Abstract
Background
As heart failure (HF)‐associated morbidity and mortality continue to escalate, enhanced focus on prevention is increasingly important. “Malignant” left ventricular (LV) hypertrophy (LVH): LVH combined with an elevated cardiac biomarker reflecting either injury (high‐sensitivity cardiac troponin T), or strain (amino‐terminal pro‐B‐type natriuretic peptide) has predicted accelerated progression to HF. We sought to determine whether malignant LVH identified community‐dwelling adults initially free of cardiovascular disease at high risk of asymptomatic decline in LV ejection fraction or a clinical cardiovascular event.
Methods and Results
A total of 4985 of 6814 individuals without prevalent cardiovascular disease underwent baseline cardiac magnetic resonance for LVH in combination with measurement of plasma high‐sensitivity cardiac troponin T and amino‐terminal pro‐B‐type natriuretic peptide as part of MESA (Multi‐Ethnic Study of Atherosclerosis) and were subsequently divided into 4 groups: (1) No LVH, no elevated biomarkers (n=2206; 44.3%); (2) No LVH, ≥1 elevated biomarkers (n=2275; 45.7%); (3) LVH, no elevated biomarkers (n=153; 3.0%); and (4) LVH, ≥1 elevated biomarkers (malignant LVH; n=351; 7.0%). Cardiac magnetic resonance was repeated 10 years later (n=2831) for assessment of LV ejection fraction <50%. Median follow‐up was 12.2 years. Malignant LVH was associated with 7.0‐, 3.5‐, and 2.6‐fold adjusted increases in incidence of HF, cardiovascular death, and asymptomatic LV dysfunction, respectively, versus group 1. New‐onset HF was predominately HF with reduced ejection fraction (9.5‐fold increase).
Conclusions
Malignant LVH is predictive of progression to asymptomatic LV dysfunction, HF (particularly HF with reduced ejection fraction), and cardiovascular death. Consequently, malignant LVH represents a high‐risk phenotype among individuals without known cardiovascular disease, which should be targeted for increased surveillance and more‐aggressive therapies.
Keywords: heart failure, left ventricular dysfunction, left ventricular hypertrophy, mortality, N‐terminal pro‐B‐type, troponin T
Subject Categories: Hypertrophy, Heart Failure, Epidemiology, Biomarkers
Clinical Perspective
What Is New?
Patients with “malignant” left ventricular hypertrophy (LVH), defined as LVH accompanied by elevations in high‐sensitivity cardiac troponin T or amino‐terminal pro‐B‐type natriuretic peptide, are at higher risk to develop asymptomatic left ventricular dysfunction and heart failure while having increased cardiovascular mortality.
Malignant LVH portends a significantly higher risk of developing heart failure with reduced ejection fraction in comparison with heart failure with preserved ejection fraction.
Malignant LVH patients who are male, as well as those with elevations in both high‐sensitivity cardiac troponin T and amino‐terminal pro‐B‐type natriuretic peptide, are at especially high risk for incident heart failure.
What Are the Clinical Implications?
Our study identifies a cohort of asymptomatic patients, previously free of cardiovascular disease, who are at high risk of development of heart failure and coincident adverse outcomes.
Patients who have been incidentally discovered to have malignant LVH may benefit from enhanced surveillance and more‐aggressive therapy.
Consideration should be given for screening for malignant LVH in order to prevent incident heart failure and associated morbidity and mortality.
Introduction
As the population continues to age, increasingly the first episode of symptomatic cardiovascular disease (CVD) manifests in the form of heart failure (HF).1 For the primary prevention of HF, there are challenges in the identification of novel risk factors outside the “traditional” factors that also apply to atherosclerotic disease, attributed to the heterogeneous nature of HF progression among asymptomatic individuals. However, preliminary evidence suggests that a multimodality cardiac‐specific biomarker approach has potential to differentiate risk beyond traditional risk factors or a single modality, such as imaging or blood tests alone.2
Left ventricular (LV) hypertrophy (LVH) is determined by noninvasive measurement by multiple modalities. Whereas patients with LVH represent a risk group for adverse outcomes, progression to reduced LV systolic function, let alone major adverse cardiac events, occurs in a minority.3 Recent studies by our group have demonstrated that presence of LVH in conjunction with elevated soluble biomarkers for myocardial injury (high‐sensitivity cardiac troponin T; hs‐cTnT) and hemodynamic stress (amino‐terminal pro‐B‐type natriuretic peptide; NT‐proBNP) can identify a “malignant” phenotype of LVH more likely to progress to HF or death in both middle‐aged and older individuals.2, 4
In the current study, we seek to further elucidate incident HF and cardiovascular mortality risk as well as to determine whether this intermediate “malignant” LVH phenotype, characterized by elevated cardiac‐specific biomarkers, leads to progression to asymptomatic reduced LV ejection fraction (LVEF) across a spectrum of age in a contemporary, racially and ethnically diverse, community‐based population free of known CVD. Application of a multimodality approach to identify patients at the highest risk for incident asymptomatic and symptomatic heart failure with reduced ejection fraction (HFrEF) while LVEF is still preserved may have significant potential for early therapeutic interventions.
Methods
Data Access
The full data set is stored at the MESA Data Coordinating Center at the University of Washington (Seattle, WA). It is available for verification of these results with approval from the MESA Publications and Presentations committee.
