Abstract
Background
Hospitalization for cardiovascular disease (CVD) is common among patients receiving maintenance dialysis, but patterns of readmissions following cardiovascular events are underexplored.
Methods and Results
In this retrospective analysis of prevalent, Medicare‐eligible patients receiving dialysis in 2012–2013, all live‐discharge hospitalizations attributed to CVD were ascertained. Rates of all‐cause, CVD‐related, and non–CVD‐related readmissions and death in the ensuing 10 and 30 days were calculated. Multinomial logistic modeling was used to assess the relationship between potential explanatory factors and outcomes of interest. Among 142 210 analyzed hospitalizations, mean age at time of index CVD hospitalization was 64.9±14.1 years; 50.4% of index hospitalizations were for women, and 41.4% were for white patients. Fully 15.6% and 34.2% of CVD hospitalizations resulted in readmission within 10 and 30 days, respectively; less than half of readmissions were CVD related (42.5%, 10 days; 43.1%, 30 days). Death within 30 days, regardless of readmission, occurred after 4.5% of index hospitalizations; 51.2% were attributed to CVD. Compared with ages 65 to 69 years, younger age tended to be associated with increased readmission risk (adjusted relative risk for ages 18–44 years: 1.55; 95% confidence interval, 1.48–1.63). Readmission risk did not differ between white and black patients, but risk of death without readmission was markedly lower for black patients (relative risk: 0.60; 95% confidence interval, 0.55–0.67).
Conclusions
Roughly 1 in 3 CVD hospitalizations resulted in 30‐day readmission; nearly 1 in 20 was followed by death within 30 days. Risk of death without readmission was higher for white than black patients, despite no difference in risk of readmission.
Keywords: cardiovascular events, dialysis, rehospitalization
Subject Categories: Cardiovascular Disease, Epidemiology
Clinical Perspective
What Is New?
Among maintenance dialysis patients, roughly 1 in 6 cardiovascular hospitalizations results in a readmission within 10 days, about 1 in 3 in readmission within 30 days, and nearly 1 in 20 in death within 30 days; only about half of readmissions are for a cardiovascular cause.
What Are the Clinical Implications?
Despite the ready access to care that characterizes the maintenance dialysis experience, readmission for both cardiovascular and noncardiovascular causes after a cardiovascular hospitalization are extremely common; how policy, regulatory, or reimbursement changes could be leveraged to forestall readmissions should be explored.
Introduction
Cardiovascular disease (CVD) is common in patients with end‐stage renal disease (ESRD) receiving maintenance dialysis. CVD is responsible for 2 of every 5 deaths in prevalent dialysis patients and remains the leading cause of hospitalization in this population.1, 2 Although CVD‐related outcomes have improved in recent years,3 CVD in dialysis patients remains a substantial burden for patients, providers, payers, and society in general.
Appropriate use of hospital admissions, for both CVD and non‐CVD indications, is a topic of immense importance. In particular, reducing readmissions, defined as hospital admissions within 30 days of a hospital discharge, constitutes a major focus for Medicare.4, 5 As part of an effort to improve patient care, develop efficiencies in the healthcare system, and contain costs, Medicare introduced the Hospital Readmissions Reduction Program (HRRP) for fiscal year 2013,6 which established a financial framework for hospitals and providers to reduce readmissions through reduced reimbursement for hospitalizations with readmission rates deemed excessive. As a sign of the importance of CVD across the healthcare system, acute myocardial infarction and congestive heart failure (CHF) were 2 of the 3 conditions initially targeted. However, understanding that readmission rates would likely be a sustained focus of Medicare, hospitals have enacted policies and procedures designed to reduce readmissions across a broad variety of medical conditions, even those not specifically targeted at present.5
Comparatively little is known about readmission of dialysis patients following a CVD hospitalization.7, 8 Given the burden of CVD in the dialysis population and the importance of reducing readmission rates, we designed a study to examine readmission rates for dialysis patients admitted for a CVD indication. Using Medicare data, we identified hospitalizations with a primary diagnosis code for CVD and followed the patients with live discharge for the ensuing 30 days to determine rates of all‐cause readmission, readmission for a CVD indication, and death. We then sought to determine key factors associated with readmission and death. Our overarching goal was to generate hypotheses about possible actionable factors that could reduce deaths and readmissions after an index CVD hospitalization.
Methods
We performed a retrospective analysis of CVD‐related hospitalizations in maintenance hemodialysis and peritoneal dialysis patients with a discharge date between January 1, 2012, and September 30, 2013. The overall study design is shown in Figure 1. We used data from the Centers for Medicare and Medicaid Services (CMS), which collects data on virtually all patients undergoing maintenance dialysis in the United States. The CMS ESRD database consists of data from the ESRD Medical Evidence Report (form CMS‐2728), the ESRD Death Notification (form CMS‐2746), Medicare Part A institutional claims (inpatient, outpatient, skilled nursing facility [SNF], home health, hospice), and Medicare Part B physician/supplier claims (inpatient, outpatient, supplier). From the CMS files, we used information on demographics (drawn from form CMS‐2728) recorded at the time of dialysis initiation. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the analysis, although the data sources (the CMS ESRD data files) are readily available, for a cost, to researchers who enter into a data use agreement with CMS.
Figure 1.
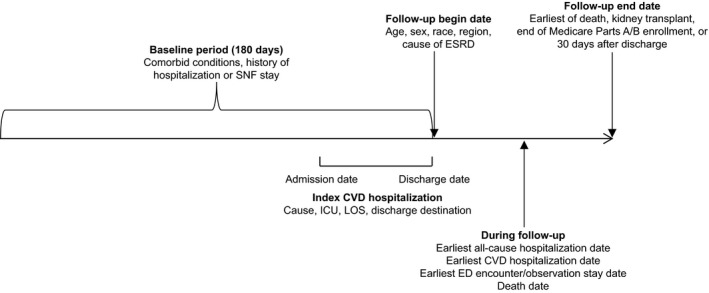
Overall study design. CVD indicates cardiovascular disease; ED, emergency department; ESRD, end‐stage renal disease; ICU, intensive care unit; LOS, length of stay; SNF, skilled nursing facility.
Hospitalizations were selected for analysis following the inclusion and exclusion requirements detailed in Figure 2. All analyzed hospitalizations occurred between the above dates, but same‐day hospitalizations (those not involving an overnight stay) were excluded. The last 3 months of 2013 (the last year for which data were available) were excluded because of the risk of incomplete data. Included hospitalizations occurred in an acute care hospital for a CVD cause (defined below). Only hospitalizations of patients on maintenance dialysis, defined as dialysis for at least 180 days before the discharge date and no history of kidney transplant, were included. The associated patients were required to have continuous Medicare Parts A and B coverage during baseline (180 days before the index hospitalization discharge date), to be aged ≥18 years at the index hospitalization discharge date, and to be discharged alive to home or an SNF.
Figure 2.
