Abstract
Background
Preterm delivery (<37 weeks gestational age) affects 11% of all pregnancies, but data are conflicting whether preterm birth is associated with long‐term adverse maternal cardiovascular outcomes. We aimed to systematically evaluate and summarize the evidence on the relationship between preterm birth and future maternal risk of cardiovascular diseases.
Methods and Results
A systematic search of MEDLINE and EMBASE was performed to identify relevant studies that evaluated the association between preterm birth and future maternal risk of composite cardiovascular disease, coronary heart disease, stroke, and death caused by cardiovascular or coronary heart disease and stroke. We quantified the associations using random effects meta‐analysis. Twenty‐one studies with over 5.8 million women, including over 338 000 women with previous preterm deliveries, were identified. Meta‐analysis of studies that adjusted for potential confounders showed that preterm birth was associated with an increased risk of maternal future cardiovascular disease (risk ratio [RR] 1.43, 95% confidence interval [CI], 1.18, 1.72), cardiovascular disease death (RR 1.78, 95% CI, 1.42, 2.21), coronary heart disease (RR 1.49, 95% CI, 1.38, 1.60), coronary heart disease death (RR 2.10, 95% CI, 1.87, 2.36), and stroke (RR 1.65, 95% CI, 1.51, 1.79). Sensitivity analysis showed that the highest risks occurred when the preterm deliveries occurred before 32 weeks gestation or were medically indicated.
Conclusions
Preterm delivery is associated with an increase in future maternal adverse cardiovascular outcomes, including a 2‐fold increase in deaths caused by coronary heart disease. These findings support the assessment of preterm delivery in cardiovascular risk assessment in women.
Keywords: cardiovascular disease risk factors, coronary heart disease risk, long‐term outcome, pregnancy and postpartum, stroke
Subject Categories: Meta Analysis, Pregnancy, Women, Risk Factors
Clinical Perspective
What Is New?
Preterm delivery is associated with a 1.4‐ to 2‐fold increase in maternal risk for future incident cardiovascular events, cardiovascular death, coronary heart disease events, coronary heart disease death, and stroke.
This increased risk is greatest in preterm births that occur before 32 weeks in gestation or in those that are delivered for medical indications such as fetal growth restriction or pre‐eclampsia.
For cardiovascular disease and coronary heart disease outcomes, the risks are higher in women with a greater number of recurrent preterm births.
What Are the Clinical Implications?
In keeping with current recommendations, our study highlights the importance of advising women with preterm births about their increased cardiovascular risk and advocating and supporting lifestyle and behavioral changes to control their modifiable risk factors.
These findings support the evaluation of preterm delivery in cardiovascular risk assessment in postnatal women.
Introduction
Globally, preterm birth affects 11% of all pregnancies, with an estimated 14.9 million babies born before 37 weeks gestational age each year.1 In addition to being the leading cause of neonatal mortality,2 there is increasing evidence to show that preterm delivery is an adverse pregnancy outcome associated with an increased risk of future maternal cardiovascular health.3, 4, 5 Cardiovascular disease is the leading cause of mortality worldwide,6 most of which is preventable by altering behavioral risk profiles and lifestyle modifications, but there may be sex‐specific cardiovascular risk factors that need to be recognized in women.7
Pregnancy is characterized by a challenge to the cardiovascular system with a doubling of blood volume, elevated coagulation and inflammatory factors, hyperlipidemia, and insulin resistance.8, 9 This physiological stress for most women is uncomplicated but for women who experience preterm birth, this adverse pregnancy outcome may serve to identify women at risk for cardiovascular disease who would not have been detected using traditional risk assessment tools at a time when it may be possible to alter their risk trajectory.10, 11, 12
It remains unclear whether preterm delivery is an independent risk factor for future cardiovascular disease or an early marker of women with background high‐risk profiles for future cardiovascular disease. As preterm birth is a heterogeneous condition with multiple causes, the pathogenesis of preterm birth remains poorly understood. The main proposed mechanisms include increased systemic inflammation, infection, or vascular diseases.13, 14, 15 The duration of pregnancy gestation has been inversely correlated to insulin resistance, blood pressure, and low‐grade inflammation in women years after delivery.16, 17, 18 In addition, women with previous preterm births, but without pre‐eclampsia or small‐for‐gestational‐age births, have higher atherogenic lipids and carotid arterial wall thickening in the decade after delivery compared with women who had term births.19 Therefore, the dysregulation in cardiometabolic factors with their common pathways to cardiovascular diseases may provide a possible explanation for the link between preterm birth and future cardiovascular diseases.20, 21
Previous studies, including a meta‐analysis, have examined the relationship between preterm delivery and future incident cardiovascular disease.3, 4, 5 The previous meta‐analysis included studies published up to 2011.3 Since then, there have been further studies including large sample sizes (ie, >100 000 participants).22, 23, 24 Some newer studies also demonstrated results inconsistent with earlier literature showing no increased risk for future stroke events.25, 26 Furthermore, previous work did not differentiate between morbidity and mortality outcomes, nor examined clinically relevant factors such as gestation at delivery, recurrence and cause of preterm births. As recent guidelines from the United States27, 28 and European Union29 recommend the inclusion of a history of preterm birth to evaluate the cardiovascular disease and stroke risk in women based on evidence from cohort studies published up to 2011,30, 31, 32, 33, 34, 35 there is a need for contemporary evidence. To this end, we conducted a systematic review and meta‐analysis to quantify the risk of maternal cardiovascular events in later life following preterm birth and contribute to future recommendations for clinical practice.
Methods
Eligibility Criteria
The data, analytic methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedure. The protocol was registered on PROSPERO an International prospective register of systematic reviews.36 We selected studies investigating postnatal cardiovascular outcomes of women with preterm delivery. Preterm delivery was defined as birth any time before 37 weeks gestation. Primary cardiovascular outcomes were composite cardiovascular disease (defined as a combination of cardiac, cerebrovascular, and peripheral vascular disease), death caused by composite cardiovascular disease, coronary heart disease, death caused by coronary heart disease, stroke, and stroke death. The International Classification of Diseases (ICD) (versions 7‐10) code definitions of the outcomes are specific to each study and are detailed in Table S2. The included studies had at least 2 groups (1 with preterm birth and 1 with term birth) and reported sufficient data to allow for accurate risk estimates to be calculated. There was no restriction based on language, cohort type, study design, or duration of follow‐up.
Data Sources and Searches
MEDLINE and EMBASE were searched using OVID SP for studies from inception to October 2017. The detailed search terms are outlined in Methods S2. Manual searching for additional articles was also conducted by reviewing the bibliography of relevant review articles and published systematic reviews.3, 4, 5, 37
Study Selection and Data Extraction
Two reviewers (P.W. and G.V.) screened all titles that met the inclusion criteria. This was followed by a screen of the remaining abstracts. The full articles were screened by the same 2 reviewers and the final decision to include studies was made by P.W. Independent double data extraction was done by 4 reviewers (P.W., C.S.K., C.W., and A.N.). Data were collected on study design, year, country, number of participants, mean age, parity, cohort characteristics, definition and ascertainment of preterm birth, ascertainment of outcomes, timing of assessment, adequacy of follow‐up, and results. The information was obtained from published data.
Study Quality Assessment
Study quality was assessed based on the recommendations of the Newcastle‐Ottawa Quality Assessment Scale for cohort studies.38 We evaluated studies that had the following characteristics as at low risk of bias: selection of exposed cohort from the general population of pregnant women; selection of nonexposed cohort from the same population; reliable ascertainment of exposure such that the likelihood of controls (term birth) being misclassified as having preterm birth when they did not or cases being wrongly classified as not having preterm birth was minimized; exclusion of women who had cardiovascular outcome of interest before or during pregnancy; comparable cohort where confounders, in particular age, pre‐eclampsia, and diabetes mellitus/insulin resistance, or any other cardiovascular risk factors such as smoking, body mass index, and cholesterol, were accounted for; assessment of outcomes prospectively or through linkage of records and/or independent blind assessment; follow‐up duration for at least 5 years postpartum; and <10% of the study participants in each cohort being lost to follow‐up.