Study Population
The MESA (Multi‐Ethnic Study of Atherosclerosis) study has been previously described.5 In short, 6814 American men and women, ranging from 45 to 84 years of age and comprising 4 self‐reported ethnicities (non‐Hispanic white, black, Hispanic, and Chinese), free of known CVD at baseline, were enrolled and gave informed consent. The study was approved by the institutional review boards of participating centers. The measurement of hs‐cTnT and NT‐proBNP for this ancillary study was approved by the University of Maryland Baltimore institutional review board.
The study sample for this analysis included all MESA participants with a complete cardiac magnetic resonance (CMR) at visit 1 (baseline), and with nonmissing cardiac‐specific biomarker measures (n=4985; Figure 1). For the subanalysis of incident asymptomatic reduced LVEF (defined as <50%), we further restricted the analysis to those participants with a CMR at visit 5 (≈10 years after baseline), who also remained free of interim HF and coronary heart disease, and who had an LVEF ≥50% at baseline (n=2831).
Figure 1.
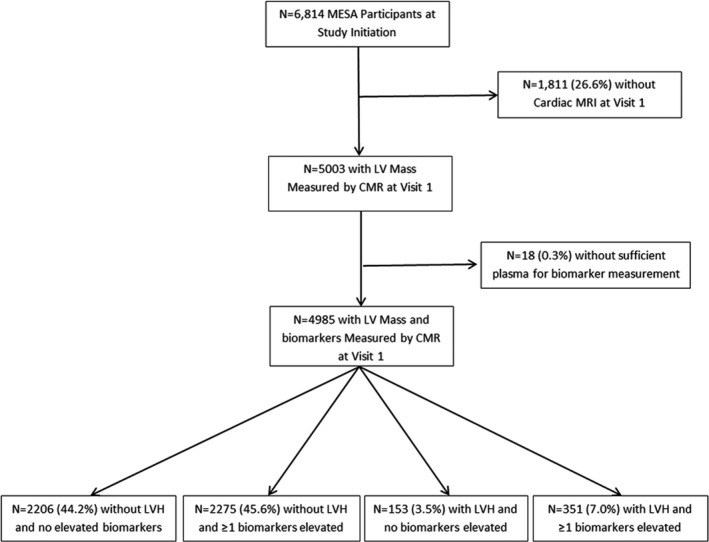
Flow diagram for inclusion of MESA participants in the present analysis. CMR indicates cardiac magnetic resonance; LV, left ventricular; LVH, left ventricular hypertrophy; MESA, The Multi‐Ethnic Study of Atherosclerosis; MRI, magnetic resonance imaging.
Biomarker Assay Measurements
hs‐cTnT and NT‐proBNP were measured in EDTA plasma previously unthawed or unthawed once that was collected at baseline in the MESA cohort (2000–2002) and measured as previously described on the Cobas e601 (Roche Diagnostics, Indianapolis, IN).6 NT‐proBNP was measured in a total of 6791 (99.6%) and hs‐cTnT in 6783 (99.5%) participants.
CMR Imaging and Image Analysis
CMR images were acquired in 5004 participants at exam 1 (2000–2002) for measures of LV mass and function as previously described.5, 7 At exam 5 (2010–2012), 3015 participants underwent a second CMR. There were 2981 participants with exams at both time points. To account for the different pulse magnetic resonance imaging sequences and subsequent variances in measurements, correction equations were used to convert fast gradient echo magnetic resonance imaging pulse sequence measurements (exam 1) into steady‐state free precession measurements (exam 5) for longitudinal measurement analyses.8
Clinical Follow‐up and Cohort Surveillance
MESA study clinical event endpoints for this analysis included incident HF and cardiovascular mortality. Events were adjudicated by the MESA study group as previously described.5, 7 Specifically, incident HF was ascertained by participant interview at annual study visits and all hospitalizations were reviewed. Expert reviewers classified potential HF events as definite, probable, or absent. Probable HF required the presence of typical symptoms, a physician‐assigned HF diagnosis, and the receipt of HF treatment. Definite HF additionally required 1 or more objective criteria for decompensated HF (such as pulmonary edema and systolic or diastolic dysfunction or dilated left ventricle on cardiac imaging). For the purposes of this analysis, events adjudicated as probable or definite HF were considered as the primary outcome. Incident HF events were further categorized as HFrEF or heart failure with preserved ejection fraction (HFpEF) based on an LVEF threshold of <50% as measured at the time of the HF event. Cardiovascular mortality was defined as death related to atherosclerotic heart disease, cerebrovascular disease, other atherosclerotic CVD, and other CVD death.
Candidate Covariates
Clinical characteristics and cardiovascular risk factors used as covariates were obtained from the MESA baseline examination and included: age, sex, race/ethnicity, smoking status (current, former, or never), systolic and diastolic blood pressure, use of antihypertensive medications, height, weight, lipid levels, diabetes mellitus, and glomerular filtration rate,5 the latter estimated from serum creatinine using the CKD‐EPI equation.9
Primary Predictor Variable
LV mass index was calculated by dividing LV mass (measured by CMR at exam 1) by estimated body surface area. LV mass index values above the sex‐specific 95th percentile of MESA participants without hypertension were considered to represent LVH, corresponding to cut‐off values of 85.3 g/m2 for women and 107.8 g/m2 among men.10 High hs‐cTnT and NT‐proBNP levels were defined as the upper tertile for each biomarker, stratified by age (per decade). The rationale for age stratification was the large difference in hs‐cTnT and NT‐proBNP distribution by age. Participants were categorized on the basis of LVH and elevated biomarkers into 4 groups: (1) No LVH, no elevated biomarkers; (2) No LVH, ≥1 elevated biomarkers; (3) LVH, no elevated biomarkers; and (4) LVH, ≥1 elevated biomarkers (“malignant” LVH).