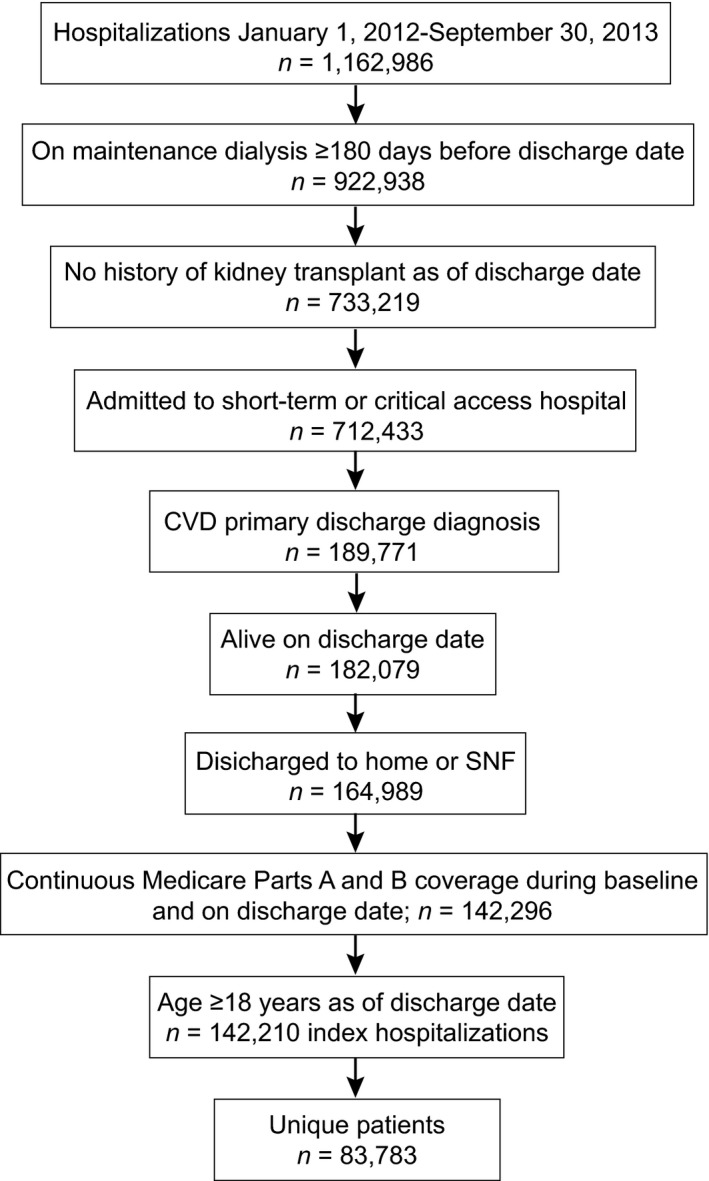
Selection of hospitalizations. CVD indicates cardiovascular disease; SNF, skilled nursing facility.
Because CMS treats every hospitalization as a new event for the purposes of reimbursement determination, patients could contribute >1 index hospitalization when >1 eligible hospitalization occurred during the study period. Follow‐up began at the date of discharge from each index hospitalization; the baseline period consisted of the 180 days before the date of discharge of each index hospitalization.
Covariates
Covariates included patient demographic characteristics (age, sex, race); dialysis duration; cause of ESRD; CMS region, defined at the discharge date of the index hospitalization (ie, the follow‐up start date); and dialysis modality (hemodialysis or peritoneal dialysis) at the discharge date of the index hospitalization. During the 180‐day baseline period, comorbid conditions, total hospitalization days, and SNF placement were assessed. To establish the most timely and complete assessment of comorbidity burden, all diagnosis codes during the baseline period (primary and secondary) were used along with secondary diagnosis codes from the index hospitalization. Because the primary diagnosis code from the index hospitalization was included as a separate covariate (cause of index hospitalization), it could not also be used to define comorbidity. To qualify as a comorbid condition, we required a relevant diagnosis code on ≥1 Part A inpatient, SNF, home health, or hospice claims or on ≥2 Part A outpatient or Part B claims.
Covariates also included characteristics of the index hospitalization, specifically primary cause (as determined by the primary diagnosis code), length of stay, requirement for an intensive care unit (ICU) for ≥1 day (yes or no, based on revenue codes for ICU care), hospitalization during the influenza season (yes or no), hospital type (short‐term or acute access hospitals), and discharge destination (home with or without home health assistance versus SNF). Determination of the influenza season is described in Data S1.
Determination of CVD
Codes used to determine reasons for CVD‐related hospitalizations are shown in Table S1. Only primary diagnoses were considered.
Outcomes
Primary outcomes were all‐cause readmission, readmission for cardiovascular indications, readmission for other causes, and death without readmission, analyzed separately within 10 or 30 days of discharge for each index hospitalization. These outcomes were defined to be mutually exclusive, for modeling purposes, so only the first event within the time period was considered. If, for example, a patient was admitted for infection and subsequently for cardiovascular indications, all within 30 days, only the first event (all‐cause readmission) was counted because that was most proximal to the index hospitalization. The first model, described below, examined all‐cause readmissions and death, in which the referent group experienced no events; the second examined CVD‐related readmissions, non–CVD‐related hospitalizations, and death, in which the referent group experienced no events. In addition to examining death without readmission, we explored, as an exploratory outcome, deaths with readmission and, separately, death regardless of readmission.
As another exploratory outcome, outpatient emergency department (ED) encounters (those not directly resulting in admission) and observation stays were quantified regardless of readmissions. ED encounters and outpatient observation stays were identified from revenue codes on outpatient claims. Any claim with any of these codes qualified as an outcome event; because some claims had codes for both, we did not study ED encounters and observation stays separately.
Statistical Analyses
Outcome events for all index CVD hospitalizations and for each subset of CVD hospitalizations (eg, acute coronary syndrome, arrhythmia, CHF [comprising heart failure, fluid overload, and cardiomyopathy], stroke, and other CVD) were calculated as percentages for 10 days and, separately, 30 days. Causes of readmissions, CVD‐ and non–CVD‐related, were also calculated as percentages. Similarly, causes of death within 30 days were calculated as percentages for all deaths and for deaths without readmission.
The principal research question was to determine factors associated with readmission following an (index) hospitalization for a cardiovascular indication. Multinomial logistic modeling was used to assess the relationship between outcomes and potential explanatory factors. To make the estimated effects of the same variable comparable between outcomes, we modeled the relative risk (RR) with “no event” as the base event (using log RR as the link function), thereby generating the probability of a given outcome relative to the probability of “no event.” The exponent of the coefficients from the model was thus the RR ratios, or the ratio of the RR for a given group to the RR for the reference group (for a categorical variable) or to the RR change with 1 U of change (for a continuous variable).
Patients could contribute multiple observations to the analysis because the unit of analysis was hospitalization. Because of the potential to induce correlation between hospitalizations for the same patient, we investigated this issue by running a cumulative logit multinomial model with and without accounting for the potential correlation and compared the parameter estimates and standard errors. Because results were virtually identical, suggesting no need to account for correlation, models were run without adjustments for correlation. Additional details regarding statistical methods appear in Data S2.
We applied to and received approval from the Human Subjects Research Committee of the Hennepin County Medical Center/Hennepin Healthcare System, Inc. The informed consent requirement was waived.
Results
We identified 142 210 hospitalizations, comprising 83 783 individual patients. Patient characteristics at the individual and hospitalization levels are shown in Table 1. At the hospitalization level, the largest age group was 65 to 69 years (14.3%; mean age: 64.9±14.1 years); 50.4% of participants were female, 41.4% were white and 39.7% black, and 95.3% were receiving hemodialysis. The largest dialysis duration group (32.5%) had been dialyzing for ≥5 years (mean dialysis duration: 4.3±3.3 years). Diabetes mellitus was the leading cause of ESRD, at slightly >50%. Comparison with patient‐level characteristics showed high similarity. Regarding numbers of contributing index CVD hospitalizations, at the patient level, 63.0% of patients had 1, 21.2% had 2, and 15.8% had ≥3.