Data Synthesis and Analysis
We used RevMan Version 5.3.5 (Nordic Cochrane Centre) to conduct random effects meta‐analysis using the inverse variance method for pooling log risk ratios (RRs). We used random effects because the studies were conducted in a wide range of settings in different populations, hence the need to take heterogeneity into account for the pooled effect estimate. Where possible, we chose to pool adjusted risk estimates from primary studies and when these data were not available, raw data were used to calculate unadjusted risk estimates. Studies were pooled in meta‐analysis with subgroups based on whether or not the study used adjustments to account for confounders. Statistical heterogeneity was assessed using the I2 statistic where I2 values of 30% to 60% represented moderate level of heterogeneity.39 Where there was greater than a moderate degree of heterogeneity, we performed leave‐1‐out analysis to identify studies that contributed to high degree of heterogeneity. In the case of an analysis where there are more than 10 studies and little evidence of heterogeneity, we planned to perform funnel plots to assess for publication bias.40 Sensitivity analysis was performed to consider the follow‐up duration of the studies (<10, 10–30, and >30 years), gestation (<32 weeks versus 32–37 weeks), and recurrence (1 recurrence versus ≥2 recurrence) of preterm births, and whether the preterm births occurred spontaneously or were medically indicated. For the sensitivity analysis on gestation, we excluded studies where the subgroups could not be categorized as either <32 weeks or 32 to 37 weeks gestation (eg, <34 weeks gestation).
Results
Description of Studies Included in Analysis
The initial MEDLINE and EMBASE search produced 653 titles and abstracts. After screening, 21 studies were included in the analysis (Figure 1) including 5 813 682 women in total (ranges from 446 to 923 686 women in each study). Studies recruiting patients from the same population were paired to avoid duplication of participant numbers.31, 32, 41, 42 Some studies assessed the same population over different time points.24, 31, 32, 41, 42, 43, 44, 45 In these cases, the study with the longest follow‐up period was used for analysis in order to obtain the highest event rate.
Figure 1.
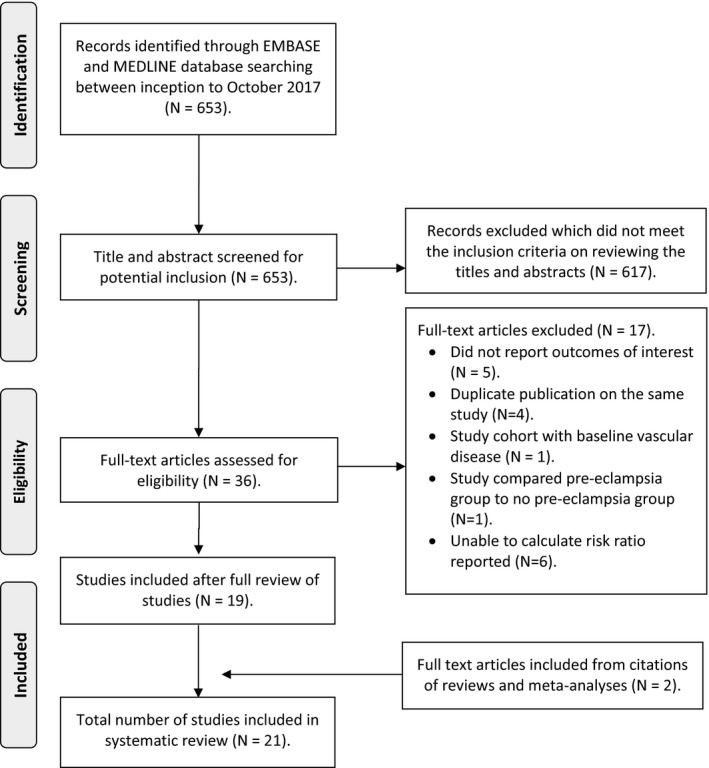
Flow diagram of study inclusion. Adapted from: Moher et al.52
Table 1 summarizes the study designs and participant characteristics. Out of the 16 studies that reported the number of women in study and control groups, 338 007 women delivered preterm while 5 261 933 delivered at term.1 Data for women with singleton pregnancies were included in 15 studies.2 At the index pregnancy, the participants had a mean or median age ranging from 23 to 31 years. The mean follow‐up period ranged from 5.2 to 57 years.
Table 1.
Study Design and Participant Characteristics
| Study ID | Study Design, Country, Year | Total No. of Participants (Preterm/Term) | Mean Age at Pregnancy (y) | Parity | Participant Selection Criteria |
|---|---|---|---|---|---|
| Bonamy 201130 | Retrospective cohort study, Sweden, 1983–2005 | 923 686 (preterm 56 893/term 866 793) | Median 26.9 | P | Women with a first singleton birth in Sweden between 1983 and 2005 |
| Catov 200746 | Cross‐sectional study, United States, 1997–2004 | 446 (preterm 27/term 419) | 23.5 | P | Women enrolled in the Health, Aging and Body Composition (Health ABC) study on 70‐ to 79‐year‐olds living in Pittsburgh during 1997 and 1998, who provided their past obstetric history |
| Catov 201043 | Retrospective cohort study, Denmark, 1973–2006 | 427 765 (preterm 26 588/term 401 177) | 25.5 | A | Women with singleton births in Denmark between 1973 and 1983 |
| Cirillo 201547 | Prospective cohort study, United States, 1959–2011 | 10 310 (preterm 1251/term 9059) | Median 26 | A | Women receiving prenatal care from the Kaiser Health Plan in California recruited to the Child Health and Development Studies (CHDS) |
| Smith 200035 | Cohort study, Finland, 1954–2000 | 3706 | Unclear | P | A cohort of singleton live births between 1954 and 1963 in Helsinki |
| Smith 200553 | Cohort study, Finland, 1973–1997 | 10 368 mothers and 22 807 fathers | Unclear | A | Parents who had children born between 1973 and 1980 in Sweden |
| Freibert 201151 | Cross‐sectional study, United States, 2006–2008 | 2882 (preterm 324/term 2558) | Unclear | A | Women from the Kentucky Women's Health Registry aged ≥50 y of age between 2006 and 2008, who provided their past obstetric history |
| Hastie 201144 | Retrospective cohort study, Scotland, 1969–2007 | 750 350 (preterm 44 743/term 705 607) | Median 24.5 | P | Women with first singleton live births in Scotland between January 1969 and July 2007 |
| Hovi 201422 | Retrospective cohort study, Finland, 1987–2012 | 152 219 mothers (preterm 8720/term 39–41 wks 143 499) and 190 996 fathers | Unclear | P | Women with first singleton births in the Finnish Medical Birth Register from 1987 to 1990 |
| Irgens 200145 | Retrospective cohort study, Norway, 1967–1992 | 602 117 (preterm 26 018/term 576 099) | Unclear | P | Women with first deliveries recorded in the Norwegian medical birth registry from 1967 to 1992 |
| Kessous 201348 | Retrospective cohort study, Israel, 1988–2010 | 47 908 (preterm 5992/term 41 916) | 29 | A | Women with singleton birth at the Soroka University Medical Center in Negev between 1988 and 1999 |
| Lykke 201041 & Lykke 201031 | Retrospective cohort study, Denmark, 1978–2007 | 755 398 (preterm 41 659/term 713 739) in Lykke 201041 or 685 594 (preterm 41 659/term 643 935) in Lykke 201031 | 26.8 | P | Women with first singleton delivery in Denmark from 1978 to 2007 |
| Nardi 200649 | Case–control study, France, 1990–2000 | 514 (preterm 76/term 438) | 55 at enrollment | P | Women born between 1925 and 1950 who had a first MI between 1990 and 2000, matched with women of similar age, year and month of inclusion in study, educational level and area of residence. All women were in a health insurance scheme primarily covering teachers who had singleton pregnancies |
| Ngo 201523 | Retrospective cohort study, Australia, 1994–2012 | 797 056 (preterm 59 563/term 737 493) | Median 31 | A | Women who had a singleton birth between July 1994 and December 2011 in New South Wales |
| Pell 200442 & Smith 200132 | Retrospective cohort study, Scotland, 1981–1999 | 199 668 (Pell 2004) or 129 920 (Smith 2001) | Median 23 | P | Women with first singleton live births in Scotland between 1981 and 1985 |
| Rich‐Edwards 201524 | Retrospective cohort study, Norway, 1967–2009 | 688 662 (preterm 40 981 [spontaneous 33 230; indicated 7751]/term 647 681 [spontaneous 550 604; indicated 97 077]) | 24.6 | P | Women with first singleton birth between 1967 and 1998 in the Medical Birth Registry of Norway |
| Tanz 201725 | Prospective cohort study, United States, 1989–2013 | 70 182 (preterm 6178, term 64 004) | 27.4 | P | Subset of women with pregnancies in the Nurses’ Health Study II, that followed registered nurses aged 25 to 42 y in 1989 |
| Wang 201126 | Retrospective cohort study, Taiwan, 2000–2008 | 4715 (preterm 1134/term 3581) | 27.8 | P | Randomly selected, frequency‐matched control women delivering in the same year as women with hypertensive disorders in pregnancy in the National Health Insurance program between 2000 and 2004 |
| Wikstrom 200550 | Cross‐sectional study; Sweden; 1973–1982 | 365 730 (preterm 17 860/term 347 870) | Median 48a | P | Women in the Swedish Medical Birth Register from 1973 to 1982 with singleton pregnancies |
A indicates any parity; MI, myocardial infarction; P, primiparous.
