Abstract
Background
Aortic stenosis is the most common valvular disease and has a dismal prognosis without surgical treatment. The aim of this meta‐analysis was to quantitatively assess the comparative effectiveness of the Perceval (LivaNova) valve versus conventional aortic bioprostheses.
Methods and Results
A total of 6 comparative studies were identified, including 639 and 760 patients who underwent, respectively, aortic valve replacement with the Perceval sutureless valve (P group) and with a conventional bioprosthesis (C group). Aortic cross‐clamping and cardiopulmonary bypass duration were significantly lower in the P group. No difference in postoperative mortality was shown for the P and C groups (2.8% versus 2.7%, respectively; odds ratio [OR]: 0.99 [95% confidence interval (CI), 0.52–1.88]; P=0.98). Incidence of postoperative renal failure was lower in the P group compared with the C group (2.7% versus 5.5%; OR: 0.45 [95% CI, 0.25–0.80]; P=0.007). Incidence of stroke (2.3% versus 1.7%; OR: 1.34 [95% CI, 0.56–3.21]; P=0.51) and paravalvular leak (3.1% versus 1.6%; OR: 2.52 [95% CI, 0.60–1.06]; P=0.21) was similar, whereas P group patients received fewer blood transfusions than C group patients (1.16±1.2 versus 2.13±2.2; mean difference: 0.99 [95% CI, −1.22 to −0.75]; P=0.001). The incidence of pacemaker implantation was higher in the P than the C group (7.9% versus 3.1%; OR: 2.45 [95% CI, 1.44–4.17]; P=0.001), whereas hemodynamic Perceval performance was better (transvalvular gradient 23.42±1.73 versus 22.8±1.86; mean difference: 0.90 [95% CI, 0.62–1.18]; P=0.001), even during follow‐up (10.98±5.7 versus 13.06±6.2; mean difference: −2.08 [95% CI, −3.96 to −0.21]; P=0.030). We found no difference in 1‐year mortality.
Conclusions
The Perceval bioprosthesis improves the postoperative course compared with conventional bioprostheses and is an option for high‐risk patients.
Keywords: aortic stenosis, prosthetic heart valve, Sutureless bioprothesis, Perceval valve
Subject Categories: Valvular Heart Disease
Clinical Perspective
What Is New?
The implantation of a Perceval (LivaNova) sutureless aortic valve is associated with a better postoperative course, as demonstrated by the lower incidence of postoperative renal failure and blood transfusions.
What Are the Clinical Implications?
The Perceval valve is a supportive option for high‐risk patients and for those at risk of patient–prosthesis mismatch.
Introduction
Aortic valve stenosis is the most common valve disease and results in poor outcomes without surgical treatment for severe and symptomatic cases.1 In these patients, aortic valve replacement is the treatment of choice. New implantation techniques have been developed to minimize the surgical risk in older patients with multiple comorbidities.2
Sutureless technology has shown promising results in terms of mortality, morbidity, and hemodynamic performance. The Perceval S (LivaNova) sutureless aortic valve is a collapsible, stent‐mounted, aortic valve bioprosthesis that can be placed in a sutureless fashion using a conventional surgical technique with cardiopulmonary bypass (CPB), aortic cross‐clamping (ACC), and aortotomy, allowing complete removal of the diseased native valve.
Several studies have shown that sutureless valves decrease the CPB and ACC times, facilitating minimally invasive cardiac surgery,3 but few studies compared this technology with conventional stented valves. To our knowledge, no randomized controlled trials have directly compared patients treated with traditional and Perceval S valve bioprostheses.
The aim of this meta‐analysis was to assess the comparative effectiveness of Perceval and conventional bioprostheses, evaluating the current, best available evidence from recent observational studies.
Methods
Data Sources and Search Strategy
We performed a systematic review and meta‐analysis in accordance with the standards set forth by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement.
We searched PubMed, Embase, and the Cochrane Collaboration databases using the following keywords: sutureless, AND aortic valve, bioprosthesis AND aortic valve, rapid deployment AND aortic valve, aortic valve AND surgery, OR operation OR replacement. In addition, we hand‐searched references of retrieved articles and used related article features in PubMed to identify studies not captured by our primary search strategy. The search was limited to trials involving human subjects, without language restriction. The last search was performed December 31, 2016.
We included studies comparing the Perceval aortic valve with conventional bioprostheses. Included studies contained data on 30‐day all‐cause mortality and the outcomes described in the Valve Academic Research Consortium standardized end point definition.4, 5
Once full articles were retrieved, studies were further excluded if there was an overlap of patients with another study within the same analysis (in such cases, the larger sample size of the 2 studies was selected). Although some patients could have been included in both the controlled and uncontrolled study analyses, they were included only once in any given analysis. Consequently, there was no overlap among patients included in our meta‐analyses.
