Abstract
Background
This nationwide population‐based cohort study evaluated the risk of and risk factors for suicide attempt in poststroke patients in Taiwan.
Methods and Results
The poststroke and nonstroke cohorts consisted of 713 690 patients and 1 426 009 controls, respectively. Adults (aged >18 years) who received new stroke diagnoses according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM; codes 430–438) between 2000 and 2011 were included in the poststroke cohort. We calculated the adjusted hazard ratio for suicide attempt (ICD‐9‐CM codes E950–E959) after adjustment for age, sex, monthly income, urbanization level, occupation category, and various comorbidities. Kaplan–Meier analysis was used to measure the cumulative incidence of suicide attempt, and the Fine and Gray method was used as a competing event when estimating death subhazard ratios and 95% confidence intervals between groups. The cumulative incidence of suicide attempt was higher in the poststroke cohort, and the adjusted hazard ratio of suicide attempt was 2.20 (95% confidence interval, 2.04–2.37) compared with that of the controls. The leading risk factors for poststroke suicide attempt were earning low monthly income (<660 US dollars), living in less urbanized regions, doing manual labor, and having a stroke before age 50 years. The attempted suicide risk did not differ significantly between male and female patients in this study.
Conclusions
These results convey crucial information to clinicians and governments for preventing suicide attempt in poststroke patients in Taiwan and other Asian countries.
Keywords: cohort study, National Health Insurance, population studies, stroke, suicide
Subject Categories: Epidemiology, Mental Health, Ischemic Stroke, Intracranial Hemorrhage
Clinical Perspective
What Is New?
This study provides evidence supporting significant predisposing factors for poststroke suicide attempt.
The leading risk factors were low monthly income, less urbanized region, manual labor, and a stroke before age 50 years.
What Are the Clinical Implications?
These results convey crucial information to clinicians and caregivers for preventing suicide in poststroke patients in Taiwan and other Asian countries.
Stroke is one of the most common neurological diseases. Regardless of pathogenesis or type, whether ischemic or hemorrhagic, the priority when treating patients with stroke is the reduction of poststroke mortality. Stroke usually causes disability or restricts daily activities, thereby resulting in long‐term reduction in quality of life for those who have had one. Stroke is listed as the most common cause of disability in adults worldwide.1, 2, 3 In Western developed countries, poststroke patients might exhibit high intolerance of their disability, and several studies have revealed notably higher rates of suicide ideation, suicide attempts, and completed suicides in poststroke patients than in the general population.4, 5, 6 In different countries, cultural heritage and socioeconomic status would usually influence a patient with disability thinking about a suicide attempt. Ethnic and cultural differences from Western countries may make Asian people more tolerant of their impairment, dependence, or disability in daily life than their European or American counterparts.7, 8, 9
Taiwan is a country located in East Asia with the ethnic and cultural heritage of the Han people, similar to that of the Chinese and Southeast Asian population.10, 11 Although the country has established a healthcare system that has covered ≈99% of the population for the previous 20 years, we do not have actual data regarding attempted suicide risk in poststroke patients.12, 13 Furthermore, we would like to identify possible risk factors that can serve as suicide markers to facilitate suicide prevention in Taiwan and other Asian countries. We used a nationwide population‐based database to study whether risk factors for suicide attempt similar to those in Western countries were noted in poststroke patients in Taiwan.
Methods
The data set used in this study is held by the Taiwan Ministry of Health and Welfare (MOHW). The MOHW must approve our application to access these data. Any researcher interested in accessing this data set can submit an application form to the MOHW requesting access. Please contact the staff of the MOHW for further assistance (Taiwan Ministry of Health and Welfare, No. 488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan [R.O.C.]; phone: +886‐2‐8590‐6848; e‐mail: stcarolwu@mohw.gov.tw). All relevant data are within the article.
Data Source
This retrospective cohort study was conducted using inpatient files from the Taiwan National Health Insurance Research Database (NHIRD) provided by the National Health Research Institute (NHRI).12 The NHRI organizes the NHIRD, which contains all claims data from the National Health Insurance (NHI) program in Taiwan. The NHI program, which started in Taiwan on March 1, 1995, covers ≈99% of Taiwan's national population. More details regarding the NHIRD have been described elsewhere.13, 14 Diagnostic codes based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) were retrieved from the NHIRD.
Ethics Statement
The NHIRD encrypts patient personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Consequently, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the institutional review board of China Medical University (CMUH104‐REC2‐115‐CR2). The institutional review board also specifically waived the consent requirement.
