03AG050930; American Geriatric Society/Society for Vascular Surgery Foundation Jahnigen Career Development Award. Brewster: NIH–National Heart, Lung, and Blood Institute (NHLBI), KO8HL119592; Society for Vascular Surgery Foundation/American College of Surgeons Mentored Clinical Scientist Research Career Development Award; Wilson: Veteran Affairs Merit Grant I01‐CX001025. This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center, Decatur GA. The funding organizations did not participate directly in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article; or the decision to submit the article for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government.
Disclosures
None.
Supporting information
Table S1. Components of Area Deprivation Index
Table S2. International Classification of Diseases, Ninth Revision Diagnosis and Current Procedural Terminology Codes Used to Define Comorbidities and Amputations
Table S3. Comparison of Missing and Nonmissing Demographics Stratified by No Versus Any Missing Data and Observations Included in and Excluded From Each Cox Proportional Hazards Models Due to Missing Data
Table S4. Cause‐Specific Cox Proportional Hazards Models for Impact of Race and Socioeconomic Status on Amputations: (1) Fully Adjusted Primary Model, (2) Fully Adjusted Model Without Medications, and (3) Fully Adjusted Model Using Chronic Kidney Disease as Defined by Estimated Glomerular Filtration Rate
(J Am Heart Assoc. 2018;7:e007425 DOI: 10.1161/JAHA.117.007425.)29330260
A small portion and earlier version of this analysis was presented at the posters session at American Heart Association Scientific Sessions, November 12 – 16, 2016, in New Orleans, LA.
References
- 1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic‐specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. [DOI] [PubMed] [Google Scholar]
- 3. Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol. 2012;60:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen LL, Hevelone N, Rogers SO, Bandyk DF, Clowes AW, Moneta GL, Lipsitz S, Conte MS. Disparity in outcomes of surgical revascularization for limb salvage: race and gender are synergistic determinants of vein graft failure and limb loss. Circulation. 2009;119:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg JB, Goodney PP, Cronenwett JL, Baker F. The effect of risk and race on lower extremity amputations among Medicare diabetic patients. J Vasc Surg. 2012;56:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rivero M, Nader ND, Blochle R, Harris LM, Dryjski ML, Dosluoglu HH. Poorer limb salvage in African American men with chronic limb ischemia is due to advanced clinical stage and higher anatomic complexity at presentation. J Vasc Surg. 2016;63:1318–1324. [DOI] [PubMed] [Google Scholar]
- 7. Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. 2013;148:617–623. [DOI] [PubMed] [Google Scholar]
- 8. Loja MN, Brunson A, Li CS, Carson JG, White RH, Romano PS, Hedayati N. Racial disparities in outcomes of endovascular procedures for peripheral arterial disease: an evaluation of California hospitals, 2005–2009. Ann Vasc Surg. 2015;29:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chew DK, Nguyen LL, Owens CD, Conte MS, Whittemore AD, Gravereaux EC, Menard MT, Belkin M. Comparative analysis of autogenous infrainguinal bypass grafts in African Americans and Caucasians: the association of race with graft function and limb salvage. J Vasc Surg. 2005;42:695–701. [DOI] [PubMed] [Google Scholar]
- 10. Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, Csiszar A, Sonntag WE. Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. J Vasc Surg. 2015;61:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loehrer AP, Hawkins AT, Auchincloss HG, Song Z, Hutter MM, Patel VI. Impact of expanded insurance coverage on racial disparities in vascular disease: insights from Massachusetts. Ann Surg. 2016;263:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphries MD, Brunson A, Li C‐S, Melnikow J, Romano PS. Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J Vasc Surg. 2016;64:1747–1755.e1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital‐related predictors of amputation for critical limb ischemia. J Vasc Surg. 2011;53:330–339.e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alter DA, Franklin B, Ko DT, Austin PC, Lee DS, Oh PI, Stukel TA, Tu JV. Socioeconomic status, functional recovery, and long‐term mortality among patients surviving acute myocardial infarction. PLoS One. 2014;8:e65130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001–2006. Ann Epidemiol. 2010;20:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arya S, Binney Z, Khakharia A, Brewster LP, Wilson PWF, Goodney PP. Peripheral arterial disease (PAD) variability in diagnosis: the Veterans Affairs (VA) population experience. Association of VA Surgeons 40th Annual Surgical Symposium. 2016.