Statistical Analysis
Baseline characteristics were compared across 4 subgroups as defined above using chi‐squared tests for categorical variables, 1‐way ANOVA for continuous variables, and Kruskall–Wallis test for skewed continuous variables.
Cumulative risk of incident HF, cardiovascular mortality, HFrEF, and HFpEF were estimated for each LVH‐biomarker subgroup using the Kaplan–Meier method and compared with the log‐rank test. Adjusted associations between the LVH‐biomarker subgroups and these 4 outcomes were estimated using separate Cox proportional hazard models. Follow‐up time was defined as time from baseline visit to incident HF or cardiovascular mortality, with censoring on noncardiovascular death or last date of observation (December 31, 2013 or last known follow‐up). For models of HFrEF and HFpEF, the primary outcome was defined as the HF subtype of interest, with censoring on HF of other subtypes (including those with unknown LVEF). Adjustment was made for the candidate confounder variables described above. Comparison of the estimated hazard ratios (HRs) for malignant LVH versus nonmalignant LVH for each outcome was performed using appropriate contrast functions. Additive interaction between LVH and elevated biomarkers on risk of HF and cardiovascular death was quantified using the relative excess risk attributed to interaction as previously described; relative excess risk attributed to interaction >0 indicates that the joint effect of LVH and elevated biomarkers is greater than that predicted from the sum of individual effects.11 Sensitivity analyses were performed explicitly modeling the competing risk with noncardiovascular mortality using the Fine‐Gray method.12 Additional sensitivity analyses were performed to compare the associations of LVH‐biomarker groups with incident HF among older (≥65 years) versus middle‐aged adults; formal interaction of LVH‐biomarker group by age was tested with multiplicative terms.
For the analysis of new systolic dysfunction, the frequency of a new LVEF <50% among those without interim HF between the initial and follow‐up CMR was compared across LVH‐biomarker subgroups using the chi‐squared test. Logistic regression was used to estimate the odds ratios for new systolic dysfunction by LVH‐biomarker subgroup, adjusting for baseline LVEF and for the same set of potential confounding risk factors described above. The analyses were performed with StataSE (v12.1; StataCorp LP, College Station, TX).
Results
Study Population
Among 6814 MESA participants enrolled at baseline, a CMR interpretable for LV mass index was completed in 5003 (73.4%). Of these, both hs‐cTnT and NT‐proBNP were measured on 4985 (99.6%). These participants were included in the analysis. LVH was present among 504 (10.1%), of whom n=351 (69.6%) also had ≥1 biomarker in the upper tertile based on age. Baseline characteristics among the 4 subgroups characterized by LVH and biomarker levels are shown in Table 1. The study sample was 48% male and had a mean (SD) age of 61.5 (10.1) years. Overall, 11.6% of the population was diabetic; 35% was on an at least 1 antihypertensive medication. The mean LVEF was 62.4% (6.2). Baseline median [interquartile range] hs‐cTnT and NT‐proBNP levels were 4.9 [<3.0, 7.1] ng/L and 51.5 [23.0, 103.4] pg/mL, respectively. Significant differences between subgroups were observed with regard to age, sex, race, diabetes mellitus status, blood pressure, antihypertensive medication, estimated glomerular filtration rate, history of smoking, low‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and LVEF.
Table 1.
Characteristics of Study Participants, by LVH‐Biomarker Group
| No LVH, No Biomarker Elevated | No LVH, ≥1 Biomarker Elevated | LVH, No Biomarker Elevated | LVH, ≥1 Biomarker Elevated | P Value | |
|---|---|---|---|---|---|
| N | 2206 | 2275 | 153 | 351 | |
| Age, y | 61.0 (10.1) | 61.6 (10.1) | 60.7 (9.8) | 64.2 (10.3) | <0.001 |
| Male | 1041 (47.2%) | 1126 (49.5%) | 46 (30.1%) | 162 (46.2%) | <0.001 |
| Race/ethnicity | |||||
| White | 751 (34.0%) | 1061 (46.6%) | 33 (21.6%) | 103 (29.4%) | <0.001 |
| Chinese‐American | 397 (18.0%) | 226 (9.9%) | 5 (3.3%) | 24 (6.8%) | |
| Black | 547 (24.8%) | 536 (25.4%) | 61 (39.9%) | 135 (38.5%) | |
| Hispanic | 511 (23.2%) | 452 (19.9%) | 54 (35.3%) | 89 (25.4%) | |
