Abstract
Background
Dapagliflozin inhibits the sodium‐glucose–linked transporter 2 in the renal proximal tubule, thereby promoting glycosuria to reduce hyperglycemia in type 2 diabetes mellitus. Because these patients may require loop diuretics, and sodium‐glucose–linked transporter 2 inhibition causes an osmotic diuresis, we evaluated the diuretic interaction between dapagliflozin and bumetanide.
Methods and Results
Healthy subjects (n=42) receiving a fixed diet with ≈110 mmol·d−1 of Na+ were randomized to bumetanide (1 mg·d−1), dapagliflozin (10 mg·d−1), or both for 7 days, followed by 7 days of both. There were no meaningful pharmacokinetic interactions. Na+ excretion increased modestly with the first dose of dapagliflozin (22±6 mmol·d−1; P<0.005) but by more (P<0.005) with the first dose of bumetanide (74±7 mmol·d−1; P<0.005), which was not significantly different from both diuretics together (80±5 mmol·d−1; P<0.005). However, Na+ excretion with dapagliflozin was 190% greater (P<0.005) when added after 1 week of bumetanide (64±6 mmol·d−1), and Na+ excretion with bumetanide was 36% greater (P<0.005) when added after 1 week of dapagliflozin (101±8 mmol·d−1). Serum urate was increased 4% by bumetanide but reduced 40% by dapagliflozin or 20% by combined therapy (P<0.05).
Conclusions
First‐dose Na+ excretion with bumetanide and dapagliflozin is not additive, but the weekly administration of one diuretic enhances the initial Na+ excretion with the other, thereby demonstrating mutual adaptive natriuretic synergy. Combined therapy reverses bumetanide‐induced hyperuricemia. This requires further study in diabetic patients with hyperglycemia who have enhanced glycosuria and natriuresis with dapagliflozin.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00930865.
Keywords: congestive heart failure, diabetes mellitus, potassium, sodium, urate
Subject Categories: Hypertension; Ion Channels/Membrane Transport; Diabetes, Type 2; Heart Failure; Pharmacology
Clinical Perspective
What Is New?
This study demonstrated that the natriuretic response to a loop diuretic (bumetanide) was enhanced in normal volunteers given a sodium‐glucose–linked transporter 2 inhibitor (dapagliflozin) for 1 week and that natriuretic response to a sodium‐glucose–linked transporter 2 inhibitor was enhanced in normal volunteers given bumetanide for 1 week.
Therefore, these 2 groups of drugs act synergistically.
What Are the Clinical Implications?
Administration of a sodium‐glucose–linked transporter 2 inhibitor may be helpful to treat loop diuretic resistance in patients with heart failure, perhaps even in those who do not have diabetes mellitus.
Dapagliflozin is a selective and competitive inhibitor of the renal proximal tubule sodium‐glucose–linked transporter 2 (SGLT2).1, 2, 3, 4 It is approved for the treatment of type 2 diabetes mellitus (T2DM), where it promotes glycosuria.1, 5 T2DM increases the incidence of congestive heart failure (CHF).6, 7 Although thiazides may be sufficient to treat mild heart failure, loop diuretics are currently widely used to manage fluid retention for more severe heart failure.8, 9 Empagliflozin was reported recently to reduce cardiovascular events and death in patients with T2DM.10 As reviewed recently, SGLT2 inhibitors are unlike other glucose‐lowering agents in that they can improve heart failure outcomes in patients with T2DM.7 Thus, SGLT2 inhibitors may become widely used in T2DM,10 but their interactions with loop diuretics have not been reported.
Bumetanide is metabolized largely by hepatic cytochrome P450, whereas dapagliflozin is metabolized largely by glucuronidation.6 Thus, there is little potential for pharmacokinetic interaction,11 but this has yet to be tested.5, 12
Prolonged administration of loop diuretics increases Na+ reabsorption at more distal nephron segments, thereby limiting Na+ loss.12, 13 This “diuretic braking phenomenon”14 ultimately leaves many patients with CHF with an expanded blood volume that predicts adverse outcomes.15 Thus, new strategies for treatment of Na+ retention are needed.13, 16, 17, 18, 19
SGLT2 accounts for a portion of proximal Na+ reabsorption5, 20, 21 and, in subjects with T2DM,22 its inhibition causes an osmotic diuresis that can enhance Na+ excretion, leading to a potentially beneficial decrease in blood pressure (BP).23 Thus, SGLT2 inhibitors may be favored in volume‐expanded patients with T2DM and hypertension or CHF, but the interaction between SGLT2 inhibitors and loop diuretics requires study. An augmented natriuresis on initial testing with 2 drugs together, compared with the most effective drug given alone, would indicate an additive interaction24; an augmented natriuresis with one diuretic when given during ongoing administration of the other would indicate an adaptive interaction, such as has been shown with loop diuretics and thiazides.13 This study tested the hypothesis that there are pharmacokinetic and pharmacodynamic interactions between these 2 classes of diuretics in normal human volunteers.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Subjects
Healthy men and nonpregnant women (aged 18–45 years) with a body mass index of 18 to 32 kg·m−2 were enrolled.
Study Design
This randomized, open‐label, parallel‐group study was conducted in accordance with the Guidelines for Good Clinical Practice of the International Committee on Harmonization and the US Code of Federal Regulations. It was approved by the Institutional Review Board of Integreview Ltd (Austin, TX). All subjects provided written informed consent.