Table 1.
Patient Characteristics by Patient and Hospitalization Levels
| Characteristics | Patients (n=83 783) | Hospitalizations (n=142 210) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Age,a y | ||||
| 18–44 | 6883 | 8.2 | 12 973 | 9.1 |
| 45–54 | 11 388 | 13.6 | 20 142 | 14.2 |
| 55–59 | 8726 | 10.4 | 15 104 | 10.6 |
| 60–64 | 10 722 | 12.8 | 18 569 | 13.1 |
| 65–69 | 12 064 | 14.4 | 20 297 | 14.3 |
| 70–74 | 11 176 | 13.3 | 18 758 | 13.2 |
| 75–79 | 9746 | 11.6 | 15 993 | 11.3 |
| 80–84 | 7420 | 8.9 | 11 752 | 8.3 |
| ≥85 | 5658 | 6.8 | 8622 | 6.1 |
| Sex | ||||
| Male | 42 538 | 50.8 | 70 544 | 49.6 |
| Female | 41 245 | 49.2 | 71 666 | 50.4 |
| Race | ||||
| White | 35 753 | 42.7 | 58 859 | 41.4 |
| Black | 32 020 | 38.2 | 56 402 | 39.7 |
| Other | 16 010 | 19.1 | 26 949 | 19.0 |
| Dialysis modalityb | ||||
| Hemodialysis | 79 092 | 94.4 | 135 406 | 95.3 |
| Peritoneal dialysis | 4662 | 5.6 | 6758 | 4.8 |
| Dialysis durationa, y | ||||
| <1 | 7799 | 9.3 | 13 281 | 9.3 |
| 1 to <2 | 13 934 | 16.6 | 24 325 | 17.1 |
| 2 to <3 | 12 427 | 14.8 | 21 659 | 15.2 |
| 3 to <5 | 21 478 | 25.6 | 36 677 | 25.8 |
| ≥5 | 28 145 | 33.6 | 46 268 | 32.5 |
| Primary cause of ESRD | ||||
| Diabetes mellitus | 42 467 | 50.7 | 72 312 | 50.9 |
| Hypertension | 25 209 | 30.1 | 43 208 | 30.4 |
| Glomerulonephritis | 5262 | 6.3 | 8840 | 6.2 |
| Other | 10 845 | 12.9 | 17 850 | 12.6 |
| CMS region | ||||
| 1: CT, MA, ME, NH, RI, VT | 2772 | 3.3 | 4480 | 3.2 |
| 2: NJ, NY, Puerto Rico, US Virgin Islands | 8201 | 9.8 | 14 514 | 10.2 |
| 3: District of Columbia, DE, MD, PA, VA, WV | 8855 | 10.6 | 15 549 | 10.9 |
| 4: AL, FL, GA, KY, MS, NC, SC, TN | 21 194 | 25.3 | 36 220 | 25.5 |
| 5: IL, IN, MI, MN, OH, WI | 14 508 | 17.3 | 25 349 | 17.8 |
| 6: AR, LA, NM, OK, TX | 12 396 | 14.8 | 20 211 | 14.2 |
| 7: IA, KS, MO, NE | 3035 | 3.6 | 4924 | 3.5 |
| 8: CO, MT, ND, SD, UT, WY | 1144 | 1.4 | 1668 | 1.2 |
| 9: American Samoa, AZ, CA, Guam, HI, Northern Mariana Islands, NV | 9832 | 11.7 | 16 331 | 11.5 |
| 10: AK, ID, OR, WA | 1846 | 2.2 | 2964 | 2.1 |
| Number of index hospitalizations | ||||
| 1 | 52 814 | 63.0 | ||
| 2 | 17 768 | 21.2 | ||
| ≥3 | 13 201 | 15.8 | ||
CMS indicates Centers for Medicare and Medicaid Services; ESRD, end‐stage renal disease.
Data from the last index hospitalization for patients with multiple index hospitalizations during the study period.
Modality data missing for 29 patients and 46 hospitalizations.
Characteristics of the index CVD hospitalizations are shown in Table 2. The single most common primary cause of index hospitalization was CHF (35.4%); the CHF category included heart failure (71.6%), fluid overload (27.9%), and cardiomyopathy (0.5%). Arrhythmia accounted for 10.5% and acute coronary syndrome for 9.2%. Mean length of stay was 5.9±4.9 days, and 5.0% of hospitalizations lasted >2 weeks. At least 1 day in an ICU was required during 41.6% of hospitalizations. Nearly one sixth (16.3%) of hospitalizations resulted in discharge to an SNF. The burden of cardiovascular comorbidity was high, as might be expected in a population selected on the basis of CVD hospitalizations, and 56.1% of hospitalizations had been preceded by a hospitalization within the previous 180 days.
Table 2.
Characteristics of Index Hospitalizations
| Characteristics | Hospitalizations (n=142 210) | |
|---|---|---|
| n | % | |
| Hospital type | ||
| Short‐term | 142 042 | 99.9 |
| Critical access | 168 | 0.1 |
| Accessed ICU during index hospitalization | ||
| No | 83 110 | 58.4 |
| Yes | 59 100 | 41.6 |
| Length of stay, d | ||
| 2–4 | 70 396 | 49.5 |
| 5–9 | 52 727 | 37.1 |
| 10–14 | 12 017 | 8.5 |
| ≥15 | 7070 | 5.0 |
| Primary cause of index hospitalization | ||
| Acute coronary syndrome | 13 018 | 9.2 |
| Arrhythmia | 14 985 | 10.5 |
| CHFa | 50 343 | 35.4 |
| Stroke | 5879 | 4.1 |
| Other cardiovascular disease | 57 985 | 40.8 |
| Discharge destination | ||
| Home, self‐care/home, home care | 119 048 | 83.7 |
| SNF | 23 162 | 16.3 |
| Index discharge during influenza season | ||
| No | 106 992 | 75.2 |
| Yes | 35 218 | 24.8 |
| Comorbid conditions 180 d before dischargeb | ||
| Arteriosclerotic heart disease | 103 923 | 73.1 |
| CHF | 108 482 | 76.3 |
| Transient ischemic attack | 38 653 | 27.2 |
| Peripheral vascular disease | 72 772 | 51.2 |
| Other cardiovascular disease | 79 246 | 55.7 |
| Chronic obstructive pulmonary disease | 60 464 | 42.5 |
| Gastrointestinal | 18 457 | 13.0 |
| Liver disease | 12 171 | 8.6 |
| Dysrhythmia | 81 131 | 57.1 |
| Cancer | 10 647 | 7.5 |
| Diabetes mellitus | 105 511 | 74.2 |
| SNF stay 180 d before dischargeb | 21 469 | 15.1 |
| History of hospitalization 180 d before dischargeb | 79 750 | 56.1 |
CHF indicates congestive heart failure; ICU, intensive care unit; SNF, skilled nursing facility.
Comprising heart failure, fluid overload, and cardiomyopathy.
Comorbidity and history of SNF stay or hospitalization were assessed during the baseline period: 180 d before discharge from the index hospitalization.