| Diabetes mellitus | |||||
| Normal | 1703 (77.3%) | 1732 (76.3%) | 98 (64.1%) | 221 (63.0%) | <0.001 |
| IFG | 297 (13.5%) | 267 (11.8%) | 21 (13.7%) | 60 (17.1%) | |
| Untreated DM | 54 (2.5%) | 50 (2.2%) | 7 (4.6%) | 11 (3.1%) | |
| Treated DM | 149 (6.8%) | 221 (9.7%) | 27 (17.7%) | 59 (16.8%) | |
| SBP, mm Hg | 121.8 (18.4) | 125.3 (21.4) | 136.3 (22.7) | 144.0 (25.4) | <0.001 |
| DBP, mm Hg | 71.3 (9.5%) | 71.3 (10.4) | 75.7 (10.2) | 76.9 (12.6) | <0.001 |
| Hypertension medication | 621 (28.2%) | 856 (37.6%) | 77 (50.3%) | 204 (58.3%) | <0.001 |
| Smoking | |||||
| Never | 1196 (54.3%) | 1126 (49.6%) | 76 (50.0%) | 159 (45.7%) | <0.001 |
| Former | 751 (34.1%) | 866 (38.2%) | 42 (27.6%) | 122 (35.1%) | |
| Current | 254 (11.5%) | 278 (12.3%) | 34 (22.4%) | 67 (19.3%) | |
| LDL‐C | 119.6 (31.3) | 115.3 (30.8) | 117.1 (28.6) | 114.0 (33.6) | <0.001 |
| HDL‐C | 50.2 (14.2) | 52.2 (15.6) | 52.2 (15.4) | 50.9 (15.2) | <0.001 |
| Triglycerides, mg/dL | 114 [78, 163] | 109 [76, 159] | 101 [78, 159] | 105 [79, 169] | 0.6 |
| eGFR, mL/min per 1.73 m2 | 80.3 (14.5) | 76.3 (16.3) | 85.1 (16.1) | 75.9 (19.7) | <0.001 |
| eGFR <60 mL/min per 1.73 m2 | 159 (7.2%) | 368 (16.2%) | 9 (5.9%) | 71 (20.2%) | <0.001 |
| hs‐cTnT, ng/L | 3.22 [<3.0, 4.47] | 6.1 [3.24, 9.30] | 3.41 [<3.00, 4.91] | 8.54 [4.98, 13.44) | |
| NT‐proBNP, pg/mL | 31.7 [15.4, 53.6] | 79.0 [41.1, 146.9] | 33.7 [18.2, 49.6] | 148.0 [67.7, 311.7] | |
| LVEF, % | 62.6 (5.6) | 62.6 (6.0) | 62.8 (6.2) | 60.1 (9.0) | <0.001 |
| LV mass, g | |||||
| Men | 156.3 (26.9) | 167.7 (30.5) | 227.8 (22.2) | 243.5 (34.1) | <0.001 |
| Women | 117.3 (20.9) | 118.8 (21.5) | 166.0 (21.2) | 172.0 (30.0) | <0.001 |
DBP indicates diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hs‐cTnT, high‐sensitivity troponin T; IFG, impaired fasting glucose; LDL, low‐density lipoprotein; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, LV hypertrophy; NT‐proBNP, amino‐terminal pro‐B‐type natriuretic peptide; SBP, systolic blood pressure.
Incident HF Events and Cardiovascular Deaths
Participants were followed for a median of 12.2 years, during which there were 177 definite or probable incident HF events and 141 cardiovascular deaths. Cumulative hazard curves for incident HF for the 4 subgroups are shown in Figure 2A. Increased incidence of HF was observed in all 3 subgroups compared with those without LVH or an elevation of a biomarker, but the increase was most dramatic in participants with LVH and elevation of ≥1 biomarker (unadjusted HR, 11.28; 95% confidence interval [CI], 7.29–17.44); this HR was significantly greater than the risk associated with LVH without elevated biomarkers. Associations were largely similar after adjustment for demographics, risk factors, and baseline LVEF (HR, 6.99; 95% CI, 3.68–9.74; Table 2). Results were also similar when regression models accounted for the competing risk of noncardiovascular death (Table S1). Cumulative risk of cardiovascular death was significantly higher in participants with LVH and elevation of ≥1 biomarker (adjusted HR, 3.48; 95% CI, 2.14–5.65; Figure 2B; Table 2), with greater risk for those with LVH and elevated versus nonelevated biomarkers. There was a significant additive interaction between LVH and ≥1 elevated biomarkers on risk of incident HF (relative excess risk attributed to interaction=3.45; 95% CI, 0.25, 6.65; P=0.035) and cardiovascular death (relative excess risk attributed to interaction=3.11; 95% CI, 1.23, 5.00; P=0.001).
Figure 2.
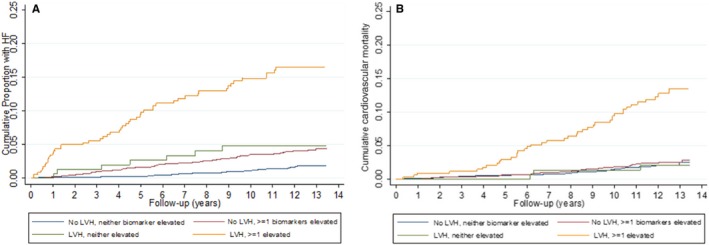
A, Cumulative incidence of HF, by LVH‐biomarker group. Kaplan–Meier curve depicting cumulative risk of HF among: (1) No LVH, no elevated biomarker; (2) No LVH, ≥1 elevated biomarker; (3) LVH, no elevated biomarker; and (4) LVH, ≥1 elevated biomarker groups over median follow‐up period of 12 years. HF indicates heart failure; LVH, left ventricular hypertrophy. B, Cumulative cardiovascular death, by LVH‐biomarker group. Kaplan–Meier curve depicting cumulative risk of cardiovascular mortality among: (1) No LVH, no elevated biomarker; (2) No LVH, ≥1 elevated biomarker; (3) LVH, no elevated biomarker; and (4) LVH, ≥1 elevated biomarker groups over median follow‐up period of 12 years. HF indicates heart failure; LVH, left ventricular hypertrophy.
Table 2.