Subjects entered the study center 2 days (day −2) before commencement of study drug administration and remained until day 15, consuming a constant diet (daily intake of sodium, <20–30 mmol; calcium, ≈1 g; phosphorus, ≈1 g; and potassium, ≈65 mmol) with 2 salt tablets (1 g each) taken 3 times daily with meals to provide ≈100 to 110 mmol·d−1 Na+ intake (equivalent to a salt‐restricted diet for CHF).
Study Drugs
Subjects were randomized to receive once‐daily bumetanide (1 mg), dapagliflozin (10 mg) or bumetanide plus dapagliflozin on days 1 through 7. All subjects received once‐daily bumetanide, 1 mg, plus dapagliflozin, 10 mg, on days 8 through 15. For pharmacokinetic studies, medication was taken after a 10‐hour overnight fast and no food was permitted for 4 hours thereafter.
Pharmacokinetic Evaluation
Blood (4‐mL) samples were collected serially over 24 hours for the determination of plasma pharmacokinetic parameters (the minimal and maximal serum concentrations, Cmin and Cmax, the time to maximal plasma concentration, Tmax and the area under the curve extrapolated to infinite time, AUCtau). Dapagliflozin and bumetanide in plasma were assayed using solid‐phase extraction and liquid chromatography–tandem mass spectrometry, respectively. Pharmacokinetic parameters were determined with noncompartmental methods using the Kinetica v 4.4.1 (Thermo Electron Corp, Philadelphia, PA) Pharmacokinetic Analysis Program.
Pharmacodynamic Evaluation
Urine was collected at 6‐hour intervals on days 1 and 8 and over 24 hours on other days for measurement of volume and electrolyte, osmolality, glucose, creatinine, and uric acid concentrations. Fasting serum samples were obtained before dosing on days 1, 8, and 15 for the determination of electrolytes, glucose, creatinine, uric acid, and plasma renin activity (PRA).
Safety Assessments
Adverse events were assessed throughout, and seated BP was taken daily.
Statistical Analysis
The primary outcome variables were the 24‐hour Na+ excretion on day 1 (first exposure) and day 8 (second drug regimen). A first‐dose additive interaction was tested by ANOVA, comparing Na+ excretion with bumetanide or dapagliflozin alone with bumetanide+dapagliflozin. An adaptive (synergistic) interaction was tested by comparing Na+ excretion with bumetanide alone (day 1) with bumetanide added during ongoing dapagliflozin administration (day 8) and similarly for dapagliflozin. Descriptive statistics were applied to the other data using Wilcoxon tests. For assessment of pharmacokinetic interactions, point estimates of the ratios of the adjusted geometric means for dapagliflozin plus bumetanide versus either bumetanide or dapagliflozin alone were calculated with respective 95% confidence intervals. Data were presented as mean±SEM. P<0.05 was accepted as significant.
Results
Study Subjects
Forty‐two healthy subjects (32 men and 10 women) were enrolled (Table S1).
Pharmacokinetics
Plasma concentration versus time curves for dapagliflozin and bumetanide were similar when given alone or when coadministered (Figure 1). There were no major changes in pharmacokinetic parameters (Table S2).
Figure 1.
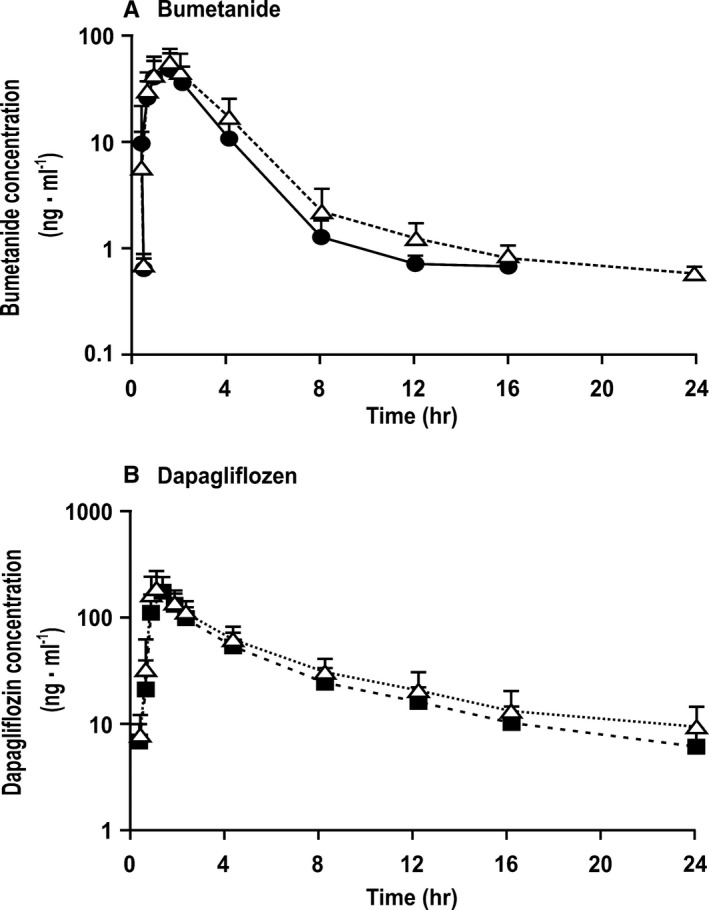
Mean±SEM values (n=14 per group) for log serum concentration vs time profiles for serum bumetanide concentration when given alone (solid circles and continuous lines) or coadministered with dapagliflozin (open triangle and dotted lines; A) or serum dapagliflozin concentration when given alone (solid squares and dashed lines) or coadministered with bumetanide (open triangle and dotted lines; B) as a function of time after dosing. There were no significant differences between test days.