Age at follow‐up.
Quality Assessment of Included Studies
The study quality was evaluated based on the recommendations of the Newcastle‐Ottawa Quality Assessment Scale (Tables S1 and S2).38 Fifteen studies were found to use reliable methods for ascertaining the preterm birth exposure, whereas 16 studies used reliable methods of obtaining cardiovascular outcomes.
Pooled Analysis of Preterm Birth and Cardiovascular Outcomes
Table 2 shows the results of the studies. A total of 8 studies were pooled and showed a 1.6‐fold significantly increased maternal risk of composite cardiovascular disease in preterm birth (RR 1.56, 95% confidence interval [CI], 1.32, 1.84, I2=93%) (Figure 2A).22, 23, 25, 30, 43, 46, 48, 51 Combining the 5 studies that adjusted for potential confounders,23, 25, 43, 46, 48 the risk was 1.4‐fold (adjusted risk ratio [aRR] 1.43, 95% CI, 1.18, 1.72; I2=89%). The potential confounding factors evaluated in the studies are shown in Table S2. All 5 studies had adjusted for age. We performed leave‐1‐out analyses to explore the sources of heterogeneity. It was mainly driven by the Catov 2010 study.43 By excluding this study, heterogeneity was reduced to 54% in the adjusted analysis (aRR 1.52, 95% CI, 1.31, 1.75). For composite cardiovascular disease death, the pooled results suggest a 1.8‐fold increase in maternal cardiovascular disease death with preterm birth (RR 1.81, 95% CI, 1.55, 2.10, I2=70%; aRR 1.78, 95% CI, 1.42, 2.21, I2=77%) (Figure 2B).24, 31, 35, 41, 43, 53 There were no common confounders in the adjusted studies as they had adjusted for different confounding factors. The heterogeneity was mainly driven by the Davey‐Smith 2005 study.53 After excluding this study, heterogeneity reduced to 0% in both overall and adjusted analyses.
Table 2.
Study Outcomes, Follow‐Up and Results
| Study ID | Definition of Preterm | Follow‐Up Duration | Definition of Outcome | Results (Preterm vs Term) |
|---|---|---|---|---|
| Bonamy 201130 | Moderately preterm (32–36 wks), very preterm (28–31 wks), extremely preterm (≤27 wks) | 11.8 y | CVD: unstable angina, acute MI, cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, transient ischemic attack, acute stroke or heart failure |
32 to 36 wks: 320/49 537 vs 3154/866 793. aHR 1.39 (1.22–1.58) 28 to 31 wks: 70/5259 vs 3154/866 793. aHR 2.57 (1.97–3.34) ≤27 wks: 24/2097 vs 3154/866 793. aHR 2.18 (1.33–3.57) |
| Catov 200746 | Delivery <37 wks gestation | 57 y | CVD: MI, angina, coronary artery bypass surgery, percutaneous transluminal angioplasty, stroke or peripheral vascular disease | 12/27 vs 120/491. aHR 2.85 (1.19–6.85) |
| Catov 201043 | Delivery <37 wks gestation | 28 y | CVD: CHD, stroke, hypertension, atherosclerosis or thrombosis |
Any preterm: 3454/26 588 vs 39 485/401 177. aHR 1.18 (1.10–1.25) 35 to 36 wks: aHR 1.26 (1.20–1.33) 33 to 34 wks: aHR 1.26 (1.16–1.37) ≤32 wks: aHR 1.36 (1.21–1.53) Recurrent 1 preterm birth: aHR 1.16 (1.09–1.25) Recurrent ≥2 preterm births: aHR 1.26 (1.05–1.51) |
| CVD deatha |
Any preterm: aHR 1.98 (1.73–2.26) 35 to 36 wks: aHR 1.87 (1.59–2.14) 33 to 34 wks: aHR 2.10 (1.73–2.78) ≤32 wks: aHR 2.10 (1.47–3.00) Recurrent 1 preterm birth: aHR 1.70 (1.33–2.16) Recurrent ≥2 preterm births: aHR 2.12 (1.22–3.68) |
|||
| CHDa |
Any preterm: 1272/26 588 vs 13 283/401 177. aHR 1.42 (1.34–1.52) 35 to 36 wks: aHR 1.41 (1.30–1.53) 33 to 34 wks: aHR 1.49 (1.32–1.68) ≤32 wks: aHR 1.38 (1.15–1.66) Recurrent 1 preterm birth: aHR 1.22 (1.09–1.36) Recurrent ≥2 preterm births: aHR 1.78 (1.40–2.27) |
|||
| Strokea |
Any preterm: 351/26 588 vs 3185/401 177. aHR 1.67 (1.48–1.89) 35 to 36 wks: aHR 1.73 (1.49–2.01) 33 to 34 wks: aHR 1.42 (1.10–1.84) ≤32 wks: aHR 1.92 (1.38–2.67) Recurrent 1 preterm birth: aHR 1.77 (1.44–2.17) Recurrent ≥2 preterm births: aHR 1.37 (0.75–2.49) |
|||
| Cirillo 201547 | Delivery <37 wks gestation | 40 y | CHD death | aHR 2.1 (1.40–3.01) |
| Smith 200035 | Delivery <37 wks gestation | Unclear | CVD death | aHR 2.06 (1.22–3.47) |
| Smith 200553 | Delivery <37 wks gestation | 20.4 y | CVD (CHD and stroke) death | aHR 1.32 (1.09–1.61) |
| CHD death | aHR 1.66 (1.20–2.29) | |||
| Stroke death | aHR 1.07 (0.77–1.49) | |||
| Freibert 201151 | Delivery between 20 and 36 wks gestation | Unclear | CVD | 110/324 vs 573/2558 |
| CHD | 37/324 vs 159/2558 | |||
| Hastie 201144 | Delivery <37 wks gestation | 22 y | CHD |
Any preterm: aHR 1.58 (1.47–1.71) Spontaneous (n=29 965): aHR 1.46 (1.33–1.61) Medically indicated (n=14 747): aHR 1.81 (1.61–2.04) |
| CHD death |
Any preterm: aHR 2.26 (1.88–2.71) Spontaneous (n=29 965): aHR 2.14 (1.70–2.70) Medically indicated (n=14 747): aHR 2.49 (1.89–3.30) |
|||
| Hovi 201422 | Delivery <37 wks gestation | 22 y | CVD: CHD and stroke |
Any preterm: 431/8720 vs 4127/143 499 34 to 36 wks: 303/6540 vs 4127/143 499. HR 1.55 (1.38–1.74) 32 to 33 wks: 50/954 vs 4127/143 499. HR 1.61 (1.22–2.13) 28 to 31 wks: 52/809 vs 4127/143 499. HR 2.12 (1.61–2.79) <28 wks: 26/417 vs 4127/143 499. HR 2.00 (1.36–2.94) |