Sampled Participants
This study focused on comparing the risk of suicide attempt between individuals with and without stroke. We recruited a poststroke and nonstroke cohort consisting of patients and controls, respectively. The poststroke cohort recruited adults (aged >18 years) who received a first‐ever diagnosis (ICD‐9‐CM codes 430–438) of either hemorrhagic stroke (ICD‐9‐CM codes 430–432) or ischemic stroke (ICD‐9‐CM codes 433–438) in NHIRD between January 1, 2000, and December 31, 2010. The admission date after the initial stroke diagnosis was set as the index date. To recruit a comparable nonstroke cohort, we randomly selected controls at ≈2 times the number of patients without a stroke diagnosis history and frequency‐matched them by sex and age (for every 5‐year span) with the poststroke patients. We set a random month and day and the same index year as that of the matched patient's index date. We excluded patients with a history of suicide attempt (ICD‐9‐CM codes E950–E959) before the index date. The baseline comorbidity history included schizophrenia (ICD‐9‐CM code 295), depression before stroke (ICD‐9‐CM codes 296.2, 296.3, 296.82, 300.4, and 311), poststroke depression (ICD‐9‐CM codes 296.2, 296.3, 296.82, 300.4, and 311), alcohol‐related illness (ICD‐9‐CM codes 291, 303, 305.00, 305.01, 305.02, 305.03, 571.0, 571.1, 571.3, 790.3, and V11.3), anxiety (ICD‐9‐CM code 300.00), mental disorders (ICD‐9‐CM codes 290–319), insomnia (ICD‐9‐CM codes 307.4 and 780.5), cognitive dysfunction (ICD‐9‐CM codes 331 and 331.83), dementia after stroke (ICD‐9‐CM codes 290, 294.1, and 331.0), diabetes mellitus (ICD‐9‐CM code 250), chronic obstructive pulmonary disease (ICD‐9‐CM codes 491, 492, and 496), asthma (ICD‐9‐CM code 493), coronary artery disease (ICD‐9‐CM code 410–414), and hypertension (ICD‐9‐CM code 401–405). These comorbidities were identified in the study cohorts and adjusted in the analysis (Figure 1).
Figure 1.
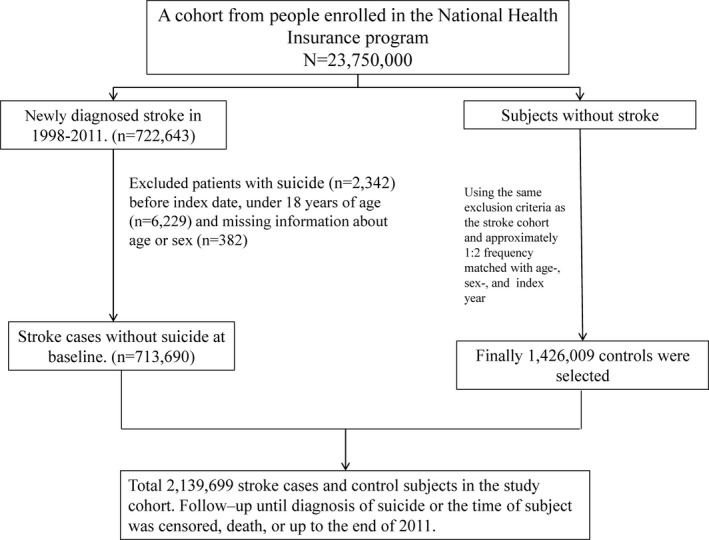
The flowchart of participant selection in this study.
Outcome Measurement
All participants enrolled in this study were followed up until a diagnosis of suicide attempt (ICD‐9‐CM codes E950–E959), death from other causes, withdrawal from the insurance system, or the end of 2011, whichever occurred earlier.
Statistical Analyses
The proportionate distributions of sociodemographic status (including sex, age, monthly income, urbanization level, and occupation category) and comorbidity of the poststroke and nonstroke cohorts were compared and examined using the χ2 test and the Student t test for categorical and continuous variables, respectively. We plotted the cumulative incidence of suicide attempt by applying the Kaplan–Meier method, and the level of statistical significance was based on the log‐rank test. The incidence density rates of attempted suicide among different risk factors stratified by age, sex, monthly income, urbanization level, occupation category, and comorbidity were calculated as the number of suicide attempt events divided by the sum of the follow‐up time (per 10 000 person‐years). Univariable and multivariable Cox proportional hazards regression models were used to calculate the hazard ratio (HR) and the 95% confidence interval (CI) of the risk of suicide attempt associated with stroke. The multivariable models were simultaneously adjusted for sociodemographic status and comorbidities. Because mortality is a vital factor affecting the estimation of suicide attempt, we also considered the event of death as a competing event to estimate sub‐HRs and 95% CIs by using the Fine and Gray method.15 These data were further analyzed to compare the risk of mortality among attempted suicide patients with and without stroke. We used SAS (v. 9.2 for Windows; SAS Institute) for all statistical analyses. P<0.05 was considered statistically significant.
Results
We recruited a poststroke cohort consisting of 713 690 patients and a nonstroke cohort comprising 1 426 009 controls (Table 1). The distributions of sex and age did not differ significantly between the poststroke and nonstroke cohorts because the patients and controls were matched. The mean age (±SD) of the poststroke cohort and nonstroke cohort was 67.9±13.9 and 67.0±14.0 years, respectively. Both the poststroke and nonstroke cohorts had average income levels between 15 000 and 19 999 New Taiwan dollars (NTD) per month (approximately equal to 495–660 US dollars [USD] per month; 49.1% versus 44.7%) and lived in higher urbanized areas (50.1% versus 54.4% in urbanization levels 1 and 2). Compared with the nonstroke controls, the poststroke patients had a higher tendency to work as manual laborers (46.6% versus 41.6%) and to have hypertension (61.9% versus 13.6%), coronary artery disease (20.3% versus 7.23%), diabetes mellitus (20.1% versus 7.21%), chronic obstructive pulmonary disease (10.4% versus 4.24%), alcohol‐related illness (2.22% versus 0.45%), insomnia (3.11% versus 0.87%), mental disorders (7.78% versus 1.31%), dementia after stroke (5.74% versus 1.43%), depression before stroke (2.31% versus 0.52%), poststroke depression (2.12% versus 0.65%), anxiety (1.45% versus 0.37%), cognitive dysfunction (1.45% versus 0.27%), and schizophrenia (0.52% versus 0.33%; Table 1).