BP and heart rate
There were no changes in systolic, diastolic, or mean BP. However, the heart rate increased by 10 to 15 minutes−1 after 1 week of administration of each diuretic individually and in combination (Table S3).
Renal excretion of fluid and glucose
Renal fluid excretion increased on the first day of each treatment but declined thereafter (Figure 2A). Glucose excretion and urine osmolality increased on the first day with dapagliflozin and combined treatments and remained elevated throughout (Figure S1).
Figure 2.
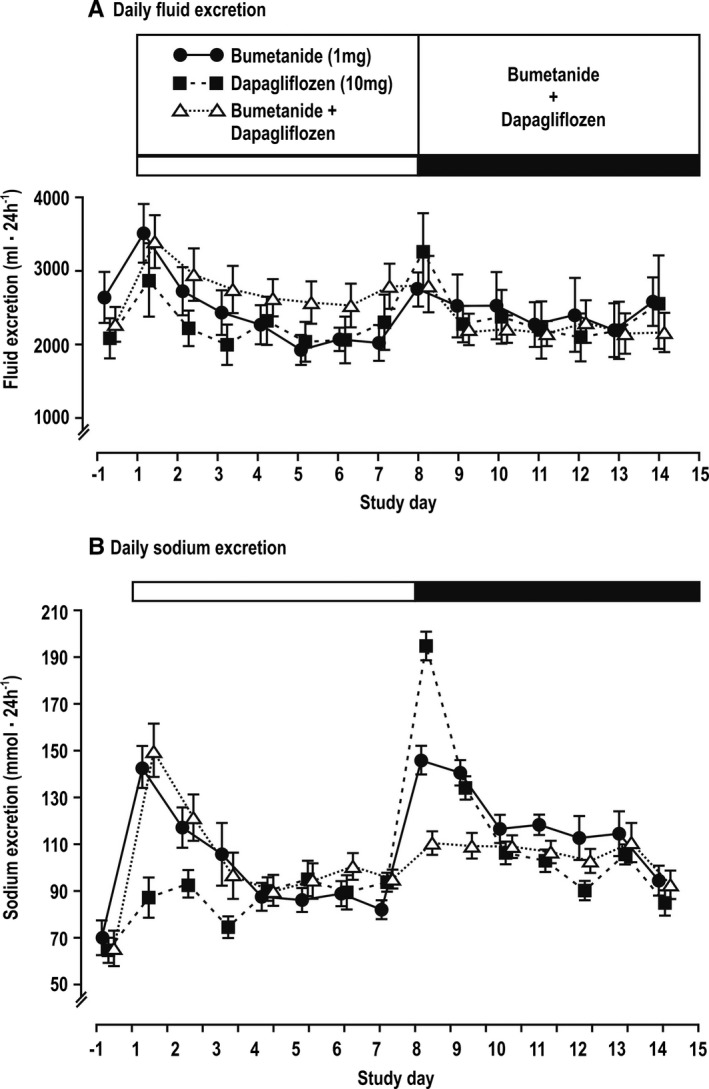
Mean±SEM values (n=14 per group) for daily renal excretion of fluid (A) and sodium (B) over time after administration of diuretics.
Renal excretion of Na+, K+, osmoles, urate, Ca2+, and Mg2+ and creatinine clearance
The first dose of dapagliflozin increased Na+, K+, osmoles, and urate excretion by 34%, 44%, 31%, and 51%, respectively (all P<0.005; Table S4). When dapagliflozin was given after 1 week of bumetanide, there was a 2‐fold greater (P<0.005) increase in Na+ excretion (74%) compared with dapagliflozin alone but a similar 24% increase in osmoles excretion (Table S4). However, K+ excretion no longer increased (Figure S3). Urate excretion remained substantially increased (85%; P<0.005) (Figure 2 and Figure S4).
The first dose of bumetanide increased Na+ excretion by 106%, which was 3‐fold more than dapagliflozin (P<0.005; Table S5). Bumetanide increased K+ excretion by 45% (P<0.005), which was similar to dapagliflozin alone (Figure S2), but reduced (P<0.05) urate excretion by 16% (Figure S3). When given after 1 week of dapagliflozin, there was a 58% greater (P<0.05) increase in Na+ excretion with bumetanide, of 168%, compared with bumetanide alone, whereas K+ excretion increased by 18%, which was less than with bumetanide alone (P<0.05). There was no longer a reduction in urate excretion.
The first dose of both diuretics given together increased Na+ and K+ excretion by 121% and 36%, respectively, which was not significantly different from bumetanide alone, but increased urate excretion by 21%, which was contrary to the effects of bumetanide alone and approximately half of the increase with dapagliflozin alone (Table S6).
The Na+ excretion in the first 6 hours after 1 week of combined diuretic administration increased less than in the first 6 hours after the first week of combined administration (Figure 3 and Table S6). Na+ excretion decreased sharply over 6 to 24 hours after bumetanide or combined treatments (Figure 3). The net effects of the exaggerated increase in 6‐hour Na+ excretion after dapagliflozin given after a week of bumetanide were somewhat offset by greater reductions in Na+ excretion in the following 6 to 24 hours (Figure 3). The mean daily excretions of fluid, Na+, and K+ over 1 week of diuretics were greater after combined diuretic administration than after dapagliflozin alone (Figure 4 and Table 1), whereas the daily excretion of fluid, glucose, and uric acid was greater after combined diuretic administration than after bumetanide alone (Figure 4 and Table 1). The mean daily Na+ excretion during the second week of combined therapy was greater than after either diuretic alone (Figure 4 and Table 1).