| Irgens 200145 | Delivery between 16 and 36 wks gestation | 13 y | CHD death | aHR 2.95 (2.12–4.11) |
| Stroke death | aHR 1.91 (1.26–2.91) | |||
| Kessous 201348 | Delivery <37 wks gestation | 10 y | CVD: hospitalization for CHD, stroke, peripheral vascular disease, hyperlipidemia, angina, hypertension, atherosclerosis, MI, heart failure, pulmonary heart disease, cardiac arrest, cardiac catheterization or cardiovascular stress test |
Any preterm: aHR 1.4 (1.2–1.6) 34 to 37 wks (n=4596): OR 1.4 (1.2–1.6). <34 wks (n=1396): OR 1.7 (1.3–2.1) Spontaneous (n=41 669): OR 1.4 (1.2–1.6) Medically indicated (n=6239): OR 1.7 (1.3–2.4) Recurrent 1 preterm birth: 261/5217 vs 1467/41 916 Recurrent ≥2 preterm births: 43/775 vs 1467/41 916 |
| Lykke 2010a41 & Lykke 2010b31 | Delivery <37 wks gestation | 14.6 y (Lykke 2010a) | CHD |
Any preterm: 589/41 659 vs 7257/713 739 32 to 36 wks: 500/35 255 vs 7257/713 739. aHR 1.32 (1.20–1.45) 28 to 31 wks: 63/4698 vs 7257/713 739. aHR 1.03 (0.80–1.34) 20 to 27 wks: 26/1706 vs 7257/713 739. aHR 1.61 (1.09–2.37) Recurrent 1 preterm birth: 71/4244 vs 4730/471 052. aHR 1.36 (1.02–1.81) |
| 14.8 y (Lykke 2010b) | CVD (CHD, stroke, hypertension, thromboembolic disease and type 2 diabetes mellitus) death | 115/41 659 vs 824/643 935. aHR 1.98 (1.64–2.40) | ||
| Nardi 200649 | Delivery <8 mo gestation | 5.2 y | CHD death | 23/76 vs 86/438 |
| Ngo 201523 | Delivery 20 to 36 wks gestation | 7.5 y | CVD: hospitalization or death for CHD, stroke, and congestive heart failure |
Any preterm: aHR 1.65 (1.50–1.83) 35 to 36 wks: aHR 1.53 (1.35–1.74) 33 to 34 wks: aHR 1.89 (1.55–2.31) 20 to 32 wks: aHR 1.83 (1.50–2.23) Spontaneous: aHR 1.53 (1.35–1.72) Medically indicated: aHR 1.93 (1.66–2.25) 1 preterm birth: aHR 1.62 (1.46–1.79). Recurrent ≥2 preterm births: aHR 2.04 (1.56–2.67) |
| CHD |
Any preterm: aHR 1.61 (1.39–1.85) 35 to 36 wks: aHR 1.49 (1.24–1.78) 33 to 34 wks: aHR 1.89 (1.43–2.51) 20 to 32 wks: aHR 1.72 (1.29–2.29) Spontaneous: aHR 1.53 (1.29–1.81) Medically indicated: aHR 1.77 (1.41–2.21) 1 preterm birth: aHR 1.54 (1.33–1.79) Recurrent ≥2 preterm births: aHR 2.31 (1.61–3.33) |
|||
| Stroke |
Any preterm: aHR 1.68 (1.46–1.95) 35 to 36 wks: aHR 1.49 (1.23–1.80) 33 to 34 wks: aHR 1.90 (1.41–2.56) 20 to 32 wks: aHR 2.13 (1.61–2.82) Spontaneous: aHR 1.49 (1.24–1.78) Medically indicated: aHR 2.12 (1.70–2.65) 1 preterm birth: aHR 1.68 (1.44–1.95) Recurrent ≥2 preterm births: aHR 1.76 (1.14–2.73) |
|||
| Pell 200442 & Smith 200132 | Delivery 24 to 36 wks gestation | 14 to 19 y (Pell 2004) | Stroke | aHR 1.91 (1.35–2.70). |
| 15 to 19 y (Smith 2001) | CHD death | HR 2.2 (0/9–5.7). aHR 1.9 (0.7–4.9) | ||
| Rich‐Edwards 201524 | Delivery <37 w gestation | 24.8 y | CVD (CHD and stroke) death |
HR 1.9 (1.7–2.2) Spontaneous: Any preterm: HR 1.7 (1.5–2.0) 35 to 36 wks: aHR 1.4 (1.0–1.8) 32 to 34 wks: aHR 1.9 (1.3–2.7) 22 to 31 wks: aHR 2.1 (1.4–3.1) Medically indicated: Any preterm: HR 3.7 (2.4–4.5) Recurrent 1 preterm birth: aHR 3.3 (2.4–4.5) |
| CHD death |
Spontaneous: Any preterm: aHR 2.1 (1.7–2.5) 35 to 36 wks: aHR 2.1 (1.6–2.7) 32 to 34 wks: aHR 2.4 (1.7–3.4) 22 to 31 wks: aHR 2.3 (1.5–3.4) Medically indicated: 35 to 36 wks: aHR 6.2 (4.2–9.3) 32 to 34 wks: aHR 3.4 (1.7–6.9) 22 to 31 wks: aHR 4.7 (2.2–9.8) |
|||
| Stroke death |
Spontaneous: Any preterm: 1.5 (1.2–1.8) 35 to 36 wks: aHR 1.3 (0.9–1.7) 32 to 34 wks: aHR 1.9 (1.3–2.8) 22 to 31 wks: aHR 1.8 (1.1–2.8) Medically indicated: Any preterm: aHR 3.0 (2.0–4.3) 35 to 36 wks: aHR 2.9 (1.7–5.1) 32 to 34 wks: aHR 1.9 (0.8–4.7) 22 to 31 wks: aHR 5.4 (2.8–10.4) |
|||
| Tanz 201725 | Delivery >20 and <37 wks gestation | 32 y | CVD: MI and stroke |
Without hypertensive disorders of pregnancy (preterm 4487 vs term 51 343): Any preterm: aHR 1.35 (1.06–1.72) 32 to <37 wks: aHR 1.12 (0.83–1.52) 20 to <32 wks: aHR 2.01 (1.38–2.93) Recurrent 1 preterm birth: aHR 1.63 (1.18–2.25) |
| CHD |
Any preterm: aHR 1.55 (1.19–2.01) 32 to <37 wks: aHR 1.36 (0.99–1.86) 20 to <32 wks: aHR 2.10 (1.38–3.21) |
|||
| Stroke |
Any preterm: aHR 1.28 (0.95–1.71) 32 to <37 wks: aHR 1.09 (0.76–1.56) 20 to <32 wks: aHR 1.84 (1.15–2.95) |
|||
| Wang 201126 | Unclear | 6.4 y | Stroke | aHR 1.51 (0.77–2.93) |
| Wikstrom 200550 | Delivery <37 wks gestation | 15 y | CHD | 145/17 860 vs 1959/347 870. aRR 1.3 (1.1–1.5) |
Data are HR/OR (95% confidence intervals). aHR indicates adjusted hazard ratio; aRR, adjusted risk ratio; CHD, coronary heart disease/ischemic heart disease; CVD, cardiovascular disease; MI, myocardial infarction; RR, risk ratio.
Data not adjusted for diabetes mellitus.
Figure 2.
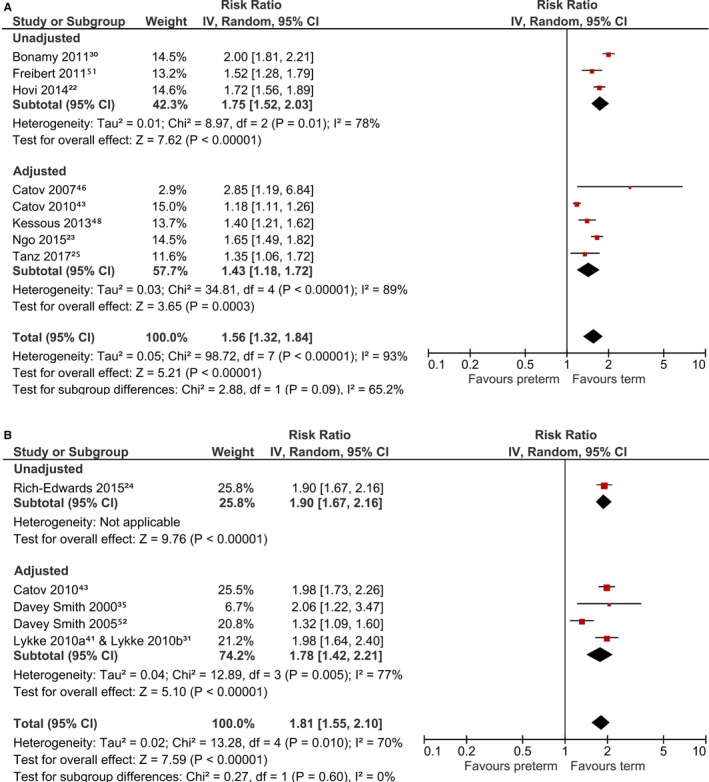
Risk of composite cardiovascular disease with preterm birth. A, Cardiovascular disease events. B, Cardiovascular disease death. CI indicates confidence interval.
