Abstract
Background
Recent trends of hospitalizations and in‐hospital mortality are not well defined in sarcoidosis. We examined aforementioned trends and prevalence of cardiovascular manifestations and explored rates of implantable cardioverter‐defibrillator implantation in hospitalizations with sarcoidosis.
Methods and Results
Using data from the National Inpatient Sample, a retrospective population cohort from 2005 to 2014 was studied. To identify sarcoidosis, an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code was used. We excluded hospitalizations with myocardial infarction, coronary artery disease, and ischemic cardiomyopathy. Cardiovascular manifestations were defined by the presence of diagnosis codes for conduction disorders, arrhythmias, heart failure, nonischemic cardiomyopathy, and pulmonary hypertension. A total of 609 051 sarcoidosis hospitalizations were identified, with an age of 55±14 years, 67% women, and 50% black. The number of sarcoidosis hospitalizations increased from 2005 through 2014 (138 versus 175 per 100 000, P trend<0.001). We observed declining trends of unadjusted in‐hospital mortality (6.5 to 4.9 per 100 sarcoidosis hospitalizations, P trend<0.001). Overall ≈31% (n=188 438) of sarcoidosis hospitalizations had coexistent cardiovascular manifestations of one or more type. Heart failure (≈16%) and arrhythmias (≈15%) were the most prevalent cardiovascular manifestations. Rates of implantable cardioverter‐defibrillator placement were ≈7.5 per 1000 sarcoidosis hospitalizations (P trend=0.95) during the study period. Black race was associated with 21% increased risk of in‐hospital mortality (odds ratio, 1.21; 95% confidence interval, 1.16–1.27 [P<0.001]).
Conclusions
Sarcoidosis hospitalizations have increased over the past decade with a myriad of coexistent cardiovascular manifestations. Black race is a significant predictor of in‐hospital mortality, which is declining. Further efforts are needed to improve care in view of low implantable cardioverter‐defibrillator rates in sarcoidosis.
Keywords: cardiovascular outcomes, implantable cardioverter‐defibrillator, sarcoidosis
Subject Categories: Cardiovascular Disease
Clinical Perspective
What Is New?
Rates of hospitalizations of sarcoidosis have increased from 2005 to 2014.
In‐hospital mortality associated with sarcoidosis has decreased over the same period.
Heart failure and arrhythmias were the most prevalent cardiovascular manifestations in sarcoidosis followed by pulmonary hypertension, nonischemic cardiomyopathy, and conduction disorder.
Notwithstanding the increasing hospitalizations with sarcoidosis, rates of implantable cardioverter‐defibrillator implantation are low, and have not shown any upward trend from 2005 through 2014.
Black race was significantly associated with in‐hospital mortality.
In a propensity match subgroup, blacks with sarcoidosis had higher in‐hospital mortality and cardiac arrest compared with whites.
What Are the Clinical Implications?
Cardiovascular manifestations are prevalent in sarcoidosis hospitalizations, and aggressive screening and risk stratification of patients with sarcoidosis for cardiovascular manifestations could be beneficial.
Implantable cardioverter‐defibrillator devices may be underutilized for prevention of sudden cardiac death in patients with sarcoidosis.
Racial disparities in outcomes may exist in sarcoidosis.
Introduction
Sarcoidosis is a systemic granulomatous disease1, 2 with a predilection towards the cardiovascular system.2 Most patients with sarcoidosis either remain clinically silent or present with nonspecific constitutional symptoms.3 Only 5% of patients with sarcoidosis present with cardiovascular manifestations attributable to cardiac sarcoidosis.4 However, biopsy‐proven cardiac sarcoidosis has been reported in up to 25% of asymptomatic sarcoidosis autopsy studies.5, 6 Patients with sarcoidosis with concomitant cardiovascular manifestations have more complications and a greater risk of sudden death.7, 8 Thus, there is considerable interest in identifying patients with sarcoidosis with concomitant cardiovascular manifestations.9, 10
In 2006, the Japan Society of Sarcoidosis and Other Granulomatous Disorders revised their diagnostic guidelines for cardiac sarcoidosis by creating “histologic” and “clinical” groups.11, 12 Rather than placing additional emphasis on histologic diagnoses of cardiac sarcoidosis, the updated guidelines highlighted the presence of cardiovascular manifestations (eg, atrioventricular block and ventricular arrhythmias) to diagnose sarcoidosis with presumed cardiac involvement.11, 12 In addition, the 2014 Heart Rhythm Society consensus statement highlighted the utilization and role of implantable cardioverter‐defibrillators (ICDs) for the primary and secondary prevention of sudden cardiac death in patients with sarcoidosis as an important direction for future research.10 Since these guidelines were implemented, data reporting hospitalization trends and outcomes in sarcoidosis are lacking.