Two reviewers (M.M. and A.M.) evaluated each article separately.6 No disagreements occurred during the study‐selection process. A possible disagreement would have been resolved by discussion with a third author (M.G.).
The study quality was examined using the method recommended by a Cochrane Collaboration tool for assessing risk of bias in the included studies. We extracted data pertaining to baseline characteristics of study participants, trial inclusion and exclusion criteria, postoperative complications, maximal length of follow‐up, and mortality.
The primary end point was 30‐day all‐cause mortality. Secondary end points were (1) CPB time, (2) ACC time, (3) postoperative acute kidney injury (AKI), (4) postoperative stroke, (5) postoperative transvalvular mean gradient, (6) postoperative paravalvular leak, (7) postoperative pacemaker implantation, and (8) 1‐year mortality.
The present meta‐analysis was conducted to analyze any difference in mortality between aortic valve replacement with the Perceval S valve (P group) and a conventional bioprosthesis (C group).
The results from all relevant studies were combined to estimate the odds ratios (ORs) and associated 95% confidence intervals (CIs) for dichotomous outcomes. With respect to the continuous outcomes, weighted mean differences and 95% CIs were estimated as the effect results. Heterogeneity was tested using the I2 statistic, and studies were classified as having low (I2: 25–49%), moderate (I2: 50–74%), or high (I2 ≥75%) heterogeneity. An I2 ≥50% indicates significant heterogeneity. In such cases, a random‐effect model was used, whereas an inverse variance method was used with the fixed‐effect model when the outcome had no significant heterogeneity (I2 <50%). Whenever heterogeneity was present, we performed sensitivity analyses to investigate the influence of a single study by excluding 1 study in each turn. Publication bias was assessed by funnel plot using mortality as an end point. P<0.05 was considered statistically significant in this meta‐analysis.
To evaluate the effect of preoperative parameters on the incidence of postoperative AKI, we performed mixed‐effects (unrestricted maximum likelihood) metaregression analyses.
The metaregression graph depicts the percentages of Perceval valves in the 2 groups, plotted as a logarithmic OR on the y‐axis and as a percentage of patients undergoing Perceval implantation. The metaregression coefficient (slope of the metaregression line) shows the estimated increase in logarithmic OR per unit increase in the covariate.
Because a logarithmic OR >0 corresponds to an OR >1 and a logarithmic OR <0 corresponds to an OR <1, a negative coefficient would indicate that as a given factor (percentages of patients in the groups undergoing Perceval implantation) increases, the OR decreases; that is, Perceval is more beneficial in reducing the outcome of interest.
All analyses were conducted using Review Manager 5.1 software (Nordic Cochrane Centre) and Open Meta Analyst.6
Kaplan–Meier curves were digitized using a dedicated software (Plot Digitizer; Free Software Foundation Europe). With this tool, the axes were defined, then the curve was read point by point. The Kaplan–Meier data reconstruction was used to derive individual patient data. We measured the recurrence rate, which is a type of time‐to‐event data, with a hazard ratio and 95% CI. Referring to Tierney et al,7 multiple forms of published hazard ratio data, which may present as a number of recurrence, as a Kaplan–Meier curve, or as another form, were processed as observed–expected events research (shown as O‐E) and variance (shown as V). We analyzed the transformed data in Revman 5.2 (Cochrane Collaboration). We used the exponential [(O‐E)/V] method to calculate hazard ratios and to implement a time‐to‐event data analysis with a fixed‐effect model.
Results
The study selection process is shown in Figure 1. After exclusion of duplicate or irrelevant references, 114 potentially relevant articles were retrieved. After detailed evaluation, 6 comparative studies8, 9, 10, 11, 12, 13 met the inclusion criteria and were considered in the present meta‐analysis. A total of 1399 patients were included, of whom 639 underwent aortic valve replacement with the Perceval sutureless valve (P group) and 760 received a conventional bioprosthesis (C group). The list of included studies and the quality assessment for each study is presented in Table S1 and Figure S1.
Figure 1.