For coronary heart disease there was a 1.5‐fold increase risk of events with preterm birth (RR 1.50, 95% CI, 1.39, 1.62, I2=51%, aRR 1.49, 95% CI, 1.38, 1.60, I2=54%) (Figure 3A). All of the 5 studies that used adjusted data had adjusted for age and socioeconomic status or education.23, 25, 43, 44, 50 The heterogeneity was mainly driven by the Hastie 2011 study.44 If this study was excluded, heterogeneity was reduced to 33% in the adjusted analysis (aRR 1.45, 95% CI, 1.33, 1.57). The 4 adjusted studies reporting coronary heart disease death showed a 2‐fold increased risk with preterm birth (aRR 2.10, 95% CI, 1.87, 2.36, I2=0%, Figure 3B).24, 44, 47, 53 There were no common confounders over these 4 studies as they had adjusted for different confounding factors.
Figure 3.

Risk of coronary heart disease with preterm birth. A, Coronary heart disease events. B, Coronary heart disease death. CI indicates confidence interval.
Figure 4 shows the pooled analysis for studies on maternal preterm birth and stroke, and illustrate the risk to be increased by 1.7‐fold in preterm birth (aRR 1.65, 95% CI, 1.51, 1.79, I2=0%).23, 25, 26, 32, 42, 43 All studies had adjusted for potential confounders that included age and socioeconomic status or education or urbanization level. The pooled result on preterm birth and stroke death was not statistically significant (aRR 1.30, 95% CI, 0.94, 1.80, I2=66%).24, 53 We did not perform funnel plots to assess for publication bias as <10 studies were included in each analysis.
Figure 4.
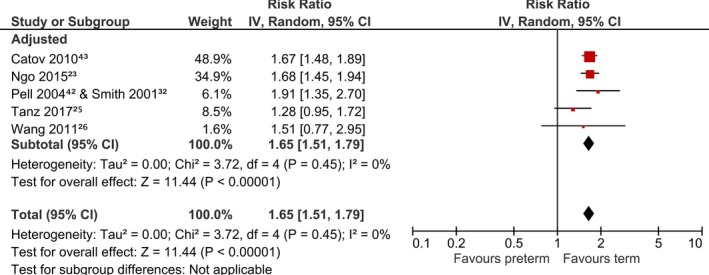
Risk of stroke with preterm birth. CI indicates confidence interval.
Sensitivity Analysis for Follow‐Up Time
We conducted sensitivity analyses to consider the effect of follow‐up time for cardiovascular outcomes that were significant in the adjusted studies (Table 3). At <10 years following preterm birth, the risks for composite cardiovascular disease (RR 1.65, 95% CI, 1.49, 1.82), coronary heart disease (RR 1.61, 95% CI, 1.40, 1.86), and stroke (RR 1.67, 95% CI, 1.45, 1.93) were already significant and similar to longer follow‐up times.
Table 3.
Sensitivity Analyses With Regard to Duration of Follow‐Up
| Outcomes | <10 Y | 10 to 30 Y | >30 Y |
|---|---|---|---|
| CVD | 1.65 [1.49, 1.82], n=1 | 1.54 [1.19, 2.01], n=4 | 1.73 [0.87, 3.46], n=2 |
| CVD death | ··· | 1.79 [1.51, 2.11], n=4 | ··· |
| CHD | 1.61 [1.40, 1.86], n=1 | 1.45 [1.32, 1.60], n=3 | 1.55 [1.19, 2.01], n=1 |
| CHD death | 1.54 [1.04, 2.28], n=1 | 2.08 [1.80, 2.40], n=3 | 2.10 [1.43, 3.08], n=1 |
| Stroke | 1.67 [1.45, 1.93], n=2 | 1.70 [1.51, 1.90], n=2 | 1.28 [0.95, 1.72], n=1 |
Data are risk ratio [95% confidence intervals], number of pooled studies. CHD indicates coronary heart disease; CVD, cardiovascular disease.
Sensitivity Analysis Considering Effect of Gestation of Preterm Birth, Recurrence of Preterm Birth, and Spontaneous Versus Medically Indicated Preterm Birth
Sensitivity analyses were performed to consider the effect of gestation, recurrence, and spontaneous onset of preterm birth in the 5 cardiovascular outcomes that were significant in the adjusted studies. These showed that the risks were higher when preterm deliveries occurred before 32 weeks gestation in all outcomes: composite cardiovascular disease (RR 1.85, 95% CI, 1.51, 2.28), composite cardiovascular disease death (RR 2.10, 95% CI, 1.61, 2.74), coronary heart disease (RR 1.62, 95% CI, 1.28, 2.04), coronary heart disease death (RR 2.30, 95% CI, 1.53, 3.46), and stroke (RR 2.00, 95% CI, 1.65, 2.43), compared with those occurring at 32 to 37 weeks gestation (Table 4).
Table 4.
Sensitivity Analysis With Regard to Gestation of Preterm Birth
| Outcomes | <32 Wks | 32 to 37 Wks |
|---|---|---|
| CVD | 1.85 [1.51, 2.28], n=6 | 1.40 [1.23, 1.59], n=5 |
| CVD death | 2.10 [1.61, 2.74], n=2 | 1.85 [1.58, 2.16], n=2 |
| CHD | 1.62 [1.28, 2.04], n=3 | 1.44 [1.35, 1.53], n=3 |
| CHD death | 2.30 [1.53, 3.46], n=1 | 2.20 [1.78, 2.71], n=1 |
| Stroke | 2.00 [1.65, 2.43], n=3 | 1.49 [1.22, 1.83], n=3 |
Data are risk ratio [95% confidence intervals], number of pooled studies. CHD indicates coronary heart disease; CVD, cardiovascular disease.
When recurrence of preterm birth was studied, the risks for composite cardiovascular disease (RR 1.58, 95% CI, 1.17, 2.12) and coronary heart disease (RR 1.95, 95% CI, 1.53, 2.50) were higher if the preterm birth recurred in 2 or more pregnancies compared with recurring once only (Table 5). The risks for all available outcomes were greatest when the preterm birth occurred as a result of medically indicated compared with spontaneous preterm birth: composite cardiovascular disease (RR 1.88, 95% CI, 1.64, 2.16), composite cardiovascular disease death (RR 3.70, 95% CI, 2.88, 4.76), coronary heart disease (RR 1.80, 95% CI, 1.62, 2.00), coronary heart disease death (RR 3.56, 95% CI, 1.74, 7.25), and stroke (RR 2.12, 95% CI, 1.70, 2.65, Table 6).
Table 5.
Sensitivity Analysis With Regard to Recurrence of Preterm Birth
| Outcomes | Recurrent 1 Preterm Birth | Recurrent ≥2 Preterm Births |
|---|---|---|
| CVD | 1.42 [1.17, 1.73], n=4 | 1.58 [1.17, 2.12], n=3 |
| CVD death | 2.35 [1.23, 4.50], n=2 | 2.12 [1.22, 3.68], n=1 |
| CHD | 1.36 [1.09, 1.71], n=2 | 1.95 [1.53, 2.50], n=2 |
| Stroke | 1.77 [1.44, 2.17], n=1 | 1.61 [1.13, 2.30], n=2 |
Data are risk ratio [95% confidence intervals], number of pooled studies. CHD indicates coronary heart disease; CVD, cardiovascular disease.
Table 6.