Table 1.
Demographic Characteristics and Comorbidities of Poststroke Patients and Nonstroke Controls
| Nonstroke (n=1 426 009) | Poststroke (n=713 690) | a P Value | |
|---|---|---|---|
| Sex | 0.76 | ||
| Female | 606 940 (42.6) | 303 918 (42.6) | |
| Male | 819 069 (57.4) | 409 772 (57.4) | |
| Age, y | 0.88 | ||
| ≤49 | 165 898 (11.6) | 82 949 (11.6) | |
| 50–64 | 360 962 (25.3) | 180 481 (25.3) | |
| ≥65 | 899 149 (63.1) | 450 260 (63.1) | |
| Age, y, mean±SDb | 67.0 (14.0) | 67.9 (13.9) | 0.001 |
| Monthly incomec | <0.001 | ||
| <15 000 | 472 116 (33.1) | 243 271 (34.1) | |
| 15 000–19 999 | 637 351 (44.7) | 350 453 (49.1) | |
| ≥20 000 | 316 542 (22.2) | 119 966 (16.8) | |
| Urbanization leveld | <0.001 | ||
| 1 (highest) | 381 567 (26.8) | 162 506 (22.8) | |
| 2 | 394 097 (27.6) | 194 932 (27.3) | |
| 3 | 229 360 (16.1) | 120 100 (16.8) | |
| 4 (lowest) | 420 985 (29.5) | 236 152 (33.1) | |
| Occupation categorye | <0.001 | ||
| Office worker | 627 244 (44.0) | 274 796 (38.5) | |
| Manual laborer | 593 210 (41.6) | 332 694 (46.6) | |
| Other | 205 555 (14.4) | 106 200 (14.9) | |
| Comorbidity | |||
| Schizophrenia | 4653 (0.33) | 3745 (0.52) | <0.001 |
| Depression before stroke | 7434 (0.52) | 16 467 (2.31) | <0.001 |
| Poststroke depression | 9280 (0.65) | 15 134 (2.12) | <0.001 |
| Alcohol‐related illness | 6432 (0.45) | 15 875 (2.22) | <0.001 |
| Anxiety | 5322 (0.37) | 10 344 (1.45) | <0.001 |
| Mental disorders | 18 657 (1.31) | 55 519 (7.78) | <0.001 |
| Insomnia | 12 439 (0.87) | 22 163 (3.11) | <0.001 |
| Cognitive dysfunction | 3918 (0.27) | 10 321 (1.45) | <0.001 |
| Dementia after stroke | 20 422 (1.43) | 40 997 (5.74) | <0.001 |
| Diabetes mellitus | 102 749 (7.21) | 143 101 (20.1) | <0.001 |
| Chronic obstructive pulmonary disease | 60 523 (4.24) | 74 144 (10.4) | <0.001 |
| Asthma | 32 099 (2.25) | 37 400 (5.24) | <0.001 |
| Coronary artery disease | 103 055 (7.23) | 144 841 (20.3) | <0.001 |
| Hypertension | 194 295 (13.6) | 441 836 (61.9) | <0.001 |
Chi‐square test.
Student t test.
In New Taiwan dollars; 1 New Taiwan dollar is equal to 0.033 US dollars.
The urbanization level was determined by dividing the population density of residential areas into 4 levels, with level 1 the most urbanized and level 4 the least urbanized.
Other occupation categories included those who were primarily retired, unemployed, and on low income.