Percentages of readmissions and death within 10 or 30 days of discharge are shown in Table 3. As noted, we counted only deaths that occurred after a patient was discharged alive from the index hospitalization. Outcomes illustrated are all‐cause readmissions and the CVD subset thereof; deaths without readmission (death within 10 or 30 days not preceded by a readmission); deaths with or after a readmission; total deaths, regardless of readmission; and other nonadmission encounters (ED encounters and observation stays), regardless of readmissions or deaths. Of 34.2% of hospitalizations followed by a readmission within 30 days, nearly half (15.6% overall) occurred within only 10 days. The rates varied only modestly by specific indication for the index CVD hospitalization, ranging from 29.1% for stroke to 37.0% for acute coronary syndrome at 30 days; the pattern at 10 days was similar. Non‐CVD causes of readmission were more common than CVD causes: 43.1% and 42.5% of readmissions were for a CVD indication at 30 and 10 days, respectively. This pattern was broadly similar by type of CVD index hospitalization. By 30 days, 1.8% of index hospitalizations resulted in death without readmission; deaths regardless of readmission occurred after fully 4.5% of index hospitalizations. Death within 30 days of discharge was particularly high after index hospitalizations for acute coronary syndrome (6.4%) and stroke (6.3%). Other encounter types, regardless of the other outcomes studied, were also frequent; 24.6% of hospitalizations were followed by an ED encounter or observation stay within 30 days that did not result in readmission. We specifically examined how readmissions and deaths varied by modality (Table S2). Generally, the percentage of patients experiencing all‐cause readmission or death without readmission differed little by modality, but the percentage experiencing cardiovascular readmission was slightly higher at both 10 and 30 days for hemodialysis patients.
Table 3.
Outcome Events Following Discharge From Index Hospitalization, by Type of Index Cardiovascular Hospitalization, Within 10 or 30 Days
| Hospitalizations, n=142 210 | Events | |||||
|---|---|---|---|---|---|---|
| All‐Cause Readmission | Cardiovascular Readmission | Death, No Readmission | Death and Readmission | Deatha | ED Visit/Observation Stay | |
| 30‐d outcomes, % | ||||||
| All cardiovascular hospitalizations | 34.2 | 14.7 | 1.8 | 2.6 | 4.5 | 24.6 |
| Acute coronary syndrome | 37.0 | 16.8 | 2.6 | 3.8 | 6.4 | 24.4 |
| Arrhythmia | 31.2 | 13.3 | 1.9 | 2.9 | 4.8 | 22.9 |
| CHFb | 34.6 | 16.1 | 1.8 | 2.8 | 4.6 | 23.7 |
| Stroke | 29.1 | 9.8 | 3.0 | 3.3 | 6.3 | 23.0 |
| Other cardiovascular disease | 34.5 | 14.0 | 1.5 | 2.1 | 3.6 | 26.1 |
| 10‐d outcomes, % | ||||||
| All cardiovascular hospitalizations | 15.6 | 6.6 | 0.9 | 0.6 | 1.5 | 11.6 |
| Acute coronary syndrome | 19.0 | 8.7 | 1.3 | 1.0 | 2.3 | 12.0 |
| Arrhythmia | 15.1 | 6.6 | 0.9 | 0.6 | 1.5 | 10.8 |
| CHFb | 15.1 | 6.9 | 0.9 | 0.6 | 1.5 | 10.6 |
| Stroke | 14.5 | 4.8 | 1.8 | 0.7 | 2.4 | 11.4 |
| Other cardiovascular disease | 15.5 | 6.2 | 0.8 | 0.5 | 1.2 | 12.5 |
CHF indicates congestive heart failure; ED, emergency department.
Regardless of readmission.
Comprising heart failure, fluid overload, and cardiomyopathy.
Individual causes of CVD (43.1%) and non‐CVD (56.9%) readmission are shown in Table 4. CHF was the single most common cause of readmission, at 39.6% of all CVD readmissions; arrhythmia (9.7%) was the second‐leading cause of CVD readmissions. A constellation of cardiovascular conditions made up a group designated as other, accounting for 39.6% of CVD readmissions; the 5 most common causes in this group (septicemia, pneumonia, hyperkalemia, respiratory failure, and access‐related complications) are shown.
Table 4.
Causes of 30‐Day Readmission
| Causes of Readmission | n | % |
|---|---|---|
| All readmissionsa | 48 604 | 100.0 |
| CVD‐related readmissions | 20 960 | 43.1 |
| CHFb | 8305 | 17.1 (39.6c) |
| Other CVD | 8291 | 17.1 (39.6c) |
| Hypertensive CKD, unspecified, with CKD stage 5 or ESRD | 1877 | 3.9 (9.0c) |
| Hypertensive CKD, malignant, with CKD stage 5 or ESRD | 1262 | 2.6 (6.0c) |
| Coronary atherosclerosis of native coronary artery | 1067 | 2.2 (5.1c) |
| Hypertensive heart and CKD, unspecified, with CHF and CKD stage 5 or ESRD | 635 | 1.3 (3.0c) |
| Atherosclerosis of native arteries of extremities with gangrene | 632 | 1.2 (3.0c) |
| Arrhythmia | 2037 | 4.2 (9.7c) |
| Acute coronary syndrome | 1672 | 3.4 (8.0c) |
| Stroke | 655 | 1.3 (3.1c) |
| Other readmissions | 27 644 | 56.9 |
| Septicemia, unspecified | 1790 | 3.7 (6.5d) |
| Pneumonia, organism unspecified | 1624 | 3.3 (5.9d) |
| Hyperkalemia | 1054 | 2.2 (3.8d) |
| Acute respiratory failure | 1024 | 2.1 (3.7d) |
| Other complications due to renal dialysis device, implant, and graft | 959 | 2.0 (3.5d) |
CHF indicates congestive heart failure; CKD, chronic kidney disease; CVD, cardiovascular disease; ESRD, end‐stage renal disease.
Index hospitalizations with all‐cause readmission as first outcome event within 30 days.
Comprising heart failure, fluid overload, and cardiomyopathy.
Percentage of CVD‐related readmissions.
Percentage of non–CVD‐related readmissions.
Causes of death, overall and without readmission, are shown in Table 5. More than half were attributed to cardiac causes, whereas nearly 1 in 7 was due to dialysis withdrawal. Notably, infection accounted for <2% of deaths without readmission but >5% of total deaths, suggesting that infections resulting in death within 3 days most often occurred on readmission.
Table 5.