Figure 3.
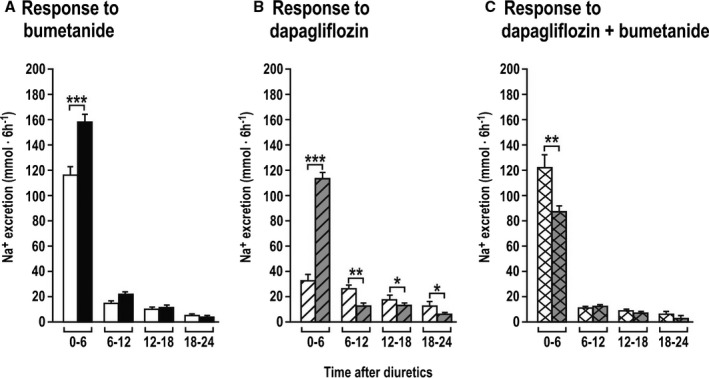
Mean±SEM values (n=14 per group) for 6 hourly sodium excretions during the 24 hours after diuretic dosing. In each panel, the first bar refers to the first administration when given alone on day 1, and the second to the first administration when given on day 8 after adaptation to the other diuretic. Response to bumetanide (A), dapagliflozin (B) and dapagliflozin + bumetanide (C) represent increases in Na+ excretion from the corresponding times of the previous days. *P<0.05, **P<0.01, ***P<0.005 (compared with day 1).
Figure 4.
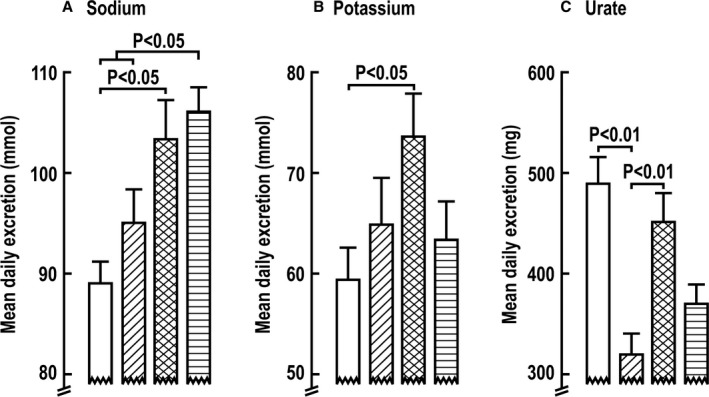
Mean±SEM values (n=14 per group) for mean daily excretion of sodium (A), potassium (B), and urate (C) over 7‐day periods during administration of dapagliflozin (open boxes), bumetanide (diagonal shading), or the combination given during 1 week (double cross‐hatched shading) or a second week (horizontal shading).
Table 1.
Total Renal Excretion During 1‐Week Periods of Diuretic Administration
| Parameter | Dapagliflozin (Day 1–7) | Bumetanide (Day 1–7) | Dapagliflozin and Bumetanide (Day 1–7) | Dapagliflozin and Bumetanide (Day 8–14) |
|---|---|---|---|---|
| Volume, mL·wk−1 | 16 082±2146 | 16 445±1612 |
20 033±1983 (P<0.05 vs dapagliflozin or bumetanide alone) |
16 272±1567 |
| Glucose, g·wk−1 | 239±20 | 0.3±0.06 |
188±20 (P<0.05 vs bumetanide) |
150±19 (P<0.05 vs bumetanide) |
| Na+, mmol·wk−1 | 625±15 | 669±46 |
723±28 (P<0.05 vs dapagliflozin alone) |
742±19 (P<0.05 vs dapagliflozin or bumetanide alone) |
| K+, mmol·wk−1 | 415±24 | 454±32 |
514±35 (P<0.05 vs dapagliflozin alone) |
442±28 |
| Urate, mg·wk−1 | 3382±187 | 2249±160 |
3163±211 (P<0.05 vs bumetanide alone) |
2598±160 |
| Ca++, mg·wk−1 | 597±76 | 667±54 | 646±33 | 707±40 |
| Mg++, mg·wk−1 | 548±37 | 613±48 | 611±42 | 552±52 |
Data are given as mean±SEM values for total renal excretion over 1 week of dapagliflozin, bumetanide, or combined therapy, and during the second week of combined therapy.
The calcium and magnesium excretion levels were similar during all periods (Figure S4), as was the creatinine clearance (Figure S5).
Serum values
There were no clinically significant changes in serum sodium, osmolality, or creatinine concentrations (Table 2). Serum glucose was reduced modestly by dapagliflozin or combined administration (Table 2), reflecting a sharp increase in urinary glucose excretion (Figure S1). Serum potassium was unchanged by dapagliflozin alone but was reduced 7% by bumetanide alone and 12% by the combination (Table 2), reflecting increases in renal K+ excretion (Figure S2). Serum urate was reduced 36% by dapagliflozin alone, was increased 3% by bumetanide alone, and was reduced 22% by the combination (Table 2). Urate excretion peaked quickly after dapagliflozin and combined administration, followed by a decline, but there was a sustained increase in renal urate clearance (Figure S3).
Table 2.