Kaplan–Meier analysis indicated that the cumulative incidence of suicide attempt was significantly higher in the poststroke cohort than in the nonstroke cohort (log‐rank P<0.001; Figure 2) throughout the 12‐year study period. The overall incidence density rates of attempted suicide were 2.38, 5.52, 7.28, and 7.02 per 10 000 person‐years in the nonstroke controls and in the poststroke patients with hemorrhagic stroke, ischemic stroke, and total stroke, respectively (Table 2). Compared with the nonstroke controls, the corresponding adjusted HRs (aHRs) for suicide attempt were 1.87 (95% CI, 1.62–2.15), 2.25 (95% CI, 2.08–2.43), and 2.20 (95% CI, 2.04–2.37) for the hemorrhagic, ischemic, and total stroke patients, respectively, after adjusting for age, monthly income, urbanization level, and comorbidities of schizophrenia, depression before stroke, poststroke depression, alcohol‐related illness, anxiety, mental disorders, insomnia, cognitive dysfunction, dementia after stroke, diabetes mellitus, chronic obstructive pulmonary disease, asthma, coronary artery disease, and hypertension. Compared with patients aged 50–64 years, the risk of attempted suicide was 1.35‐fold higher in those aged ≤49 years (95% CI, 1.22–1.50). The low monthly income group (<15 000 NTD, ≈495 USD) had an aHR of 1.64 (95% CI, 1.46–1.85), and the middle monthly income group (15 000–19 999 NTD, ≈495–660 USD) had an aHR of 1.46 (95% CI, 1.31–1.63) for suicide attempt compared, respectively, with the high monthly income group (≥20 000 NTD, ≈660 USD). The aHRs of suicide attempt were 1.58 (95% CI, 1.43–1.74), 1.49 (95% CI, 1.34–1.66), and 1.37 (95% CI, 1.24–1.51) for those patients living in regions with urbanization level 4 (the lowest urbanized region), level 3, and level 2, respectively, compared with those living in regions with the urbanization level 1 (the highest urbanized region). The risk of attempted suicide was higher among manual laborers than office workers (aHR: 1.31; 95% CI, 1.20–1.43). Moreover, the risk of attempted suicide was higher in specific patients with schizophrenia (aHR: 1.98; 95% CI, 1.46–2.70), depression before stroke (aHR: 3.37; 95% CI, 2.91–3.89), poststroke depression (aHR: 2.81; 95% CI, 2.44–3.24), alcohol‐related illness (aHR: 2.09; 95% CI, 1.72–2.52), anxiety (aHR: 1.72; 95% CI, 1.42–2.08), insomnia (aHR: 1.79; 95% CI, 1.54–2.08), diabetes mellitus (aHR: 1.28; 95% CI, 1.18–1.39), chronic obstructive pulmonary disease (aHR: 1.35; 95% CI, 1.20–1.52), asthma (aHR: 1.33; 95% CI, 1.15–1.53), coronary artery disease (aHR: 1.23; 95% CI, 1.13–1.35), and hypertension (aHR: 1.11; 95% CI, 1.03–1.20) compared with others without these comorbidities (Table 2).
Figure 2.
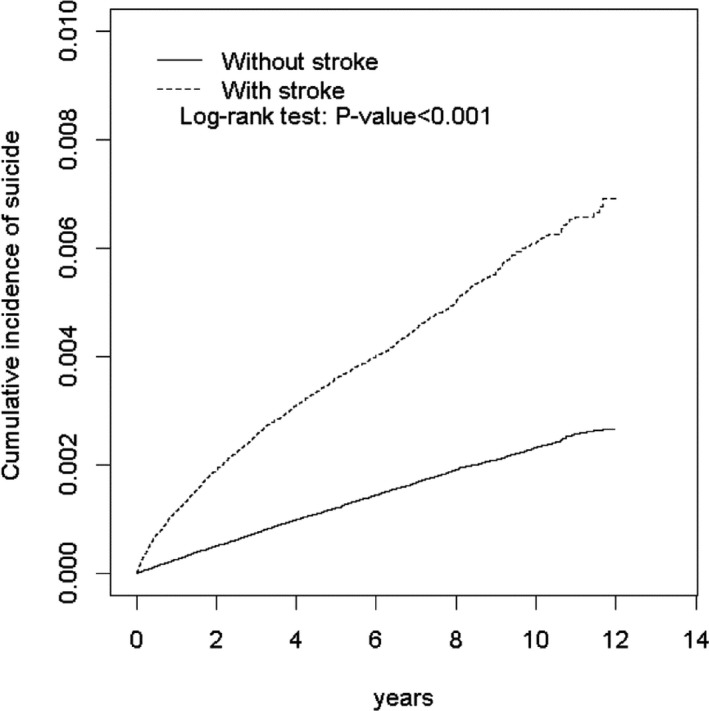
Comparison of cumulative incidence of attempted suicide in the poststroke and nonstroke cohorts.
Table 2.
Incidence of and Risk Factors for Suicide Attempt
| Variable | Event | Person‐Years | Ratea | Crude HR (95% CI) | Adjusted HRb (95% CI) |
|---|---|---|---|---|---|
| Stroke | |||||
| No | 1925 | 8 096 035 | 2.38 | 1.00 | 1.00 |
| Hemorrhagic stroke | 246 | 445 868 | 5.52 | 2.27 (1.99, 2.59)c | 1.87 (1.62, 2.15)c |
| Ischemic stroke | 1894 | 2 602 413 | 7.28 | 2.99 (2.81, 3.19)c | 2.25 (2.08, 2.43)c |
| Total | 2140 | 3 048 280 | 7.02 | 2.89 (2.71, 3.07)c | 2.20 (2.04, 2.37)c |
| Age group, y | |||||