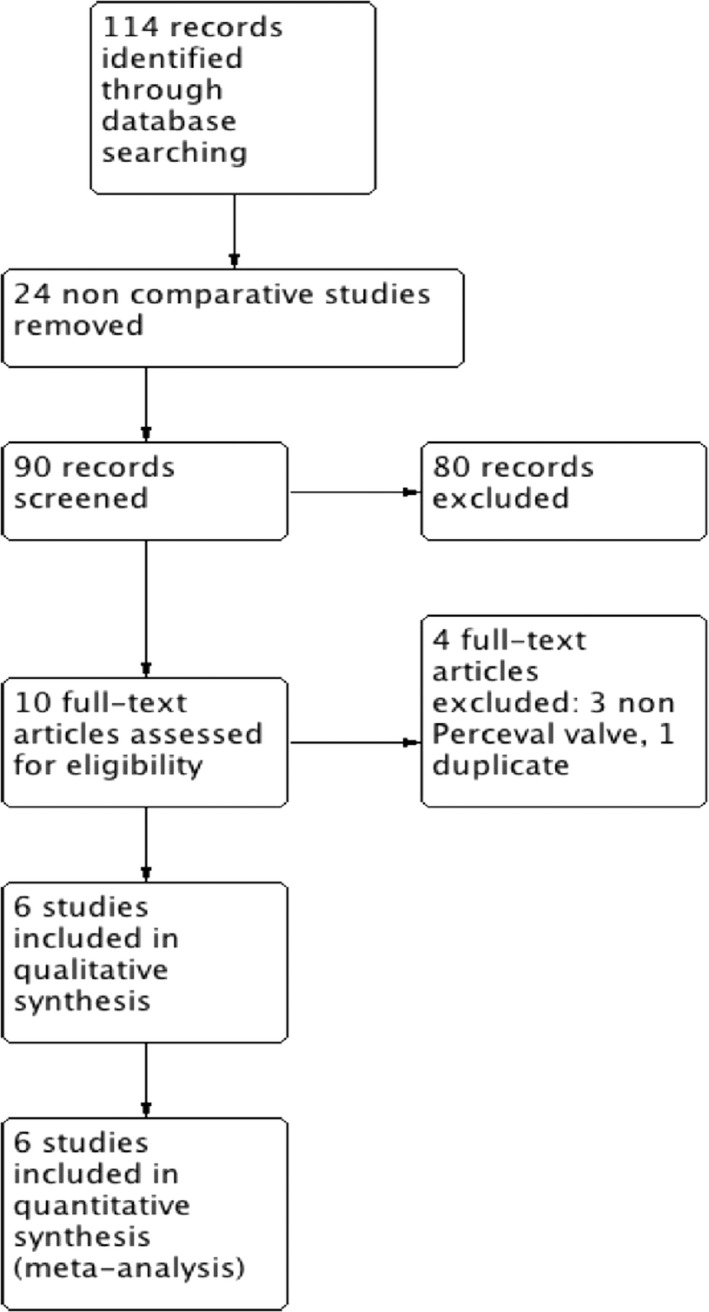
Study selection process.
Preoperative characteristics are shown in Table 1. The preoperative risk, calculated using the logistic EuroSCORE I, was comparable between the 2 groups. The percentage of patients with diabetes mellitus was significantly higher in the P group. No other preoperative differences were observed between groups.
Table 1.
Preoperative Characteristics of the Patients Included in the Meta‐Analysis
| P Group | C Group | OR/WMD† (95% CI) | P Overall Effect | |
|---|---|---|---|---|
| Logistic EuroSCORE | 12.5±6.9 | 11.3±6.2 | 1.24 (−0.48 to 2.87) | 0.16 |
| Age, y | 77.7±4.6 | 75.9±5.9 | 1.45 (−0.36 to 3.27) | 0.12 |
| Female, % | 56 | 56 | 1.05 (0.83–1.33) | 0.67 |
| Diabetes mellitus, % | 27.1 | 20.9 | 1.33 (1.01–1.75) | 0.04* |
| Chronic renal failure, % | 14.3 | 14.1 | 1.02 (0.72–1.43) | 0.93 |
| COPD, % | 15.2 | 14.1 | 0.93 (0.67–1.51) | 0.68 |
| CAD, % | 9.1 | 9.1 | 1.01 (0.66–1.51) | 0.93 |
| Cerebrovascular accidents, % | 10.2 | 8.6 | 1.21 (0.81–1.82) | 0.35 |
| NYHA class 3/4, % | 55.5 | 54.7 | 1.12 (0.82–1.52) | 0.47 |
| Hypertension, % | 73.7 | 69.6 | 1.20 (0.88–1.63) | 0.24 |
| LVEF | 57.2±8 | 56.8±7.6 | 0.11 (−2.02 to 2.24) | 0.92 |
| Body mass index | 27.7±4.9 | 26.9±4.4 | −0.08 (−1.08 to 0.92) | 0.88 |
CAD indicates coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; OR, odds ratio; WMD, weighted mean difference.
*Indicates statistically significant.
†Data as percentage are odds ratios, mean values are weighted mean differences.
Postoperative Outcomes
All postoperative data are reported in Table 2. Meta‐analysis of the data showed that the pooled postoperative CPB (67.4±20.2 versus 93.2±25.8 minutes for the P and C groups, respectively; P=0.001) and ACC times (39.6±14.2 versus 66±17.4 minutes for the P and C group, respectively; P=0.001,) were significantly lower in the P group.