In view of the gaps in the existing literature base, a retrospective study was conducted to determine trends of hospitalizations and outcomes in sarcoidosis during 2005–2014. Furthermore, we examined rates of ICD, cardiac resynchronization therapy (CRT), and permanent pacemaker (PPM) placement, and factors associated with in‐hospital mortality. We also examined racial disparities in outcomes in sarcoidosis hospitalizations in a propensity matched subgroup.
Methods
Data, Materials, and Code Disclosure Statement
The National Inpatient Sample (NIS) database is publicly available online at the following link (URL: https://www.distributor.hcup-us.ahrq.gov/). Additional information on the data, analytic methods, and study materials will be made available on request from the corresponding author to other researchers for purposes of reproducing the results or replicating the procedure.
Data Source
Our study records were derived from the NIS.13, 14 The details regarding the NIS data have been previously published.15 The NIS is a subset of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality.16 The NIS is the largest publicly available all‐payer inpatient care database in the United States, including data on ≈7 to 8 million discharges per year, and is a stratified 20% sample of discharges from the United States community hospitals, excluding rehabilitation and long‐term acute‐care hospitals.17 Our study sample spans from 2005 through 2014. To represent national estimates, weights were calculated using discharge weights provided by the sponsor (ie, Agency for Healthcare Research and Quality). The NIS constitutes data from 44 states participating in the Healthcare Cost and Utilization Project, representing more than 95% of the United States hospitalizations.
The study cohort was derived from a deidentified publicly available database. Hence, the study was considered exempt from formal review by the University of Alabama at Birmingham's (Birmingham, AL) institutional review board.
Study Population
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) was used to identify our study population. We queried the NIS database using the ICD‐9‐CM diagnostic code of 135 for sarcoidosis in the primary or secondary diagnosis fields.9, 18, 19 Hospitalizations with any present or past history of myocardial infarction, coronary artery disease, or ischemic cardiomyopathy were excluded using ICD‐9‐CM diagnostic codes to avoid influence of ischemic heart disease on trends and outcomes (Table S1).
We examined sarcoidosis hospitalizations free of prevalent ischemic heart disease. Cardiovascular manifestations were defined by the presence of the following disease classes in the primary or secondary diagnosis fields: conduction disorders, arrhythmias, heart failure, nonischemic cardiomyopathy, and pulmonary hypertension (Table S2).18, 20, 21, 22 The NIS variables were used to identify demographic and baseline characteristics of sarcoidosis hospitalizations. Comorbidities used in our study were derived from Elixhauser method for quantification of comorbidities.14 A cardiac arrest (ICD‐9‐CM diagnosis code 427.5) was identified from the disease class of arrhythmias.23 We identified all discharges who came or required new ICD implantation or replacement while hospitalized (ICD‐9‐CM procedure codes‐37.94, 37.95, and 37.96), CRT implantation or replacement (ICD‐9‐CM procedural code‐00.50, 00.51), or PPM implantation (ICD‐9‐CM procedural code‐37.7, 37.8) by using primary and secondary procedural fields.
Outcomes
The primary outcomes of our study were the trends of hospitalizations, and in‐hospital mortality associated with sarcoidosis. Trends of hospitalizations and in‐hospital mortality associated with sarcoidosis. Additional analyses performed in sarcoidosis were: (1) trends of ICD, CRT, and PPM use; (2) factors associated with in‐hospital mortality; and (3) propensity score–matched analyses between blacks and whites in a subgroup of the overall sarcoidosis hospitalizations.
Statistical Analysis
Primary analyses
SAS 9.4 (SAS Institute Inc) was used to analyze the NIS database. The initial study cohort derived from the NIS was 20% stratified. To generate nationally representative estimates, discharge weights provided by the sponsor (ie, Agency for Healthcare Research and Quality) were utilized. All analyses were performed in the weighted study cohort to minimize biases. Categorical data were presented as weighted frequency in percentages. Continuous data were presented as mean±SD. Trends of sarcoidosis hospitalizations were calculated using number of sarcoidosis hospitalizations divided by total number of hospitalizations in a given year per 100 000. Furthermore, trends of in‐hospital mortality were calculated as total number of events per 100 sarcoidosis hospitalizations. The trend analyses were performed using Jonckheere‐Terpstra test. Rates of ICD, CRT, and PPM implantation were calculated by number of these procedures per 1000 sarcoidosis hospitalizations. In addition, we identified factors associated with in‐hospital mortality by using a mixed effect logistic regression model.
Subgroup analyses
A propensity score–matched model was generated to compare in‐hospital outcomes between whites and blacks. Hospitalizations identified from a different race other than black or white were removed before propensity matching. A logistic regression model was performed for age, sex, and all baseline characteristics to calculate a propensity score for each hospitalization. Next, we matched all hospitalizations using a one‐to‐one scheme without replacement using the nearest number matching method. The primary outcome of incident in‐hospital mortality was assessed using McNemar test. Development of in‐hospital cardiac arrest in whites and blacks was also compared using the McNemar test. Paired t test was used to calculate hospital stay.