| ≤49 | 605 | 1 471 834 | 4.11 | 1.31 (1.19, 1.45)c | 1.35 (1.22, 1.50)c |
| 50–64 | 992 | 3 153 473 | 3.15 | 1.00 | 1.00 |
| ≥65 | 2468 | 6 519 009 | 3.79 | 1.17 (1.09, 1.26)c | 1.06 (0.98, 1.15) |
| Sex | |||||
| Female | 1727 | 4 830 122 | 3.58 | 1.00 | 1.00 |
| Male | 2338 | 6 314 193 | 3.70 | 1.03 (0.97, 1.10) | – |
| Monthly incomed | |||||
| <15 000 | 1311 | 3 577 081 | 3.66 | 1.71 (1.54, 1.89)c | 1.64 (1.46, 1.85)c |
| 15 000–19 999 | 2241 | 5 155 646 | 4.35 | 2.04 (1.85, 2.24)c | 1.46 (1.31, 1.63)c |
| ≥20 000 | 513 | 2 411 588 | 2.13 | 1.00 | 1.00 |
| Urbanization levele | |||||
| 1 (highest) | 678 | 2 861 571 | 2.37 | 1.00 | 1.00 |
| 2 | 1097 | 3 081 302 | 3.56 | 1.50 (1.37, 1.65)c | 1.37 (1.24, 1.51)c |
| 3 | 721 | 1 813 307 | 3.98 | 1.68 (1.51, 1.86)c | 1.49 (1.34, 1.66)c |
| 4 (lowest) | 1569 | 3 388 134 | 4.63 | 1.95 (1.79, 2.14)c | 1.58 (1.43, 1.74)c |
| Occupation categoryf | |||||
| Office worker | 1325 | 4 788 374 | 2.77 | 1.00 | 1.00 |
| Manual laborer | 2154 | 4 816 219 | 4.47 | 1.62 (1.51, 1.73)c | 1.31 (1.20, 1.43)c |
| Other | 586 | 1 539 723 | 3.81 | 1.37 (1.24, 1.51)c | 1.01 (0.91, 1.13) |
| Comorbidity | |||||
| Schizophrenia | |||||
| No | 4023 | 11 105 894 | 3.62 | 1.00 | 1.00 |
| Yes | 42 | 38 421 | 10.9 | 2.96 (2.18, 4.01)c | 1.98 (1.46, 2.70)c |
| Depression before stroke | |||||
| No | 3844 | 11 043 613 | 3.48 | 1.00 | 1.00 |
| Yes | 221 | 100 703 | 22.0 | 6.11 (5.33, 6.99)c | 3.37 (2.91, 3.89)c |
| Poststroke depression | |||||
| No | 3852 | 10 990 993 | 3.50 | 1.00 | 1.00 |
| Yes | 213 | 153 323 | 13.9 | 4.05 (3.53, 4.65)c | 2.81 (2.44, 3.24)c |
| Alcohol‐related illness | |||||
| No | 3936 | 11 051 763 | 3.56 | 1.00 | 1.00 |
| Yes | 129 | 92 552 | 13.9 | 3.81 (3.19, 4.54)c | 2.09 (1.72, 2.52)c |
| Anxiety | |||||
| No | 3944 | 11 070 435 | 3.56 | 1.00 | 1.00 |
| Yes | 121 | 73 880 | 16.4 | 4.52 (3.77, 5.42)c | 1.72 (1.42, 2.08)c |
| Mental disorders | |||||
| No | 3939 | 10 890 990 | 3.62 | 1.00 | 1.00 |
| Yes | 126 | 253 325 | 4.97 | 1.29 (1.08, 1.55)g | 0.81 (0.67, 1.00) |
| Insomnia | |||||
| No | 3858 | 11 000 255 | 3.51 | 1.00 | 1.00 |
| Yes | 207 | 144 061 | 14.4 | 3.95 (3.44, 4.55)c | 1.79 (1.54, 2.08)c |
| Cognitive dysfunction | |||||
| No | 4042 | 11 099 183 | 3.64 | 1.00 | 1.00 |
| Yes | 23 | 45 133 | 5.10 | 1.30 (0.86, 1.96) | 0.76 (0.50, 1.15) |
| Dementia after stroke | |||||
| No | 3983 | 10 830 139 | 3.68 | 1.00 | 1.00 |
| Yes | 82 | 314 176 | 2.61 | 0.71 (0.57, 0.88)g | 0.42 (0.33, 0.52)c |
| Diabetes mellitus | |||||
| No | 3292 | 9 730 782 | 3.38 | 1.00 | 1.00 |
| Yes | 773 | 1 413 534 | 5.47 | 1.64 (1.52, 1.78)c | 1.28 (1.18, 1.39)c |
| Chronic obstructive pulmonary disease | |||||
| No | 3682 | 10 668 608 | 3.45 | 1.00 | 1.00 |
| Yes | 383 | 475 707 | 8.05 | 2.21 (1.99, 2.46)c | 1.35 (1.20, 1.52)c |
| Asthma | |||||
| No | 3825 | 10 873 179 | 3.52 | 1.00 | 1.00 |
| Yes | 240 | 271 137 | 8.85 | 2.42 (2.12, 2.76)c | 1.33 (1.15, 1.53)c |
| Coronary artery disease | |||||
| No | 3370 | 10 125 179 | 3.33 | 1.00 | 1.00 |
| Yes | 695 | 1 019 136 | 6.82 | 1.98 (1.83, 2.15)c | 1.23 (1.13, 1.35)c |
| Hypertension | |||||
| No | 2411 | 8 422 637 | 2.86 | 1.00 | 1.00 |
| Yes | 1654 | 2721 678 | 6.08 | 2.06 (1.93, 2.19)c | 1.11 (1.03, 1.20)g |
CI indicates confidence interval; HR, hazard ratio; NTD, New Taiwan dollars.
Incidence rate per 10 000 person‐years.