Table 2.
Postoperative Results
| P Group | C Group | OR/WMD† (95% CI) | P Overall Effect | |
|---|---|---|---|---|
| Minimally invasive, % | 96.53 | 43.86 | 301 (112–805) | 0.001* |
| 30‐d mortality, % | 2.8 | 2.7 | 0.99 (0.52–1.88) | 0.980 |
| Renal failure, % | 2.7 | 5.5 | 0.45 (0.25–0.80) | 0.007* |
| Respiratory failure, % | 5.4 | 6.8 | 0.83 (0.45–1.52) | 0.540 |
| Stroke, % | 2.3 | 1.7 | 1.34 (0.56–3.21) | 0.510 |
| Pacemaker, % | 7.9 | 3.1 | 2.45 (1.44–4.17) | 0.001* |
| Paravalvular leak, % | 3.1 | 1.6 | 2.52 (0.60–1.06) | 0.210 |
| Blood transfusions | 1.16+1.2 | 2.13+2.2 | 0.99 (−1.22 to −0.75) | 0.001* |
| CPB time, min | 67.4+20.2 | 93.2+25.8 | −25.28 (−32.0 to −18.4) | 0.001* |
| ACC time, min | 39.6+14.2 | 66+17.4 | −26.26 (−30 to −22.48) | 0.001* |
| Mechanical ventilation time, h | 7.6±2.3 | 11±7.5 | −1.05 (−1.43 to 0.67) | 0.001* |
| ICU stay, d | 1.7±1.5 | 1.9±2.2 | 0.2 (0.66–0.25) | 0.38 |
| Transfused RBCs, U | 3.5±3.8 | 6.4±6.7 | −0.99 (−1.22 to −0.75 | 0.001* |
| Prosthesis size, mm | 23.42+1.73 | 22.8+1.86 | 0.90 (0.62–1.18) | 0.001* |
| Mean gradient, mm Hg | 10.98+5.7 | 13.06+6.2 | −2.08 (−3.96 to −0.21) | 0.030* |
ACC indicates aortic cross‐clamping; CI, confidence interval; CPB, cardiopulmonary bypass; ICU, intensive care unit; OR, odds ratio; RBCs, red blood cells; WMD, weighted mean difference.
*Indicates statistically significant.
†Data as percentage are odds ratios, mean values are weighted mean differences.
Five studies8, 9, 10, 11, 12 reported the incidence of postoperative AKI, and meta‐analysis of the data showed that the pooled postoperative AKI in the P group was significantly lower compared with the C group (Figure 2A). The patients in the P group had less need for blood transfusions.
Figure 2.
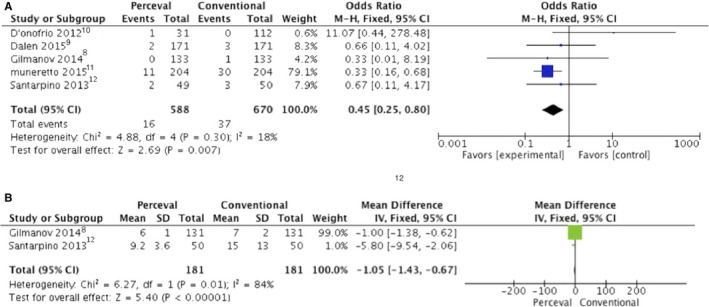
Forrest plot of the odds ratio or mean difference of (A) acute kidney injury and (B) length of mechanical ventilation after Perceval valve vs conventional bioprosthesis implantation. CI indicates confidence interval; IV, inverse variance; M‐H, Mantel–Haenszel.
The incidence of postoperative respiratory failure was similar in both groups, whereas the duration of mechanical ventilation was significantly less in the P group (Figure 2B).
The incidence of postoperative pacemaker implantation for atrioventricular block was significantly higher in the P group (Figure 3A), whereas we found no difference in the incidence of postoperative stroke (Table 2) and paravalvular leak (Figure 3B) between groups.
Figure 3.