Results
Trends of Sarcoidosis Hospitalizations and In‐Hospital Mortality
We identified 740 762 sarcoidosis hospitalizations from 2005 to 2014. All sarcoidosis hospitalizations with any present or past history of myocardial infarction, coronary artery disease, or ischemic cardiomyopathy were excluded (n=131 711). A total of 609 051 sarcoidosis hospitalizations were included in our final study cohort (Figure 1). Of which 188 438 hospitalizations (≈31%) were coded as having cardiovascular manifestations of one or more type.
Figure 1.
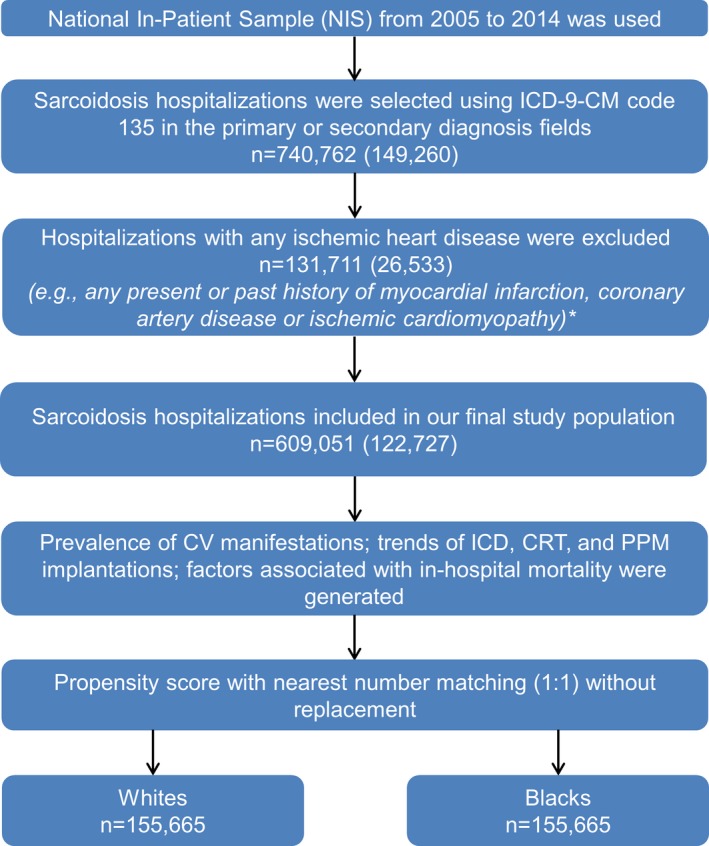
Flow chart of the study design and cohort selection. Sample size presented in weighted numbers (unweighted numbers). *Presence of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 410.1‐410.9, 411.1, 411.8, 412, 414.00‐414.07, and 414.8 in the primary or secondary diagnosis fields. CRT indicates cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator; NIS, National Inpatient Sample; PPM, permanent pacemaker.
Table 1 demonstrates baseline characteristics of sarcoidosis hospitalizations. The mean age of the overall cohort with sarcoidosis was 55±14 years. However, the mean age of the cohort with cardiovascular manifestations was higher (59±14 years) compared with the cohort without cardiovascular manifestations (53±13 years) (Table 1). Among the study cohort with sarcoidosis, ≈67% were women and ≈33% were men. The distribution of sex was similar in sarcoidosis hospitalizations with or without cardiovascular manifestations. Sarcoidosis hospitalizations were more frequent (49%) in blacks as compared with the other races (Table 1). Both hospitalization groups with and without cardiovascular manifestations had a preponderance of blacks (≈54% and ≈47%). Comorbidities including diabetes mellitus, hypertension, fluid and electrolyte disorders, coagulopathy, anemia, pulmonary circulation disorder, and renal failure were more prevalent in sarcoidosis hospitalizations who had co‐existing cardiovascular manifestations (Table 1). The Southern United States (Table 1) reported the majority (≈40%) of sarcoidosis hospitalizations. Approximately 91% of sarcoidosis hospitalizations occurred in urban hospitals (Table 1).
Table 1.