Serum or Plasma Values Before and After 1 or 2 Weeks of Diuretic Administration
| Parameter | Before (Day ‐1) | Dapagliflozin (Day 8) | Before (Day ‐1) | Bumetanide (Day 8) | Before (Day ‐1) | Dapagliflozin+Bumetanide (Day 8) | Dapagliflozin+Bumetanide (Day 14) |
|---|---|---|---|---|---|---|---|
| SNa, mmol·L−1 | 137.9±0.5 | 138.1±0.4 | 137.7±0.3 | 137.8±0.3 | 138.1±0.4 | 137.1±0.4* | 138.3±0.5 |
| Sosm, mOsmol·L−1 | 283.5±0.8 | 285.4±0.7* | 283.9±1.0 | 285.4±0.9 | 284.4±0.9 | 283.3±0.9 | 284.3±1.0 |
| Serum glucose, mg·dL−1 | 86.3±2.0 | 82.3±2.0† | 88.4±2.2 | 85.5±1.8* | 87.2±1.4 | 82.4±2.5* | 84.0±1.7* |
| SK, mmol·L−1 | 4.5±0.09 | 4.4±0.08 | 4.4±0.07 | 4.1±0.09* | 4.6±0.1 | 4.1±0.07‡ | 4.0±0.008‡ |
| Surate, mmol·L−1 | 5.5±0.3 | 3.5±0.2‡ | 5.9±0.3 | 6.1±0.4* | 5.4±0.4 | 4.2±0.3† | 4.3±0.3* |
| SCr, mg·dL−1 | 0.9±0.05 | 1.0±0.04‡ | 0.9±0.04 | 1.0±0.05* | 0.9±0.05 | 1.0±0.05‡ | 1.0±0.05‡ |
| Plasma renin activity, ng·mL−1·h−1 | 3.4±0.7 | 3.4±0.9 | 3.6±0.8 | 7.8±1.8* | 5.6±1.2 | 9.8±2.0 | 6.2±1.1 |
Data are given as mean±SEM values before and after 1 week of dapagliflozin alone (10 mg·d−1), or 1 week of bumetanide alone (mg·d−1), or after 1 and 2 weeks of dapagliflozin and bumetanide combined. SCr indicates serum creatinine; SK, serum potassium; SNa, serum sodium; Sosm, serum osmoles; and Surate, serum urate.
*P<0.05, † P<0.01, ‡ P<0.005 (compared with before).
Plasma renin activity
PRA was increased by 117% (P<0.05) after 1 week of bumetanide alone and remained increased after additional dapagliflozin (Table 2 and Figure S6). PRA was unchanged by dapagliflozin but was increased after additional bumetanide. PRA was not significantly changed by 1 week of combined bumetanide and dapagliflozin.
Adverse events
No deaths or serious adverse events occurred, but 81 mild adverse events were reported, which were equivalent in each group (Table S7). One subject experienced syncope on the third day of combined administration, accompanied by some orthostatic hypotension. One required oral potassium chloride to treat hypokalemia at completion.
Discussion
Dapagliflozin increased excretion of glucose and urate substantially and of fluid, osmoles, K+, and Na+ modestly in these euglycemic subjects, whereas bumetanide caused a 3‐fold larger increase in initial Na+ excretion, a similar increase in fluid and K+ excretion, but a reduction in urate excretion.25 The main new findings are that the initial Na+ excretion after bumetanide plus dapagliflozin was no greater than after bumetanide alone, indicating no first‐dose synergy. However, dapagliflozin administration for 1 week enhanced the Na+ excretion with bumetanide, and bumetanide administration for 1 week enhanced the Na+ excretion with dapagliflozin. Thus, there was significant 2‐way adaptive natriuretic synergy. This resulted in a greater Na+ excretion during the second week when both diuretics were given together than during the first week of dapagliflozin or bumetanide alone. Because the Na+ excretion over 6 hours after the first exposure to combined diuretics was no greater than after first exposure to bumetanide alone, prior diuretic administration was required to evoke this synergistic natriuretic interaction. The early increased Na+ excretion after bumetanide was followed by a sharp reduction over the next 18 hours, similar to prior reports for bumetanide25 and furosemide.14 Na+ excretion over the 6 hours after combined bumetanide and dapagliflozin was reduced after 1 week of combined administration, indicating diuretic tolerance. Thus, the adaptive synergistic responses that developed after 1 week of prior administration of the other drug were moderated by diuretic tolerance. These results translated into a greater mean daily excretion of Na+ during the second week of combined therapy than after dapagliflozin or bumetanide alone.
The modest natriuresis with dapagliflozin in euglycemic subjects is consistent with prior studies in normal subjects.2, 26 However, dapagliflozin increased glycosuria significantly, suggesting enhanced delivery of fluid and Na+ out of the proximal tubule that should have increased reabsorption in the loop of Henle by the Na+/K+/2Cl, which is load dependent. However, any increased Na+ delivery from the proximal tubule was likely modest because natriuresis with the first dose of dapagliflozin was not enhanced by blockade of Na+/K+/2Cl with bumetanide. Similarly, blockade of Na+ reabsorption in the proximal tubule by acetazolamide caused only a minor first‐dose additive increase in Na+ excretion with furosemide.27 We conclude that there is no significant additive natriuresis with dapagliflozin and bumetanide on first administration. However, there is an adaptive natriuresis over 1 week that is reminiscent of the synergy between loop and thiazide diuretics in subjects adapted to loop diuretics.13, 24 Interestingly, despite rather modest natriuresis, administration of dapagliflozin to patients with T2DM reduced the blood volume, whereas treatment with a thiazide was not effective. It is possible that the relatively better volume‐depleting effects of dapagliflozin were attributable to volume loss without increase in renin.