- 17. McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez‐Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kind AJH, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Ann Intern Med. 2014;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fincke BG, Miller DR, Turpin R. A classification of diabetic foot infections using ICD‐9‐CM codes: application to a large computerized medical database. BMC Health Serv Res. 2010;10:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007;45:55–59. [DOI] [PubMed] [Google Scholar]
- 23. Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. 2011;54:420–426, 426.e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, Gibbons GH, Vaccarino V, Din‐Dzietham R, Quyyumi AA. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META‐Health) study. Metab Syndr Relat Disord. 2012;10:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muennig P, Sohler N, Mahato B. Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: evidence from NHANES. Prev Med. 2007;45:35–40. [DOI] [PubMed] [Google Scholar]
- 26. Sidawy AN, Schweitzer EJ, Neville RF, Alexander EP, Temeck BK, Curry KM. Race as a risk factor in the severity of infragenicular occlusive disease: study of an urban hospital patient population. J Vasc Surg. 1990;11:536–543. [PubMed] [Google Scholar]
- 27. He C, Yang JG, Li YM, Rong J, Du FZ, Yang ZG, Gu M. Comparison of lower extremity atherosclerosis in diabetic and non‐diabetic patients using multidetector computed tomography. BMC Cardiovasc Disord. 2014;14:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selvarajah S, Black JH III, Haider AH, Abularrage CJ. Racial disparity in early graft failure after infrainguinal bypass. J Surg Res. 2014;190:335–343. [DOI] [PubMed] [Google Scholar]
- 29. Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower‐extremity vascular amputations? Ann Surg. 2009;250:424–431. [DOI] [PubMed] [Google Scholar]
- 30. Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–626. [DOI] [PubMed] [Google Scholar]
- 31. Safford MM, Gamboa CM, Durant RW, Brown TM, Glasser SP, Shikany JM, Zweifler RM, Howard G, Muntner P. Race‐sex differences in the management of hyperlipidemia: the REasons for Geographic and Racial Differences in Stroke study. Am J Prev Med. 2015;48:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim LK, Swaminathan RV, Minutello RM, Gade CL, Yang DC, Charitakis K, Shah A, Kaple R, Bergman G, Singh H, Wong SC, Feldman DN. Trends in hospital treatments for peripheral arterial disease in the United States and association between payer status and quality of care/outcomes, 2007–2011. Catheter Cardiovasc Interv. 2015;86:864–872. [DOI] [PubMed] [Google Scholar]
- 34. Ferguson HJM, Nightingale P, Pathak R, Jayatunga AP. The influence of socio‐economic deprivation on rates of major lower limb amputation secondary to peripheral arterial disease. Eur J Vasc Endovasc Surg. 2010;40:76–80. [DOI] [PubMed] [Google Scholar]
- 35. Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285:297–303. [DOI] [PubMed] [Google Scholar]
- 36. Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. J Am Coll Cardiol. 2004;43:778–784. [DOI] [PubMed] [Google Scholar]
- 37. Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar‐Zadeh K. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation. 2015;132:1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suckow BD, Newhall KA, Bekelis K, Faerber AE, Gottlieb DJ, Skinner JS, Stone DH, Goodney PP. Hemoglobin A1c testing and amputation rates in black, Hispanic, and white Medicare patients. Ann Vasc Surg. 2016;36:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colleran K, Harding E, Kipp BJ, Zurawski A, MacMillan B, Jelinkova L, Kalishman S, Dion D, Som D, Arora S. Building capacity to reduce disparities in diabetes: training community health workers using an integrated distance learning model. Diabetes Educ. 2012;38:386–396. [DOI] [PubMed] [Google Scholar]
- 40. Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–1336. [DOI] [PubMed] [Google Scholar]
- 41. Rhodes ET, Laffel LMB, Gonzalez TV, Ludwig DS. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care. 2006;30:141. [DOI] [PubMed] [Google Scholar]
- 42. Hirsch A, Criqui M, Treat‐Jacobson D, Regensteiner J, Creager M, Olin J, Krook S, Hunninghake D, Comerota A, Walsh M, McDermott M, Hiatt W. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 43. Hoeks SE, Scholte op Reimer WJM, van Gestel YRBM, Schouten O, Lenzen MJ, Flu W‐J, van Kuijk J‐P, Latour C, Bax JJ, van Urk H, Poldermans D. Medication underuse during long‐term follow‐up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2:338–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Components of Area Deprivation Index
Table S2. International Classification of Diseases, Ninth Revision Diagnosis and Current Procedural Terminology Codes Used to Define Comorbidities and Amputations
Table S3. Comparison of Missing and Nonmissing Demographics Stratified by No Versus Any Missing Data and Observations Included in and Excluded From Each Cox Proportional Hazards Models Due to Missing Data
Table S4. Cause‐Specific Cox Proportional Hazards Models for Impact of Race and Socioeconomic Status on Amputations: (1) Fully Adjusted Primary Model, (2) Fully Adjusted Model Without Medications, and (3) Fully Adjusted Model Using Chronic Kidney Disease as Defined by Estimated Glomerular Filtration Rate