Risk of Incident HF, Cardiovascular Death, and HF Subtype by LVH‐Biomarker Subgroup
| Outcome | Unadjusted | Demographic‐Adjusted* | Risk Factor–Adjusted† | RF+LVEF–Adjusted‡ |
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | ||||
| Heart failure | ||||
| No LVH, no biomarker elevated | Reference | Reference | Reference | Reference |
| No LVH, ≥1 biomarker elevated | 2.49 (1.68, 3.71) | 2.24 (1.50, 3.35) | 2.11 (1.40, 3.18) | 2.10 (1.39, 3.17) |
| LVH, no biomarker elevated | 3.01 (1.33, 6.80) | 3.38 (1.48, 7.68) | 2.80 (1.21, 6.47) | 2.74 (1.12, 6.32) |
| LVH, ≥1 biomarker elevated | 11.28 (7.29, 17.44) | 9.55 (6.13, 14.87) | 7.37 (4.57, 11.87)* | 6.99 (3.68, 9.74)* |
| Cardiovascular death | ||||
| No LVH, no biomarker elevated | Reference | Reference | Reference | Reference |
| No LVH, ≥1 biomarker elevated | 1.20 (0.80, 1.79) | 1.08 (0.73, 1.62) | 0.99 (0.65, 1.50) | 0.97 (0.64, 1.47) |
| LVH, no biomarker elevated | 0.99 (0.31, 3.17) | 1.05 (0.32, 3.39) | 0.95 (0.29, 3.12) | 0.91 (0.28, 3.00) |
| LVH, ≥1 biomarker elevated | 6.52 (4.25, 10.01) | 5.12 (3.31, 7.92) | 4.05 (2.52, 6.51)a | 3.48 (2.14, 5.65)a |
| HFrEF | ||||
| No LVH, no biomarker elevated | Reference | Reference | Reference | Reference |
| No LVH, ≥1 biomarker elevated | 3.40 (1.73, 6.65) | 2.95 (1.50, 5.77) | 2.68 (1.36, 5.30) | 2.61 (1.32, 5.16) |
| LVH, no biomarker elevated | 5.33 (1.70, 16.75) | 5.75 (1.82, 18.23) | 4.98 (1.55, 16.03) | 4.67 (1.45, 15.02) |
| LVH, ≥1 biomarker elevated | 23.58 (11.9, 46.5) | 18.99 (9.56, 37.74) | 14.54 (7.01, 30.16)a | 9.53 (4.52, 20.12)b |
| HFpEF | ||||
| No LVH, no biomarker elevated | Reference | Reference | Reference | Reference |
| No LVH, ≥1 biomarker elevated | 1.74 (1.02, 2.98) | 1.59 (0.92, 2.73) | 1.55 (0.89, 2.71) | 1.55 (0.88, 2.70) |
| LVH, no biomarker elevated | 2.09 (0.62, 6.99) | 2.33 (0.68, 7.92) | 1.83 (0.52, 6.39) | 1.83 (0.52, 6.49) |
| LVH, ≥1 biomarker elevated | 4.07 (1.96, 8.44) | 3.45 (1.65, 7.24) | 2.63 (1.19, 5.79)b | 2.73 (1.23, 6.04)b |
HF indicates heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; RF, risk factors.
Adjustment covariates: *Age, race, sex. †Demographics and: systolic and diastolic blood pressure, antihypertensive medications, weight, height, LDL‐C, HDL‐C, triglycerides, diabetes mellitus, smoking, and estimated glomerular filtration rate. ‡Risk factors and LVEF by baseline cardiac magnetic resonance.
Significant difference (P<0.05) comparing LVH with elevated biomarker to LVH without elevated biomarker.
No significant difference comparing LVH with elevated biomarker to LVH without elevated biomarker levels.
Among those with incident HF a determination of HFrEF was made in 87 (49.2%), HFpEF in 72 (40.6%), and unknown LVEF in 18 (10.2%). Incident HFrEF was relatively common in participants with ≥1 elevated biomarker level and LVH, occurring in 10.6% (95% CI, 7.6–14.8) at 10 years. This translated into a nearly 24‐fold increment in unadjusted risk and 9.5‐fold greater adjusted risk compared with those with neither LVH nor an elevated biomarker level (Figure 3A; Table 2). The groups with either LVH or ≥1 elevated biomarker level also had a significant increased risk of HFrEF, but to a significantly (P<0.05) lesser extent than the group with the combination of the 2. In contrast, incident HF attributed to HFpEF was qualitatively less differentiated based on the presence of LVH and/or an elevated biomarker level (Figure 3B). Once adjusted, only the group with LVH combined with an elevated biomarker level remained at significantly higher risk of HFpEF compared with those without LVH or an elevated biomarker level (HR=2.73 [95% CI, 1.23–6.04]), although this risk was not significantly different from the risk associated with LVH without elevated biomarkers.
Figure 3.
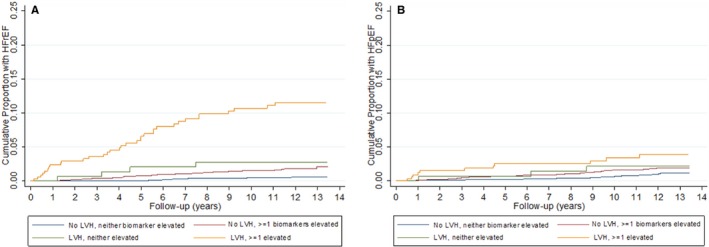
A, Cumulative risk of HFrEF, by LVH‐biomarker group. Kaplan–Meier curve depicting cumulative risk of HFrEF among: (1) No LVH, no elevated biomarker; (2) No LVH, ≥1 elevated biomarker; (3) LVH, no elevated biomarker; and (4) LVH, ≥1 elevated biomarker groups over median follow‐up period of 12 years. B, Cumulative risk of HFpEF, by LVH‐biomarker group. Kaplan–Meier curve depicting cumulative risk of HFpEF among: (1) No LVH, no elevated biomarker; (2) No LVH, ≥1 elevated biomarker; (3) LVH, no elevated biomarker; and (4) LVH, ≥1 elevated biomarker groups over median follow‐up period of 12 years. HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVH, left ventricular hypertrophy.