Thomson et al5 reported that dapagliflozin increased sodium excretion (UNaV) by 3‐ to 4‐fold in a rat model of early type 1 diabetes mellitus. However, this was dissipated during regular administration, despite a maintained reduction in proximal tubule fluid reabsorption of ≈24%5 because of an adaptive increase in NaCl reabsorption by the loop of Henle via the Na+/K+/2Cl luminal transporter. This adaptation could underlie the increased Na+ excretion with bumetanide observed in this study in subjects adapted to a week of dapagliflozin.
Volume depletion, as produced by 1 week of loop diuretics, enhances proximal Na+ reabsorption.28 An adaptive increase in proximal tubular Na+ transporters could account for the increased Na+ excretion after blockade of proximal SGLT2 by dapagliflozin after adaptation to bumetanide. PRA was doubled by 1 week of diuretics, as previously described,14, 18, 25, 29 whereas it was unchanged by dapagliflozin alone or by addition of dapagliflozin to bumetanide. This may be important because an increase in PRA and angiotensin II can increase protein excretion in CHF that enhances the activity of the collecting duct epithelial sodium channel and thereby enhances Na+ reabsorption.30 Indeed, dapagliflozin reduces proteinuria in diabetic patients and rat models,29 independent of the renin‐angiotensin system.31
Daily renal K+ excretion was greater during combined therapy than during dapagliflozin alone. Accordingly, serum potassium was reduced more when administered with bumetanide. Hypokalemia with loop diuretics has been attributed variously to flow‐mediated K+ secretion in the distal nephron32 or to effects of K+ arginine vasopressin33 or hyperaldosteronism.33, 34 The greater K+ excretion and hypokalemia with combined therapy may be a consequence of hyperaldosteronism because there were high levels of PRA. Serum potassium should be monitored during combined therapy.
Dapagliflozin enhanced urate excretion and urate clearance and reduced serum urate substantially,35, 36 whereas bumetanide alone reduced urate clearance. An enhanced proximal urate reabsorption with loop diuretics has been ascribed to volume depletion.37 Moreover, both uric acid and loop diuretics are substrates for the apical proximal tubule human sodium phosphate transporter 4 (SLC17A3)38 and the proximal multidrug resistance protein 4.39 Thus, loop diuretics might compete for the renal secretion of uric acid. Glucose reabsorption enhances urate reabsorption via the sugar‐related human uric acid transporter/channel.40 Thus, an increase in proximal tubule fluid glucose delivery with dapagliflozin may decrease proximal tubular urate transport and thereby increase urate clearance.41
There was no apparently significant pharmacokinetic interaction between dapagliflozin and bumetanide. There was a relatively high frequency of nausea, vomiting, and dizziness that may have been related to the administration of the salt tablets.
We acknowledge some limitations. First, we measured Na+ excretion in 6‐hour, 24‐hour, and 1‐week periods rather than changes from baseline because the Na+ excretion on the predosing day was low because subjects were fasted to undertake pharmacokinetic studies. More important, all 42 subjects consumed identical meals throughout the 16 days. Therefore, between‐group comparisons would not be prejudiced. We elected to undertake this initial study in a homogeneous group of normal subjects. The effects were more modest than would be anticipated in patients with T2DM who have an increased filtered load of glucose and therefore an enhanced excretion of glucose, osmoles, and Na+.42 Nevertheless, substantial adaptive changes were apparent, despite the relatively small natriuretic effect of dapagliflozin in these euglycemic subjects.
In conclusion, the combination of bumetanide and dapagliflozin was generally well tolerated, although dapagliflozin enhanced the hypokalemia with bumetanide. There was no apparent first‐dose additive natriuresis with dapagliflozin and bumetanide. However, 1 week of accommodation to dapagliflozin increased in Na+ excretion with bumetanide, likely reflecting an adaptive increase in Na+/K+/2Cl after inhibition of Na+/glucose cotransport in the upstream proximal tubule. Moreover, 1 week of accommodation to bumetanide increased Na+ excretion with dapagliflozin, likely reflecting enhanced proximal tubule Na+ reabsorption after some volume depletion after 1 week of bumetanide administration. Further studies will be required to probe the clinical significance of these interactive effects in patients with CHF and T2DM.
Perspective
An SGLT2 inhibitor given to patients with T2DM reduces cardiovascular events10 and BP,23 which have been attributed to volume loss.23 The positive natriuretic effect of bumetanide added to dapagliflozin in subjects adapted to a loop diuretic therefore may extend the volume‐depleting and antihypertensive actions of loop diuretics, which could be beneficial for patients with resistant CHF and hypertension, as shown recently for patients with nephrotic edema.43 Dapagliflozin's uricosuric action more than compensated for the reduction in urate excretion with bumetanide when given in combination. Thus, dapagliflozin should address favorably both inadequate correction of blood volume and hypertension and 2 adverse metabolic consequences of loop diuretics: hyperglycemia and hyperuricemia.
Sources of Funding
This study was funded by AstraZeneca and Bristol‐Myers Squibb. Wilcox is supported by funds from the George E. Schreiner Chair of Nephrology, the Georgetown University Hypertension Research Center, the Smith‐Kogard Family Foundation, and the Gildenhorn‐Spiesman Family Foundation.
Disclosures
Wilcox is a scientific consultant for Bristol‐Myers Squibb and Astra Zenica. He helped design and analyze results from this study and write the manuscript but received no compensation for these tasks. Boulton is an employee/shareholder of AstraZeneca. X. Liu, S. Kasichayanula, A. Bui, Leslie, and Griffen were employees/shareholders of Bristol‐Myers Squibb at the time the analysis was conducted.