Sensitivity Analysis With Regard to Spontaneous or Medically Indicated Preterm Birth
| Outcomes | Spontaneous | Medically Indicated |
|---|---|---|
| CVD | 1.47 [1.34, 1.62], n=2 | 1.88 [1.64, 2.16], n=2 |
| CVD death | 1.70 [1.47, 1.96], n=1 | 3.70 [2.88, 4.76], n=1 |
| CHD | 1.48 [1.36, 1.60], n=2 | 1.80 [1.62, 2.00], n=2 |
| CHD death | 2.12 [1.82, 2.45], n=2 | 3.56 [1.74, 7.25], n=2 |
| Stroke | 1.49 [1.24, 1.79], n=1 | 2.12 [1.70, 2.65], n=1 |
Data are risk ratio [95% confidence intervals], number of pooled studies. CHD indicates coronary heart disease; CVD, cardiovascular disease.
The full cardiovascular risk factor profile of the preterm birth and the control term birth population is described in Table S3. Significant differences in age,44, 48 ethnicity,48 education,43 socioeconomic class,44 obesity,48 hypertension,44 pre‐eclampsia,43, 44 and small‐for‐gestational‐age fetus43 between the preterm and term birth groups were detected at the index pregnancy in 3 studies. Although these only contributed to 21% of total participant women, the cardiovascular risk factor profiles at the index birth were not available in the majority of participants within this systematic review and meta‐analysis. Additional sensitivity analyses were performed where studies were stratified by singleton pregnancies, year of the study, quality of the study, location of the study, and pre‐existing cardiovascular diagnosis in participants (Tables S4 through S8). We found that the results did not vary substantially.
Discussion
This meta‐analysis examined 96 341 474 women years and included 338 007 women with preterm birth out of 5 813 682 study participants in 21 studies. We found that preterm delivery is associated with an increased maternal risk for future incident cardiovascular events, cardiovascular death, coronary heart disease events, coronary heart disease death, and stroke. The adjusted risk ranged between 1.4‐ and 2‐fold compared with those without a history of preterm birth. This increased risk is greatest in preterm births that occur before 32 weeks in gestation or in those that are delivered for medical indications such as fetal growth restriction or pre‐eclampsia. For the composite cardiovascular disease and coronary heart disease outcomes, the risks are higher in women with a greater number of recurrent preterm births. Preterm delivery is a significant event in a woman's reproductive history with a good recall rate including high specificity.54, 55, 56 Therefore, it may be considered as a potential risk factor for future cardiovascular disease in women, as recommended by the current guidelines from the American Heart Association and European Society of Cardiology.27, 28, 29
By including an additional 1.7 million participants to the previous meta‐analysis in this field, our results are consistent with earlier literature showing an increased risk in coronary heart disease, stroke, and composite cardiovascular disease.3 Although our risk estimate for composite cardiovascular disease was lower than previously reported, this may be because of the difference in data analysis. In the previous meta‐analysis, there was no distinction between adjusted and unadjusted data nor between morbidity and mortality outcomes. There are 2 other systematic reviews without meta‐analysis of the literature, which also support our findings.4, 5 Unique to this study, we conducted sensitivity analyses to consider the duration of follow‐up, gestation at birth, recurrent preterm birth, and spontaneous or medically indicated preterm birth.
Because of the multifactorial nature of preterm birth causes, several pathognomonic mechanisms have been hypothesized.13, 57 These include vascular and metabolic factors,58, 59, 60 as well as pre‐eclampsia and fetal growth restriction that have both been independently associated with future adverse cardiovascular outcomes.61, 62, 63 Moreover, preterm birth markers, such as proinflammatory cytokines, matrix metalloproteinase, fibrinolysis, prostaglandin cascade,8, 59, 64, 65, 66, 67, 68 and dyslipidemia,59, 66, 69 are also involved in atherosclerosis and endothelial dysfunction.34, 70, 71, 72, 73 Therefore, preterm birth shares common risk factors with cardiovascular disease74, 75 and the association we identified may have been an epiphenomenon in women with high cardiovascular risk profiles that predispose them to both preterm birth and cardiovascular diseases. In contrast, other longitudinal studies have shown no difference in lipid profile, blood pressure, and inflammatory markers between preterm and term deliveries.17, 76
There may also be other possible hypotheses for the association of preterm delivery and long‐term adverse cardiovascular outcomes. One third of normotensive preterm births exhibit placental abnormalities commonly seen in pre‐eclampsia and placental insufficiency,77, 78 while ≈17% of preterm births are medically indicated.79 Common medical indications for preterm birth include pre‐eclampsia and placental insufficiency causing fetal growth restriction, which may have confounded any relationships reported in the literature. Moreover, diabetes mellitus is more common in women with previous preterm deliveries, which may have confounded our findings.80 Although smoking has not been universally agreed upon as a risk factor for preterm birth,81, 82 the causative relationship between smoking and cardiovascular diseases is well established.83, 84, 85, 86 Other possible confounders include obesity and socioeconomic status, both of which have been linked to increased risks of preterm birth32, 87, 88, 89 and cardiovascular disease in women.90, 91, 92
Although the majority of the included studies (n=16) have attempted to adjust for some potential confounders,3 none of the studies have adequately adjusted for all relevant risk factors that form the basis of many of the established cardiovascular risk prediction scores (eg, cholesterol and family history of cardiovascular disease). There was also limited overlap between the adjusted confounding factors among the studies. As many key confounders for cardiovascular diseases were not adjusted for in the included studies, it is possible that the relationships that we have reported are entirely driven by differences in cardiovascular risk factor profiles at baseline. In the studies (48% of total participants) that presented the baseline cardiovascular risk factor profiles, the majority did not calculate whether there were any differences between the preterm and term birth groups. In the 3 studies (21% of total participants) that calculated this difference, all of them showed significant baseline risk factor profile differences between the preterm birth and the term birth populations.43, 44, 48
The 2011 American Heart Association guidelines for cardiovascular disease prevention in women advised healthcare professionals to inquire about adverse pregnancy outcomes, including preterm delivery, as a part of any cardiovascular risk assessment in women. However, there was a lack of additional specific guidance as preterm birth was not considered a major cardiovascular disease risk factor.28 The 2014 guidelines from the American Heart Association and American Stroke Association for the prevention of stroke in women also recognized preterm birth as a factor associated with increased stroke risks after pregnancy, but did not make further recommendations because of the lack of evidence in the literature.27 More recently, the 2016 European Society of Cardiology guidelines recommended the consideration of periodic screening for hypertension and diabetes mellitus in women with a history of preterm birth.29 In line with these recommendations, we suggest a detailed evaluation of a screening program for cardiovascular disease in women with a history of preterm birth, particularly in women who delivered because of any medical indications or before 32 weeks gestation (ie, the very or extremely preterm as defined by the World Health Organization). An opportune time for this screening is at the 6‐week postpartum visit suggested in the World Health Organization recommendations on postnatal care.93
The strength of our study lies in the large sample size with a total of 96 341 474 patient‐years follow‐up. We used a search strategy without limiting the study design, language, and used independent reviewers for performing double data extractions and data analysis. All of the studies were designed to assess future cardiovascular diseases as their main outcome.
The limitations of this study include the risk of confounding and being unable to attribute causality of future cardiovascular disease to preterm delivery. These are because of the longitudinal nature of the epidemiological studies we included in this meta‐analysis. As with any meta‐analysis, there may be inherent publication bias, where studies with positive findings are more likely to be published compared with those showing neutral or negative outcomes. Over half of the included studies were retrospective in design. Therefore, there was limited control over the quality of data collected. As such, the preterm birth exposure could have been prone to recall bias or inaccuracies in historical data collection. Furthermore, the cardiovascular outcomes were determined by subjective self‐reporting in 3 studies.25, 46, 51 Heterogeneity may have arisen because of differences in the study population, research methodology, period of conducting the study, and inherent differences between the studies. Two studies were conducted in ethnically diverse populations26, 48 in contrast to the other studies that were performed in white populations. Six studies examined women of any parity,23, 43, 47, 48, 51, 53 while the others studied primiparous women. Specific populations were analyzed in 2 studies, which were Nardi et al49 (women covered by a particular health insurance program) and Tanz et al25 (nurses). As shown in Table 1, there was a mixture of retrospective, prospective, cross‐sectional, and case–control studies. Because of the variation in duration of follow‐up in the studies, the index preterm birth could have occurred in 1954 or in 2011. There has been both a change in obstetric practice, cardiovascular screening, and management of cardiovascular risk factors over these 57 years, which could have contributed toward differences between the studies. In the composite cardiovascular disease outcome, the heterogeneity was mainly driven by the Catov 2010 study.43 Out of the pooled adjusted studies, this was the only study conducted in Europe as the others were conducted in the United States, Australia, or Israel.