Baseline Characteristics of Sarcoidosis Hospitalizations Stratified by With and Without Cardiovascular Manifestations
| Variable Name | Overall Sarcoidosis (N=609 051) | With Cardiovascular Manifestations (n=188 438) | Without Cardiovascular Manifestations (n=420 613) |
|---|---|---|---|
| Age, y | 55±14 | 59±14 | 53±13 |
| Sex, % | |||
| Male | 32.8 | 33.9 | 32.3 |
| Female | 67.2 | 66.1 | 67.7 |
| Race, % | |||
| White | 43.9 | 40.5 | 45.4 |
| Black | 49.5 | 54.3 | 47.3 |
| Other | 6.6 | 5.2 | 7.3 |
| Comorbidities, % | |||
| Diabetes mellitus | 24.9 | 29.3 | 22.9 |
| Hypertension | 52.9 | 58.5 | 50.4 |
| Liver disease | 4.5 | 4.2 | 4.7 |
| Fluid and electrolyte disorder | 24.2 | 29.2 | 22 |
| Neurological disorder | 6.8 | 6.6 | 6.9 |
| Coagulopathy | 4.6 | 5.7 | 4.1 |
| Chronic pulmonary disease | 29.0 | 35.8 | 25.9 |
| Anemia | 18.1 | 20.8 | 16.9 |
| Solid tumor with metastasis | 1.4 | 1.3 | 1.4 |
| Metastatic cancer | 1.6 | 1.2 | 1.8 |
| Pulmonary circulation disorders | 6.3 | 18.9 | 0.6 |
| Renal failure | 12.2 | 19.4 | 9.0 |
| Hospital region, % | |||
| Northeast | 25.1 | 23.5 | 25.7 |
| Midwest | 24.1 | 24.9 | 23.8 |
| South | 40.4 | 40.8 | 40.2 |
| West | 10.4 | 10.8 | 10.3 |
| Hospital type, % | |||
| Urban | 91.3 | 91.8 | 91.0 |
| Rural | 8.7 | 8.2 | 9.0 |
Data are presented as mean±SD or percentage.
Overall, hospitalizations for sarcoidosis substantially increased from 2005 to 2014 (P trend<0.001) (Figure 2). The number of sarcoidosis hospitalizations increased from ≈138 to ≈175 per 100 000 (relative increase ≈26%) (Figure 2). However, rates of in‐hospital mortality of sarcoidosis significantly decreased from 2005 to 2014 (6.5 versus 4.9 per 100 sarcoidosis hospitalizations) (relative decrease ≈26%, P trend<0.001) (Figure 2).
Figure 2.

Rate of hospitalizations and in‐hospital mortality in sarcoidosis hospitalizations from 2005 to 2014. Figure shows estimated sarcoidosis hospitalizations per 100 000 (red square), and unadjusted in‐hospital mortality per 100 sarcoidosis hospitalizations (blue triangle) in the given year. The error bars represent percentage errors. Trend P<0.001 by Jonckheere‐Terpstra test for both.
Prevalence of Cardiovascular Manifestations and Rates of ICD, CRT, and PPM Implantation
The percentage prevalence of cardiovascular disease class in sarcoidosis is shown in Figure 3. Overall, we found ≈31% (n=188 438) of sarcoidosis hospitalizations (n=609 051) had one or more of these cardiovascular manifestations. The overall prevalence of heart failure was ≈15.5%, any arrhythmias ≈14.7%, pulmonary hypertension 8.6%, nonischemic cardiomyopathy 6.7%, and conduction disorder 2.5% among sarcoidosis hospitalizations (Figure 3). Rates of ICDs were steady at ≈7.5 per 1000 sarcoidosis admissions during the study period (P trend=0.95). We observed decreased utilization of CRT (P trend=0.007), and increased placement of PPMs (P trend<0.001) during the study period (Figure 4).
Figure 3.

Prevalence of cardiovascular manifestations in sarcoidosis. Data are represented in the percentage. Overall, ≈31% (n=188 438) of sarcoidosis hospitalizations (n=609 051) had cardiovascular manifestations. Individual cardiovascular manifestation may have overlapped with the other. Prevalence of cardiovascular manifestations in sarcoidosis was classified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes (Table S2).
Figure 4.
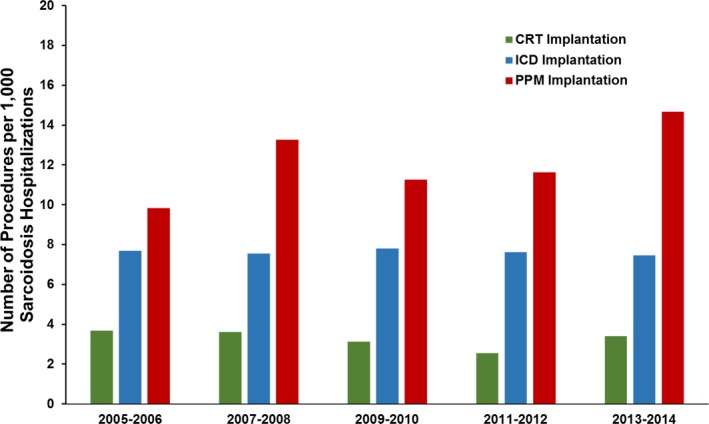
Implantable cardioverter‐defibrillator (ICD), cardiac resynchronization therapy (CRT), and permanent pacemaker (PPM) implantations per 1000 hospitalizations of sarcoidosis from 2005 to 2014. Trend P=0.95 for ICD implantation; trend P=0.007 for CRT implantation; and trend P<0.001 for PPM implantation by Jonckheere‐Terpstra test.