Supporting information
Table S1. Baseline Characteristics of Study Subjects
Table S2. Pharmacokinetic Parameters
Table S3. Blood Pressure and Heart Rate
Table S4. Renal Excretion During First Day of Administration of Dapagliflozin and After 1 Week of Adaptation to Bumetanide
Table S5. Renal Excretion During First Day of Administration of Bumetanide and After 1 Week of Adaptation to Dapagliflozin
Table S6. Renal Excretion During First Day of Administration of Dapagliflozin+Bumetanide and After 1 Week of Adaptation to the Combined Diuretics
Table S7. Summary of Adverse Events
Figure S1. Daily glucose excretion and serum glucose concentration.
Figure S2. Daily Potassium excretion and serum potassium concentration.
Figure S3. Daily urate excretion, serum urate concentration and renal urate clearance.
Figure S4. Daily calcium and magnesium excretion.
Figure S5. Creatinine clearance.
Figure S6. Plasma retin activity.
Acknowledgments
Medical writing assistance was provided by Robert Axford‐Gatley, MD, and Jean Turner of PPSI (a PAREXEL company) and was funded by AstraZeneca. We thank Xiaoni Liu, PhD (Bristol‐Myers Squibb, Princeton, NJ), Sreeneeranj Kasichayanula, PhD (Bristol‐Myers Squibb; Current address: Amgen, Thousand Oaks, CA), and Anh Bui, BSN (Bristol‐Myers Squibb), for technical assistance with this project.
(J Am Heart Assoc. 2018;7:e007046 DOI: 10.1161/JAHA.117.007046.)29440005
References
- 1. Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, Wu G, Sher PM, Morrison EP, Biller SA, Zahler R, Deshpande PP, Pullockaran A, Hagan DL, Morgan N, Taylor JR, Obermeier MT, Humphreys WG, Khanna A, Discenza L, Robertson JG, Wang A, Han S, Wetterau JR, Janovitz EB, Flint OP, Whaley JM, Washburn WN. Discovery of dapagliflozin: a potent, selective renal sodium‐dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51:1145–1149. [DOI] [PubMed] [Google Scholar]
- 2. Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, Komoroski B, Kasichayanula S, Discenza L, Washburn W, Meng W, Ellsworth BA, Whaley JM, Humphreys WG. In vitro characterization and pharmacokinetics of dapagliflozin (BMS‐512148), a potent sodium‐glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38:405–414. [DOI] [PubMed] [Google Scholar]
- 3. Peene B, Benhalima K. Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab. 2014;5:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghezzi C, Yu AS, Hirayama BA, Kepe V, Liu J, Scafoglio C, Powell DR, Huang SC, Satyamurthy N, Barrio JR, Wright EM. Dapagliflozin binds specifically to sodium‐glucose cotransporter 2 in the proximal renal tubule. J Am Soc Nephrol. 2017;28:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brater DC. Clinical pharmacology of loop diuretics. Drugs. 1991;41:14–22. [DOI] [PubMed] [Google Scholar]
- 7. Fitchett DH, Udell JA, Inzucchi SE. Heart failure outcomes in clinical trials of glucose‐lowering agents in patients with diabetes. Eur J Heart Fail. 2017;19:43–53. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto K. Pharmacological treatment of heart failure with preserved ejection fraction. Yonago Acta Med. 2017;60:71–76. [PMC free article] [PubMed] [Google Scholar]
- 9. Hardin EA, Grodin JL. Diuretic strategies in acute decompensated heart failure. Curr Heart Fail Rep. 2017;14:127–133. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 11. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. [DOI] [PubMed] [Google Scholar]
- 12. Hoorn EJ, Wilcox CS, Ellison DH. Diuretics In: Brenner BM, Rector FC, eds. Brenner & Rector's the Kidney. 10th ed Philadelphia, PA: Elsevier; 2016:1702–1733. [Google Scholar]
- 13. Loon NR, Wilcox CS, Unwin RJ. Mechanism of impaired natriuretic response to furosemide during prolonged therapy. Kidney Int. 1989;36:682–689. [DOI] [PubMed] [Google Scholar]
- 14. Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF. Response of the kidney to furosemide, I: effects of salt intake and renal compensation. J Lab Clin Med. 1983;102:450–458. [PubMed] [Google Scholar]
- 15. Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93:1254–1259. [DOI] [PubMed] [Google Scholar]
- 16. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trullas JC, Morales‐Rull JL, Casado J, Freitas Ramirez A, Manzano L, Formiga F. Rationale and design of the “Safety and Efficacy of the Combination of Loop with Thiazide‐type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial”: a double‐blind, randomized, placebo‐controlled study to determine the effect of combined diuretic therapy (loop diuretics with thiazide‐type diuretics) among patients with decompensated heart failure. J Card Fail. 2016;22:529–536. [DOI] [PubMed] [Google Scholar]
- 18. Wilcox CS, Guzman NJ, Mitch WE, Kelly RA, Maroni BJ, Souney PF, Rayment CM, Braun L, Colucci R, Loon NR. Na+, K+, and BP homeostasis in man during furosemide: effects of prazosin and captopril. Kidney Int. 1987;31:135–141. [DOI] [PubMed] [Google Scholar]
- 19. Almeshari K, Ahlstrom NG, Capraro FE, Wilcox CS. A volume‐independent component to post‐diuretic sodium retention in man. J Am Soc Nephrol. 1993;3:1878–1883. [DOI] [PubMed] [Google Scholar]