Cumulative Risk of HF by Age, Sex, and Race/Ethnicity
Of the 177 HF events, 125 (73.5%) occurred in participants aged ≥65 years. A subgroup analysis based on age is presented in Table S2. The combination of LVH and biomarkers markedly differentiated the risk of incident HFrEF in older adults. For example, in the absence of LVH and elevation of a biomarker level, the incidence of HFrEF is only 0.6 in 1000 patient‐years, which is comparable to middle‐aged adults with similar characteristics (0.3 in 1000 patient‐years). In contrast, older adults with both LVH and an elevated cardiac biomarker level (n=173 [8.5%]) have an incidence rate of 15.2 HFrEF events per 1000 patient‐years (adjusted HR, 23.2; 95% CI, 8.78–61.32) versus those with neither LVH nor an elevated biomarker. However, there was no significant interaction by age for incident HF (P=0.96) nor cardiovascular death (P=0.3).
Adjusted HRs for incident HF and HFrEF were greater for men with LVH and ≥1 elevated biomarker compared with women with the same findings. Men with LVH and ≥1 elevated biomarker had an approximately 32‐fold increased risk of HFrEF compared with a 4‐fold increased risk for women when compared with those without LVH or an elevated biomarker level (Table S3). There was significant interaction based on sex for all incident HF (P=0.02). Despite qualitatively greater differences among men than women for differentiation of cardiovascular mortality risk, there was no significant interaction based on sex (P=0.2). In addition, no significant interaction was found for risk of incident HF or cardiovascular mortality across the four represented races/ethnicities (data not shown).
Risk of HF by Specific Soluble Cardiac Biomarker and Alternate Cutoffs Combined With LVH
Combining LVH and the presence of an elevated hs‐cTnT level identifies a higher risk for incident HF compared with LVH and an elevated NT‐proBNP level (12.5 versus 6.8 HF events/1000 patient‐years; P=0.009). The highest incidence rate of HF occurred in the presence of LVH and both biomarkers being elevated (25.0 HF events per 1000 patient‐years; Table S4). However, in a fully adjusted model, these differences in incident HF risk were no longer significantly different between subgroups defined by elevations in individual versus both cardiac‐specific biomarkers.
We also evaluated hs‐cTnT by using non‐age‐based tertile values for the MESA cohort. As anticipated, once adjusted for age, the HR for both cardiovascular death and HF were similar to the age‐based tertiles (Table S5), with significantly greater risk of both outcomes for LVH with elevated biomarkers compared with LVH alone. The age‐based upper tertile values for hs‐cTnT and NT‐proBNP are shown in Table S6.
Progression to an Asymptomatic Abnormal LVEF
An evaluation for incident LVEF <50% in participants without interim HF or coronary heart disease events was examined in 2831 participants who underwent baseline and follow‐up CMR with a baseline LVEF ≥50%. There were a total of 109 participants with new asymptomatic abnormal LVEF over 10 years. There was a progressively greater incidence of new abnormal LVEF across the 4 subgroups, with the lowest incidence (3.1%) in those without LVH nor elevated biomarkers and greatest incidence (7.4%) in those with LVH and ≥1 elevated biomarker (P=0.04 across subgroups). After adjustment, those with LVH and an elevated level of ≥1 biomarker had a more than 2.5‐fold greater odds of progression to an abnormal LVEF compared with asymptomatic participants without LVH nor an elevated biomarker level (adjusted OR, 2.58; 95% CI, 1.20–5.56; Figure 4; Table S7).
Figure 4.
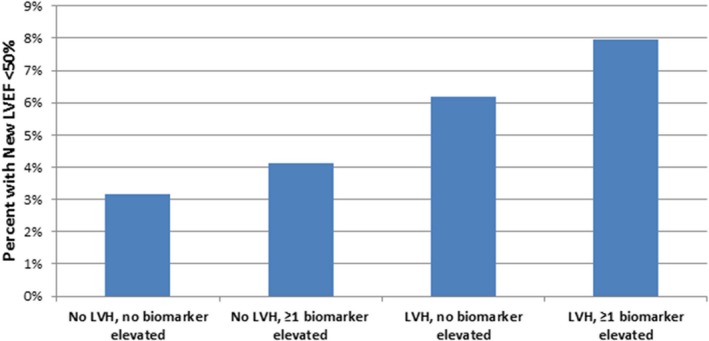
Incidence of new‐onset asymptomatic LV dysfunction by LVH‐biomarker group over 10 years. Bar graph depicts incidence of new LVEF <50% in patients with initial LVEF >50% among: (1) No LVH, no elevated biomarker; (2) No LVH, ≥1 elevated biomarker; (3) LVH, no elevated biomarker; and (4) LVH, ≥1 elevated biomarker group. LVEF indicates left ventricular ejection fraction; LVH, left ventricular hypertrophy.