Factors Associated With In‐Hospital Mortality
Factors associated with in‐hospital mortality are shown in Table 2. Age was an independent factor associated with in‐hospital mortality (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.03–1.04 [P<0.001]). After considering white race as a reference, black race was associated with a 21% higher risk of in‐hospital mortality (OR, 1.21; 95% CI, 1.16–1.27 [P<0.001]) among sarcoidosis (Table 2). Fluid and electrolyte disorders, liver disease, neurological disorders, coagulopathy, history of heart failure, metastatic cancer, history of pulmonary circulation disorder, and renal failure were all associated with a greater risk of in‐hospital mortality (Table 2). In contrast, female sex was associated with 17% lower risk of in‐hospital mortality (OR, 0.83; 95% CI, 0.80–0.87 [P<0.001]). Chronic diseases such as diabetes mellitus, hypertension, and anemias were associated with lower risk of in‐hospital mortality (Table 2). Assuming Northeast region as a reference, other regions had significantly lower risk of in‐hospital mortality among sarcoidosis (Table 2).
Table 2.
Factors Associated With In‐Hospital Mortality in Sarcoidosis Hospitalizations
| Variable Name | OR (95% CI) | P Value |
|---|---|---|
| Agea | 1.03 (1.03–1.04) | <0.001 |
| Female vs male | 0.83 (0.80–0.87) | <0.001 |
| Race | ||
| White | Referent | |
| Black | 1.21 (1.16–1.27) | <0.001 |
| Other | 1.25 (1.19–1.32) | <0.001 |
| Comorbiditiesb | ||
| Diabetes mellitus | 0.88 (0.84–0.92) | <0.001 |
| Hypertension | 0.60 (0.58–0.63) | <0.001 |
| Liver disease | 1.44 (1.34–1.54) | <0.001 |
| Fluid and electrolyte disorder | 2.69 (2.59–2.79) | <0.001 |
| Neurological disorder | 1.34 (1.26–1.43) | <0.001 |
| Coagulopathy | 3.14 (2.98–3.31) | <0.001 |
| Chronic pulmonary disease | 1.10 (1.05–1.14) | <0.001 |
| Congestive heart failure | 1.98 (1.89–2.08) | <0.001 |
| Anemia | 0.80 (0.77–0.84) | <0.001 |
| Solid tumor with metastasis | 1.58 (1.41–2.78) | <0.001 |
| Metastatic cancer | 3.76 (3.49–4.09) | <0.001 |
| Pulmonary circulation disorders | 2.39 (2.27–2.52) | <0.001 |
| Renal failure | 1.54 (1.46–1.61) | <0.001 |
| Hospital region | ||
| Northeast | Referent | |
| Midwest | 0.79 (0.75–0.84) | <0.001 |
| South | 0.92 (0.88–0.97) | <0.001 |
| West | 0.91 (0.85–0.97) | 0.005 |
Data are presented in odds ratio with 95% confidence interval.
CI indicates confidence interval; OR, odds ratio.
Age is a continuous variable.
Reference group is not present for these variables.
C statistic 0.78.
Propensity‐Matched Analyses of in‐Hospital Outcomes Between Blacks and Whites
As black race was an independent factor associated with in‐hospital mortality in sarcoidosis, we compared in‐hospital outcomes in propensity‐matched whites (n=155 665) and blacks (n=155 665) (Table 3). Black race was associated with higher in‐hospital mortality (1.8% versus 1.5%) (OR, 1.20; 95% CI, 1.14–1.27 [P<0.001]) and cardiac arrest (0.5% versus 0.3%) (OR, 1.67; 95% CI, 1.49–1.87 [P<0.001]) compared with white race (Table 3). Moreover, median length of hospitalization stay was higher in blacks compared with whites (median 4 [interquartile range, 2–6] days versus 3 [interquartile range, 2–6] days, P<0.001) (Table 3).
Table 3.