Cause of Death (Without Readmission) Within 30 Days of Discharge From Index Hospitalization
| Cause of Death | Death Without Readmission | Death Regardless of Readmission | ||
|---|---|---|---|---|
| n | % | n | % | |
| All | 2593 | 100.0 | 6352 | 100.0 |
| Cardiac | 1342 | 51.8 | 3251 | 51.2 |
| Withdrawal from dialysis | 410 | 15.8 | 822 | 12.9 |
| Infection | 47 | 1.8 | 349 | 5.5 |
| Liver disease/gastrointestinal | 19 | 0.7 | 101 | 1.6 |
| Vascular | 13 | 0.5 | 38 | 0.6 |
| Metabolic/endocrine | 5 | 0.2 | 22 | 0.4 |
| Other | 210 | 8.1 | 525 | 8.3 |
| Unknown | 547 | 21.1 | 1244 | 19.6 |
Variables potentially associated with all‐cause readmissions, CVD‐related readmissions, other readmissions, and death without readmission within 30 days are shown in Table 6. This table presents results from 2 multinomial models, with the following outcomes: all‐cause readmission and death without readmission, both compared with no event (first model); and CVD‐related readmission, other readmission, and death without readmission, all 3 compared with no event (second model). Compared with the referent group (age 65–69 years), younger age tended to be associated with increased RRs of all types of readmissions; for example, in patients aged 18 to 44 years, the adjusted RRs (aRRs) were 1.5 to 1.6 times higher than in the referent group. However, the effect of age diminished, such that there were no major differences in RRs of readmission in groups older than the referent group. RRs of death were substantially lower in the youngest age groups (eg, aRR ratio for ages 18–44 years: 0.76; 95% confidence interval [CI], 0.59–0.98) and substantially higher in the oldest (eg, aRR ratio for ages ≥85 years: 2.17; 95% CI, 1.86–2.55). Compared with RRs for white patients, RRs for black patients were similar for all types of readmissions but markedly lower for death without readmission (aRR ratio: 0.60; 95% CI, 0.55–0.67). Hemodialysis patients had higher risk of all‐cause readmissions (aRR ratio: 1.11; 95% CI, 1.05–1.17), other readmissions (aRR ratio: 1.27; 95% CI, 1.19–1.35), and death without readmission (aRR ratio: 1.35; 95% CI, 1.11–1.63) but lower risk of CVD‐related readmissions (aRR ratio: 0.91; 95% CI, 0.84–0.98). RRs of death without readmission were higher for patients with diabetes mellitus as the cause of ESRD (aRR ratio: 1.12; 95% CI, 1.01–1.25) than for patients with hypertension as the cause of ESRD, but RRs of readmission did not differ by cause of ESRD. There was evidence of geographic variation (see Figure S1 for reference): in region 6, for example, RRs were lower for all‐cause readmission (aRR ratio: 0.89; 95% CI, 0.85–0.94), CVD readmission (aRR ratio: 0.85; 95% CI 0.80–0.91), and other readmission (aRR ratio: 0.93; 95% CI, 0.88–0.99) compared with region 3. RRs for death were higher in region 8 (aRR ratio: 1.76; 95% CI, 1.29–2.40) and region 10 (aRR ratio: 1.44; 95% CI, 1.12–1.86) compared with region 3. Unadjusted rates of readmissions by region are shown in Table S3, demonstrating the range of differences between regions for the outcomes studied.
Table 6.
Multinomial Modeling Results: RR Ratios With 95% CIs, by Patient and Hospitalization Characteristics Associated With Each Index Hospitalization
| First Event Within 30 d | ||||
|---|---|---|---|---|
| All‐Cause Readmission | CVD‐Related Readmission | Other Readmission | Death Without Readmission | |
| Patient characteristicsa | ||||
| Age, y | ||||
| 18–44 | 1.55 (1.48–1.63) | 1.53 (1.43–1.64) | 1.58 (1.49–1.68) | 0.76 (0.59–0.98) |
| 45–54 | 1.15 (1.10–1.20) | 1.12 (1.06–1.19) | 1.17 (1.11–1.24) | 0.72 (0.60–0.87) |
| 55–59 | 1.12 (1.07–1.17) | 1.09 (1.02–1.16) | 1.14 (1.08–1.20) | 0.88 (0.73–1.06) |
| 60–64 | 1.06 (1.02–1.11) | 1.03 (0.97–1.09) | 1.09 (1.03–1.15) | 0.96 (0.81–1.12) |
| 65–69 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 70–74 | 1.04 (1.00–1.09) | 1.04 (0.98–1.10) | 1.05 (0.99–1.10) | 1.20 (1.03–1.39) |
| 75–79 | 0.98 (0.93–1.02) | 0.97 (0.91–1.03) | 0.98 (0.93–1.04) | 1.23 (1.05–1.43) |
| 80–84 | 1.02 (0.97–1.07) | 1.03 (0.96–1.10) | 1.01 (0.95–1.08) | 1.74 (1.50–2.02) |
| ≥85 | 1.02 (0.96–1.08) | 1.01 (0.94–1.09) | 1.03 (0.96–1.10) | 2.17 (1.86–2.55) |
| Male | 0.97 (0.94–0.99) | 0.97 (0.94–1.00) | 0.96 (0.94–0.99) | 1.16 (1.07–1.26) |
| Race | ||||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Black | 0.99 (0.96–1.02) | 1.02 (0.98–1.06) | 0.97 (0.93–1.00) | 0.60 (0.55–0.67) |
| Other | 0.96 (0.93–1.00) | 1.00 (0.95–1.05) | 0.94 (0.90–0.98) | 0.60 (0.53–0.69) |
| Dialysis modality | ||||
| Peritoneal dialysis | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Hemodialysis | 1.11 (1.05–1.17) | 0.91 (0.84–0.98) | 1.27 (1.19–1.35) | 1.35 (1.11–1.63) |
| Dialysis duration, y | ||||
| <1 | 0.97 (0.93–1.02) | 0.99 (0.93–1.05) | 0.96 (0.90–1.01) | 0.97 (0.82–1.13) |
| 1 to <2 | 0.98 (0.94–1.01) | 0.98 (0.93–1.04) | 0.97 (0.92–1.02) | 1.01 (0.88–1.16) |
| 2 to <3 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 3 to <5 | 0.97 (0.93–1.00) | 0.98 (0.93–1.03) | 0.96 (0.92–1.00) | 0.98 (0.86–1.11) |
| ≥5 | 0.91 (0.88–0.94) | 0.88 (0.84–0.92) | 0.93 (0.89–0.97) | 1.13 (1.00–1.28) |
| Primary cause of ESRD | ||||
| Diabetes mellitus | 0.97 (0.94–1.00) | 0.91 (0.88–0.95) | 1.02 (0.98–1.06) | 1.12 (1.01–1.25) |
| Hypertension | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Glomerulonephritis | 0.98 (0.94–1.04) | 0.95 (0.89–1.02) | 1.01 (0.95–1.08) | 0.85 (0.69–1.04) |
| Other | 1.02 (0.98–1.06) | 0.94 (0.89–0.99) | 1.09 (1.04–1.14) | 1.03 (0.90–1.18) |
| CMS region | ||||
| 1: CT, MA, ME, NH, RI, VT | 1.00 (0.93–1.07) | 1.04 (0.94–1.14) | 0.97 (0.89–1.06) | 0.81 (0.64–1.03) |
| 2: NJ, NY, Puerto Rico, US Virgin Islands | 1.01 (0.96–1.06) | 1.07 (1.00–1.14) | 0.96 (0.90–1.02) | 0.79 (0.66–0.93) |
| 3: District of Columbia, DE, MD, PA, VA, WV | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 4: AL, FL, GA, KY, MS, NC, SC, TN | 0.96 (0.92–1.00) | 0.96 (0.91–1.02) | 0.96 (0.92–1.01) | 1.08 (0.94–1.25) |
| 5: IL, IN, MI, MN, OH, WI | 1.01 (0.97–1.05) | 0.97 (0.92–1.03) | 1.04 (0.99–1.10) | 0.90 (0.78–1.05) |
| 6: AR, LA, NM, OK, TX | 0.89 (0.85–0.94) | 0.85 (0.80–0.91) | 0.93 (0.88–0.99) | 1.13 (0.96–1.33) |