Discussion
Among community dwelling adults free of CVD, the presence of LVH and a modest elevation of 1 of 2 cardiac specific biomarker levels identified a marked increased risk of new‐onset HF and high cardiovascular mortality. This finding persisted across a broad spectrum of age in a contemporary multiethnic, community‐based cohort. For the outcome of incident HF (particularly HFrEF) and cardiovascular mortality, the presence of LVH with a modest elevation of ≥1 biomarker significantly differentiated an often markedly higher risk than LVH alone. Furthermore, we found there was a significant additive interaction between LVH and cardiac‐specific biomarker levels for the risk of incident HF and cardiovascular death. There are novel findings in this study of the MESA cohort that build upon our past work in the Dallas Heart Study and the Cardiovascular Health Study, where we first coined the term “malignant” LVH.2, 4 First, we differentiated that malignant LVH is a potent risk factor for HFrEF, qualitatively more so than for HFpEF, across a spectrum of age in participants without known CVD. This finding suggests that biochemical measures of myocyte loss and strain may best predict a process of adverse remodeling (represented by HFrEF) and, to a lesser extent, a process of progressive fibrosis (represented by HFpEF). This may also be attributed to the fact that HFpEF reflects both cardiac and noncardiac factors that play variable roles across a heterogeneous group of patients. We found a significant interaction with sex and the presence of malignant LVH for the risk of incident HF, particularly HFrEF, with a 32‐fold increased risk in men versus those with neither LVH nor an elevated biomarker. No such interaction was observed across race/ethnicity based on the presence of malignant LVH. Second, we provide insight into the pathophysiology of hypertrophy coupled with biochemical evidence of low‐level injury or strain by showing a 2.5‐fold greater risk of decline to an asymptomatic abnormal LVEF over 10 years compared with those with neither baseline feature. This supports the concept that hypertrophy can be associated with accelerated myocyte cell loss, replacement fibrosis, and adverse remodeling.13 The pathophysiological differences between sex for progression to HF symptoms in the presence of LVH and ≥1 elevated biomarker level are not certain, but men in the MESA study have almost a 6‐fold higher risk for the presence of CMR defined replacement fibrosis compared with women.14 We also noted that, after adjustment, neither cardiac‐specific biomarker nor the combination of the 2 was superior for prediction of incident HF, suggesting significant overlap in pathophysiology represented by these biomarkers for identifying LVH patients at highest risk and that measurement of both NT‐proBNP and hs‐cTnT should be considered to identify those with malignant LVH.
The implications of our study are multifold. Stage B HF is defined as the presence of structural heart disease in the absence of symptoms of HF and represents the tip of the iceberg compared with the morbidity, mortality, and cost associated with stage C disease.15, 16 Currently, routine cardiac imaging to screen for LVH is not recommended, attributed, in part, to the observation that the progression of LVH to symptomatic HF is heterogeneous.17 Malignant LVH defines a subgroup of LVH patients, representing 7% of MESA participants, by definition free of known CVD, and independent of the presence of hypertension, who may potentially benefit from increased surveillance. Surveillance with biomarkers has some precedence, previously being efficacious among primary care patients in a randomized, controlled trial of BNP monitoring.18 We show that combining LVH with biomarker elevation identifies participants at a 2.5‐ to 4‐fold greater risk than just biomarker elevation alone.
Our finding that malignant LVH has strong predisposition for HFrEF over HFpEF is of particular importance and makes a compelling case to consider this multimodality cardiac‐specific risk stratification. HF has a heterogeneous etiology with near equivalent incidences of HFpEF and HFrEF.19 Unfortunately, the lack of effective treatment strategies for HFpEF, combined with the lack of clinical and echocardiographic characteristics allowing for differentiation of progression to HFpEF versus HFrEF, makes it difficult to identify patients who are most likely to derive benefit from a specific intervention in the absence of a low LVEF. Low LVEF is infrequent in an asymptomatic community‐based population, making screening for it an unattractive strategy.20 Consequently, the finding that malignant LVH is more strongly predictive of HFrEF than LVH without elevated biomarkers, but is not significantly more predictive of HFpEF, may allow for both earlier and more‐appropriate initiation of the multiple therapeutic modalities, which have demonstrated efficacy in HFrEF (but not HFpEF).21, 22 A prospective study is needed to determine whether the same medical therapies (renin‐angiotensin aldosterone system inhibitors and beta‐blockers) that have efficacy for reducing progression to symptomatic HF in patients with asymptomatic LV systolic dysfunction would also have comparable efficacy in patients with malignant LVH. Recently, it has been suggested that it is possible to subgroup HFpEF into different phenotypes using machine learning or other algorithms.23, 24 It remains to be determined whether the malignant LVH phenotype may be also able to identify specific patient phenotypes that would be at greater risk to progress to HFpEF among the overall heterogeneous HFpEF cohort. The converse is that the absence of LVH and lower levels of both biomarker, representing 44.3% of the MESA population, identifies a subgroup at a reassuring low‐risk for progression to HF even in those with advanced age.
Limitations
Our findings should be interpreted in the setting of several methodological limitations. With regard to CMR, it should be noted that no CMR was available in ≈30% of the MESA cohort and that generally patients without CMR were older and less healthy. We recognize that detection of LVH with CMR, though accurate would be limited by both cost and accessibility. Our past work has shown that detection of LVH by ECG and echocardiography combined with these same biomarkers also detects a malignant LVH that identifies individuals at much higher risk for progression to HF hospitalization and death.2, 4 The current analysis in MESA with a later second CMR exam provided the opportunity to also accurately show the progression of an asymptomatic decline in LVEF in addition to clinical events. Although we did not observe that malignant LVH was more strongly associated with HFpEF than LVH alone (in contrast to HFrEF), this may have represented a type II error and does not provide conclusively inference that malignant LVH is more predictive of HFrEF. Furthermore, it should be emphasized that only 2 biomarkers were included in our analysis and that other biomarkers may have better prognostic utility for both HF and, in particular, HFpEF. It should be noted that measurements of LVEF during sentinel HF events were incomplete and not adjudicated by a core echocardiography laboratory, which may have biased the results of associations with a specific HF subtype.