Propensity Score Matched Cohort (1:1) of Sarcoidosis Hospitalizations Between Blacks and Whites
| Variable Name | Blacks (n=155 665) | Whites (n=155 665) | P Value |
|---|---|---|---|
| Age, y | 54.2±12.3 | 54.3±12.6 | 0.11 |
| Sex, % | |||
| Female | 75.8 | 75.8 | 0.71 |
| Male | 24.2 | 24.2 | |
| Comorbidities, % | |||
| Diabetes mellitus | 24.2 | 24.2 | 0.71 |
| Hypertension | 52.5 | 52.8 | 0.09 |
| Liver disease | 3.1 | 3.2 | 0.32 |
| Fluid and electrolyte disorder | 22.1 | 22.1 | 0.88 |
| Neurological disorder | 5.2 | 5.4 | 0.17 |
| Coagulopathy | 3.0 | 2.8 | 0.40 |
| Chronic pulmonary disease | 27.6 | 27.5 | 0.33 |
| Congestive heart failure | 6.6 | 7.0 | 0.22 |
| Anemia | 14.8 | 15 | 0.06 |
| Solid tumor with metastasis | 1.4 | 1.3 | 0.70 |
| Metastatic cancer | 1.6 | 1.7 | 0.15 |
| Pulmonary circulation disorders | 3.8 | 3.9 | 0.16 |
| Renal failure | 9.3 | 9.4 | 0.28 |
| In‐hospital outcomes | |||
| In‐hospital mortality | 1.8 | 1.5 | <0.001 |
| Length of stay, median (IQR)a | 4 (2–6) | 3 (2–6) | <0.001 |
| Cardiac arrest, % | 0.5 | 0.3 | <0.001 |
| In‐hospital outcomes, OR (95% CI) | |||
| In‐hospital mortality | 1.20 (1.14–1.27) | <0.001 | |
| Cardiac arrest | 1.67 (1.49–1.87) | <0.001 | |
Data are presented as mean±SD or number (percentage) or percentage. CI indicates confidence interval; OR, odds ratio.
Interquartile range (IQR) is from Q1 to Q3.
Discussion
Our study highlights numerous key trends in the overall sarcoidosis population. By exploring the largest national hospitalization database available, we found that sarcoidosis hospitalizations are more frequent in urban as compared with rural hospitals, blacks as compared with other races, and in Southern United States as compared with other regions. We observed a ≈26% increase in hospitalizations of sarcoidosis from 2005 to 2014. Notwithstanding the increasing hospitalizations, we observed a ≈26% reduction in the rates of in‐hospital mortality in sarcoidosis over the same time period. We found that heart failure and arrhythmias were most prevalent cardiovascular manifestations in sarcoidosis hospitalizations. Furthermore, we observed a steady rate of implantation of ICDs, increasing trends of PPM implantation, and decreasing trends of implantation of CRT among sarcoidosis hospitalizations. Lastly, we observed evidence of racial disparities, with blacks having higher odds of in‐hospital cardiac arrest and mortality than whites in a propensity‐matched cohort derived from the study cohort.
We noted that sarcoidosis hospitalizations are more prevalent in blacks. It follows that the geographical variation may be attributable to the higher concentrations of blacks in the Southern United States.24, 25 The rural‐urban hospital variations of sarcoidosis observations may be somewhat expected given that urban hospitals are more likely to have an advanced referral network of primary and secondary care centers. They may also have more diagnostic resources (eg, magnetic resonance imaging and positron emission tomography) for the early detection of sarcoidosis.
There are several possible reasons for the hospitalization statistics of sarcoidosis in the past decade. The publication of 2 sets of formalized guidelines10, 11 during the study period (2005–2014) offered clinicians a more organized and algorithmic method for the diagnostic workup of sarcoidosis, especially with cardiovascular involvement. The availability of these guidelines has also been complemented with rapid and landmark advances in myocardial imaging modalities. The development of the guidelines and imaging techniques has undoubtedly served to enhance the early detection of sarcoidosis, thus increasing the incidence of this disease.10, 11, 26, 27
There are multiple mechanistic explanations for the mortality trends and statistics seen in our investigation. It is well recognized that age is perhaps the most significant role player in the development of clinical cardiovascular disease.28, 29 Thus, it follows with sarcoidosis. Moreover, the decreasing trend (from 2005 to 2014) of in‐hospital mortality in sarcoidosis can partly be explained by the overall increasing survival from cardiovascular diseases especially from cardiac arrest. This is probably attributable to a combination of enhanced public awareness of cardiac arrest and sudden cardiac death, multiple important process‐ and systems‐based advances in cardiac arrest care in health care, and the publication of at least 3 sets of targeted consensus guidelines addressing cardiopulmonary resuscitation and post‐arrest care.30, 31, 32 Hence, patients with longstanding sarcoidosis are now living longer, and potentially experiencing more complications from sarcoidosis that require admission to hospital settings.