Multivariable analysis included age, monthly income, urbanization level, and comorbidity of schizophrenia, depression before stroke, poststroke depression, alcohol‐related illness, anxiety, mental disorders, insomnia, cognitive dysfunction, dementia after stroke, diabetes mellitus, chronic obstructive pulmonary disease, asthma, coronary artery disease, and hypertension.
P<0.001.
In NTD, where 1 NTD is equal to 0.03 US dollars.
The urbanization level of residential areas was determined by dividing the population density into 4 levels, with level 1 the most urbanized and level 4 the least urbanized.
Other occupation categories included those who were primarily retired, unemployed, and on low income.
P<0.01.
Table 3 presents a sensitivity analysis for the estimation of the risk of suicide in the study cohorts by considering the competing risk of death. After adjustment for confounding factors and the competing risk of death, the poststroke cohort remained more associated with significantly higher risk of new‐onset stroke, and specifically of hemorrhagic stroke, than the nonstroke cohort (adjusted sub‐HR: 2.15 [95% CI, 1.99–2.32] and 2.01 [95% CI, 1.87–2.16]; Table 3). We further analyzed the mortality rate in ≈3 years after suicide attempts were recorded. The mortality rate is, unexpectedly, slightly lower in the poststroke patients than in the nonstroke controls (24.9% versus 26.2%); however, there was no significant difference in mortality rates between the poststroke patients and controls.
Table 3.
SHR for Suicide in the Poststroke and Nonstroke Cohorts, Estimated Using Competing‐Risks Regression Models
| Competing‐Risks Regression Models | ||
|---|---|---|
| Nonstroke | Poststroke | |
| Stroke | ||
| Crude SHR (95% CI) | 1 (Reference) | 2.88 (2.71–3.06)a |
| Adjusted SHRb (95% CI) | 1 (Reference) | 2.15 (1.99–2.32)a |
| Hemorrhagic stroke | ||
| Crude SHR (95% CI) | 1 (Reference) | 3.02 (2.83–3.21)a |
| Adjusted SHRb (95% CI) | 1 (Reference) | 2.01 (1.87–2.16)a |
| Ischemic stroke | ||
| Crude SHR (95% CI) | 1 (Reference) | 2.32 (2.04–2.65)a |
| Adjusted SHRb (95% CI) | 1 (Reference) | 1.09 (0.96–1.25) |
CI indicates confidence interval; SHR, subhazard ratio.
P<0.001.
Adjusted SHR: multivariable analysis considered age, monthly income, urbanization level, and comorbidity of schizophrenia, depression before stroke, poststroke depression, alcohol‐related illness, anxiety, mental disorders, insomnia, cognitive dysfunction, dementia after stroke, diabetes mellitus, chronic obstructive pulmonary disease, asthma, coronary artery disease, and hypertension.
In addition, we analyzed the different ways of attempting suicide in our cohorts. The poststroke patients were 4.22‐fold more likely to jump from high places (95% CI, 2.08–8.56), 2.28‐fold more likely to develop liquid or solid poisoning (95% CI, 2.09–2.49), 1.93‐fold more likely to cut or pierce themselves (95% CI, 1.53–2.44), 1.73‐fold more likely to hang themselves (95% CI, 1.16–2.57), and 2.25‐fold more likely to attempt suicide in other ways (95% CI, 1.72–2.94) than the nonstroke controls who attempted suicide (Table 4).
Table 4.
Incidence and HRs of Different Ways of Attempting Suicide in the Poststroke and Nonstroke Cohorts
| Outcome | Nonstroke | Poststroke | Crude HR (95% CI) | Adjusted HRb (95% CI) | ||
|---|---|---|---|---|---|---|
| Event | Ratea | Event | Ratea | |||
| Liquid or solid poisoning (ICD‐9‐CM code E950) | 1377 | 1.70 | 1607 | 5.27 | 3.02 (2.81–3.25)c | 2.28 (2.09–2.49)c |
| Self‐poison using motor vehicle exhaust gas (ICD‐9‐CM code E952) | 86 | 0.11 | 62 | 0.20 | 1.88 (1.36–2.61)c | 1.42 (0.94–2.14) |
| Hanging (ICD‐9‐CM code E953) | 78 | 0.10 | 69 | 0.23 | 2.29 (1.66–3.17)c | 1.73 (1.16–2.57)d |
| Cutting/piercing (ICD‐9‐CM code E956) | 213 | 0.26 | 208 | 0.68 | 2.53 (2.09–3.06)c | 1.93 (1.53–2.44)c |
| Jumping from high places (ICD‐9‐CM code E957) | 16 | 0.02 | 33 | 0.11 | 5.29 (2.91–9.62)c | 4.22 (2.08–8.56)c |
| Other (ICD‐9‐CM codes E951, E954, E955, E958, and E959) | 155 | 0.19 | 161 | 0.53 | 2.76 (2.21–3.44)c | 2.25 (1.72–2.94)c |
CI indicates confidence interval; HR, hazard ratio; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Incidence rate per 10 000 person‐years.
Multivariable analysis considered age, monthly income, urbanization level, and comorbidity of schizophrenia, depression before stroke, poststroke depression, alcohol‐related illness, anxiety, mental disorders, insomnia, cognitive dysfunction, dementia after stroke, diabetes mellitus, chronic obstructive pulmonary disease, asthma, coronary artery disease, and hypertension.