Our finding of an association between preterm delivery and the future development of incident cardiovascular disease has important implications for women and health policy. Women who experience a preterm delivery are at a higher risk of cardiovascular events and this suggests that a formal cardiovascular risk assessment using established risk scores should be considered in these women.94, 95 In addition, clinicians may find it pertinent to educate women regarding their increased cardiovascular risk and potentially motivate women toward controlling any modifiable risk factors. The perinatal period is a valuable time for opportunistic advice, education, intervention, and monitoring in at‐risk women. However, there is little awareness regarding the long‐term cardiovascular consequences of pregnancy complications among healthcare professionals. A survey showed that only 5% of internists inquired about pre‐eclampsia during history taking, while primary care data showed that 50% of women who had pre‐eclampsia did not receive any further postnatal follow‐up after 3 months.96, 97 Cardiovascular disease presents differently between men and women,28, 98 and most cardiac sudden deaths in women occur without prior history of heart disease.99, 100 Therefore, it would be appropriate to utilize past obstetric history to comprehensively assess cardiovascular risk profiles in women. Our findings support the current guidelines from the American Heart Association27, 28 and the European Society of Cardiology29 to assess preterm delivery as part of the cardiovascular disease risk assessment in women.
Conclusions
Our large meta‐analysis that included 5 813 682 women, 338 007 of whom had experienced a preterm delivery, demonstrated that preterm birth is associated with a 1.4‐ to 2‐fold increase in future adverse cardiovascular outcomes. In keeping with current recommendations, our study highlights the importance of advising women with preterm births about their increased cardiovascular risk and advocating and supporting lifestyle and behavioral changes to control their modifiable risk factors. These findings support the assessment of preterm delivery in cardiovascular risk assessment in women, with the 6‐week postpartum visit the ideal place for this to occur.
Sources of Funding
This work was supported by a grant from the North Staffordshire Heart Committee. Wu is funded by a National Institute for Health Research (NIHR) Transitional Research Fellowship and Kwok is funded by a NIHR Academic Clinical Fellowship. This article presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosures
None.
Supporting information
Data S1. Search terms.
Table S1. Study Quality Assessment Overview
Table S2. Study Quality Assessment in Detail
Table S3. Cardiovascular Risk Factor Profile of Preterm Birth and Term Birth Groups in the Included Studies
Table S4. Sensitivity Analysis With Regard to Singleton and Multiple Pregnancies
Table S5. Sensitivity Analysis With Regard to the Year Each Study Was Commenced
Table S6. Sensitivity Analysis With Regard to Study Quality Score
Table S7. Sensitivity Analysis With Regard to Study Location
Table S8. Sensitivity Analysis With Regard to Whether the Study Excluded Women With Pre‐Existing Cardiovascular Disease
Figure S1. PRISMA checklist.
(J Am Heart Assoc. 2018;7:e007809 DOI: 10.1161/JAHA.117.007809.)29335319
Notes
References
- 1. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. [DOI] [PubMed] [Google Scholar]
- 3. Heida KY, Velthuis BK, Oudijk MA, Reitsma JB, Bots ML, Franx A, van Dunné FM. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2015;23:253–263. [DOI] [PubMed] [Google Scholar]
- 4. Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol. 2014;210:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rich‐Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The World Health Organisation . The global health observatory (GHO). 2017.
- 7. Gulati M. Improving the cardiovascular health of women in the nation. Circulation. 2017;135:495–498. [DOI] [PubMed] [Google Scholar]
- 8. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hytten F, Leitch I. The physiology of human pregnancy. 2nd edition, Blackwell Scientific Publications, Oxford: 1971. [Google Scholar]
- 10. Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci. 2007;334:291–295. [DOI] [PubMed] [Google Scholar]
- 11. Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622. [DOI] [PubMed] [Google Scholar]
- 12. Rich‐Edwards JW, McElrath TF, Karumanchi A, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;56:331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(suppl 3):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqui N, Hladunewich M. Understanding the link between the placenta and future cardiovascular disease. Trends Cardiovasc Med. 2011;21:188–193. [DOI] [PubMed] [Google Scholar]
- 15. Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal‐medial thickness: the CARDIA study. Hypertension. 2013;61:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hastie CE, Smith GC, Mackay DF, Pell JP. Association between preterm delivery and subsequent C‐reactive protein: a retrospective cohort study. Am J Obstet Gynecol. 2011;205:556.e1–556.e4. [DOI] [PubMed] [Google Scholar]
- 18. Perng W, Stuart J, Rifas‐Shiman SL, Rich‐Edwards JW, Stuebe A, Oken E. Preterm birth and long‐term maternal cardiovascular health. Ann Epidemiol. 2015;25:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catov JM, Dodge R, Barinas‐Mitchell E, Sutton‐Tyrrell K, Yamal JM, Piller LB, Ness RB. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt). 2013;22:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. [DOI] [PubMed] [Google Scholar]
- 21. Williams D. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol. 2003;15:465–471. [DOI] [PubMed] [Google Scholar]
- 22. Hovi P, Turkka S, Näsänen‐Gilmore S, Vääräsmäki M, Gissler M, Pouta A, Kajantie E. Parental cardiovascular morbidity in families with a preterm child, a national register study [abstract]. Arch Dis Child. 2014;99:A103. [Google Scholar]
- 23. Ngo AD, Chen JS, Figtree G, Morris JM, Roberts CL. Preterm birth and future risk of maternal cardiovascular disease—is the association independent of smoking during pregnancy? BMC Pregnancy Childbirth. 2015;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rich‐Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long‐term risk of coronary heart disease and stroke mortality in women: a population‐based study. Am J Obstet Gynecol. 2015;213:518.e1–518.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, Mukamal KJ, Rich‐Edwards JW. Preterm delivery and maternal cardiovascular disease in young and middle‐aged adult women. Circulation. 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang IK, Chang SN, Liao CC, Liang CC, Chang CT, Lin HH, Liu JH, Liu YL, Chuang FR, Hsu CY, Huang CC, Sung FC. Hypertensive disorders in pregnancy and preterm delivery and subsequent stroke in Asian women: a retrospective cohort study. Stroke. 2011;42:716–721. [DOI] [PubMed] [Google Scholar]
- 27. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Pina IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd‐Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris‐Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra‐Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness‐based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 30. Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846. [DOI] [PubMed] [Google Scholar]
- 31. Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff‐Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type‐ii diabetes in the mother. BJOG. 2010;117:274–281. [DOI] [PubMed] [Google Scholar]
- 32. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. [DOI] [PubMed] [Google Scholar]
- 33. Banerjee M, Cruickshank JK. Pregnancy as the prodrome to vascular dysfunction and cardiovascular risk. Nat Clin Pract Cardiovasc Med. 2006;3:596–603. [DOI] [PubMed] [Google Scholar]
- 34. Sattar N. Do pregnancy complications and CVD share common antecedents? Atheroscler Suppl. 2004;5:3–7. [DOI] [PubMed] [Google Scholar]
- 35. Smith GD, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356:2066–2067. [DOI] [PubMed] [Google Scholar]
- 36. Wu P, Gulati M, Kwok C, Wong C, Narain A, O'Brien S, Kadam U, Mamas M. Preterm birth and maternal cardiovascular outcome: a systematic review and meta‐analysis. PROSPERO: International prospective register of systematic reviews. 2017;CRD42017068455 Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017068455. Accessed December 27, 2017. [Google Scholar]
- 37. Park K, Wei J, Minissian M, Merz CNB, Pepine CJ. Adverse pregnancy conditions, infertility, and future cardiovascular risk: implications for mother and child. Cardiovasc Drugs Ther. 2015;29:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2000. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Octoober 12, 2017.