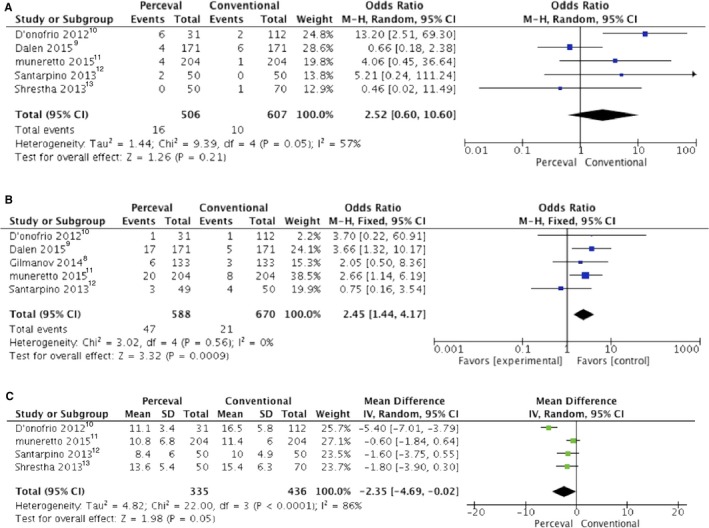
Forrest plot of the odds ratio or mean difference of (A) postoperative pacemaker implantation, (B) paravalvular leak, and (C) mean transvalvular gradient after Perceval valve vs conventional bioprosthesis implantation. CI indicates confidence interval; IV, inverse variance; M‐H, Mantel–Haenszel.
Prosthesis size was significantly larger and the postoperative mean transvalvular gradient was significantly lower in the P group (Figure 3C).
Patients who underwent Perceval valve implantation were more likely to receive a minimally invasive cardiac surgery approach. There was no difference in postoperative mortality between groups (Figure 4A).
Figure 4.
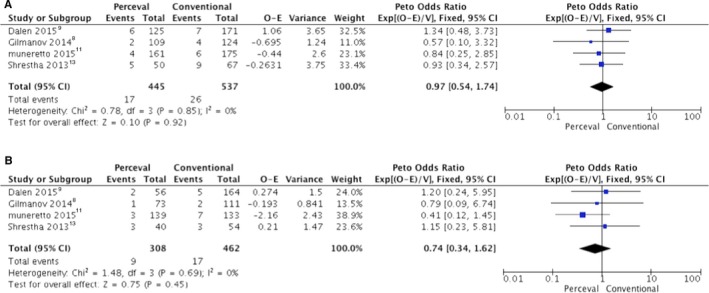
Forrest plot of the Peto odds ratio of (A) postoperative mortality, of (B) 1‐year survival. CI indicates confidence interval; O‐E, observed–expected events; V, variance; Exp, exponential
Follow‐up Data
All follow‐up data at 1 year are reported in Figure 4B. Mean follow‐up of the patients in the P and C groups was 18.25+10.5 and 38.3+13.1 months, respectively. One‐year survival was 95.5+1.6% in the P group and 95.5+1.1% in the C group, without significant differences between groups.
The mean transvalvular gradient remained lower in sutureless valves, even during follow‐up, without reaching statistical significance (12.3±6.2 versus 13.2±7.1 mm Hg; mean difference: −1.14; [95% CI, −2.79 to 0.50; P=0.18).
Inspection of the funnel plot (Figure S2) did not show significant asymmetry for all‐cause mortality. Trim‐and‐fill analysis indicated that no studies were missing. These results suggest that publication bias was not a significantly influencing factor.
Discussion
This meta‐analysis showed that the Perceval sutureless bioprosthesis is safe. The use of the Perceval valve reduced CPB and ACC times and the amount of blood transfused and showed lower incidence of postoperative AKI. Moreover, the use of the Perceval valve allowed for implantation of larger diameter prostheses, resulting in lower transvalvular gradient, and there was no difference in the frequency of postoperative strokes and paravalvular leaks between groups.
The incidence of postoperative pacemaker implantation was significantly higher in the P group. The incidence of permanent pacemaker implantation after aortic valve replacement with conventional bioprostheses is reported to be highly variable, ranging between 3% and 8.5%.14, 15
Many preoperative and intraoperative factors contribute to the final result. Preoperative right or left bundle‐branch block and first‐degree atrioventricular block were identified as important risk factors for the development of postoperative conduction disturbances.17 Moreover, the conduction tissue may be directly damaged by extensive annular debridement.17
Vogt et al8 reported a high incidence (10.5%) of postoperative pacemaker implantation after Perceval valve implantation and identified only preoperative right bundle‐branch block as a risk factor but showed high variability in the rates of postoperative pacemaker implantation for the 2 surgeons who performed the operations. This factor suggests the key role of the surgeon's technique and experience in influencing this complication.
Moreover, the large intra‐annular sealing coil of the Perceval bioprosthesis may be the cause of atrioventricular conduction disorders because the prosthesis frame delivers an outward force that affects the aortic annulus during balloon dilatation.18 To overcome this issue, Yanagawa et al described a simple modification of the surgical technique, placing the guiding sutures at the nadir of each cusp and not 2 or 3 mm below.16 This technique allows for targeting postoperative permanent pacemaker implantation in their cohort. The positioning of the Perceval valve some millimeters lower than the aortic annulus may cause the compression of the conduction system by the nitinol stent, causing atrioventricular block.19 None of the studies included in this meta‐analysis reported the presence of preoperative conduction disturbances or quantified preoperative aortic annular calcification; therefore, we could not know whether patients in the P group had a higher risk of pacemaker implantation.