- 20. Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2: delineation of the major renal reabsorptive mechanism for D‐glucose. J Clin Invest. 1994;93:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahman A, Kittikulsuth W, Fujisawa Y, Sufiun A, Rafiq K, Hitomi H, Nakano D, Sohara E, Uchida S, Nishiyama A. Effects of diuretics on sodium‐dependent glucose cotransporter 2 inhibitor‐induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J Hypertens. 2016;34:893–906. [DOI] [PubMed] [Google Scholar]
- 22. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oliva RV, Bakris GL. Blood pressure effects of sodium‐glucose co‐transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8:330–339. [DOI] [PubMed] [Google Scholar]
- 24. Brater DC, Pressley RH, Anderson SA. Mechanisms of the synergistic combination of metolazone and bumetanide. J Pharmacol Exp Ther. 1985;233:70–74. [PubMed] [Google Scholar]
- 25. Wilcox CS, Loon NR, Ameer B, Limacher MC. Renal and hemodynamic responses to bumetanide in hypertension: effects of nitrendipine. Kidney Int. 1989;36:719–725. [DOI] [PubMed] [Google Scholar]
- 26. Ly JP, Onay T, Sison K, Sivaskandarajah G, Sabbisetti V, Li L, Bonventre JV, Flenniken A, Paragas N, Barasch JM, Adamson SL, Osborne L, Rossant J, Schnermann J, Quaggin SE. The Sweet Pee model for Sglt2 mutation. J Am Soc Nephrol. 2011;22:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brater DC, Kaojaren S, Chennavasin P. Pharmacodynamics of the diuretic effects of aminophylline and acetazolamide alone and combined with furosemide in normal subjects. J Pharmacol Exp Ther. 1983;227:92–97. [PubMed] [Google Scholar]
- 28. Wilcox CS, Baylis C, Wingo CS. Glomerular‐tubular balance and proximal regulation In: Seldin DW, Giebisch G, eds. The Kidney: Physiology and Pathophysiology. 2nd ed New York, NY: Raven Press; 1992:1807–1841. [Google Scholar]
- 29. Shin SJ, Chung S, Kim SJ, Lee EM, Yoo YH, Kim JW, Ahn YB, Kim ES, Moon SD, Kim MJ, Ko SH. Effect of sodium‐glucose co‐transporter 2 inhibitor, dapagliflozin, on renal renin‐angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11:e0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng H, Liu X, Sharma NM, Li Y, Pliquett RU, Patel KP. Urinary proteolytic activation of renal epithelial Na+ channels in chronic heart failure. Hypertension. 2016;67:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heerspink HJ, Johnsson E, Gause‐Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin‐angiotensin blockers. Diabetes Obes Metab. 2016;18:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen D, Cornelius RJ, Sansom SC. Interacting influence of diuretics and diet on BK channel‐regulated K homeostasis. Curr Opin Pharmacol. 2014;15:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilcox CS, Mitch WE, Kelly RA, Friedman PA, Souney PF, Rayment CM, Meyer TW, Skorecki KL. Factors affecting potassium balance during frusemide administration. Clin Sci. 1984;67:195–203. [DOI] [PubMed] [Google Scholar]
- 34. Mitch WE, Wilcox CS. Disorders of body fluids, sodium and potassium in chronic renal failure. Am J Med. 1982;72:536–550. [DOI] [PubMed] [Google Scholar]
- 35. Lytvyn Y, Skrtic M, Yang GK, Yip PM, Perkins BA, Cherney DZ. Glycosuria‐mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–F83. [DOI] [PubMed] [Google Scholar]
- 36. Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125:181–189. [DOI] [PubMed] [Google Scholar]
- 37. Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14:179–188. [DOI] [PubMed] [Google Scholar]
- 38. El‐Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008;155:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. El‐Sheikh AA, van den Heuvel JJ, Krieger E, Russel FG, Koenderink JB. Functional role of arginine 375 in transmembrane helix 6 of multidrug resistance protein 4 (MRP4/ABCC4). Mol Pharmacol. 2008;74:964–971. [DOI] [PubMed] [Google Scholar]
- 40. Lipkowitz MS, Leal‐Pinto E, Cohen BE, Abramson RG. Galectin 9 is the sugar‐regulated urate transporter/channel UAT. Glycoconj J. 2004;19:491–498. [DOI] [PubMed] [Google Scholar]
- 41. Cheeseman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens. 2009;18:428–432. [DOI] [PubMed] [Google Scholar]
- 42. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519. [DOI] [PubMed] [Google Scholar]
- 43. Imai T, Akimoto T, Ito C, Masuda T, Nagata D. Management of diabetes associated with nephrotic syndrome: therapeutic potential of dapagliflozin for protracted volume retention. Drug Target Insights. 2015;9:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Study Subjects
Table S2. Pharmacokinetic Parameters
Table S3. Blood Pressure and Heart Rate
Table S4. Renal Excretion During First Day of Administration of Dapagliflozin and After 1 Week of Adaptation to Bumetanide
Table S5. Renal Excretion During First Day of Administration of Bumetanide and After 1 Week of Adaptation to Dapagliflozin
Table S6. Renal Excretion During First Day of Administration of Dapagliflozin+Bumetanide and After 1 Week of Adaptation to the Combined Diuretics
Table S7. Summary of Adverse Events
Figure S1. Daily glucose excretion and serum glucose concentration.
Figure S2. Daily Potassium excretion and serum potassium concentration.
Figure S3. Daily urate excretion, serum urate concentration and renal urate clearance.
Figure S4. Daily calcium and magnesium excretion.
Figure S5. Creatinine clearance.
Figure S6. Plasma retin activity.