P<0.001.
P<0.01.
Discussion
In this study, our results indicated, surprisingly, that patients who had hemorrhagic stroke (rather than ischemic stroke) had a higher risk of a next stroke compared with those who had not had a stroke. As in the studies in Western countries,5 our poststroke patients had a significantly higher risk of suicide attempt than did the nonstroke controls. In addition, the leading risk factors for poststroke suicide attempt were having a stroke at before age 50 years, earning low or middle monthly income (<20 000 NTD, ≈660 USD), living in regions with less urbanization, and doing manual labor. The attempted suicide risk did not differ significantly between male and female poststroke patients in this study. The ethnic and cultural heritage of the Han people in Taiwan seems not to cause differences in study results regarding suicide attempts by Taiwanese poststroke patients and those in Western countries.
The socioeconomic status and social support of patients with disability should be thought as the major factors affecting long‐term outcome and life span.1, 16 Patients who survive a stroke may be frustrated to manage the new experience of disability or impairment by themselves. Poststroke patients need health‐related rehabilitation to achieve optimal interaction with their environments and participation in various activities, and financial support to health‐care system may be needed to provide or improve those services. Health‐related rehabilitation services are often insufficiently funded and provided in many respects, particularly in Asian and developing societies. While considering the cost effectiveness of a high‐volume rehabilitation service, governments may try to avoid investing large funds for primary care, especially in communities of developing countries.17 With a similar cultural heritage, Yamauchi et al reported that poststroke suicide risk was higher in young and male patients in Japan, a neighboring and more developed country in East Asia.18 Those findings were similar to the studies from Western countries and to ours, even with different ethnic backgrounds. Compared with our study, we conclude that having stroke at a young age and having low socioeconomic status, such as earning less monthly income, living in a less urbanized region, and doing manual labor, are the leading predisposing factors for poststroke patients to attempt suicide in Taiwan.
A stroke might cause physical damage that affects the cognitive and interpretive abilities of a patient, and such damage is strongly associated with depression, anxiety, mental or sleep disorders, cognitive dysfunction, or even dementia after a stroke. Poststroke patients may feel like they have no hope, are a burden on others, or are losing their personal dignity and thus may have a desire for death.19, 20, 21 Our study provides information for suicide prevention by suggesting that those who received a depression diagnosis before a stroke are likely at higher risk of attempting suicide than those diagnosed with depression after a stroke. Among the various comorbidities, depression before and after stroke and alcohol‐related illness are the most likely to increase poststroke suicide attempts, whereas the risk of suicide attempt would not be increased by mental disorders, cognitive dysfunction, or poststroke dementia.
The four common ways of poststroke patients attempting suicide identified in this study were jumping from high places, liquid or solid poisoning, cutting or piercing, and hanging. Among them, jumping from high places was the most common way in Taiwan. This finding is different from those in Western countries, where firearms and self‐poisoning are the 2 most common ways of poststroke patients attempting suicide.22, 23 High‐rise buildings are more common than firearms in populated Asian countries, thus it may be that jumping from a high place is an easily accessible way for poststroke patients to attempt suicide attempt.
Because we adjusted for comorbid psychiatric disorders to reduce their major confounding effects on suicide in this study, and because this study was a nationwide, representative, population‐based study with a low possibility of recall and selection bias, the results indicated that the risk factors were related to poststroke suicide attempt in Taiwan. However, some limitations of this study should be noted. First, although the NHI performs quarterly expert reviews to ensure the accuracy of claim files, and any false diagnostic reports are liable to a severe penalty, occasional miscoding (eg, underdiagnosis of stroke and suicide attempt in the NHIRD) and the omission of some suicide events in our study groups may still have occurred. Second, only suicidal actions were recorded in the NHIRD. We could not directly contact the study participants; their actual coping skills and the degree of social support available were vague and might be confounding factors for their suicidal ideation. We had no way of determining this. The prevalence of suicidal ideation should be even higher than the frequency of attempted suicide and could be underdiagnosed in clinical practice. Third, we could not ascertain if the suicidal actions were performed in a conscious state or in a delirious or unclear state due to the adverse effects of poststroke medications. Finally, although our study design included adequate controls for numerous confounding factors, unmeasured or unknown confounders may have generated bias. However, our statistical results indicated that the sample size was sufficient for achieving a significant interpretation of the phenomena and getting valuable information from this study.
Conclusion
Suicide prevention is a crucial aspect of public health, but no large representative data set from healthcare systems regarding suicide is yet available in Asian countries. This study provides evidence supporting significant predisposing factors for poststroke suicide attempt. Moreover, we should specifically prevent poststroke suicide attempts by preventing jumping from high places. These results convey crucial information to clinicians and caregivers for preventing suicide in poststroke patients in Taiwan and other Asian countries.
Author Contributions
Conception/Design: Tomor Harnod, Chia‐Hung Kao. Provision of study material and patients: Chia‐Hung Kao. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Article writing: All authors. Final approval of article: All authors.