Despite significant technical improvements over time, CPB remains a crucial factor in determining postoperative morbidity and mortality. Contact of blood components with the artificial surface of the bypass circuit causes a systemic inflammatory response syndrome associated with CPB,17 resulting in diffuse microcirculatory damage leading to postoperative organ dysfunction. Many factors, including CPB duration, have been associated with AKI after cardiac surgery. The impact of AKI on poor outcomes after cardiac surgery is remarkable, especially if it leads to continuous renal replacement therapy.20 Several studies associated longer CPB with postoperative AKI.21, 22, 23
Based on these premises, the lower frequency of postoperative AKI in the P group might be explained by a shorter duration of CPB with Perceval valve implantation. However, we have to consider that the study by Muneretto et al11 could have influenced the incidence rate for postoperative AKI: This study is the largest included in the meta‐analysis, and the authors reported very high incidence of postoperative AKI. This fact could be explained by the large number of patients at increased risk for postoperative complications, as explained by the high mean EuroSCORE.
In the Perceval group, we reported a lower duration of CPB. CPB is associated with impaired hemostatic function in the mechanisms of hemodilution, activation,24, 25 and consumption.26 Moreover, CPB duration is significantly associated with postoperative nonsurgical bleeding27 and increased transfusion of blood products28—well‐known risk factors for postoperative morbidity and mortality in cardiac surgery.29 The lower CPB time may at least partly explain the lower amount of postoperative transfusion of blood products.
Our meta‐analysis showed that implantation of a Perceval valve increases the likelihood that the surgery will be performed with a minimally invasive approach. In fact, our meta‐analysis showed that the percentage of patients undergoing minimally invasive surgery was significantly higher in the P group, but this finding is not straightforward. Indeed, the variability of minimally invasive procedures within groups is very high, between 0% to 100%, suggesting that the choice of a minimally invasive approach is influenced by the habits and experience of the surgeon.
It is still under discussion whether the minimally invasive aortic valve replacement surgery could significantly influence strong outcomes. Two large meta‐analyses30, 31 showed shorter intensive care unit and hospital stays for patients who underwent minimally invasive aortic valve replacement compared with conventional surgery, but both studies reported longer CPB and ACC times for the minimally invasive approach.
Moreover, all studies comparing the minimally invasive and conventional approaches reported a significant increase in CPB times when conventional valves were implanted.32 Consequently, it seems reasonable to argue that the increase in CPB time could mask, in some way, the potential advantages of minimally invasive cardiac surgery, especially in high‐risk patients who would benefit from a minor surgical insult.
Dalén et al9 and Miceli et al33 showed that the use of the Perceval valve in minimally invasive aortic valve replacement is associated with a significant decrease in CPB time compared with conventional valves. Therefore, the ease of implantation and the consequent reduction of the CPB time could make the Perceval valve the first choice for high‐risk patients undergoing minimally invasive cardiac surgery.
This meta‐analysis showed that the use of Perceval valve allowed the implantation of valves of greater diameter with better hemodynamic performance (as indicated by the lower transvalvular gradient). The conventional valves have a sewing ring, and, for any given valve size, at least some part of the external valve area is taken up by this sewing ring. This may lead to patient–prosthesis mismatch, particularly in patients with small aortic roots. Incidence of prosthesis–patient mismatch ranges between 2% and 11% after aortic valve replacement and leads to lack of regression of left ventricular hypertrophy,34 persistent symptoms, and reduced survival.33 Implantation of the Perceval valve may a viable alternative for patients who need conventional aortic root enlargement to treat a small aortic annulus and to avoid patient–prosthesis mismatch, especially in older patients, who typically benefit from the quick implantation process.35 Sutureless valves have been proposed recently as an ideal solution for this subset of patients13: Given the absence of a sewing ring, these valves are almost “stentless,” with a greater valve effective orifice area for any given size.
Our meta‐analysis did not show any mortality benefit for patients who underwent implantation of the Perceval valve versus conventional bioprostheses for aortic stenosis. However, the reduction of blood transfusions, the lower incidence of postoperative AKI, and the better hemodynamic profile of the Perceval valve make it an option worth considering for patients at high operative risk and at risk of patient–prosthesis mismatch. Randomized controlled trials are warranted to expand reliable evidence in this field, ensuring appropriate selection of the aortic prosthesis on the basis of each patient's characteristics.