Demographic factors such as older age,29 male sex,33 and black race9 were associated with in‐hospital mortality. Our findings are in agreement with an informative study that examined sarcoidosis mortality from 1988 to 2007 at a population level using data from the National Center for Health Statistics.34 Comorbidities such as liver diseases, fluid and electrolyte imbalance, neurological disorders, coagulopathy, cancer, and renal failure were significant factors associated with in‐hospital morality. Unexpectedly, we observed that hypertension, diabetes mellitus, and anemia were associated with reduced risk of in‐hospital mortality. We believe that these observations are likely attributable to coding errors in the reporting of chronic comorbidities, and have been inadvertently seen as protective in previous NIS‐based investigations.35, 36
Our findings also add to the existing literature on sarcoidosis. Gerke et al19 reported a 2‐fold increase in hospitalizations among sarcoidosis using the NIS from 1998 to 2008. By quantifying prevalence of cardiovascular manifestations in sarcoidosis, we added more precision on clinical reasoning of increasing sarcoidosis hospitalizations. Our investigation helps to reiterate some of the prior findings in the field and estimate the prevalence of these conditions in the contemporary era.20
Our investigation also addresses important issues relating to racial disparities in sarcoidosis. Mirsaeidi et al9 previously reported that blacks have a mortality rate that is 12 times higher than whites with sarcoidosis in their age‐adjusted model. Their investigation used the Centers for Disease Control and Prevention database with evaluating death certificate (the immediate cause of death) in patients with sarcoidosis.9 We expand on their work by using the NIS with propensity matching on a number of demographic and clinical variables to help further define the increased risk of mortality in blacks with sarcoidosis. Mirsaiedi et al also reported that incident cardiac arrest occurred at a higher rate in blacks compared with whites.9 This is consistent with our study findings in a much larger sample, making it more generalizable.
The results of our investigation have important implications for clinical practice and public health. On the most basic level, we hope to inform clinicians that the co‐occurring of cardiovascular manifestations in sarcoidosis are devastating and could be a leading cause of death. Our investigation also highlights that aggressive primary and secondary prevention of comorbid cardiovascular and pulmonary conditions is critical for patients with sarcoidosis. Our investigation also serves as a stark reminder of the need to aggressively pursue these preventive measures to ameliorate these longstanding cardiovascular racial disparities. We hope that our investigation offers an impetus for clinical groups to achieve the aforementioned prevention through greater clinical collaboration by forming specialist sarcoidosis with cardiovascular manifestations clinics and/or specialist centers. Finally, our data again emphasize that clinicians and investigators alike have an important responsibility to proactively investigate this condition. Consequently, we hope that the need and motivation for further observational and randomized controlled trial data are renewed through our investigation, consistent with the Heart Rhythm Society's visions for the future.10
Limitations
We recognize that our analysis also has limitations. Retrospective studies have well‐known limitations. However, the use of a retrospective analysis in this investigation affords us the opportunity to access a large group of hospitalizations with a disease that has relatively low incidence and prevalence rates. Large administrative databases such as the NIS are lacking readmission status and cause of death and are prone to coding errors. There can be misinterpretation of procedure volumes and underreporting of comorbid conditions.23, 35 We have identified prevalence of cardiovascular manifestations in sarcoidosis by presence of concurrent diagnosis codes. Although we excluded all hospitalizations with old or present myocardial infarction, coronary artery disease, and ischemic cardiomyopathy, we cannot validate that the cardiovascular manifestations were caused by cardiac sarcoidosis in the absence of histologic evidence. Also, the ICD‐9‐CM procedural code 37.94 is used to describe both the implantation and replacement of ICDs. The ICD‐9‐CM procedural code 00.51 is used for implantation and replacement of CRT. Similarly, ICD‐9‐CM procedural code 37.7 and 37.8 are used for implantation and replacement of PPM. We may not know whether the admission was for an ICD or CRT or PPM implantation or replacement. Elective implantation of an ICD, CRT, or PPM may not have been captured completely in our investigation.
Conclusions
This NIS‐based retrospective investigation shows that hospitalizations of sarcoidosis have increased considerably over the past decade with declining in‐hospital mortality. Furthermore, our study summarizes the prevalence of cardiovascular manifestations, rate of device placement, and factors associated with in‐hospital mortality in sarcoidosis hospitalizations. Subgroup analyses in a propensity‐matched cohort show that blacks had a higher incidence of in‐hospital mortality and cardiac arrest compared with whites. Aggressive screening of patients with sarcoidosis for cardiovascular manifestations should be considered for prevention of cardiac arrest in sarcoidosis.
Sources of Funding
This work was supported in part by the Walter B. Frommeyer, Junior Fellowship in Investigative Medicine, which was awarded to Dr Arora by the University of Alabama at Birmingham. Dr Patel is supported by National Institutes of Health grant number 1T32HL129948‐01A1. Dr Bajaj is supported by National Institutes of Health grant number 5T32HL094301‐07.
Disclosures
None.