- 39. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, United Kingdom: Wiley; 2008. [Google Scholar]
- 40. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lykke JA, Langhoff‐Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non‐cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24:323–330. [DOI] [PubMed] [Google Scholar]
- 42. Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am J Epidemiol. 2004;159:336–342. [DOI] [PubMed] [Google Scholar]
- 43. Catov JM, Wu CS, Olsen J, Sutton‐Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre‐term delivery: retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol. 2011;40:914–919. [DOI] [PubMed] [Google Scholar]
- 45. Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre‐eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Catov JM, Newman AB, Roberts JM, Kelsey SF, Sutton‐Tyrrell K, Harris TB, Colbert L, Rubin SM, Satterfield S, Ness RB; Health ABCS . Preterm delivery and later maternal cardiovascular disease risk. Epidemiology. 2007;18:733–739. [DOI] [PubMed] [Google Scholar]
- 47. Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50‐year follow‐up of the child health and development studies pregnancy cohort. Circulation. 2015;132:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kessous R, Shoham‐Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long‐term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209:368.e1–368.e8. [DOI] [PubMed] [Google Scholar]
- 49. Nardi O, Zureik M, Courbon D, Ducimetiere P, Clavel‐Chapelon F. Preterm delivery of a first child and subsequent mothers’ risk of ischaemic heart disease: a nested case‐control study. Eur J Cardiovasc Prev Rehabil. 2006;13:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–1491. [DOI] [PubMed] [Google Scholar]
- 51. Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt). 2011;20:287–293. [DOI] [PubMed] [Google Scholar]
- 52. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith GD, Sterne J, Tynelius P, Lawlor DA, Rasmussen F. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiology. 2005;16:563–569. [DOI] [PubMed] [Google Scholar]
- 54. Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. J Clin Epidemiol. 1998;51:399–405. [DOI] [PubMed] [Google Scholar]
- 55. Tomeo CA, Rich‐Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and validity of maternal recall of pregnancy‐related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- 56. Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophr Res. 2004;71:417–426. [DOI] [PubMed] [Google Scholar]
- 57. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, Roberts JM. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:610.e1–610.e17. [DOI] [PubMed] [Google Scholar]
- 59. Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–1319. [DOI] [PubMed] [Google Scholar]
- 60. Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, Goulet L, Seguin L, Dassa C, Lydon J, McNamara H, Dahhou M, Genest J. Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol. 2009;38:715–723. [DOI] [PubMed] [Google Scholar]
- 61. Magnussen EB, Vatten LJ, Lund‐Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre‐eclampsia: population based cohort study. BMJ. 2007;335:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ray JG, Diamond P, Singh G, Bell CM. Brief overview of maternal triglycerides as a risk factor for pre‐eclampsia. BJOG. 2006;113:379–386. [DOI] [PubMed] [Google Scholar]
- 63. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 64. Behman R, Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 65. Catov JM, Bodnar LM, Hackney D, Roberts JM, Simhan HN. Activation of the fibrinolytic cascade early in pregnancy among women with spontaneous preterm birth. Obstet Gynecol. 2008;112:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Edison RJ, Berg K, Remaley A, Kelley R, Rotimi C, Stevenson RE, Muenke M. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics. 2007;120:723–733. [DOI] [PubMed] [Google Scholar]
- 67. Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid‐pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012;91:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang J, Villar J, Sun W, Merialdi M, Abdel‐Aleem H, Mathai M, Ali M, Yu KF, Zavaleta N, Purwar M, Nguyen TN, Campodonico L, Landoulsi S, Lindheimer M, Carroli G. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:162.e1–162.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Steffen KM, Cooper ME, Shi M, Caprau D, Simhan HN, Dagle JM, Marazita ML, Murray JC. Maternal and fetal variation in genes of cholesterol metabolism is associated with preterm delivery. J Perinatol. 2007;27:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJR, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 71. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. [DOI] [PubMed] [Google Scholar]
- 72. Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. [DOI] [PubMed] [Google Scholar]
- 73. DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007;25:40–51. [DOI] [PubMed] [Google Scholar]
- 74. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. [DOI] [PubMed] [Google Scholar]
- 75. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. [DOI] [PubMed] [Google Scholar]
- 76. Fraser A, Nelson SM, Macdonald‐Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. [DOI] [PubMed] [Google Scholar]
- 78. Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. [DOI] [PubMed] [Google Scholar]
- 79. Tucker JM, Goldenberg RL, Davis RO, Copper RL, Winkler CL, Hauth JC. Etiologies of preterm birth in an indigent population: is prevention a logical expectation? Obstet Gynecol. 1991;77:343–347. [PubMed] [Google Scholar]
- 80. Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta‐analysis. Obes Rev. 2014;15:804–811. [DOI] [PubMed] [Google Scholar]
- 81. Kyrklund‐Blomberg NB, Cnattingius S. Preterm birth and maternal smoking: risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179:1051–1055. [DOI] [PubMed] [Google Scholar]
- 82. Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy. Their effects on preterm birth. JAMA. 1986;255:82–84. [PubMed] [Google Scholar]
- 83. McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A, Prabhakaran D, Almahmeed W, Rumboldt Z, Budaj A, Dans AL, Gerstein HC, Teo K, Anand SS. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–589. [DOI] [PubMed] [Google Scholar]
- 84. Haire‐Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care. 1999;22:1887–1898. [DOI] [PubMed] [Google Scholar]
- 85. Al‐Delaimy WK, Manson JE, Solomon CG, Kawachi I, Stampfer MJ, Willett WC, Hu FB. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med. 2002;162:273–279. [DOI] [PubMed] [Google Scholar]
- 86. Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420–1432. [DOI] [PubMed] [Google Scholar]
- 87. Morgen CS, Bjork C, Andersen PK, Mortensen LH, Nybo Andersen AM. Socioeconomic position and the risk of preterm birth: a study within the Danish National Birth Cohort. Int J Epidemiol. 2008;37:1109–1120. [DOI] [PubMed] [Google Scholar]
- 88. Torloni MR, Betran AP, Daher S, Widmer M, Dolan SM, Menon R, Bergel E, Allen T, Merialdi M. Maternal BMI and preterm birth: a systematic review of the literature with meta‐analysis. J Matern Fetal Neonatal Med. 2009;22:957–970. [DOI] [PubMed] [Google Scholar]
- 89. McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta‐analyses. BMJ. 2010;341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thurston RC, Kubzansky LD, Kawachi I, Berkman LF. Is the association between socioeconomic position and coronary heart disease stronger in women than in men? Am J Epidemiol. 2005;162:57–65. [DOI] [PubMed] [Google Scholar]
- 91. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 92. Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. [DOI] [PubMed] [Google Scholar]
- 93. World Health Organization . WHO recommendations on postnatal care of the mother and newborn. 2013. [PubMed]
- 94. Bang H, Edwards AM, Bomback AS, Ballantyne CM, Brillon D, Callahan MA, Teutsch SM, Mushlin AI, Kern LM. Development and validation of a patient self‐assessment score for diabetes risk. Ann Intern Med. 2009;151:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–731. [DOI] [PubMed] [Google Scholar]
- 96. Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy. 2012;31:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nijdam ME, Timmerman MR, Franx A, Bruinse HW, Numans ME, Grobbee DE, Bots ML. Cardiovascular risk factor assessment after pre‐eclampsia in primary care. BMC Fam Pract. 2009;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI‐sponsored women's ischemia syndrome evaluation (WISE) study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender‐optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. [DOI] [PubMed] [Google Scholar]
- 99. Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. [DOI] [PubMed] [Google Scholar]
- 100. Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search terms.
Table S1. Study Quality Assessment Overview
Table S2. Study Quality Assessment in Detail
Table S3. Cardiovascular Risk Factor Profile of Preterm Birth and Term Birth Groups in the Included Studies
Table S4. Sensitivity Analysis With Regard to Singleton and Multiple Pregnancies
Table S5. Sensitivity Analysis With Regard to the Year Each Study Was Commenced
Table S6. Sensitivity Analysis With Regard to Study Quality Score
Table S7. Sensitivity Analysis With Regard to Study Location
Table S8. Sensitivity Analysis With Regard to Whether the Study Excluded Women With Pre‐Existing Cardiovascular Disease
Figure S1. PRISMA checklist.