Limitations of this meta‐analysis merit careful consideration. There were few trials, and the absence of randomized controlled trials comparing sutureless and conventional bioprostheses represents the main limitation of our meta‐analysis. Three studies used a propensity score matching technique. Despite the absence of overt biases, we cannot consider these studies equivalent to randomized controlled trials; therefore, the results should be interpreted carefully when they are included in systematic reviews and meta‐analysis. Particular concerns arise with respect to differences between patients in different intervention groups (selection bias) and studies that did not explicitly report a protocol (reporting bias). Unlike randomized trials, it would usually be appropriate to analyze adjusted, rather than unadjusted, effect estimates (ie, analyses that attempt to control for confounding). To reduce the effect of treatment selection bias and potential confounding in observational studies, rigorous adjustment for significant differences in patients’ baseline characteristics should be conducted. Furthermore, adjusted estimates have to be pooled in a meta‐analysis that includes observational studies. In the present meta‐analysis, we strictly selected and then included only adjusted ORs or hazard ratios for all‐cause mortality, using appropriate statistical methods from observational studies. Our results may be influenced by a publication bias favoring sutureless valve. This risk was minimized through an exhaustive search of the available literature in our analysis. Because the statistical tests did not indicate publication bias, there is limited power to detect such bias, given the small number of studies examined.
Conclusions
The Perceval bioprostheis ensures a better postoperative course than coventional bioprostheses and deserves consideration in patients at high surgical risk. Future RCTs are required to enlight its possible role in reducing mortality.
Disclosures
Dr Glauber, Dr Miceli and Dr Ferrarini are consultants for educational activities for Sorin. The remaining authors have no disclosures to report.
Supporting information
Table S1. List of Included Studies
Figure S1. Risk of bias summary: Review authors’ judgments about each risk‐of‐bias item for the included study.
Figure S2. Funnel plot for the meta‐analysis of mortality with the Perceval valve compared with conventional prostheses.
(J Am Heart Assoc. 2018;7:e006091 DOI: 10.1161/JAHA.117.006091.)29453309
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W‐K, Stevenson WG, Yancy CW; American College of Cardiology, American College of Cardiology/American Heart Association, American Heart Association . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148:e1–e132. [DOI] [PubMed] [Google Scholar]
- 2. Aoyagi S, Fukunaga S, Arinaga K, Tomoeda H, Akasu K, Ueda T. Heart valve surgery in octogenarians: operative and long‐term results. Heart Vessels. 2010;25:522–528. [DOI] [PubMed] [Google Scholar]
- 3. Bakhtiary F, Schiemann M, Dzemali O, Wittlinger T, Doss M, Ackermann H, Moritz A, Kleine P. Stentless bioprostheses improve postoperative coronary flow more than stented prostheses after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2006;131:883–888. [DOI] [PubMed] [Google Scholar]
- 4. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration; 2011. Available at: http://handbook.cochrane.org. Accessed December 10, 2017. [Google Scholar]
- 5. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB; Valve Academic Research Consortium‐2 . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 6. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilmanov D, Miceli A, Ferrarini M, Farneti P, Murzi M, Solinas M, Glauber M. Aortic valve replacement through right anterior minithoracotomy: can sutureless technology improve clinical outcomes? Ann Thorac Surg. 2014;98:1585–1592. [DOI] [PubMed] [Google Scholar]
- 9. Dalén M, Biancari F, Rubino AS, Santarpino G, Glaser N, De Praetere H, Kasama K, Juvonen T, Deste W, Pollari F, Meuris B, Fischlein T, Mignosa C, Gatti G, Pappalardo A, Svenarud P, Sartipy U. Aortic valve replacement through full sternotomy with a stented bioprosthesis versus minimally invasive sternotomy with a sutureless bioprosthesis. Eur J Cardiothorac Surg. 2016;49:220–227. [DOI] [PubMed] [Google Scholar]
- 10. D'Onofrio A, Rizzoli G, Messina A, Alfieri O, Lorusso R, Salizzoni S, Glauber M, Di Bartolomeo R, Besola L, Rinaldi M, Troise G, Gerosa G. Conventional surgery, sutureless valves, and transapical aortic valve replacement: what is the best option for patients with aortic valve stenosis? A multicenter, propensity‐matched analysis J Thorac Cardiovasc Surg. 2013;146:1065–1070. [DOI] [PubMed] [Google Scholar]
- 11. Muneretto C, Bisleri G, Moggi A, Di Bacco L, Tespili M, Repossini A, Rambaldini M. Treating the patients in the “grey‐zone” with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg. 2015;20:90–95. [DOI] [PubMed] [Google Scholar]