Conclusions
Malignant LVH as measured by CMR defined LVH in combination with elevation of soluble cardiac biomarkers for myocardial injury (hs‐cTnT), and myocardial hemodynamic stress (NT‐proBNP) was associated with an increased incidence of asymptomatic LV dysfunction, HF (specifically HFrEF), and cardiovascular mortality among a contemporary multiethnic cohort of middle‐ to older‐aged individuals without prevalent CVD. These findings define a subpopulation, without symptomatic CVD, who may benefit from enhanced surveillance and intervention to prevent progression to symptomatic HF and death.
Sources of Funding
This research was supported by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from NCRR. Roche Diagnostics supported the measurement of hs‐cTnT and additional NT‐proBNP through an investigator‐initiated grant (PI: deFilippi).
Disclosures
deFilippi receives grant support from Roche Diagnostics and Abbott Diagnostics, consulting for Alere, Roche Diagnostics, Siemens Healthcare Diagnostics, Metanomics, Thermo‐Fisher and Ortho Clinical Diagnostics, and participates on the endpoint review committee for Radiometer. Seliger has received grant support from Roche Diagnostics. Christenson receives grant support from Roche Diagnostics, Ortho Clinical Diagnostics and Abbott Diagnostics, consulting for Siemens Healthcare and Ortho Clinical Diagnostics. Daniels has received speaking fees from Roche Diagnostics and consulting for Siemens Healthcare Diagnostics. de Lemos has received grant support and consulting income from Roche Diagnostics and Abbott Diagnostics, and has participated on endpoint review committees for Siemen's Health Care Diagnostics and Radiometer. The remaining authors have no disclosures to report.
Supporting information
Table S1. Rates of Incident HF, by LVH‐Biomarker Subgroup, Accounting for Competing Risk of Noncardiovascular Mortality
Table S2. Risk of Incident HF and HF Subtype, by LVH‐Biomarker and Age Subgroup
Table S3. Risk of Incident HF, Cardiovascular Death, and HFrEF, Among Men and Women
Table S4. Rates of Incident HF, by LVH and Elevation in Each Biomarker
Table S5. Association of LVH‐Biomarker With Incident HF and Cardiovascular Death (Non‐Age‐Based Biomarker Tertiles)
Table S6. Age‐Specific Cut‐Points of hs‐cTnT and NT‐proBNP Which Define Elevated Biomarkers
Table S7. Odds of New LVEF <50% at Follow‐Up CMR, by Initial LVH‐Biomarker Group
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2018;7:e006619 DOI: 10.1161/JAHA.117.006619.)29437599
References
- 1. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T, de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension (LIFE): a randomized trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 4. Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J, deFilippi C. Old adults, “Malignant” left ventricular hypertrophy and associated cardiac specific biomarker phenotypes to identify the differential risk of new‐onset reduced versus preserved ejection fraction heart failure: CHS (Cardiovascular Health Study). JACC Heart Fail. 2015;3:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 6. Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JA, de Lemos JA, Bertoni AG, deFilippi CR. High sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ambale Venkatesh B, Volpe GJ, Donekal S, Mewton N, Liu CY, Shea S, Liu K, Burke G, Wu C, Bluemke DA, Lima JA. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the MESA study. Hypertension. 2014;64:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, Siscovick D, Bertoni AG, Shlipak MG. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2008;52:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. VanderWeele TJ, Knol MJ. A tutorial on interaction with SAS and Stata code: a tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13. Piek A, de Boer RA, Silljé HH. The fibrosis‐cell death axis in heart failure. Heart Fail Rev. 2016;21:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turkbey EB, Nacif MS, Guo M, McClelland RL, Teixeira PB, Bild DE, Barr RG, Shea S, Post W, Burke G, Budoff MJ, Folsom AR, Liu CY, Lima JA, Bluemke DA. Prevalence and correlates of myocardial scar in a US cohort. JAMA. 2015;314:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah A, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among older adults in the community: the Atherosclerosis Risks in Communities Study. Circulation. 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–2860. [DOI] [PubMed] [Google Scholar]
- 17. Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. [DOI] [PubMed] [Google Scholar]
- 18. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 19. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2013;355:251–259. [DOI] [PubMed] [Google Scholar]
- 20. Atherton JJ. Screening for left ventricular systolic dysfunction: is imaging a solution? JACC Cardiovasc Imaging. 2010;3:421–428. [DOI] [PubMed] [Google Scholar]
- 21. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 22. Massie BM, Carson PE, McMurray JJ. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 23. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rates of Incident HF, by LVH‐Biomarker Subgroup, Accounting for Competing Risk of Noncardiovascular Mortality
Table S2. Risk of Incident HF and HF Subtype, by LVH‐Biomarker and Age Subgroup
Table S3. Risk of Incident HF, Cardiovascular Death, and HFrEF, Among Men and Women
Table S4. Rates of Incident HF, by LVH and Elevation in Each Biomarker
Table S5. Association of LVH‐Biomarker With Incident HF and Cardiovascular Death (Non‐Age‐Based Biomarker Tertiles)
Table S6. Age‐Specific Cut‐Points of hs‐cTnT and NT‐proBNP Which Define Elevated Biomarkers
Table S7. Odds of New LVEF <50% at Follow‐Up CMR, by Initial LVH‐Biomarker Group