| 7: IA, KS, MO, NE | 0.93 (0.87–1.00) | 0.89 (0.81–0.98) | 0.97 (0.89–1.06) | 1.13 (0.91–1.42) |
| 8: CO, MT, ND, SD, UT, WY | 0.89 (0.79–1.00) | 0.70 (0.59–0.83) | 1.04 (0.91–1.19) | 1.76 (1.29–2.40) |
| 9: American Samoa, AZ, CA, Guam, HI, Northern Mariana Islands, NV | 0.98 (0.93–1.03) | 0.93 (0.87–0.99) | 1.02 (0.96–1.09) | 0.90 (0.75–1.08) |
| 10: AK, ID, OR, WA | 0.99 (0.91–1.08) | 0.89 (0.79–1.01) | 1.07 (0.97–1.19) | 1.44 (1.12–1.86) |
| Index hospitalization characteristics | ||||
| Hospital type | ||||
| Short term | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Long term | 0.82 (0.58–1.16) | 0.84 (0.52–1.38) | 0.80 (0.52–1.23) | 1.01 (0.37–2.77) |
| Discharge destination | ||||
| Home self care/home care | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| SNF | 1.08 (1.04–1.12) | 0.82 (0.78–0.87) | 1.27 (1.22–1.33) | 2.60 (2.36–2.87) |
| Access ICU (yes vs no) | 1.02 (1.00–1.05) | 1.06 (1.03–1.10) | 0.99 (0.97–1.02) | 1.00 (0.92–1.08) |
| Length of stay, d | ||||
| 2–4 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 5–9 | 1.14 (1.11–1.16) | 1.04 (1.00–1.07) | 1.22 (1.19–1.26) | 1.40 (1.27–1.53) |
| 10–14 | 1.31 (1.26–1.37) | 1.11 (1.04–1.17) | 1.48 (1.41–1.56) | 1.86 (1.63–2.13) |
| ≥15 | 1.63 (1.54–1.72) | 1.15 (1.06–1.24) | 2.01 (1.89–2.14) | 2.50 (2.15–2.91) |
| Primary cause of hospitalization | ||||
| Acute coronary syndrome | 1.14 (1.10–1.19) | 1.16 (1.10–1.23) | 1.14 (1.08–1.20) | 1.35 (1.18–1.55) |
| Arrhythmia | 0.89 (0.85–0.92) | 0.83 (0.79–0.88) | 0.93 (0.89–0.98) | 0.90 (0.78–1.04) |
| CHFb | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Stroke | 0.86 (0.80–0.91) | 0.71 (0.64–0.78) | 0.97 (0.90–1.05) | 1.20 (1.00–1.45) |
| Other CVD | 1.04 (1.01–1.07) | 0.96 (0.93–1.00) | 1.10 (1.06–1.14) | 0.91 (0.83–1.01) |
| Index discharge during flu season (yes vs no) | 1.05 (1.02–1.08) | 1.03 (0.99–1.07) | 1.06 (1.03–1.10) | 1.16 (1.05–1.28) |
| Comorbid conditionsc | ||||
| Arteriosclerotic heart disease | 1.15 (1.12–1.19) | 1.27 (1.22–1.33) | 1.08 (1.04–1.11) | 0.99 (0.89–1.11) |
| CHF | 1.21 (1.17–1.25) | 1.43 (1.37–1.50) | 1.08 (1.04–1.12) | 1.38 (1.22–1.55) |
| Transient ischemic attack | 1.09 (1.06–1.12) | 1.11 (1.07–1.15) | 1.07 (1.04–1.11) | 1.12 (1.02–1.22) |
| Peripheral vascular disease | 1.19 (1.16–1.22) | 1.14 (1.11–1.18) | 1.22 (1.19–1.26) | 1.32 (1.21–1.44) |
| Other CVD | 1.21 (1.18–1.24) | 1.34 (1.29–1.38) | 1.12 (1.09–1.15) | 1.21 (1.11–1.32) |
| COPD | 1.21 (1.19–1.24) | 1.21 (1.17–1.25) | 1.22 (1.19–1.26) | 1.19 (1.09–1.29) |
| Gastrointestinal disease | 1.19 (1.15–1.23) | 1.11 (1.06–1.16) | 1.25 (1.20–1.30) | 1.20 (1.08–1.34) |
| Liver disease | 1.07 (1.03–1.12) | 1.00 (0.95–1.05) | 1.13 (1.08–1.19) | 1.34 (1.17–1.52) |
| Dysrhythmia | 1.18 (1.15–1.21) | 1.19 (1.15–1.23) | 1.18 (1.14–1.21) | 1.36 (1.24–1.50) |
| Cancer | 1.07 (1.03–1.12) | 0.95 (0.90–1.01) | 1.16 (1.11–1.22) | 1.29 (1.14–1.47) |
| Diabetes mellitus | 1.10 (1.06–1.13) | 1.07 (1.02–1.11) | 1.12 (1.08–1.17) | 1.01 (0.90–1.14) |
| SNF stayc | 1.13 (1.09–1.17) | 1.08 (1.03–1.13) | 1.16 (1.12–1.21) | 1.38 (1.25–1.53) |
| History of hospitalizationc | 1.54 (1.50–1.57) | 1.40 (1.35–1.45) | 1.65 (1.60–1.70) | 1.27 (1.16–1.39) |
CHF indicates congestive heart failure; CI, confidence interval; CMS, Centers for Medicare and Medicaid Services; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESRD, end‐stage renal disease; ICU, intensive care unit; RR, relative risk; SNF, skilled nursing facility.
At the time of discharge from the index hospitalization.
Comprising heart failure, fluid overload, and cardiomyopathy.
180 d before discharge.
Other factors significantly associated with outcomes were discharge destination, length of stay, and comorbid conditions. Compared with discharge to home (with or without home health care), discharge to an SNF was associated with higher risk of all‐cause readmission (aRR ratio: 1.08; 95% CI, 1.04–1.12) and death (aRR ratio: 2.60; 95% CI, 2.36–2.87). Longer lengths of stay were associated with higher aRRs of all causes of readmission and death compared with stays of ≤4 days. As might be expected, RRs for CVD readmissions generally tended to be higher for major cardiovascular comorbid conditions than for non‐CVD conditions, whereas the reverse was true for non‐CVD readmissions. Findings were virtually identical when 10‐day events were modeled (data not shown).
In addition, we performed a sensitivity analysis by selecting the first qualifying CVD hospitalization per patient within the study period. By excluding any future hospitalizations, we reduced the impact on our findings of patients with frequent readmissions. We found that results were similar (data not shown).
Discussion
In this study examining short‐term outcomes in dialysis patients admitted for CVD events, we found that more than one third of hospitalizations were followed by readmission within 30 days. Nearly half of these readmissions occurred within 10 days of discharge. In addition, other types of healthcare encounters were also frequent; nearly a quarter of hospitalizations were followed by a subsequent ED encounter or observation stay. Hospitalization for a CVD event appeared to confer substantial risk of mortality: Nearly 1 in 20 hospitalizations were followed by death within 30 days. Collectively, these findings demonstrate the extremely high morbidity and mortality associated with CVD events in patients receiving maintenance dialysis.
This study extends previous findings in the dialysis population by specifically examining readmissions after CVD events. Although the 30‐day readmission rate we report is comparable to rates in previous studies of the dialysis population examining readmissions after all‐cause8 and infectious9 index events, it is substantially higher than rates in the general population. For example, using data from a similar era, readmission rates of ≈22% were reported for CMS‐targeted conditions (2 of which, acute myocardial infarction and CHF, were included in our case definition) and about 15% for all other (nontargeted) conditions.5 This itself is unsurprising, given the high burden of both CVD‐ and non–CVD‐related comorbid conditions in dialysis patients.