- 12. Santarpino G, Pfeiffer S, Concistré G, Grossmann I, Hinzmann M, Fischlein T. The Perceval S aortic valve has the potential of shortening surgical time: does it also result in improved outcome? Ann Thorac Surg. 2013;96:77–81. [DOI] [PubMed] [Google Scholar]
- 13. Shrestha M, Maeding I, Höffler K, Koigeldiyev N, Marsch G, Siemeni T, Fleissner F, Haverich A. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg. 2013;17:778–782; discussion 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawkins S, Hobson AR, Kalra PR, Tang ATM, Monro JL, Dawkins KD. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann Thorac Surg. 2008;85:108–112. [DOI] [PubMed] [Google Scholar]
- 15. Lewis JW Jr, Webb CR, Pickard SD, Lehman J, Jacobsen G. The increased need for a permanent pacemaker after reoperative cardiac surgery. J Thorac Cardiovasc Surg. 1998;1:74–81. [DOI] [PubMed] [Google Scholar]
- 16. Yanagawa B, Cruz J, Boisvert L, Bonneau D. Surgical technique: acquired a simple modification to lower incidence of heart block with sutureless valve implantation. J Thorac Cardiovasc Surg. 2016;152:630–632. [DOI] [PubMed] [Google Scholar]
- 17. Fransen E, Maessen J, Dentener M, Senden N, Geskes G, Buurman W. Systemic inflammation present in patients undergoing CABG without extracorporeal circulation. Chest. 1998;5:1290–1295. [DOI] [PubMed] [Google Scholar]
- 18. Vogt F, Pfeiffer S, Dell'Aquila AM, Fischlein T, Santarpino G. Sutureless aortic valve replacement with Perceval bioprosthesis: are there predicting factors for postoperative pacemaker implantation? Interact Cardiovasc Thorac Surg. 2016;22:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miceli A, Lio A, Glauber M. Size, position, and timing: a mixture of success. J Thorac Cardiovasc Surg. 2016;152:633–634. [DOI] [PubMed] [Google Scholar]
- 20. Frost L, Pedersen RS, Lund O, Hansen OK, Hansen HE. Prognosis and risk factors in acute, dialysis‐requiring renal failure after open‐heart surgery. Scand J Thorac Cardiovasc Surg. 1991;25:161–166. [DOI] [PubMed] [Google Scholar]
- 21. Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. [DOI] [PubMed] [Google Scholar]
- 22. Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta‐analysis. J Cardiothorac Vasc Anesth. 2012;26:64–69. [DOI] [PubMed] [Google Scholar]
- 23. Rahmanian PB, Kwiecien G, Langebartels G, Madershahian N, Wittwer T, Wahlers T. Logistic risk model predicting postoperative renal failure requiring dialysis in cardiac surgery patients. Eur J Cardiothorac Surg. 2011;40:701–707. [DOI] [PubMed] [Google Scholar]
- 24. Musial J, Niewiarowski S, Hershock D, Morinelli TA, Colman RW, Edmunds LH. Loss of fibrinogen receptors from the platelet surface during simulated extracorporeal circulation. J Lab Clin Med. 1985;105:514–522. [PubMed] [Google Scholar]
- 25. Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–857. [PubMed] [Google Scholar]
- 26. Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci. 2010;47:197–212. [DOI] [PubMed] [Google Scholar]
- 27. Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:814–822. [DOI] [PubMed] [Google Scholar]
- 28. Despotis GJ, Joist JH. Anticoagulation and anticoagulation reversal with cardiac surgery involving cardiopulmonary bypass: an update. J Cardiothorac Vasc Anesth. 1999;13:18–29. [PubMed] [Google Scholar]
- 29. Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478–485. [DOI] [PubMed] [Google Scholar]
- 30. Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg. 2009;137:670–679. [DOI] [PubMed] [Google Scholar]
- 31. Murtuza B, Pepper JR, DeL Stanbridge R, Jones C, Rao C, Darzi A, Athanasiou T. Minimal access aortic valve replacement: is it worth it? Ann Thorac Surg. 2008;85:1121–1131. [DOI] [PubMed] [Google Scholar]
- 32. Glauber M, Ferrarini M, Miceli A. Minimally invasive aortic valve surgery: state of the art and future directions. Ann Cardiothorac Surg. 2015;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miceli A, Murzi M, Gilmanov D, Fugà R, Ferrarini M, Solinas M, Glauber M. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg. 2014;148:133–137. [DOI] [PubMed] [Google Scholar]
- 34. Mohty D, Dumesnil JG, Echahidi N, Mathieu P, Dagenais F, Voisine P, Pibarot P. Impact of prosthesis‐patient mismatch on long‐term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol. 2009;53:39–47. [DOI] [PubMed] [Google Scholar]
- 35. Phan K, Xie A, Di Eusanio M, Yan TD. A meta‐analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg. 2014;98:1499–1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of Included Studies
Figure S1. Risk of bias summary: Review authors’ judgments about each risk‐of‐bias item for the included study.
Figure S2. Funnel plot for the meta‐analysis of mortality with the Perceval valve compared with conventional prostheses.