Sources of Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW106‐TDU‐B‐212‐113004), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10601010036), Taiwan Clinical Trial Consortium for Stroke (MOST 106‐2321‐B‐039‐005), Tseng‐Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. No additional external funding was received for this study.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e007830 DOI: 10.1161/JAHA.117.007830.)29321162
References
- 1. Howard‐Wilsher S, Irvine L, Fan H, Shakespeare T, Suhrcke M, Horton S, Poland F, Hooper L, Song F. Systematic overview of economic evaluations of health‐related rehabilitation. Disabil Health J. 2016;9:11–25. [DOI] [PubMed] [Google Scholar]
- 2. Di Rienzo F, Collet C, Hoyek N, Guillot A. Impact of neurologic deficits on motor imagery: a systematic review of clinical evaluations. Neuropsychol Rev. 2014;24:116–147. [DOI] [PubMed] [Google Scholar]
- 3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartoli F, Pompili M, Lillia N, Crocamo C, Salemi G, Clerici M, Carrà G. Rates and correlates of suicidal ideation among stroke survivors: a meta‐analysis. J Neurol Neurosurg Psychiatry. 2017;88:498–504. [DOI] [PubMed] [Google Scholar]
- 5. Eriksson M, Glader EL, Norrving B, Asplund K. Poststroke suicide attempts and completed suicides: a socioeconomic and nationwide perspective. Neurology. 2015;84:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pompili M, Venturini P, Campi S, Seretti ME, Montebovi F, Lamis DA, Serafini G, Amore M, Girardi P. Do stroke patients have an increased risk of developing suicidal ideation or dying by suicide? An overview of the current literature. CNS Neurosci Ther. 2012;18:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santamaría‐Vázquez M, Guijo‐Blanco V. Cultural influence in the development of functional independence. Cadernos de Terapia Ocupacional. 2016;24:663–671. [Google Scholar]
- 8. Chen KL, Tseng MH, Hu FC, Koh CL. Pediatric evaluation of disability inventory: a cross‐cultural comparison of daily function between Taiwanese and American children. Res Dev Disabil. 2010;31:1590–1600. [DOI] [PubMed] [Google Scholar]
- 9. Greenfield PM, Suzuki LK. Culture and human development: implications for parenting, education, pediatrics, and mental health In: Damon W, Sigel IE, Renninger KA, eds. Handbook of Child Psychology: Child Psychology in Practice, Vol. 4, 5th ed. Hoboken, NJ: John Wiley & Sons Inc; 1998:1059–1109. [Google Scholar]
- 10. Tu WM. Cultural China: the periphery as the center. Daedalus. 1991:1–32. [Google Scholar]
- 11. Ying YW, Han M. Cultural orientation in Southeast Asian American young adults. Cultur Divers Ethnic Minor Psychol. 2008;14:29–37. [DOI] [PubMed] [Google Scholar]
- 12. Database NHIR. Taiwan. Available at: http://nhird.nhri.org.tw/en/index.html Accessed 2015/7/15.
- 13. Hu WS, Lin CL. CHA2DS2‐VASc score in the prediction of ischemic bowel disease among patients with atrial fibrillation: insights from a nationwide cohort. Int J Cardiol. 2017;235:56–60. [DOI] [PubMed] [Google Scholar]
- 14. Chiu CH, Wang YC, Lin CL, Lee FY, Kao CH. Leptospirosis and depression: a nationwide cohort analysis. J Clin Psychiatry. 2017;78:e398–e403. [DOI] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16. Neumayer E, Plümper T. Inequalities of income and inequalities of longevity: a cross‐country study. Am J Public Health. 2016;106:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan F, Owolabi MO, Amatya B, Hamzat TK, Ogunniyi A, Oshinowo H, Elmalik A, Galea MP Challenges and barriers for implementation of the World Health Organization Global Disability Action Plan in low‐ and middle‐income countries. J Rehabil Med. 2014; doi:10.2340/16501977‐2276. [DOI] [PubMed] [Google Scholar]
- 18. Yamauchi T, Inagaki M, Yonemoto N, Iwasaki M, Inoue M, Akechi T, Iso H, Tsugane S; JPHC Study Group . Death by suicide and other externally caused injuries after stroke in Japan (1990–2010): the Japan public health center‐based prospective study. Psychosom Med. 2014;76:452–459. [DOI] [PubMed] [Google Scholar]
- 19. Meier DE, Emmons CA, Wallenstein S, Quill T, Morrison RS, Cassel CK. A national survey of physician‐assisted suicide and euthanasia in the United States. N Engl J Med. 1998;338:1193–1201. [DOI] [PubMed] [Google Scholar]
- 20. Breitbart W, Rosenfeld BD. Physician‐assisted suicide: the influence of psychosocial issues. Cancer Control. 1999;6:146–161. [DOI] [PubMed] [Google Scholar]
- 21. Chochinov HM, Hack T, Hassard T, Kristjanson LJ, McClement S, Harlos M. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23:5520–5525. [DOI] [PubMed] [Google Scholar]
- 22. Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. NCHS Data Brief. 2016;241:1–8. [PubMed] [Google Scholar]
- 23. Ahmedani BK, Simon GE, Stewart C, Beck A, Waitzfelder BE, Rossom R, Lynch F, Owen‐Smith A, Hunkeler EM, Whiteside U, Operskalski BH, Coffey MJ, Solberg LI. Health care contacts in the year before suicide death. J Gen Intern Med. 2014;29:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]