Our model revealed several notable findings with regard to demographic factors such as age, race, and geography. We found that the youngest patients had a 50% increased risk of readmission compared with a much older reference group. Although the relationship between age and readmission is nuanced, relatively younger age has often been associated with increased risk of readmission generally10 and following heart failure specifically.11, 12 The reason for our finding may lie in the discharge destination, as this age effect disappeared when discharges to SNFs alone were considered (and remained when discharges only to home were modeled separately). Our data demonstrate that younger patients were much less likely to be discharged to an SNF, suggesting the possibility that younger patients who may benefit from SNF care may not be receiving it. Conceivably, a milieu of patient preferences, societal expectations, and system effects results in underuse of SNF care for younger dialysis patients, although this is merely speculative.
The 30‐day risk of death for black patients was roughly half that for white patients, despite no notable difference in risk of readmission—a finding similar to that reported by Dalrymple et al in a study of incident dialysis patients.9 Our finding of lower readmission risk for black patients is in contrast to findings from the general population, in which black, compared with white, race has been associated with increased risk of readmission both generally10 and following a heart failure hospitalization.13, 14 Reasons for this are uncertain but may relate to the controversially termed phenomenon known as reverse epidemiology,15 in which nonwhite adult patients receiving dialysis have generally more favorable outcomes than white adult patients. Further examination should be undertaken and findings leveraged to improve the care of patients of all races.
We found regional variation in both readmissions and deaths. In some regions (eg, region 6), risk of readmission was significantly lower than average, but risk of death without readmission was average; the reverse was true in other regions (eg, region 2). This suggests that readmissions and deaths do not necessarily “compete” with each other over the short term (ie, that areas with high death rates invariably have lower readmission rates, and vice versa), and that readmissions and deaths may be separate issues requiring individualized approaches to improvement. Nevertheless, because US regions invariably encompass large areas, our findings regarding geographic variation in care can be considered only hypothesis generating. More granular treatment of geography would be needed before any more definitive conclusions could be rendered or policy recommendations proposed.
Consideration of dialysis modality also revealed noteworthy findings. Risks of all‐cause readmissions and death were higher for hemodialysis than for peritoneal dialysis patients; this finding is not unexpected because the latter typically represent a highly selected group, at least in the United States. However, for hemodialysis patients, risk of CVD‐related hospitalization was lower and risk of non‐CVD readmissions was higher. This may be because infection‐related hospitalizations, which would be expected to be higher in hemodialysis patients, were competing with CVD‐related hospitalizations. Given the lack of more granular information, including current access type in the hemodialysis patients, this potential explanation can only be considered speculative.
Our findings contribute to the current debate about how best to achieve reductions in readmissions. Although such attempts are imperative in the dialysis population, they are fraught with challenges. A critical element of any readmission‐prevention strategy would likely involve timely follow‐up, given that physician visit frequency is associated with readmission risk.16 Dialysis patients already have many opportunities to interface with the healthcare system. Hemodialysis patients, for example, typically have direct thrice‐weekly contact with medical personnel in their outpatient dialysis units, whereas peritoneal dialysis patients generally have ready access to specialized nurses in peritoneal dialysis clinics. However, even this high degree of contact seems insufficient, suggesting need for more intense paradigms of follow‐up care. A potential follow‐up mechanism might involve dedicated discharge coordinators stationed in outpatient dialysis units. These coordinators could shepherd the transition from the inpatient to the outpatient environment by facilitating communication between inpatient physicians (typically cardiologists and nephrologists) and outpatient dialysis providers, by rendering additional scrutiny of at‐risk patients (eg, calling the patient at home on nondialysis days), and by performing dedicated tasks such as medication reconciliation. An even more intense level of scrutiny might use dedicated healthcare providers who round on recently discharged patients in the dialysis unit. Chair‐side encounters immediately following a discharge might permit the inpatient treating team's recommendations, such as increasing the ultrafiltration goals in a patient admitted for heart failure, to be implemented more effectively. Although these interactions would require a higher degree of flexibility and responsiveness than is typical in outpatient dialysis units, their effect could readily be tested in a pragmatic clinical trial with patients randomized to intense follow‐up versus standard of care.
Policy, regulatory, or reimbursement changes could, in theory, also be leveraged to decrease readmission rates. Such efforts are already under way at the level of the hospital,5 but including dialysis units has been proposed. Nevertheless, incentivizing optimal care while avoiding unintended consequences presents a major challenge. An extensive discussion of the potential pitfalls of the dialysis readmission metric proposed by CMS for inclusion in the ESRD Quality Improvement Program was recently published.17 In addition to technical arguments about lack of demonstrated reliability and validity of the proposed measure—a serious concern about a measure used to impose financial penalties on dialysis units with high readmission rates—many objections with strong intuitive clinical appeal were raised. For example, the original version of the metric did not account for sociodemographic factors, a flaw that might provide a disincentive for dialysis units to accept complex patients with social or economic risk factors. Readmissions for vascular access were included in the metric, which could, perversely, provide a disincentive to optimize dialysis access performance. Perhaps the most philosophical objection is that dialysis itself may be a much more modest determinant of readmission risk than the discharging hospital and treating physicians, suggesting that the financial risks associated with readmissions should be shared among many stakeholders.17 Finally, any metric that tracks only hospital readmissions may substantially underestimate the intensity of interaction with the healthcare system and the burden of acute disease patients’ experience, as shown by high rates of ED encounters and observation stays in the present study and in others.8
In alignment with previous work,9 we found that more than half of readmissions were for non‐CVD indications, most commonly related to infection (septicemia and pneumonia), hyperkalemia, respiratory failure, and dialysis access. Non–CVD‐related hospitalizations are likely tied to underlying comorbidity or to posthospitalization syndrome, a well‐described phenomenon in the general population.18 This suggests that when following up recently discharged patients, nephrologists and other care providers would ideally remain vigilant about overall health status rather than focusing solely on the reason for the recent hospitalization. Even so, the high rate of “discordant” readmissions (ie, readmissions apparently medically unrelated to the initial hospitalization) calls into question whether it is realistic to anticipate and forestall them.17, 19
A strength of our study is the analytic design. Studying incident patients, as has been done by others,9 yields particular insights regarding the effects of dialysis initiation on hospitalization and death. However, our approach was designed to specifically address the main aims of the HRRP, as least as we interpreted them. We studied prevalent patients because the HRRP judges hospitals for readmission rates among “all comers,” regardless of how long they were covered by Medicare or had a given medical condition, and because the HRRP appears to treat all readmissions similarly, with no distinction between patients with a single readmission or with many. To closely mimic clinical reality, we permitted patients to contribute >1 index hospitalization to the analysis but undertook careful preliminary analyses to determine that interdependence of observations would not have a measurable effect on our modeled results. Finally, we focused on CVD hospitalizations because 2 of 3 initially targeted conditions under the HRRP are cardiovascular in nature, suggesting that these are worthy of particular scrutiny.