Supporting information
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Used to Exclude Sarcoidosis Hospitalizations with Ischemic Heart Diseases
Table S2. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Included to Identify Cardiovascular Manifestations in Sarcoidosis
(J Am Heart Assoc. 2018;7:e007844 DOI: 10.1161/JAHA.117.007844.)29358190
References
- 1. Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28:36–52. [DOI] [PubMed] [Google Scholar]
- 2. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 3. Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Archiv Med Sci. 2011;7:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saidha S, Sotirchos ES, Eckstein C. Etiology of sarcoidosis: does infection play a role? Yale J Biol Med. 2012;85:133–141. [PMC free article] [PubMed] [Google Scholar]
- 5. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 6. Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–172. [PubMed] [Google Scholar]
- 7. Kim JS, Judson MA, Donnino R, Gold M, Cooper LT Jr, Prystowsky EN, Prystowsky S. Cardiac sarcoidosis. Am Heart J. 2009;157:9–21. [DOI] [PubMed] [Google Scholar]
- 8. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 9. Mirsaeidi M, Machado RF, Schraufnagel D, Sweiss NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 11. Soejima K, Yada H. The work‐up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–583. [DOI] [PubMed] [Google Scholar]
- 12. Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 13. HCUP National (Nationwide) Inpatient Sample (NIS) . Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on April 13, 2017. [Google Scholar]
- 14. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 15. Whalen D, Houchens R, Elixhauser A. 2004 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Method Series Report # 2007‐2003. U.S. Agency for Healthcare Research and Quality. Available at: http://www.hcup-us.ahrq.gov/reports/methods/methods_topic.jsp. Accessed December 2, 2007.
- 16. Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project (HCUP). Available at: https://www.ahrq.gov/research/data/hcup/index.html. Accessed on May 10, 2017. [PubMed]
- 17. Overview of the National (Nationwide) Inpatient Sample (NIS). Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed on January 13, 2017.
- 18. Erdal BS, Clymer BD, Yildiz VO, Julian MW, Crouser ED. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir Med. 2012;106:893–899. [DOI] [PubMed] [Google Scholar]
- 19. Gerke AK, Yang M, Tang F, Cavanaugh JE, Polgreen PM. Increased hospitalizations among sarcoidosis patients from 1998 to 2008: a population‐based cohort study. BMC Pulm Med. 2012;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. [DOI] [PubMed] [Google Scholar]
- 21. Froehlich W, Bogun FM, Crawford TC. Cardiac sarcoidosis. Circulation. 2015;132:e137–e138. [DOI] [PubMed] [Google Scholar]
- 22. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, Sweiss N, Crouser E, Morgenthau AS, Lower EE, Azuma A, Ishihara M, Morimoto S, Tetsuo Yamaguchi T, Shijubo N, Grutters JC, Rosenbach M, Li HP, Rottoli P, Inoue Y, Prasse A, Baughman RP; Organ Assessment Instrument Investigators TW . The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 23. Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, Rabinstein AA. Post‐cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. [DOI] [PubMed] [Google Scholar]
- 24. United States Census Bureau . Census Shows Black Population has Highest Concentration in the South. 2010. Available at: https://www.census.gov/newsroom/releases/archives/2010_census/cb11-cn185.html. Accessed on May 16, 2017.
- 25. Rybicki BA, Major M, Popovich J Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5‐year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. [DOI] [PubMed] [Google Scholar]
- 26. Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, Endo K, Yokoyama T, Suzuki T, Kurabayashi M. Usefulness of fasting 18F‐FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 27. Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 29. Zhou Y, Lower EE, Li HP, Costea A, Attari M, Baughman RP. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest. 2017;151:139–148. [DOI] [PubMed] [Google Scholar]
- 30. ECC Committee, Subcommittees and Task Forces of the American Heart Association . 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(24 suppl):IV1–IV203. [DOI] [PubMed] [Google Scholar]
- 31. Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O'Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: executive summary. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S640–S656. [DOI] [PubMed] [Google Scholar]
- 32. Neumar RW, Shuster M, Callaway CW, Gent LM, Atkins DL, Bhanji F, Brooks SC, deCaen AR , Donnino MW, Ferrer JME, Kleinman ME, Kronick SL, Lavonas EJ, Link MS, Mancini ME, Morrison LJ, O'Connor RE, Samson RA, Schexnayder SM, Singletary EM, Sinz EH, Travers AH, Wyckoff MH, Hazinski MF. Part 1: Executive Summary. 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S315–S367. [DOI] [PubMed] [Google Scholar]
- 33. Martusewicz‐Boros MM, Boros PW, Wiatr E, Kempisty A, Piotrowska‐Kownacka D, Roszkowski‐Sliz K. Cardiac sarcoidosis: is it more common in men? Lung. 2016;194:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swigris JJ, Olson AL, Huie TJ, Fernandez‐Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis‐related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jencks SF, Williams DK, Kay TL. Assessing hospital‐associated deaths from discharge data. The role of length of stay and comorbidities. JAMA. 1988;260:2240–2246. [PubMed] [Google Scholar]
- 36. Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among US hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol. 2015;13:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Used to Exclude Sarcoidosis Hospitalizations with Ischemic Heart Diseases
Table S2. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Included to Identify Cardiovascular Manifestations in Sarcoidosis