Our study also has several important limitations. First, we studied only Medicare‐insured individuals, but this is likely only a minor weakness because >80% of US dialysis patients are insured by Medicare.20 Second, our results may not be generalizable to non‐US dialysis populations, given the numerous differences between US and non‐US populations. Third, we elected to study all CVD hospitalization among prevalent patients, meaning that our results are not directly comparable to other study designs in which only incident patients or first hospitalizations are studied. Despite these differences, our findings are generally concordant with those of other investigators. In addition, we relied on Medicare claims rather than on granular data from the electronic health record; because the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) taxonomy does not always map neatly to clinical entities, as clinicians understand them, some degree of misclassification of hospitalization causes almost certainly occurred. With this acknowledgement, claims‐based analyses are widely used and permit large cohorts to be assembled, increasing statistical power and overall generalizability; in our study, such an analysis is particularly appropriate because CMS assesses whether outcomes are achieved through use of claims submitted for reimbursement. Finally, because ICU use was unintutively high, we are uncertain whether ICU stays represent true critical care hospitalizations in a clinical sense. Because conditions for ICU care likely vary substantially by hospital, ICU claims can be considered only a crude potential marker of illness severity.
In summary, in patients receiving maintenance dialysis, we found high rates of hospital readmissions, as well as use of ED encounters and observation stays, in the 30 days following hospitalization for a cardiovascular event. Nearly half of readmissions occurred within only 10 days of discharge. Furthermore, 30‐day death rates were high, with more than half of deaths within 30 days occurring on or after a readmission. Reasons for readmissions were roughly evenly split between CVD and non–CVD related. Our findings, coupled with others, challenge the nephrology community to investigate ways to most effectively render care to patients recently discharged after a CVD event and to debate the role of quality‐of‐care metrics, such as those intended to minimize lengths of stay and readmission rates, in the care of dialysis patients.
Sources of Funding
This study was funded by the Peer Kidney Care Initiative. Financial support for the Peer Kidney Care Initiative is provided by 12 participating dialysis provider organizations: American Renal Associates, Atlantic Dialysis Management Services, Centers for Dialysis Care, DaVita HealthCare Partners, Dialysis Clinic, Inc. (DCI), Fresenius Medical Care, Independent Dialysis Foundation, Northwest Kidney Centers, Satellite Healthcare, the Rogosin Institute, US Renal Care, and Wake Forest‐Emory Universities. The funders had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Disclosures
Wetmore is on the Speakers’ Bureau for OPKO Renal. Herzog has provided consultation to AbbVie, Amgen, DaVita Clinical Research, GlaxoSmithKline, FibroGen, KBP Biosciences, Matinas BioPharma, Relypsa(Vifor), Sanifit, and ZS Pharma; owns equity interest in Boston Scientific; has received honoraria from UpToDate; and has provided research support to Amgen, Peer KCI, and Zoll. Collins is employed by NxStage, with stock options, and has provided consultation to NxStage, Amgen, Hospira, Relypsa, Bayer, and ZS Pharma. Gilbertson has provided statistical consultation to DaVita Clinical Research. The remaining authors have no disclosures to report.
Supporting information
Data S1. Details regarding determination of influenza season.
Data S2. Supplemental statistical methods.
Table S1. Codes Used to Determine Reasons for Cardiovascular Disease–Related Admissions
Table S2. Outcome Events Following Discharge From Index Hospitalization, Within 10 or 30 Days, by Dialysis Modality
Table S3. Outcome Events Following Discharge From Index Hospitalization, by Region, Within 10 or 30 Days
Figure S1. Map of Centers for Medicare and Medicaid Services regions.
Acknowledgments
The authors thank Chronic Disease Research Group colleagues Kimberly Nieman and Beth Forrest for overall project management of the Peer Kidney Care Initiative, Anne Shaw for article preparation, and Nan Booth, MSW, MPH, ESL, for article editing.
(J Am Heart Assoc. 2018;7:e007231 DOI: 10.1161/JAHA.117.007231.)29440035
References
- 1. Peer Kidney Care Initiative . Peer Report: Dialysis Care and Outcomes in the United States. 2016 ed Minneapolis, MN: Chronic Disease Research Group; 2016. Available at: http://www.peerkidney.org/download-the-peer-report/. Accessed August 22, 2017. [Google Scholar]
- 2. United States Renal Data System . 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2016 ed Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 3. Wetmore JB, Liu J, Li S, Hu Y, Peng Y, Gilbertson DT, Collins AJ. The Healthy People 2020 Objectives for Kidney Disease: how far have we come, and where do we need to go? Clin J Am Soc Nephrol. 2017;12:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 5. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374:1543–1551. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services . Readmissions Reduction Program (HRPP). 2012. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed August 22, 2017.
- 7. Perkins RM, Rahman A, Bucaloiu ID, Norfolk E, DiFilippo W, Hartle JE, Kirchner HL. Readmission after hospitalization for heart failure among patients with chronic kidney disease: a prediction model. Clin Nephrol. 2013;80:433–440. [DOI] [PubMed] [Google Scholar]
- 8. Harel Z, Wald R, McArthur E, Chertow GM, Harel S, Gruneir A, Fischer HD, Garg AX, Perl J, Nash DM, Silver S, Bell CM. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalrymple LS, Mu Y, Romano PS, Nguyen DV, Chertow GM, Delgado C, Grimes B, Kaysen GA, Johansen KL. Outcomes of infection‐related hospitalization in Medicare beneficiaries receiving in‐center hemodialysis. Am J Kidney Dis. 2015;65:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strom JB, Kramer DB, Wang Y, Shen C, Wasfy JH, Landon BE, Wilker EH, Yeh RW. Short‐term rehospitalization across the spectrum of age and insurance types in the United States. PLoS One. 2017;12:e0180767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dharmarajan K, Hsieh A, Dreyer RP, Welsh J, Qin L, Krumholz HM. Relationship between age and trajectories of rehospitalization risk in older adults. J Am Geriatr Soc. 2017;65:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arora S, Patel P, Lahewala S, Patel N, Patel NJ, Thakore K, Amin A, Tripathi B, Kumar V, Shah H, Shah M, Panaich S, Deshmukh A, Badheka A, Gidwani U, Gopalan R. Etiologies, trends, and predictors of 30‐day readmission in patients with heart failure. Am J Cardiol. 2017;119:760–769. [DOI] [PubMed] [Google Scholar]
- 13. Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. 2016;4:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziaeian B, Heidenreich PA, Xu H, DeVore AD, Matsouaka RA, Hernandez AF, Bhatt DL, Yancy CW, Fonarow GC. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2017;5:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalantar‐Zadeh K. What is so bad about reverse epidemiology anyway? Semin Dial. 2007;20:593–601. [DOI] [PubMed] [Google Scholar]
- 16. Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J. Physician visits and 30‐day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25:2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fishbane S, Wish JB. Quality measurement in wonderland: the curious case of a dialysis readmissions measure. Clin J Am Soc Nephrol. 2016;11:190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krumholz HM. Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wish JB. The role of 30‐day readmission as a measure of quality. Clin J Am Soc Nephrol. 2014;9:440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. United States Government Accountability Office (GAO) . Report to the Subcommittee on Health, Committee on Ways and Means, House of Representatives. End‐stage renal disease: Medicare payment refinements could promote increased use of home dialysis. GAO‐16‐125. October 2015. Available at: http://www.gao.gov/assets/680/673293.pdf. Accessed August 22, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Details regarding determination of influenza season.
Data S2. Supplemental statistical methods.
Table S1. Codes Used to Determine Reasons for Cardiovascular Disease–Related Admissions
Table S2. Outcome Events Following Discharge From Index Hospitalization, Within 10 or 30 Days, by Dialysis Modality
Table S3. Outcome Events Following Discharge From Index Hospitalization, by Region, Within 10 or 30 Days
Figure S1. Map of Centers for Medicare and Medicaid Services regions.


